Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin †
Abstract
:1. Introduction
2. Experimental Section
Materials and Methods
Materials
Methods
| Polymer | Nanoparticle yield (%) | Lutein loading content (%) | Entrapment efficacy (%) | Particle mean size (%) |
|---|---|---|---|---|
| Chitosan-HA-Lutein | 33 ± 9 | 10 ± 3 | 32 ± 5 | 458 ± 14 |
| Amorphous Chitin-HA- Lutein | 31 ± 10 | 18 ± 3 | 40 ± 5 | 355 ± 13 |
| Crystal-Chitin HA (CN) Lutein | 42 ± 9 | 35 ± 3 | 66 ± 6 | 185 ± 13 |
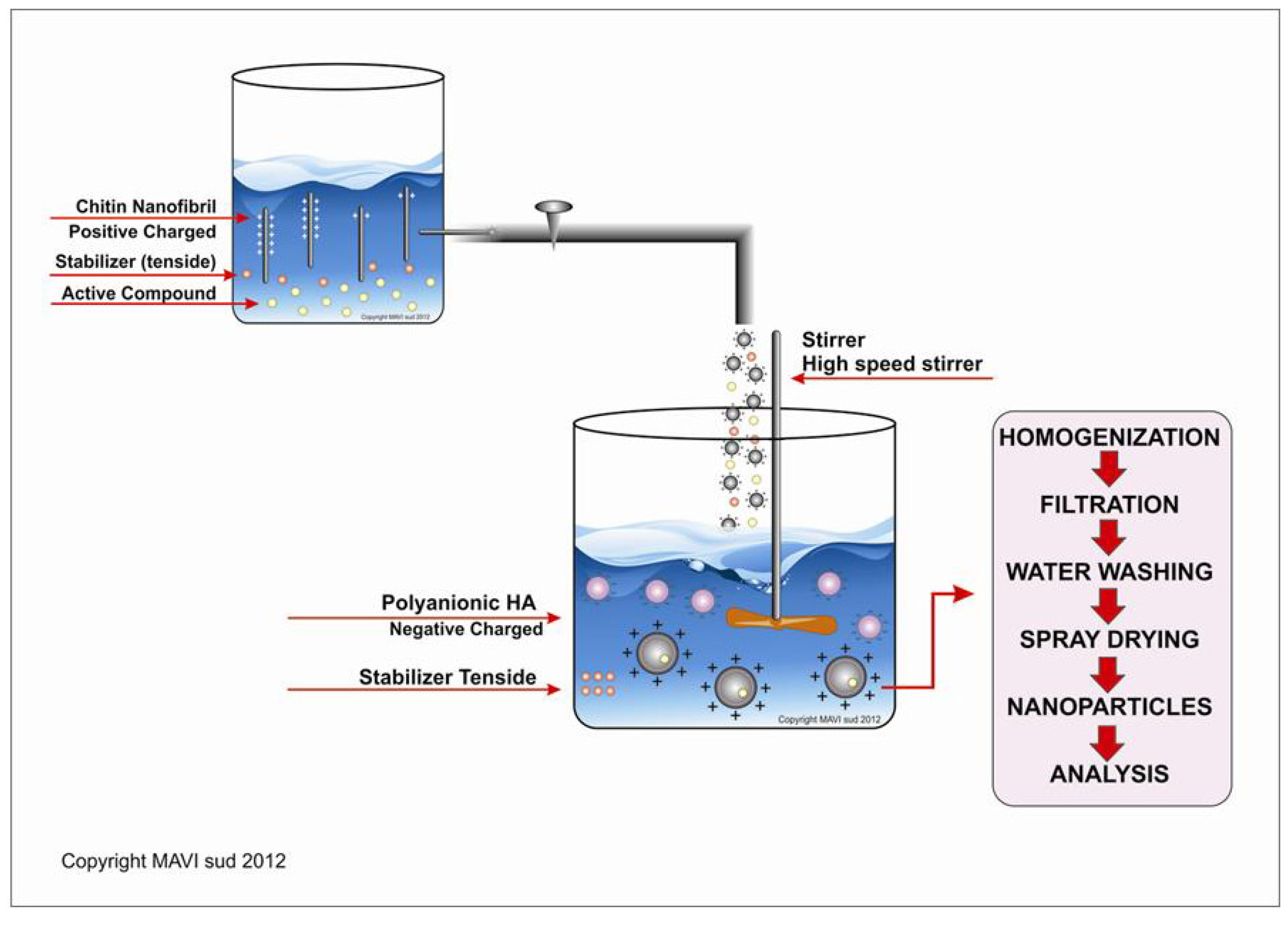
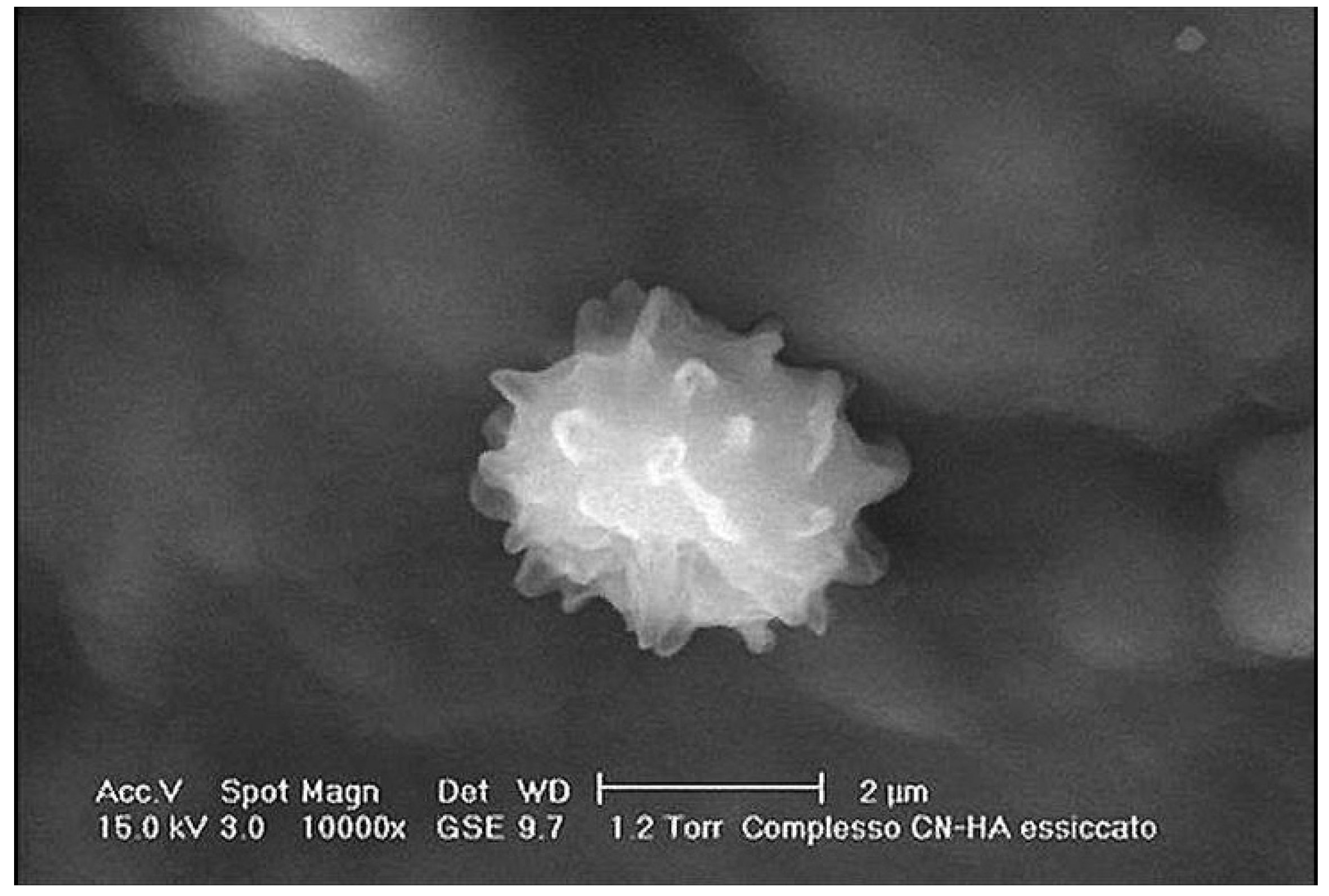
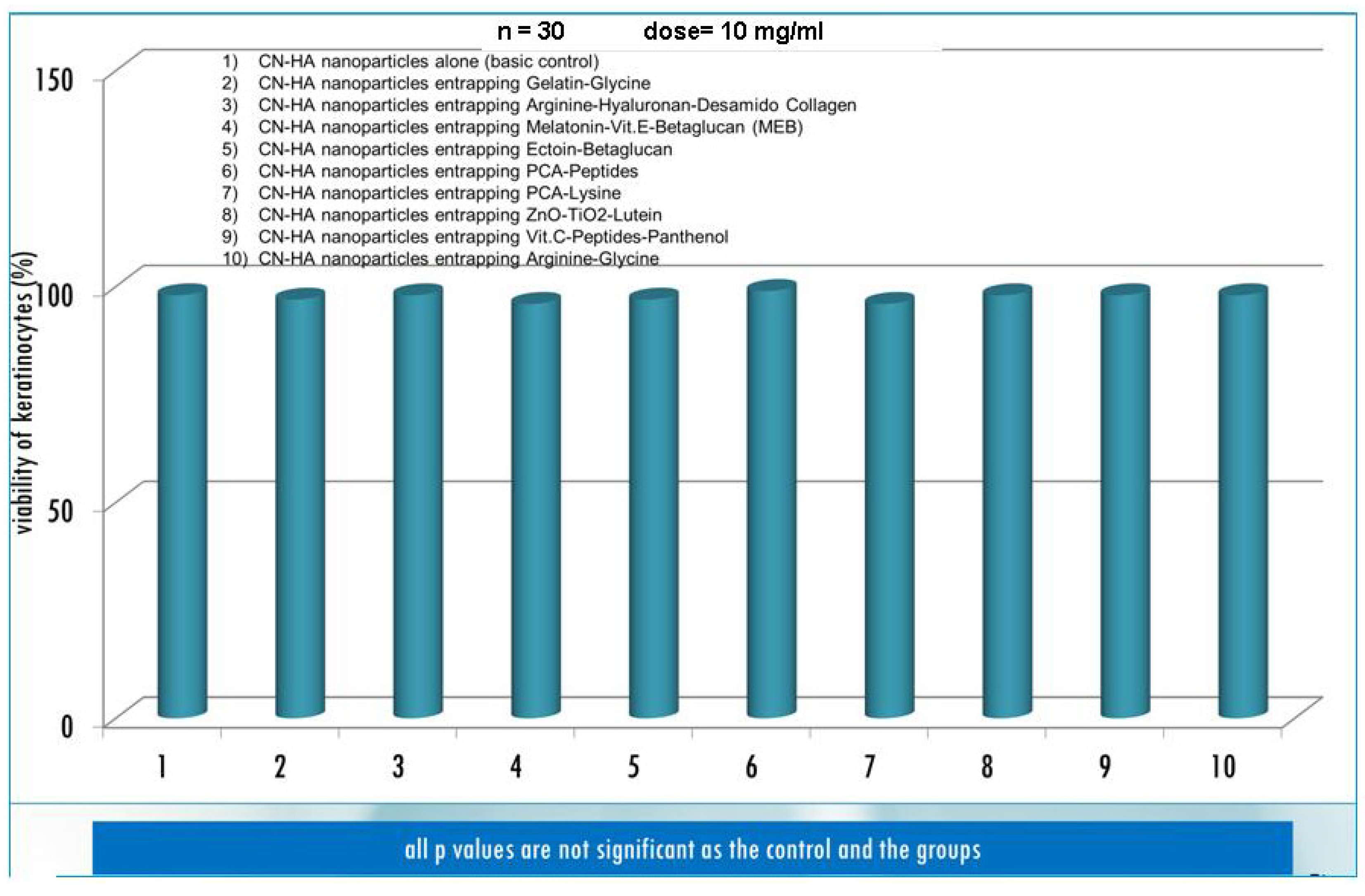
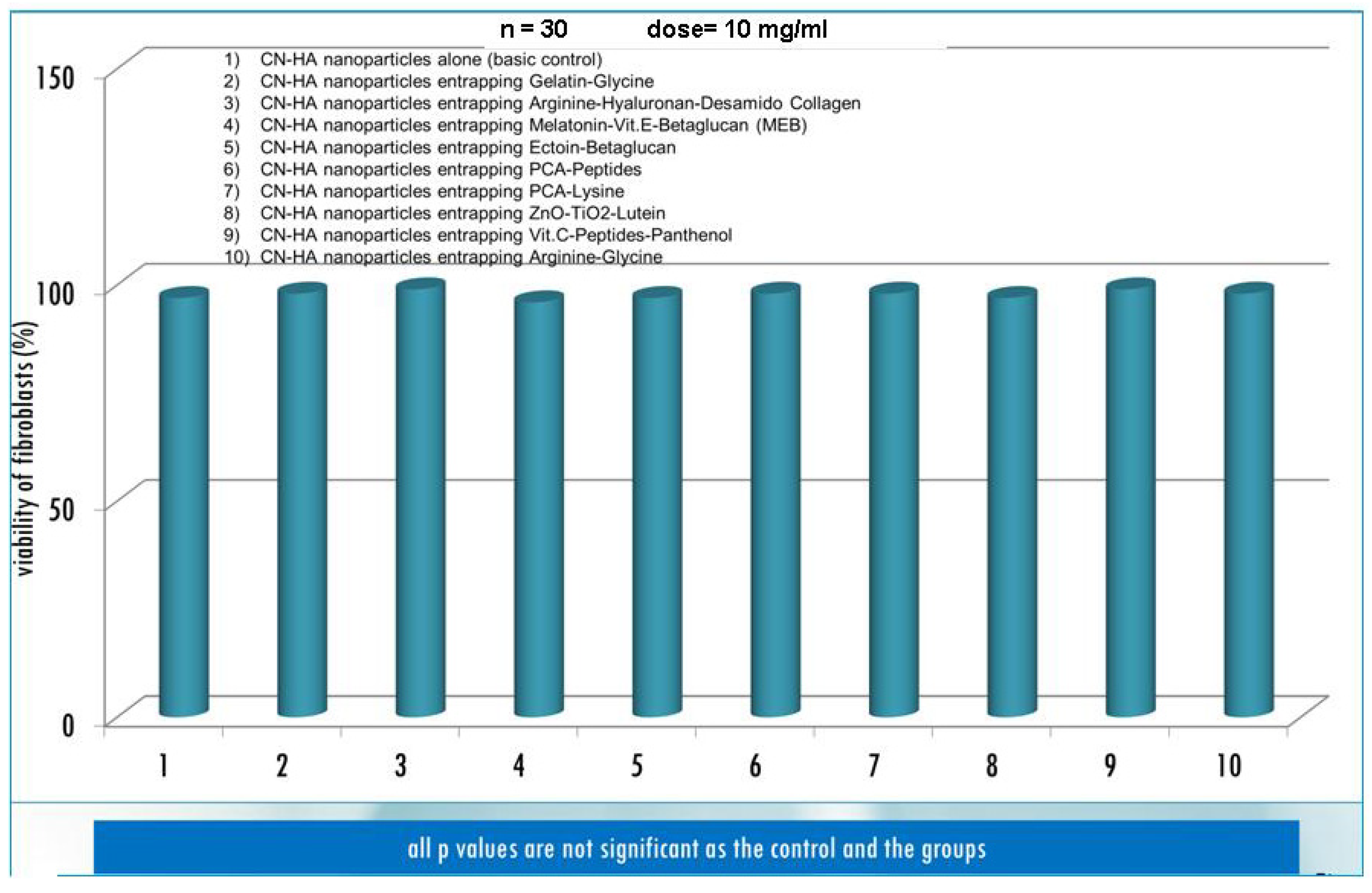
3. Results and Discussion
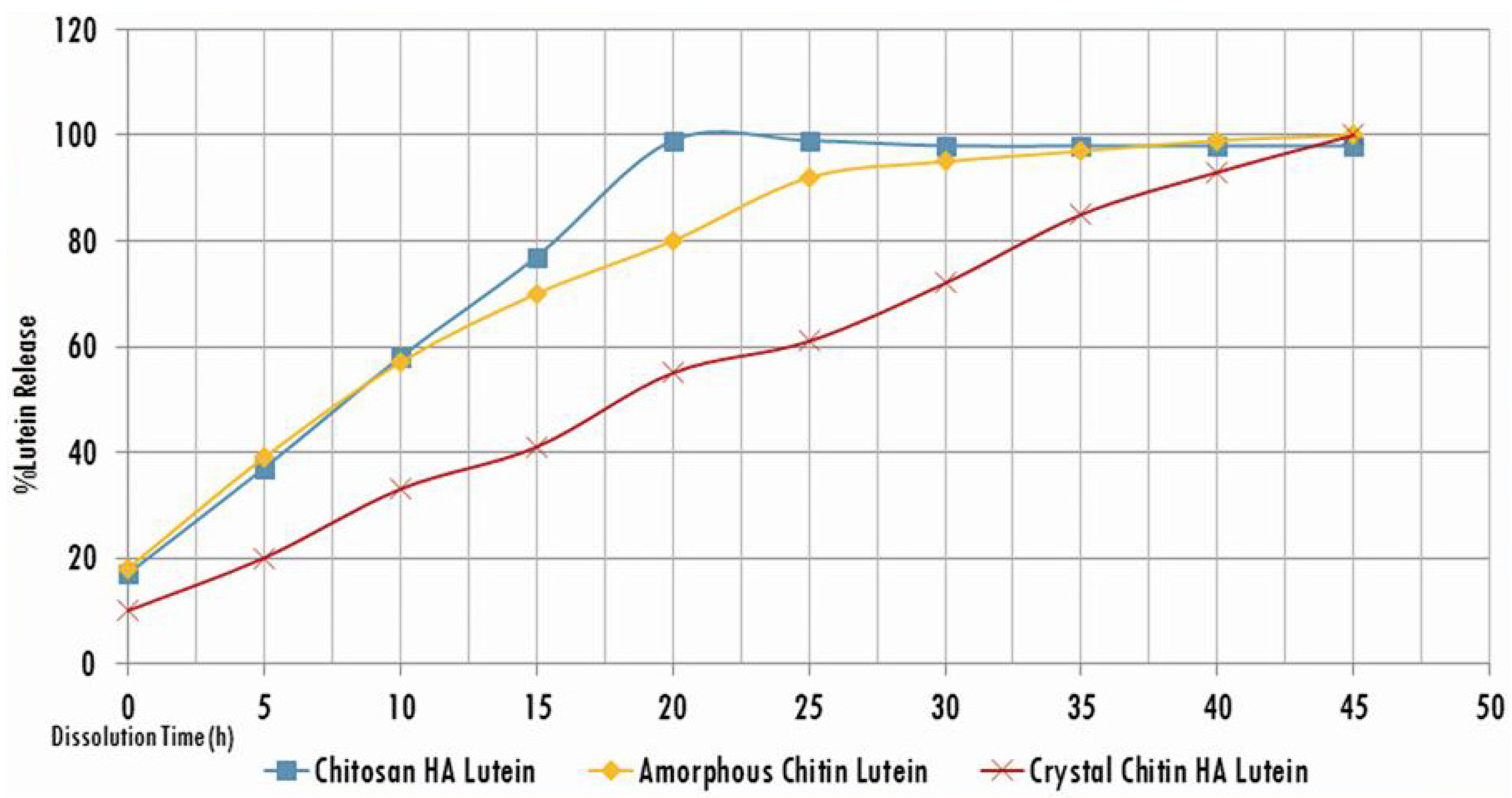
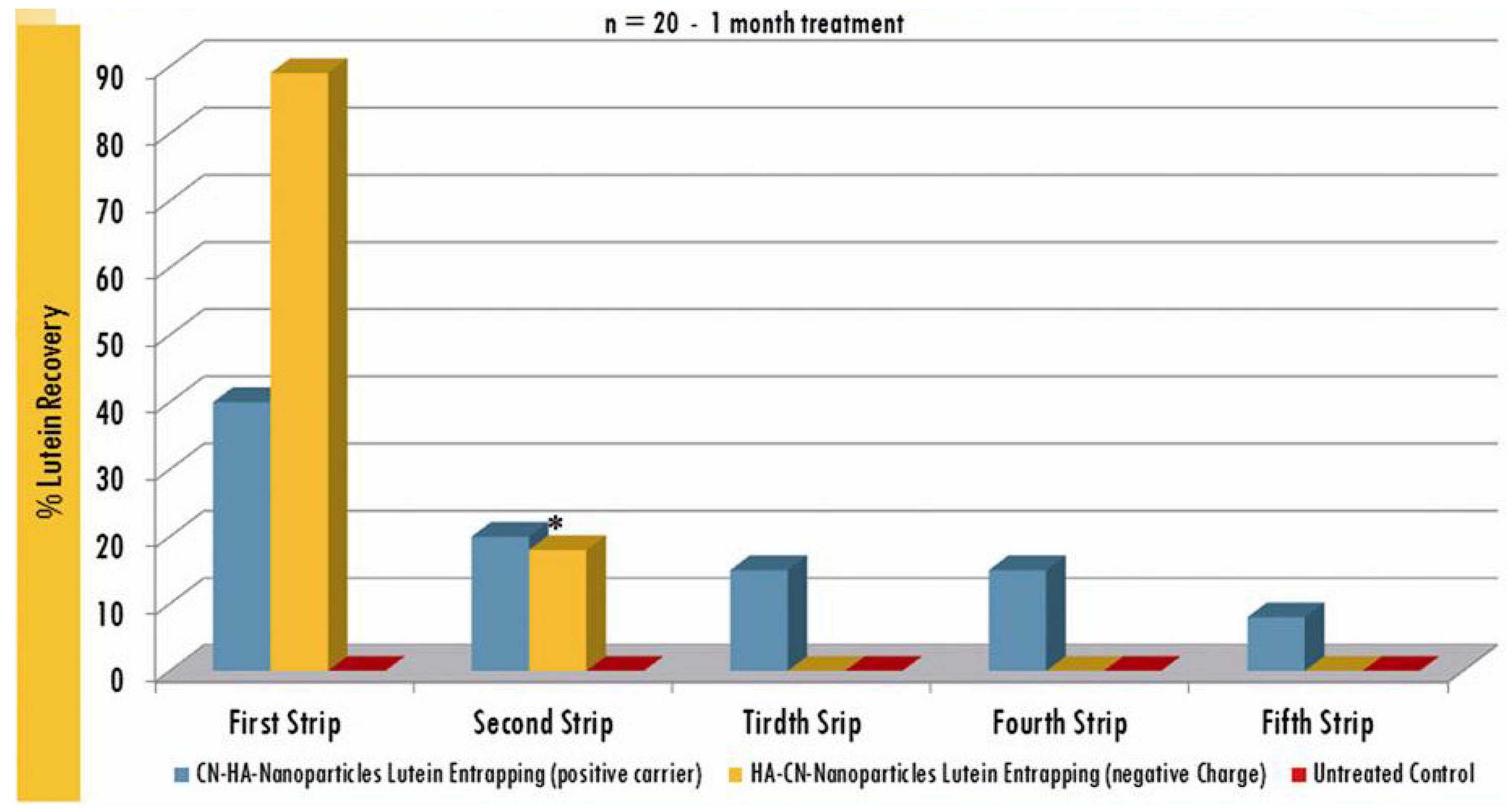
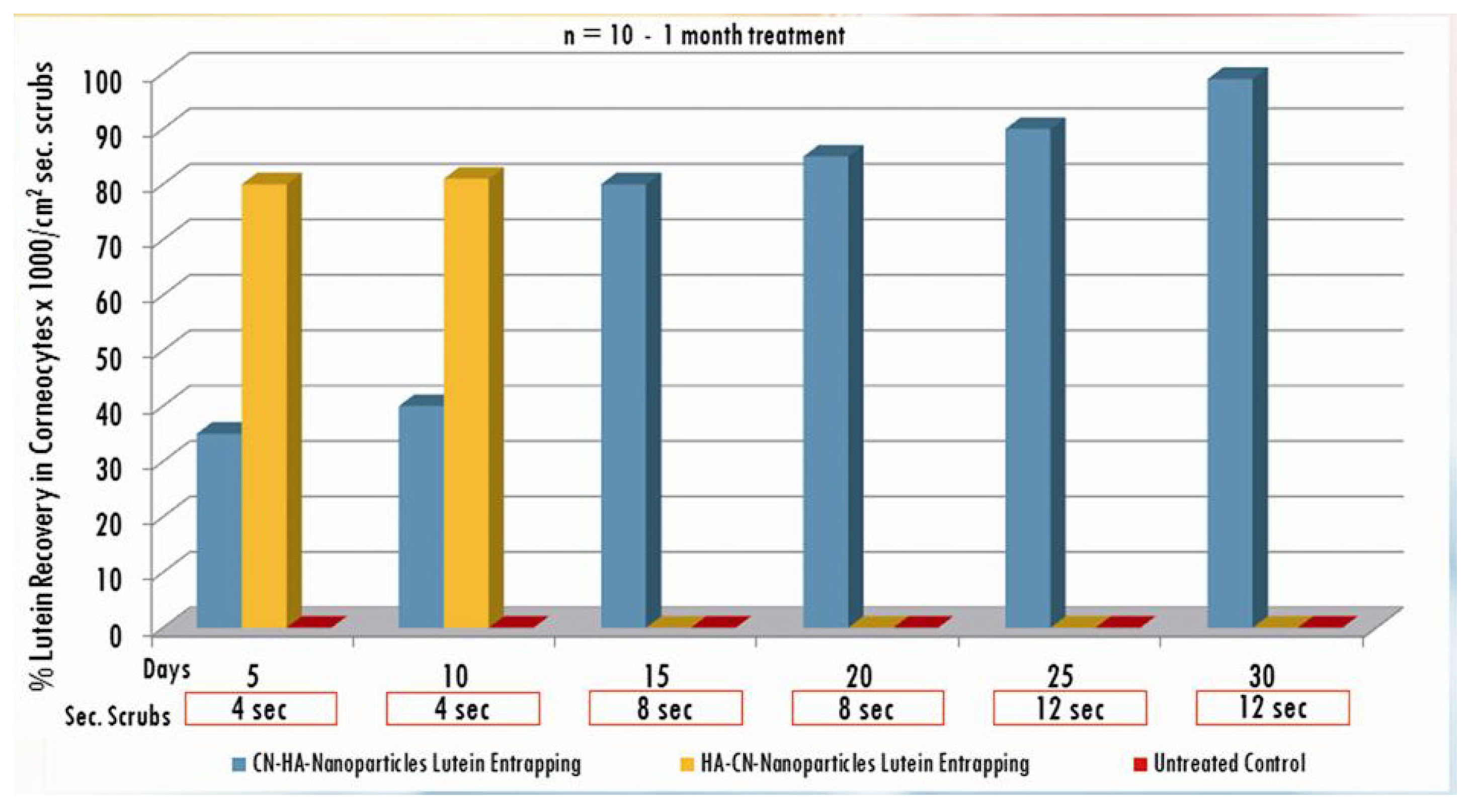
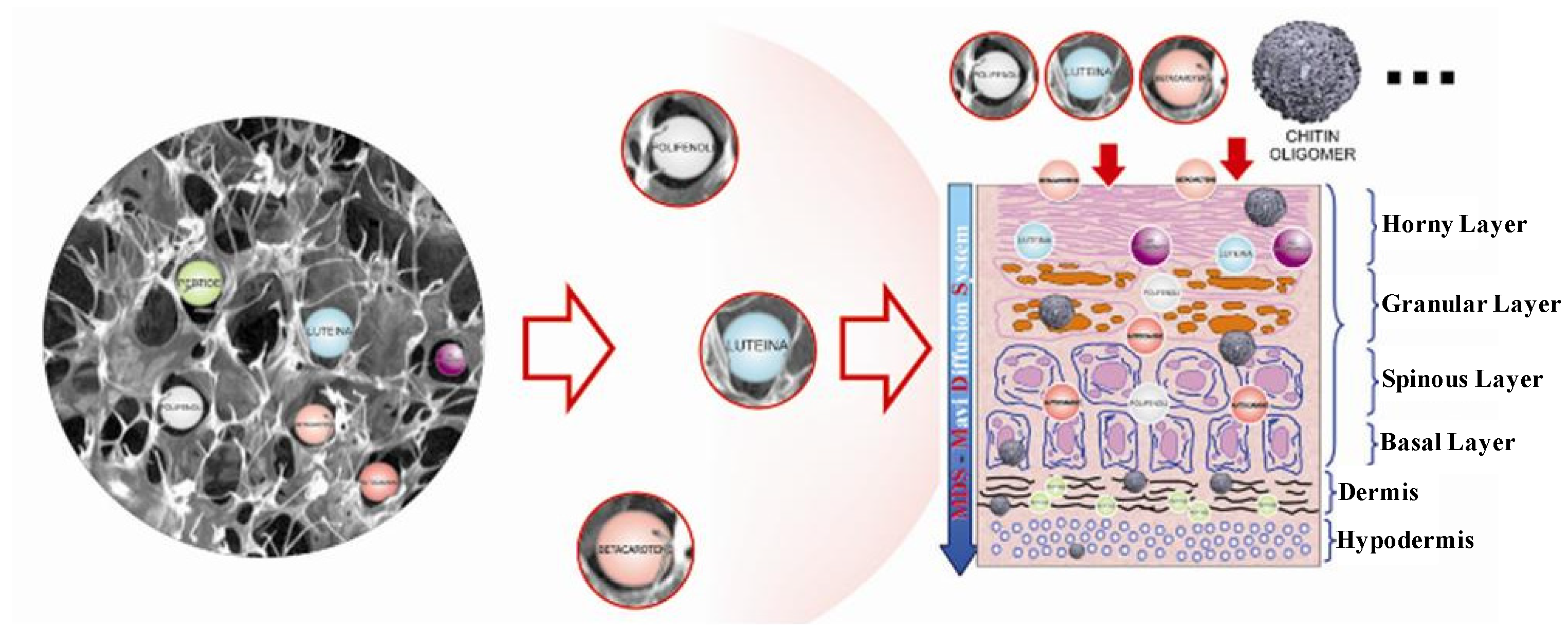
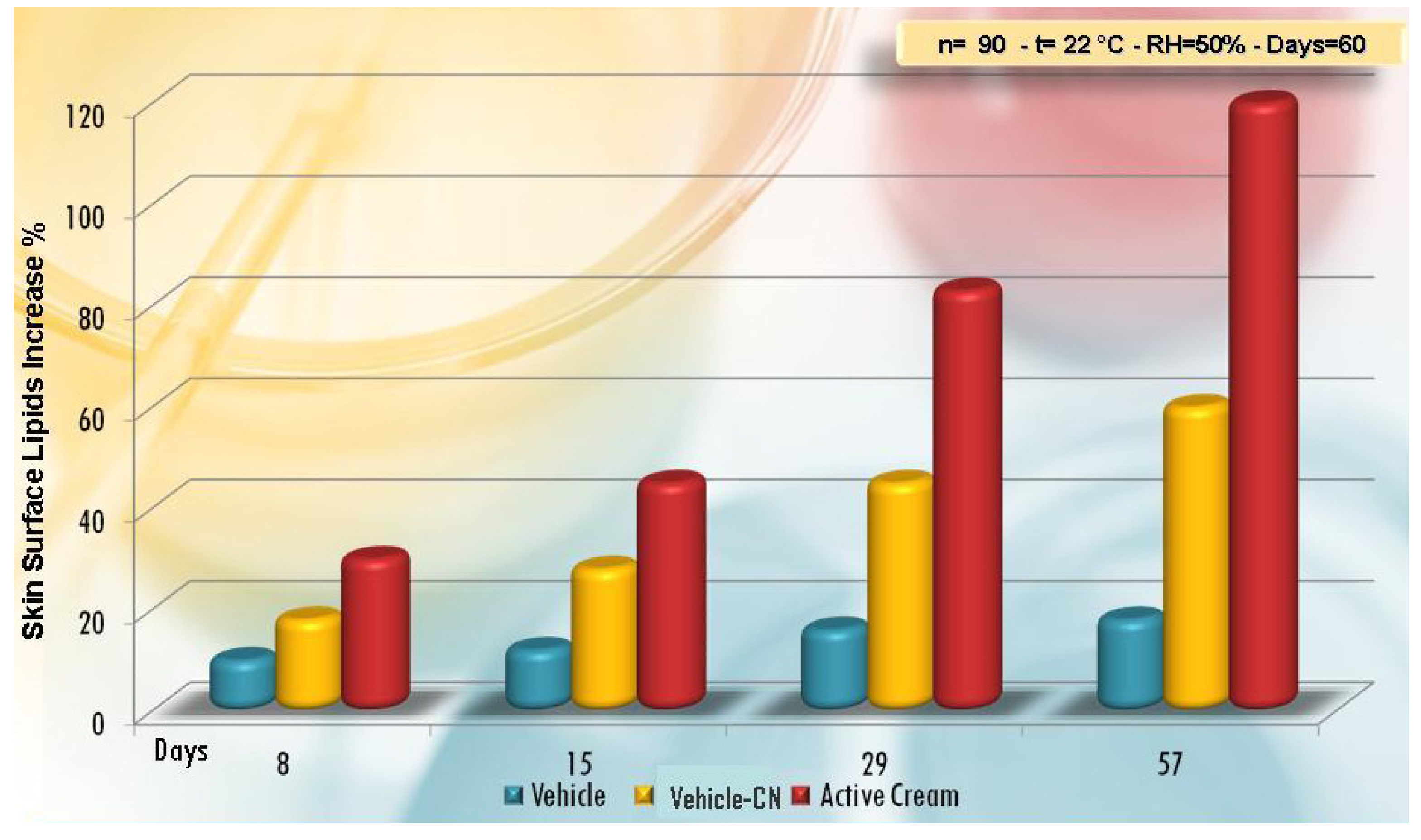
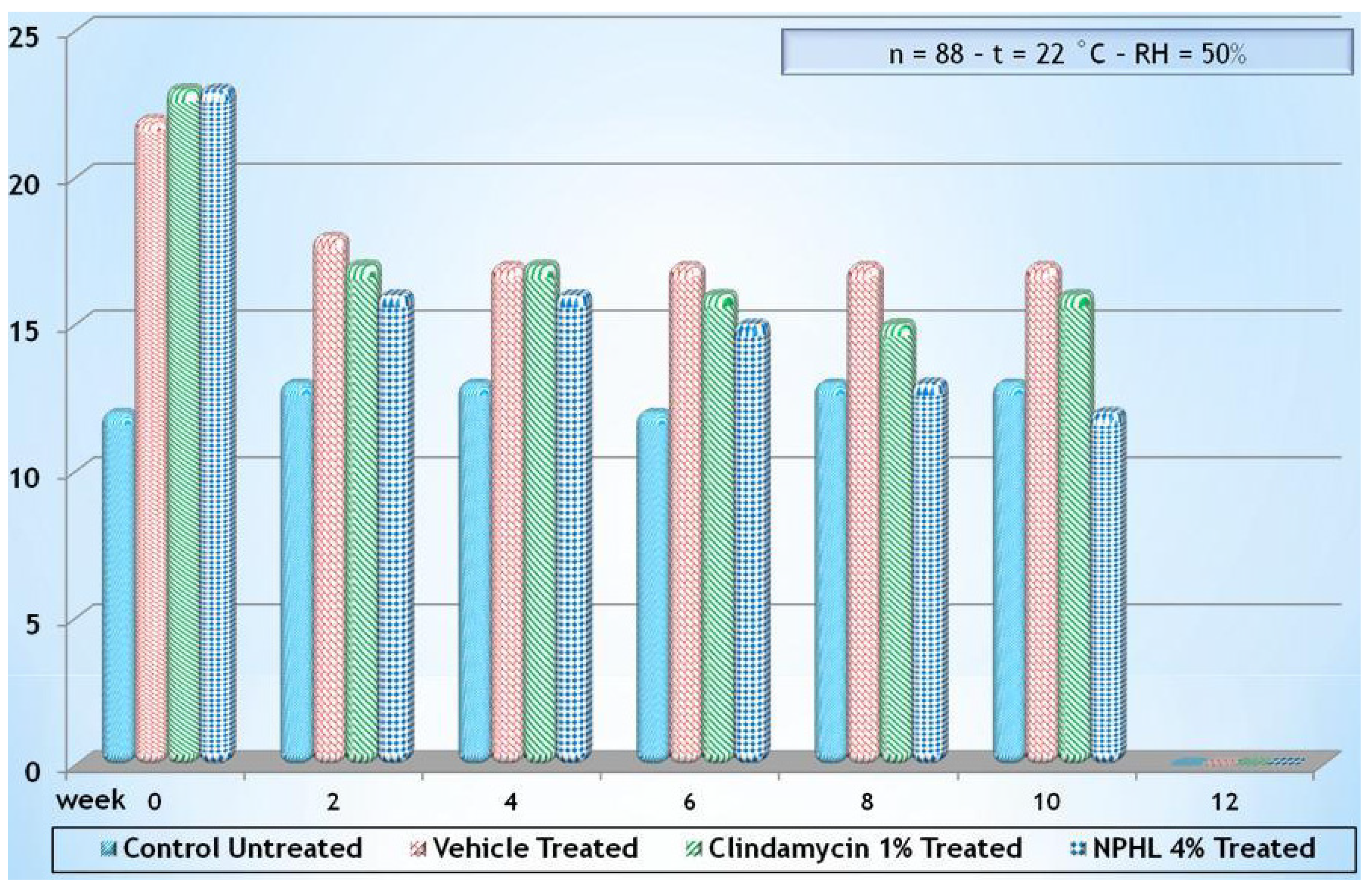
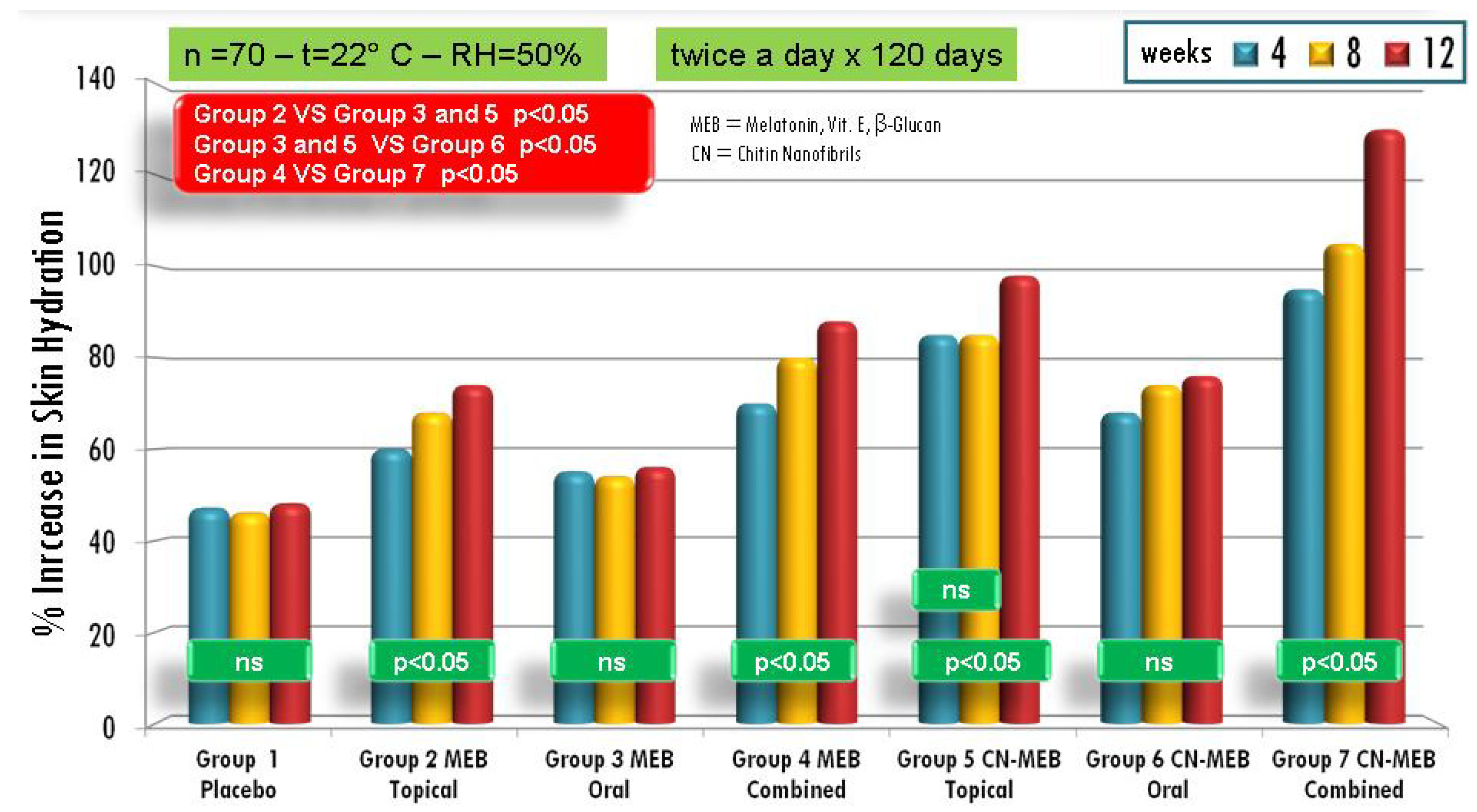
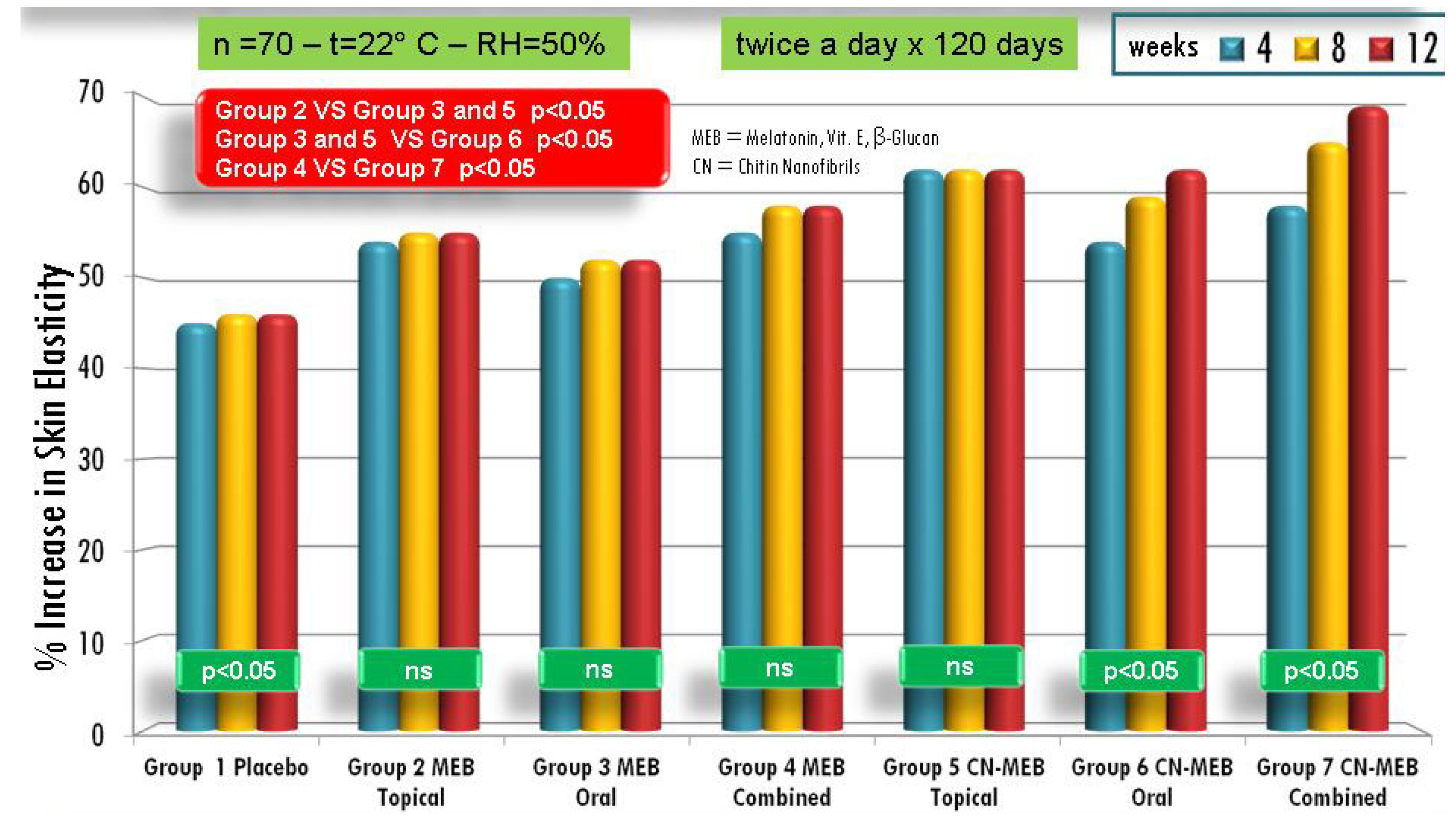
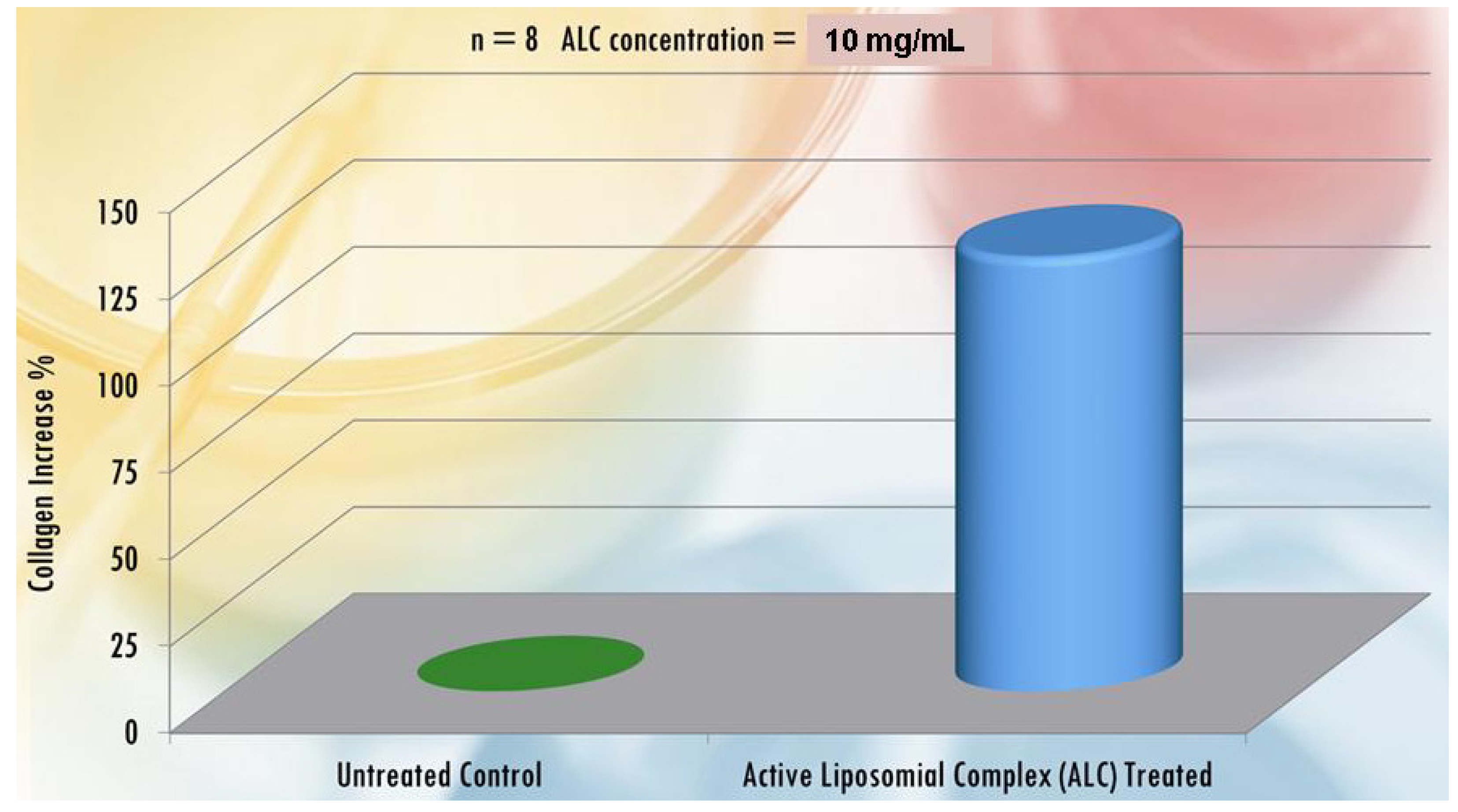
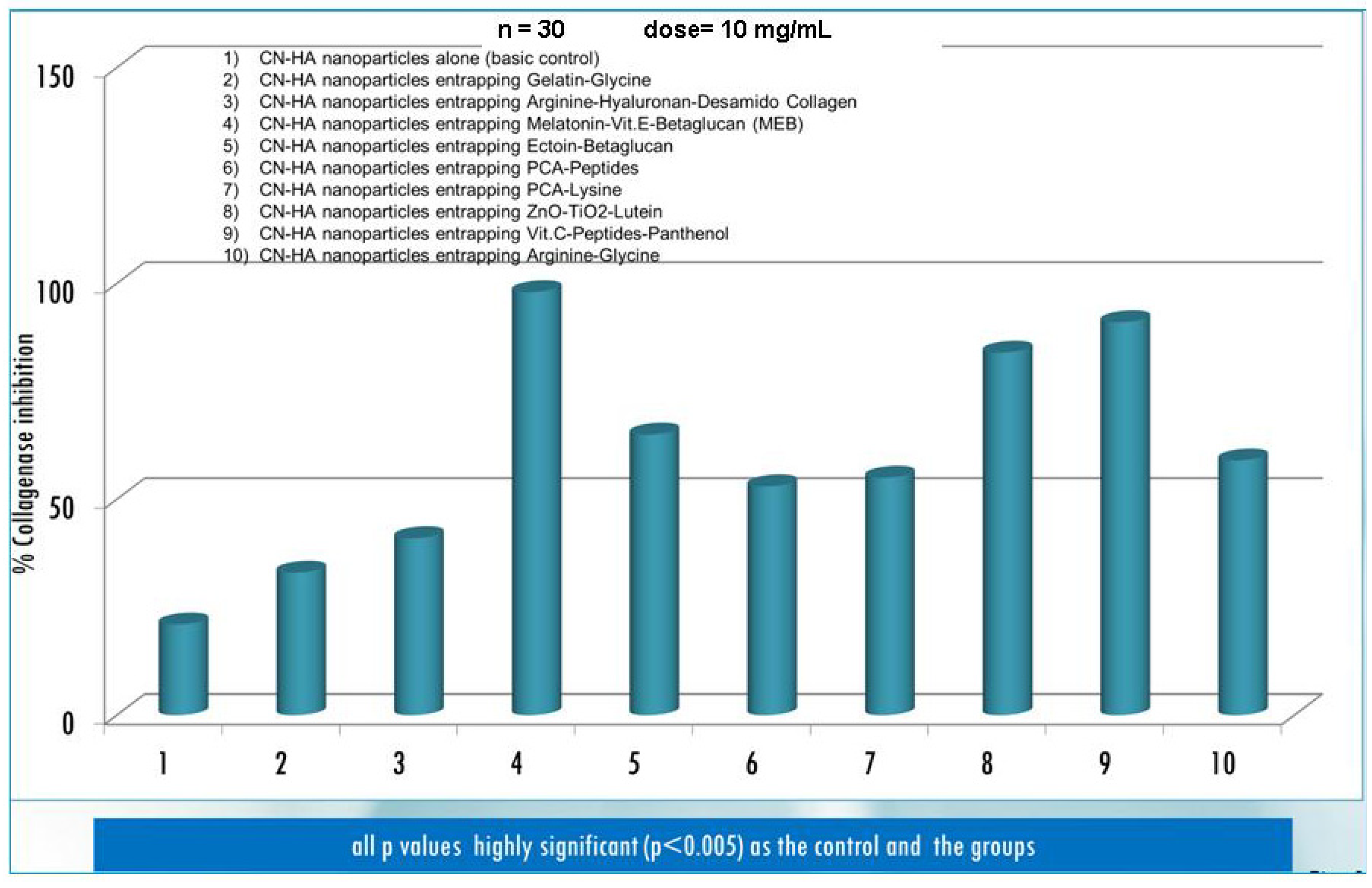
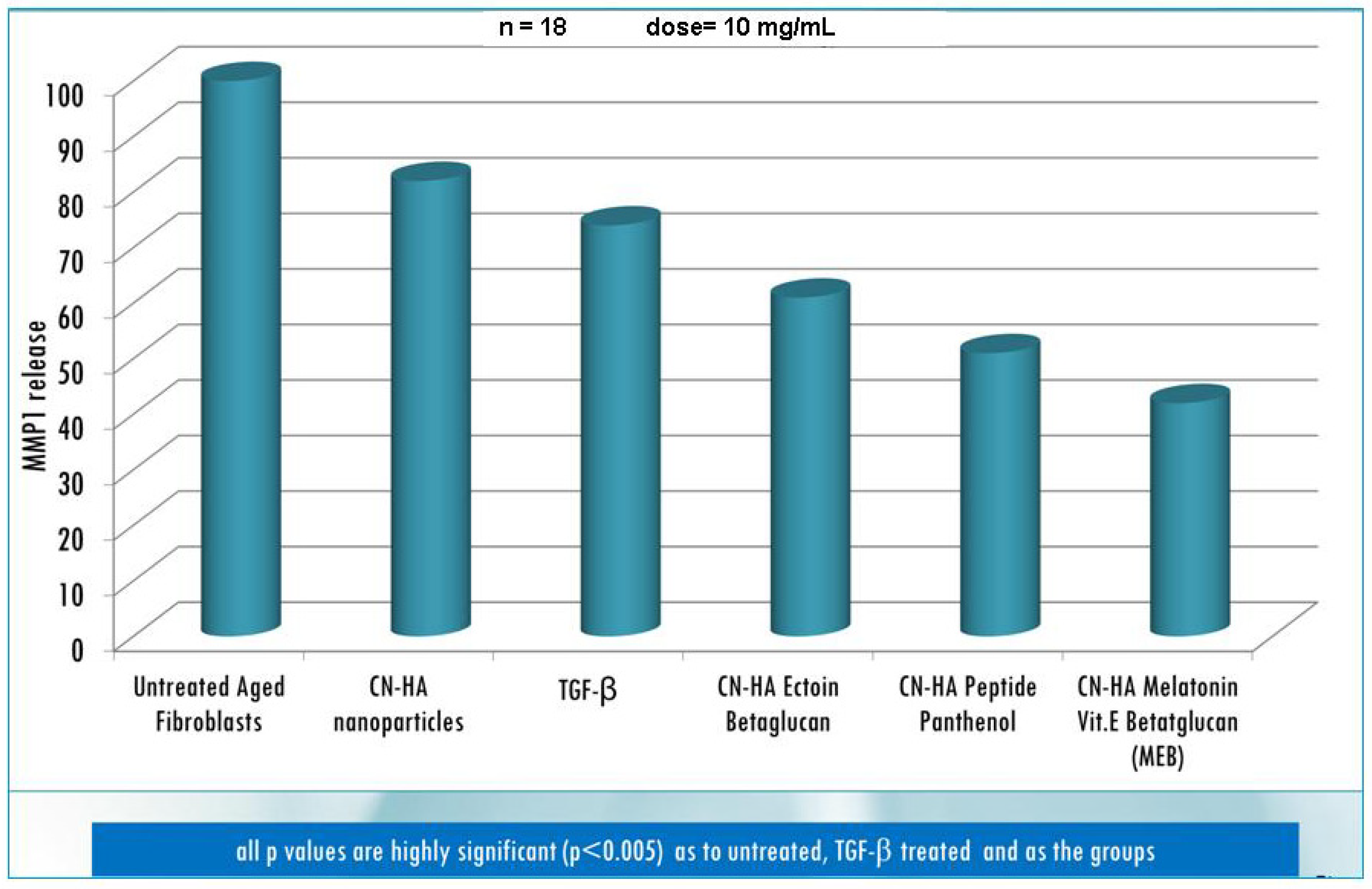
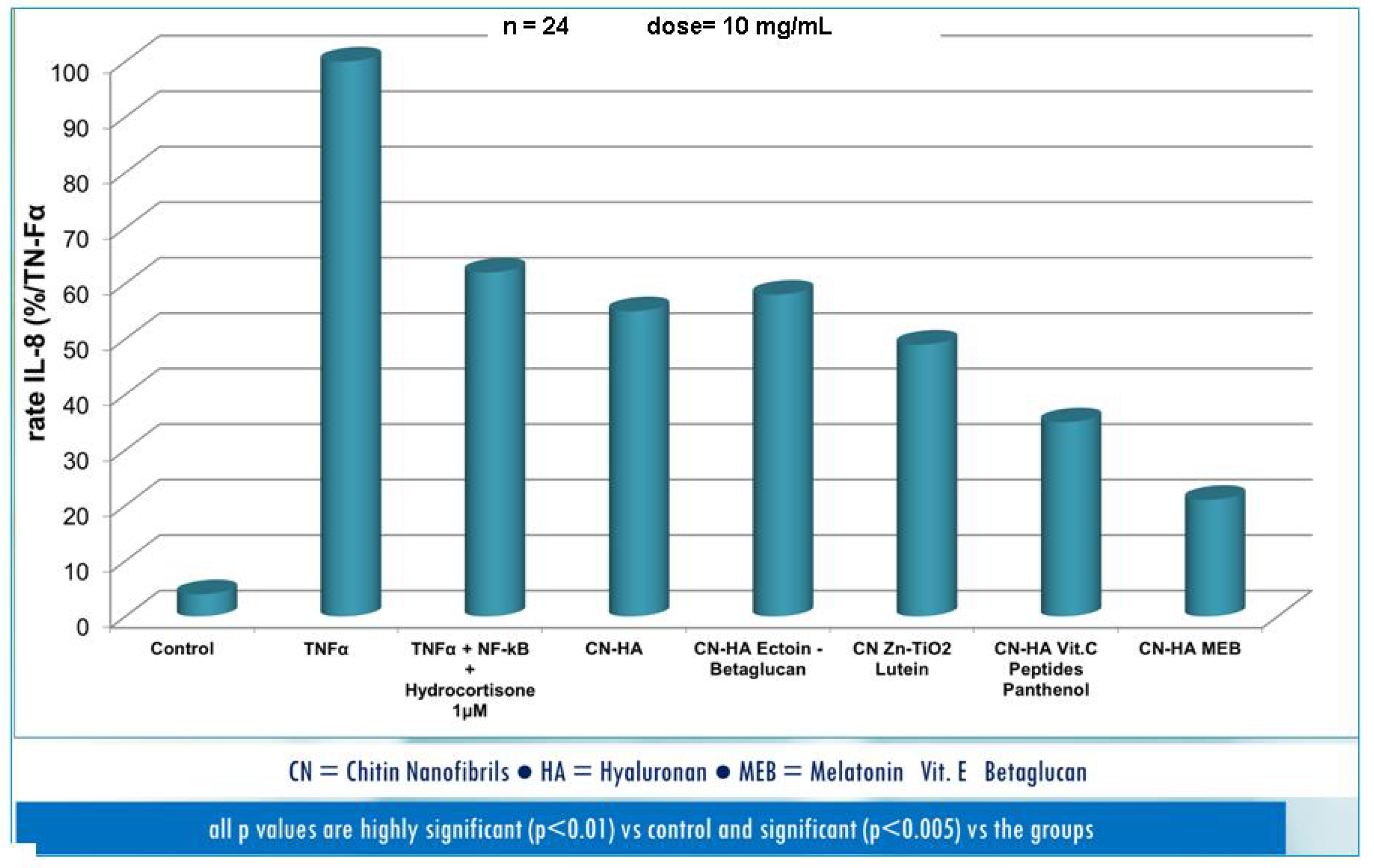
| Active compounds | SPF (Sun Protection Factor) | UVA-PF (UVA Protection Factor) |
|---|---|---|
| Zn-TiO2 Alone (control) | 20 ± 1.8 | 7 ± 0.8 |
| Zn-TiO2 CH-HA entrapped | 30 ± 2.3 | 10 ± 2 |
| Zn-TiO2 Lutein CH-HA entrapped | 50 ± 3.4 | 21 ± 4 |
| Zn-TiO2 β-Carotene CN-HA entrapped | 40 ± 2.9 | 13 ± 3 |
| Zn-TiO2 Lycopene CN-HA entrapped | 45 ± 2.5 | 20 ± 4 |
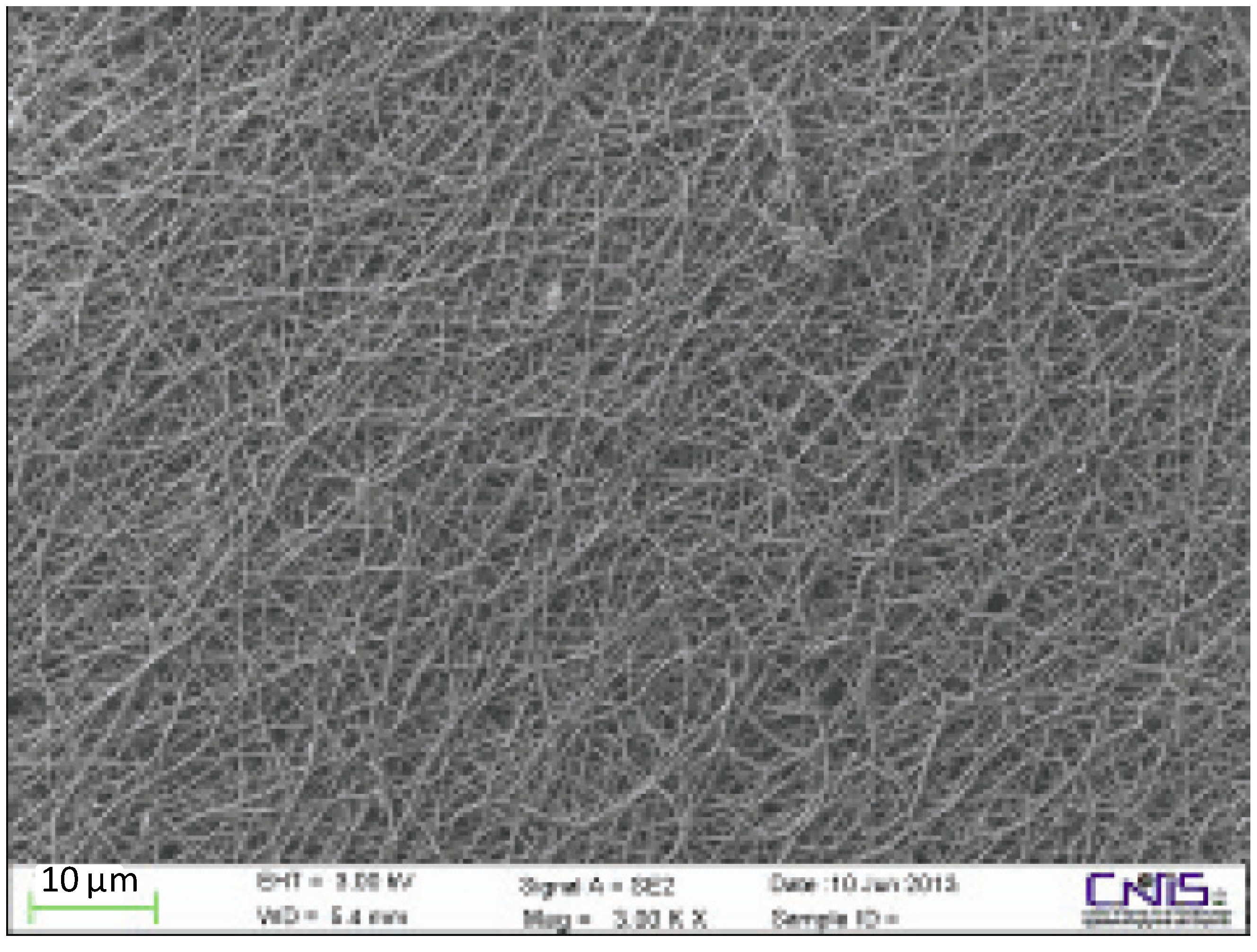
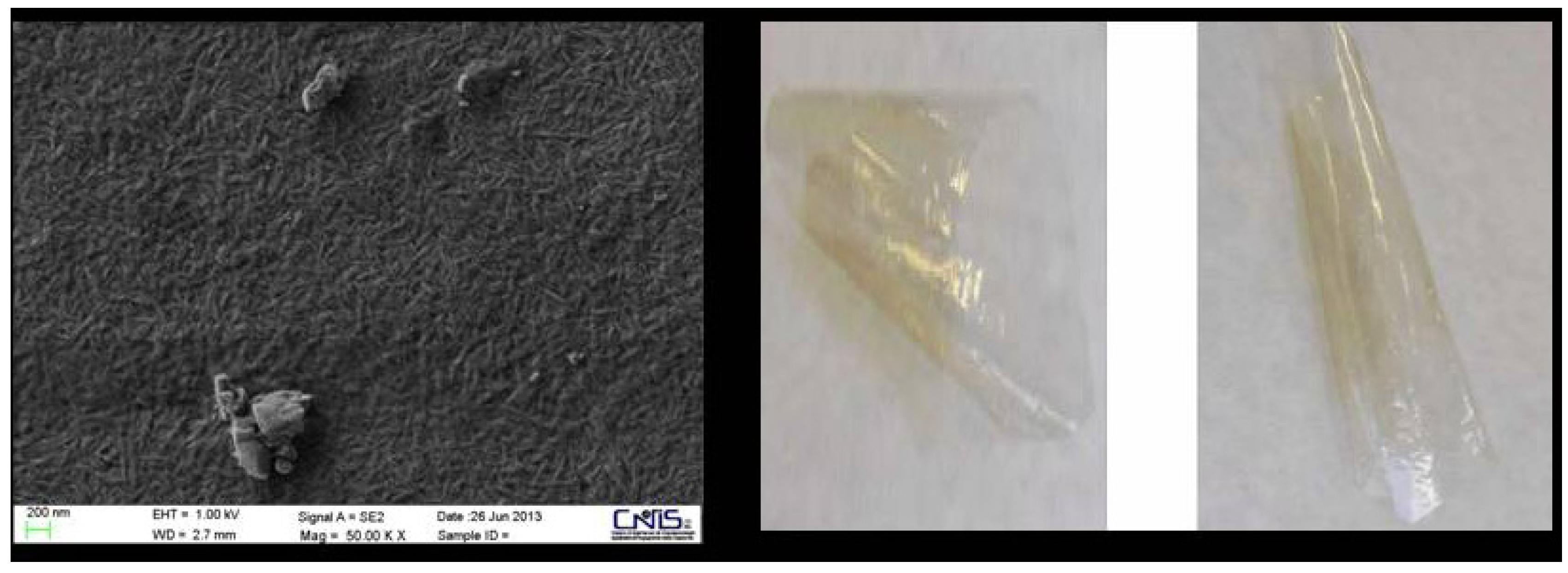
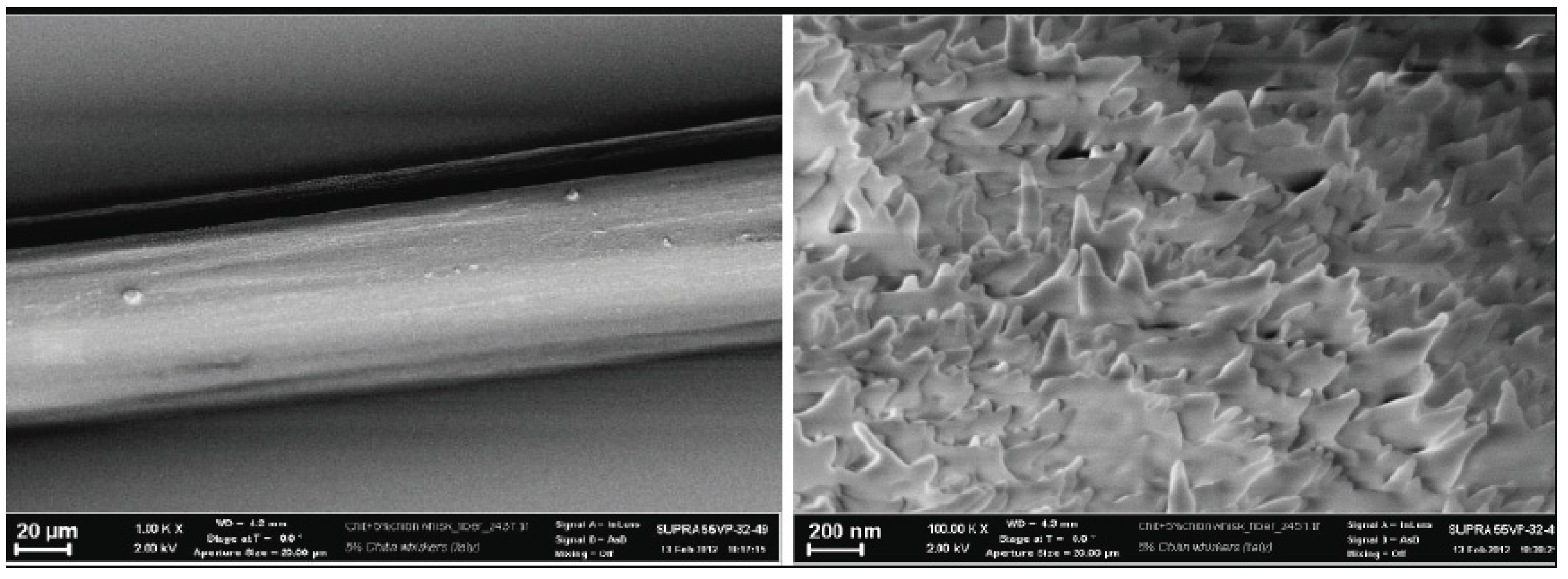
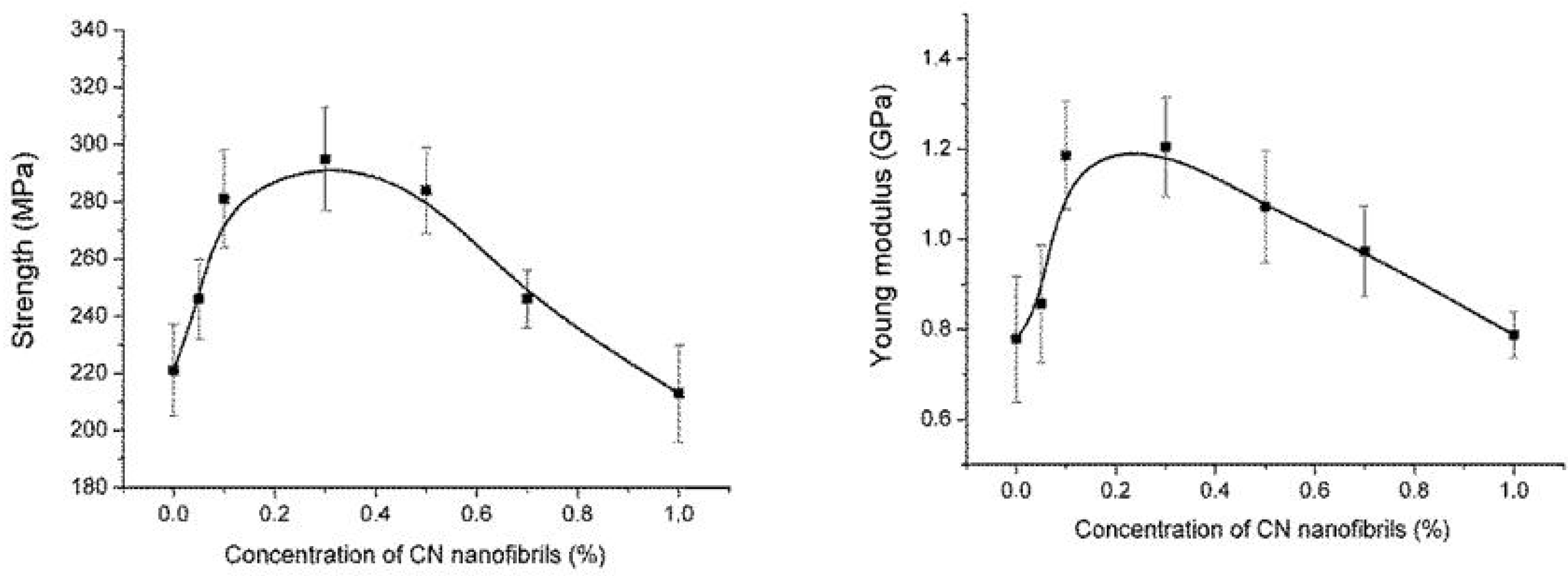
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sprintz, M. Editorial: Nanotechnology for advanced therapy and diagnosis. Biomed. Microdevices 2004, 6, 101–103. [Google Scholar] [CrossRef]
- Devalapally, H.; Chakilam, A.; Amiji, M.M. Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhza, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. 2010, 3, 5–13. [Google Scholar] [CrossRef]
- Weissleder, R.; Kelly, K.; Sun, E.Y.; Shtatland, T.; Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005, 23, 1418–1423. [Google Scholar] [CrossRef]
- Euliss, L.E.; Dupont, J.A.; Gratton, S.; DeSimone, J. Imparting size, shape, and composition control of materials for nanomedicine. Chem. Soc. Rev. 2006, 35, 1095–1104. [Google Scholar] [CrossRef]
- Jana, N.R. Shape effect in nanoparticle self-assembly. Angew. Chem. 2004, 116, 1562–1566. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napie, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. 2008, 105, 11613–11618. [Google Scholar]
- Clarke, S.J.; Hollmann, C.A.; Aldaye, F.A.; Nadeau, J. Effect of ligand density on the spectral, physics and biological characteristics of CdSe/ZnS quantum dots. Bioconjugate Chem. 2008, 19, 562–568. [Google Scholar]
- Haun, J.B.; Hammer, D.A. Quantifying nanoparticle adhesion mediated by specific molecular interactions. Langmuir 2008, 24, 8821–8832. [Google Scholar] [CrossRef]
- Moore, T.; Graham, E.; Mattix, B.; Alexis, F. Nanoparticles to Cross Biological Barriers. In Biomaterials Science: An Integrated Clinical and Engineering Approach; Rosen, Y., Elman, N., Eds.; CRC-Press: New York, NY, USA, 2012; pp. 85–121. [Google Scholar]
- Morganti, P.; Morganti, G.; Morganti, A. Transforming nanostructured chitin from crustacean waste into beneficial health products: A must of our society. Nanotechnol. Sci. Appl. 2011, 4, 123–129. [Google Scholar] [CrossRef]
- Morganti, P.; Li, Y.H. From Waste Materials Skin-Friendly Nanostructured Products to Save Humans and the Environment. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 99–105. [Google Scholar]
- Morganti, P.; Morganti, A. Chitin nanofibrils: A natural nanostructured compound to save the environment. NBT 2011, 7, 50–52. [Google Scholar]
- Morganti, P.; Li, Y.H. Healthy products from waste materials. Euro Cosmet. China Spec. Issue 2012, 20, 60–63. [Google Scholar]
- Pu, Y.; Kosa, M.; Kalluri, U.C.; Tuskan, G.A.; Ragauskas, A.J. Challenges of the utilization of wood polymers: How can they overcome? Appl. Microbiol. Biotechnol. 2011, 91, 1525–1536. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Functionalized Polymers from Lignocellulosic Biomass: State of the Art. Polymers 2013, 5, 600–642. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G. Chitin Nanofibrils for Advanced Cosmeceuticals. Clin. Dermatol. 2008, 26, 334–240. [Google Scholar] [CrossRef]
- Morganti, P. Chitin Nanofibrils for Cosmetic Delivery. Cosmet. Toilet. USA 2010, 125, 36–39. [Google Scholar]
- Morganti, P.; Carezzi, F.; del Ciotto, P.; Morganti, G. Chitin nanoparticles as innovative delivery system. Pers. Care Eur. 2012, 5, 95–98. [Google Scholar]
- Morganti, P.; del Ciotto, P.; Gao, X.H. Skin Delivery and Controlled Release of Active Ingredients by Chitin Nanofibrils: A New Approach. Cosmet. Sci. Technol. 2012, 20, 136–142. [Google Scholar]
- Morganti, P.; Palombo, M.; Chen, H.D.; Gao, X.H. Medical Textile and Nanotechnology. Cosmet. Sci. Technol. 2013, 21, 128–138. [Google Scholar]
- Morganti, P.; Tishchenko, G.; Palombo, M.; Kelnar, L.; Brozova, L.; Spirkova, M.; Pavlova, E.; Kobera, L.; Carezzi, F. Chitin Nanofibrils for Biomimetic Products: Nanoparticles and Nanocomposite Chitosan Films in Health-Care. In Marine Biomaterials: Isolation, Characterization and Application; Kim, S.K., Ed.; CRC-Press: New York, NY, USA, 2013; pp. 681–715. [Google Scholar]
- Boochemal, K.; Briancon, S.; Fessi, H.; Chevalier, Y.; Bonnet, I.; Perrier, E. Simultaneous emulsification and interfacial Polycondensation for the preparation of colloidal suspension of nanocapsules. Mater. Sci. Eng. 2006, 26, 478–480. [Google Scholar]
- Morganti, P.; del Ciotto, P.; Fabrizi, G.; Guarneri, F.; Cardillo, A.; Palombo, M.; Morganti, G. Safety and Tolerability of Chitin Nanofibril/Hyaluronic Acid Nanoparticles Entrapping Lutein. Note I. Nanopartices Characterization, Bioavailability and Biodegradability. SOFW J. 2013, 139, 12–23. [Google Scholar]
- Characterization of Chitin Nanofibril-Hyaluronan Block Polymer Characterised. Available online: http://www.personalcaremagazine.com/Story.aspx?Story=11805 (accessed on 26 June 2014).
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, F.; Slovacchia, F.; Cardillo, A.; del Ciotto, P.; Carezzi, F.; Morganti, G. New Insights on Anti-Aging Activity of Chitin Nanofibril-Hyaluronan Block Copolymers Entrapping Active Ingredients: In Vitro and in Vivo Study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Morganti, P.; Guarracino, M. Industrial EU Research Projects Performed by Biomaterials and Nanotechnology to Save the Environment. Euro Cosmet. 2013, 22, 19–24. [Google Scholar]
- Morganti, P. Biomimetic Materials Mimicking Nature at the Base of EU Projects. J. Sci. Res. Rep. 2014, 3, 532–544. [Google Scholar]
- Morganti, P. Nanoparticles and Nanostructured Man-Made or Naturally Recovered: The Biomimetic Activity of Chitin Nanofibrils. J. Nanomater. Mol. Nanotechnol. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, P.; Palombo, M.; Fabrizi, G.; Slovacchia, F.; Guevara, L.; Mezzana, P. A phosphatidylcholine-hyaluronic acid-chitin nanofibrils complex for a fast skin remodelling and a rejuvenating look. Clin. Cosmet. Investig. Dermatol. 2012, 5, 213–220. [Google Scholar]
- Morganti, P.; del Ciotto, P.; Carezzi, F.; Morganti, G.; Chen, H.D. From Waste Material A New Anti Aging Compound: A Chitin Nanofibril Complex. SOFW J. 2012, 138, 28–36. [Google Scholar]
- Baratt, G.M. Therapeutic applications of colloidal drug carriers. Pharm. Sci. Thecnol. Today 2000, 3, 163–171. [Google Scholar] [CrossRef]
- Schlieker, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Characterization of a homologous series of d,l-lactic oligomers: A mechanistic study on the degradation kinetics in vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef]
- Grung, S. Polymers in drug delivery—State of the art future trends. Adv. Eng. Mater. 2011, 13, B61–B87. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoocheheri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar]
- Jansen, L.H.; Hoiyo-Tomako, M.T.; Kligman, A.M. Improved fluorescent staining technique for estimating turnover of the human stratum corneum. Br. J. Dermatol. 1974, 90, 9–12. [Google Scholar]
- Roberts, D.; Marks, R. The determination of regional and age variations in the rate of desquamation: A comparison of four techniques. J. Invest. Dermatol. 1980, 74, 13–16. [Google Scholar] [CrossRef]
- Morganti, P.; Fabrizi, G.; Guarneri, F.; Palombo, M.; Palombo, P.; Cardillo, A.; Ruocco, E.; del Ciotto, P.; Morganti, G. Repair activity of skin barrier by chitin-nanofibrils complexes. SOFW J. 2011, 5, 10–23. [Google Scholar]
- Morganti, P.; Berardesca, E.; Guarneri, B.; Guarneri, F.; Fabrizi, G.; Palombo, P.; Palombo, M. Topical clindamicyn 1% vs. phosphatidylcholine linoleic acid-rich and nicotinamide 4% in the treatment of acne: A multicenter-randomized trial. Int. J. Cosmet. Sci. 2010, 33, 1–10. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, M.; Guarneri, F.; Cardillo, A.; Morganti, G. New chitin complexes and their anti-aging activity from inside out. J. Nutr. Health Aging 2012, 16, 242–245. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 32, 3657–3666. [Google Scholar]
- Morganti, P.; Chen, H.D.; Gao, X.H.; del Ciotto, P.; Carezzi, F.; Morganti, G. Nano-Particles of Chitin Nanofibril-Hyaluronan Block Polymer Entrapping Lutein as UVA Protective Compound. In Carotenoids: Food Source, Production and Health Benefits; Miyamaguchi, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 237–259. [Google Scholar]
- Morganti, P.; Fabrizi, G.; del Ciotto, P.; Palombo, P.; Palombo, M.; Cardillo, A.; Morganti, G. The boosting activity of chitin nanofibrils. Eurocosmetics 2011, 19, 22–26. [Google Scholar]
- Yudin, V.E.; Dobrovolskayia, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kovaa, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef]
- Morganti, P.; Chen, H.D.; Gao, X.H.; Li, Y.H.; Jackbson, C.; Arct, J.; Fabianowski, W. Nanoscience Challenging Cosmetics, Healthy Food & Biotextiles. SOFW J. 2009, 135, 32–41. [Google Scholar]
- Morganti, P. Natural Products Work in Multiple Ways. In Nutritional Cosmetics Beauty from Within; Tabor, A., Blair, R., Eds.; William Andrew: New York, NY, USA, 2009; pp. 95–112. [Google Scholar]
- Morganti, P.; Palombo, M.; Palombo, P.; Fabrizi, G.; Cardillo, A.; Carezzi, F.; Morganti, G.; Ruocco, E.; Dziergowski, S. Cosmetic Science in Skin Aging: Achieving the Efficacy by Chitin Nano-Structured Crystallites. SOFW J. 2010, 136, 14–24. [Google Scholar]
- Morganti, P. Chitin Nanofibrils and Their Derivatives as Cosmeceuticals. In Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Application; SeKwon, K., Ed.; CRC-Press: New York, NY, USA, 2010; pp. 531–542. [Google Scholar]
- Morganti, P. Uso biomedico delle nanofibrille di chitina. Caratteristiche e meccanismo d’azione di una molecola naturale. Natural 2011, 103, 40–43. (In Italian) [Google Scholar]
- Morganti, P.; Chen, H.D. Una nanoemulsione innovativa per il trattamento della xerosi cutanea e dell’alterazione della barriera. Cosmet. Technol. 2011, 14, 29–37. (In Italian) [Google Scholar]
- Morganti, P.; del Ciotto, P.; Morganti, G.; Fabien-Soule, V. Application of Chitin Nanofibrils and Collagen of Marine Origin as Bioactive Ingredients. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.K., Ed.; CRC Press: New York, NY, USA, 2012; pp. 267–289. [Google Scholar]
- Morganti, P.; Chen, H.D. NICE melody for innovative mind-body skin care. Cosmet. Sci. Technol. 2011, 19, 49–59. [Google Scholar]
- Morganti, P.; Li, Y.H.; Chen, H.D. Skin Cell Management: The NICE Approach. Pers. Care 2011, 4, 29–36. [Google Scholar]
- Morganti, P.; Chen, H.D.; Li, Y.H. Concetto NICE mente-corpo. Una melodia innovativa per la cura della pelle. Cosmet. Technol. 2011, 14, 27–33. (In Italian) [Google Scholar]
- Morganti, P.; Chen, H.D. Trillions of Signals from the Net Architecture of the Skin Cells: The NICE-TCM Approach for Innovative Cosmeceuticals. Eurocosmetics 2012, 20, 18–21. [Google Scholar]
- Morganti, P.; Chen, H.D.; Gao, X.H.; Morganti, G. Cento trilioni di Connessioni Cellulari del Nostro Corpo: Approccio NICE-TCM con molecole naturali nano strutturate. Cosmet. Technol. 2012, 15, 31–37. (In Italian) [Google Scholar]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Belmonte, M.M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Zizzi, A.; Lucarini, G.; Giantomassi, F.; Biagini, G.; Tucci, G.; Orlando, F.; Provinciali, M.; Carezzi, F.; Morganti, P. Chitosan-linked to chitosan glycolate as Spray, Gel, and Gauze Preparations for Wound Repair. J. Bioact. Compat. Polym. 2007, 22, 525–538. [Google Scholar] [CrossRef]
- Mezzana, P. Clinical efficacy of a new nanofibrils-based gel in wound healing. Acta Chir. Investig. Dermatol. 2008, 3, 5–13. [Google Scholar]
- Morganti, P.; Paglialunga, S. EU borderline cosmetic products review of current regulatory status. Clin. Dermatol. 2008, 26, 392–397. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics 2014, 1, 140-158. https://doi.org/10.3390/cosmetics1030140
Morganti P, Palombo M, Tishchenko G, Yudin VE, Guarneri F, Cardillo M, Del Ciotto P, Carezzi F, Morganti G, Fabrizi G. Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics. 2014; 1(3):140-158. https://doi.org/10.3390/cosmetics1030140
Chicago/Turabian StyleMorganti, Pierfrancesco, Marco Palombo, Galina Tishchenko, Vladimir E. Yudin, Fabrizio Guarneri, Maria Cardillo, Paola Del Ciotto, Francesco Carezzi, Gianluca Morganti, and Giuseppe Fabrizi. 2014. "Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin" Cosmetics 1, no. 3: 140-158. https://doi.org/10.3390/cosmetics1030140
APA StyleMorganti, P., Palombo, M., Tishchenko, G., Yudin, V. E., Guarneri, F., Cardillo, M., Del Ciotto, P., Carezzi, F., Morganti, G., & Fabrizi, G. (2014). Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics, 1(3), 140-158. https://doi.org/10.3390/cosmetics1030140





