Abstract
Sunscreen products often contain combinations of ultraviolet (UV)-filters in order to achieve broad spectrum protection from exposure to sunlight. The inclusion of both chemical and physical UV-filters in these products, however, increases the possibility for both photolytic and photocatalytic reactions to occur. This study investigated the effect of titanium dioxide (TiO2) particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane (BMDM) and octocrylene (OC) formulated in a microemulsion. The International Conference on Harmonisation (ICH) Guideline Q1B for photostability testing of new active substances and medicinal products was applied. BMDM and OC in the microemulsion were irradiated with simulated sunlight in the presence of nano- (<25 nm) and micro-TiO2 (~0.6 μm) and their concentrations determined using a validated high performance liquid chromatography (HPLC) method. For the combination of BMDM and OC, the photodegradation for BMDM was found to be 12% higher in the presence of nano-TiO2 as compared to that of the micro-TiO2. This enhanced photodegradation is attributed to the larger surface area of the nano-TiO2 and the increased generation of reactive oxygen species (ROS). Because of these findings, sunscreen products containing chemical UV-filters and nano-TiO2 should be regarded with caution, due to the potential loss of photoprotection.
1. Introduction
Sunscreen products often contain a combination of ultraviolet (UV)-filters to achieve broad spectrum UV-protection. TiO2 is a physical filter offering protection from both UVB- (290–320 nm) and UVA I (320–340 nm)-light. Butyl methoxy dibenzoylmethane (BMDM, Figure 1) is a chemical UV-filter, protecting from UVA I- and UVA II (340–400 nm)-light, while octocrylene (OC, Figure 1) is a UVB-filter. Photostability of UV-filters is essential to ensure protection from dangerous health effects such as skin cancer or immunosuppression, caused by excessive exposure to UV-light [1]. Although TiO2 is photostable, some chemical UV-filters such as BMDM are susceptible to photodegradation on UV-irradiation [2]. Photodegradation results in a loss of UV-protection, since most photodegradants show UV-absorption at lower wavelengths than their parent compounds. These photodegradants may also cause allergic skin reactions and other toxic effects. For example, it was shown that exposure of octyl methoxycinnamate to UV-light increased the toxicity to mouse cells [3]. The photodegradants of BMDM also resulted in cytotoxic effects towards the amino acid arginine and photosensitive effects using the local lymphonode assay, a typical in vivo test for skin sensitization [4]. Sunscreen products are listed in the Australian Register of Therapeutic Goods and regarded as over-the-counter (OTC) drugs in the USA and as such, photostability testing as described by the International Conference on Harmonisation (ICH) Guideline Q1B [5], is not mandatory in either of these countries [6,7].
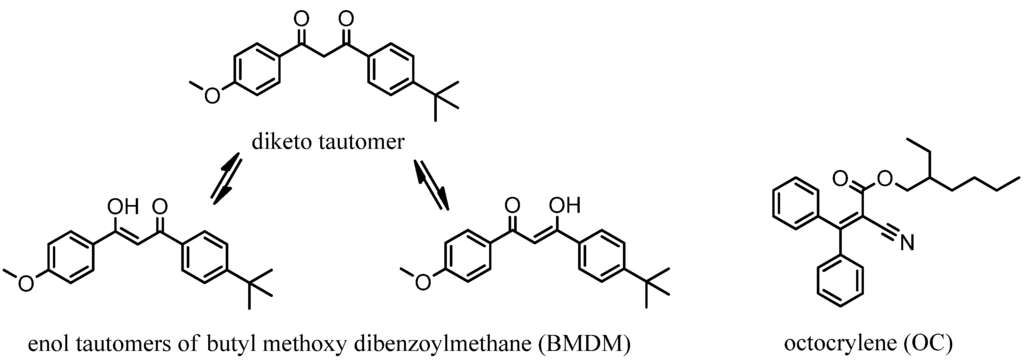
Figure 1.
Chemical structures of butyl methoxy dibenzoylmethane (BMDM) and octocrylene (OC).
TiO2 is a widely used in sunscreen products and often in combination with chemical UV-filters [6,7]. Two crystalline forms, anatase and rutile, are available in different particle sizes, with particles under 100 nm classified as nanoparticles, and those larger particles as microparticles. Micro-sized TiO2 appears white on the skin and is thus not very popular with consumers, while nano-sized TiO2 is nowadays more often used because of its transparency and improved aesthetic appearance [8]. However, TiO2 has the potential to compromise the photostability of chemical UV-filters in combination sunscreen products. In particular, it has been shown that TiO2 increases their photodegradation due to its photocatalytic properties and ability to generate reactive oxygen species (ROS) [2,9]. These results are not surprising as TiO2 is often used as a photocatalyst in water treatment, to induce the photodegradation of organic pollutants [10]. To address this issue TiO2 used for sunscreen products is often coated, for example with silica, dimethicone or aluminium hydroxide [11,12,13]. Uncoated TiO2 material for the use in sunscreen products is also available on the market [14,15,16] and still used, which may be due to potential cost implications of using the coated material [14]. Sunscreen manufacturers are also not required to use only the coated material or even state on the label whether or not coated TiO2 was employed in the final formulation [6,7,17]. TiO2 extracted from several commercially available sunscreen products caused the photodegradation of azur B and the oxidation of α-terpinene, attributed to the generation of singlet oxygen [11] and resulted in significant cellular damage of cultured cell lines [18]. This is possibly due to the presence of uncoated TiO2 in the formulation or that the integrity of the coating may have been compromised.
In Europe, sunscreen products containing nanomaterials are required to be clearly labeled with the word “nano” after the name of the ingredient [17], while in Australia and the USA this is not a requirement [6,7]. Despite this labeling requirement in Europe, the photoreactivity of different-sized TiO2 particles has thus far not been compared and therefore its effects on the stability of other ingredients in the sunscreen are unknown. The current study was therefore undertaken to examine the effect of TiO2 particle size (nano- and micro-TiO2) and coating (non- and silica coated TiO2) on the photostability of BMDM and OC. Due to lack of available photostability protocols for sunscreens, the internationally recognized ICH Guideline Q1B was followed. This is appropriate, as the complete exposure dose recommended in these guidelines (21 MJ/m2) is in the same range than the daily solar exposure in Australia in summer (20–28 MJ/m2) [19].
Previous photostability studies of UV-filters undertaken used an equivalent UVA irradiation dose of 90 [20,21] or 60 [22,23] min of sunshine on the French Riviera (Nice) in summer at noon or a full day in Scandinavia [24,25]. Equivalents of standard erythemal doses (SED) to solar radiation at midsummer noon in central Europe [26] or of minimal erythemal doses (MED) of half-day solar emission close to the equator [27] have also been reported.
2. Experimental Section
2.1. Materials
UV-filters, 4-tert-butyl-4’-methoxy dibenzoylmethane (butyl methoxy dibenzoylmethane, Eusolex® 9020), 2-ethylhexyl-2-cyano-3,3-diphenylacrylate (octocrylene, Eusolex® OCR) and the silica-coated titanium dioxide (Eusolex® T-AVO; 100% rutile, particle size ~119 nm) were purchased from Merck (Darmstadt, Germany). Anatase nano- (99.7% trace metals basis, particle size <25 nm) and micro-TiO2 (≥99% trace metals basis, particle size ~0.6 μm) were purchased from Sigma Aldrich (St. Louis, MI, USA). High performance liquid chromatography (HPLC)-grade methanol was obtained from RCI Labscan (Bankok, Thailand) and reverse osmosis water was prepared with a Millipore® Elix 10 from Millipore SAS (Molsheim, France). Xanthan gum and Mygliol® 812 (caprylic/capric triglycerides) were acquired from PCCA (Houston, TX, USA), glycerol oleate from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and oleth-20 (Brij® 98) from Acros Organics (Geel, Belgium). Other chemicals and solvents of reagent grade were used without further purification.
2.2. Methods
2.2.1. Microemulsion Preparation
An oil in water (O/W) microemulsion was prepared according to a procedure described by Montenegro et al. [28], using the phase inversion temperature (PIT) method [29]. The component oil and water phases were however modified and a thickener was added to achieve an appropriate viscosity for a sunscreen product. The oil, Mygliol® 812 (10.0% w/w), glycerol oleate (4.4% w/w), oleth-20 (11.0% w/w), containing BMDM (1.0% w/w) and OC (1.5% w/w) and water phases were heated to 85–90 °C, combined and then cooled to 40 °C, after the formation of the microemulsion. Phenoxyethanol (1.0% w/w), TiO2 (2.0% w/w) and xanthan gum (0.6% w/w) were then added with constant stirring and the final weight adjusted with water to 10.0 g. The oil soluble UV-filters BMDM and OC were dissolved in the internal phase, while TiO2 was evenly dispersed in the external phase.
2.2.2. Photostability Studies
Irradiations of UV-filters in the microemulsion were undertaken in a SunTest XLS+(I) solar simulator from Atlas Material Testing Technology GmbH (Linsengericht, Germany) according to the ICH Guideline Q1B [5]. The solar simulator was equipped with a xenon arc lamp (2.2 kVA) and a UV-glass-filter D65, which transmits wavelengths above 290 nm and is recognised as the internationally standard for outdoor daylight [5]. About 20 mg (accurately weighed) of the microemulsion was evenly spread onto a glass surface of 10 cm2 and maintained at <40 °C during irradiation at 400 W/m2 for 14.6 h (1.2 million lux hours), which is equivalent to 21 MJ/m2. After irradiation the sample was transferred using 10 mL of methanol to a 25 mL volumetric flask, sonicated and then made up to volume. Samples were then filtered through a 15 mm syringe filter with a 0.45 μm PTFE (polytetrafluoroethylene) membrane (Phenomenex Inc., Sydney, Australia) prior to determining the concentration of BMDM and OC using the validated HPLC method [30]. Dark controls were prepared to confirm the quantitative recovery of the UV-filters from the microemulsion. All experiments were undertaken in triplicate to demonstrate reproducibility.
2.2.3. Dark Adsorption Studies
Dark experiments to determine the adsorption of BMDM and OC onto coated, micro- and nano-TiO2 were conducted in triplicate. A standard UV-filter solution (60 μg/mL) in methanol was prepared (nominated to 100%) and coated, micro- or nano-TiO2 (100 μg/mL) was added. Aliquots of 10 mL were withdrawn and shaken protected from light at a speed of 600 osc/min on a Stuart® flask shaker, SF1 (Bibby Scientific Ltd., Staffordshire, UK). After one, six and 24 h samples were centrifuged at 4000 rpm for 30 min using an Eppendorf centrifuge 5810R (Eppendorf South Pacific, Sydney, Australia), filtered as described above and analysed by HPLC.
2.2.4. HPLC Analysis
All samples were analysed using a validated HPLC method [30]. A Varian ProStar® HPLC system (Varian Inc., Melbourne, Australia), consisting of a 240 quaternary solvent delivery module, 410 autosampler and a 330 photodiode array detector (PDA) was used. Data collection was undertaken with the Star Chromatography Workstation System Control version 6.41, which was equipped with the PolyView 2000™ spectral Processing Application (Varian Inc., Melbourne, Australia). A SunFire™ C18 column from Waters (4.6 × 250 mm, 5 μm) maintained at 30 °C was used with an isocratic mobile phase containing methanol/water/acetic acid (89/10/1% v/v). Samples (10 μL) were injected in triplicate and the flow rate was 1 mL/min. The UV-filter BMDM was detected at 358 nm and OC at 303 nm. BMDM and OC recovery percentages were calculated from the peak area and the mean% recovery of three experiments reported ± the standard deviation (SD). Limit of detection (LOD) was determined for both BMDM and OC to be 0.03 μg/mL, while the limit of quantification (LOQ) for BMDM and OC was 0.15 μg/mL and 0.10 μg/mL, respectively. For statistical analysis a One-way ANOVA (analysis of variance) was calculated using the IBM® SPSS® statistics software Version 20 (IBM Corporation, Armonk, NY, USA), with the level of significance at p <0.05. For equal variances the Bonferroni Post Hoc test was undertaken, while for unequal variances the Games-Howell Post Hoc test was applied.
3. Results and Discussion
Figure 2 shows the UV-absorption spectra of BMDM and OC in methanol with the molar absorption coefficient (ε) plotted against the wavelength (λ). The dominant enol-form of BMDM exhibits a maximum at 358 nm and for OC the maximum is at 303 nm. TiO2 absorbs UV-light in the UVB and UVA II range, but is less effective in the UVA I range, with absorption depending on particle size [1]. The solar simulator, using the D65 filter, emits wavelengths ≥290 nm, including UVB-, UVA I- and UVA II-light, which allows effective excitation of all UV-filters.
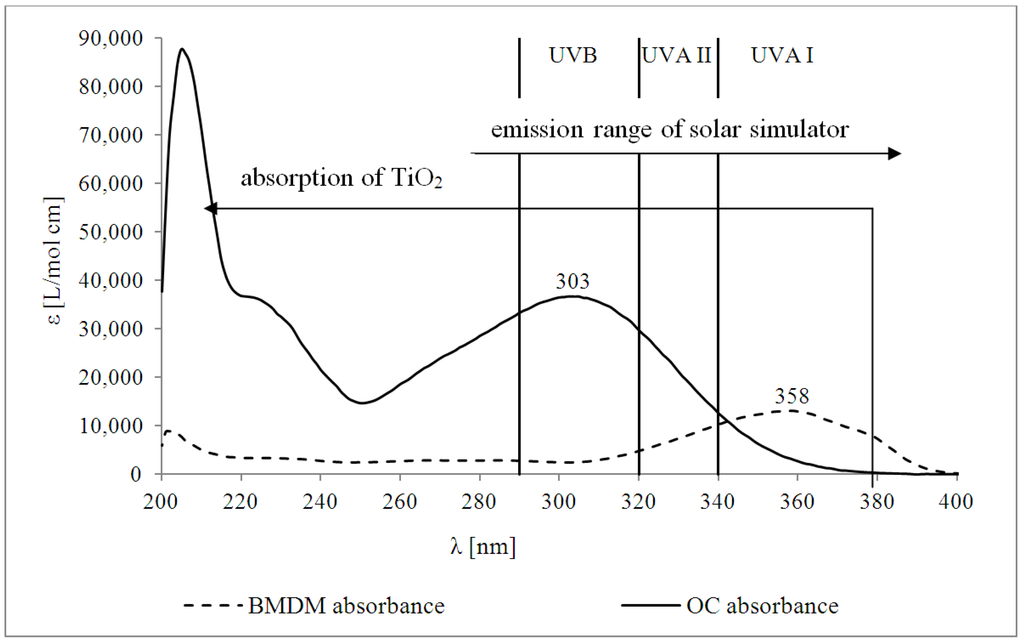
Figure 2.
Molar absorption coefficients (ε) at λ (nm) for BMDM and OC in methanol.
All irradiations were performed on the microemulsion, representing a typical formulation used for the topical application of sunscreens. To validate the extraction method used for the recovery of the UV-filters, their concentrations were compared to a standard. After extraction, the measured OC concentration was 101.83% and the BMDM concentration 104.94% of the calculated value. These results confirm the suitability of the extraction method for quantitative recovery of both UV-filters.
3.1. Dark Adsorption Studies
Dark experiments conducted in methanol showed no adsorption of either BMDM or OC onto coated, micro- or nano-TiO2. After one, six and 24 h, both UV-filter concentrations remained between 99% and 102%, compared to a reference sample. This confirms that adsorption does not contribute to the removal of the UV-filters. In general, TiO2 particles show a strong affinity for highly polar or ionisable groups [31], which are not present in either of the chemical UV-filters.
3.2. Photostability Studies
This study investigated the influence of the particle size and surface coating of TiO2 on the photostability of BMDM, OC and their combinations. Following the ICH Guideline Q1B, the UV-filters were irradiated separately and in combination, in the absence or presence of TiO2 in a solar simulator. Recovery percentages of BMDM and OC after an irradiation time of 14.6 h are listed in Table 1 and shown in Figure 3. All experiments showed excellent reproducibility as the SD (standard deviation) did not exceed 5.97%. In general, the recovery of BMDM was lower than that of OC, in particular when irradiated individually, confirming that OC is the more stable UV-filter [2]. The generation of ROS for the coated TiO2 is reported to be reduced compared to uncoated TiO2 [12]. In this study, this protective effect was confirmed as the presence of silica coated TiO2 resulted in no significant difference in UV-filter degradation (entries 2, 6 and 10) compared to experiments conducted in the absence of TiO2 (entries 1, 5 and 9). The small variations observed most likely resulted from the scattering of light by the TiO2 particles [32].

Table 1.
% Recovery ± SD (standard deviation) of butyl methoxy dibenzoylmethane (BMDM) and octocrylene (OC) after irradiation in the solar simulator.
| Entry | UV-filter combination | % Recovery ± SD | |
|---|---|---|---|
| BMDM | OC | ||
| 1 | BMDM | 3.81 ± 1.15 | – |
| 2 | BMDM + coated TiO2 | 3.43 ± 0.83 | – |
| 3 | BMDM + micro-TiO2 | 2.05 ± 0.73 | – |
| 4 | BMDM + nano-TiO2 | 0.00 * | – |
| 5 | OC | – | 98.54 ± 2.35 |
| 6 | OC + coated TiO2 | – | 99.98 ± 4.26 |
| 7 | OC + micro-TiO2 | – | 96.71 ± 2.78 |
| 8 | OC + nano-TiO2 | – | 88.33 ± 0.77 |
| 9 | BMDM + OC | 16.08 ± 2.04 | 101.57 ± 1.37 |
| 10 | BMDM + OC + coated TiO2 | 16.00 ± 1.32 | 98.23 ± 5.97 |
| 11 | BMDM + OC + micro-TiO2 | 12.59 ± 3.13 | 94.98 ± 1.96 |
| 12 | BMDM + OC + nano-TiO2 | 0.64 ± 0.52* | 92.45 ± 3.86 |
* measured concentration below limit of quantification (LOQ).
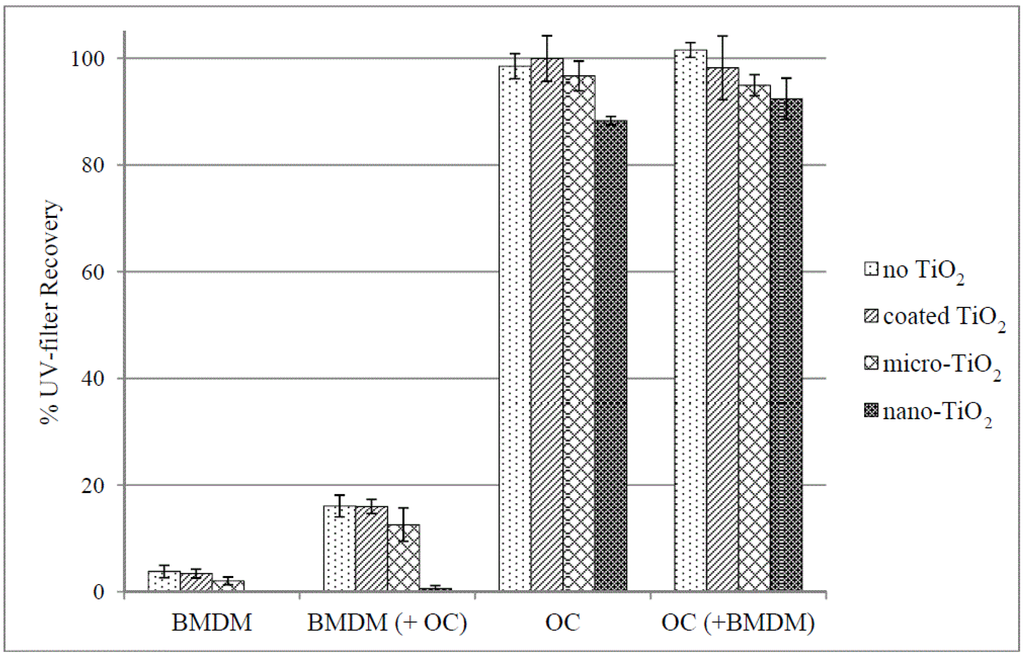
Figure 3.
% Recovery ± SD of BMDM and OC after irradiation in the solar simulator.
Irradiations of BMDM alone resulted in less than 4% recovery without TiO2, with coated and micro-TiO2 (entries 1–3). The documented photolability of BMDM was thus confirmed in the microemulsion [33]. TiO2 further accelerated photodecomposition, despite the light scattering effects of its particles. Following this trend, complete photodegradation of BMDM occurred in the presence of nano-TiO2, with no BMDM detected after irradiation (entry 4).
In combination with OC, the photostability of BMDM generally improved and recovery without TiO2 and with coated and micro-TiO2 was increased to more than 12% (entries 9–11). Despite this improvement, this percentage recovery does not lie within the acceptable range (90%–120%) required for the UV-filter concentration in a sunscreen product [6]. Photostability again decreased in the presence of nano-TiO2 and less than 1% of BMDM remained after the irradiation period (entry 12).
In contrast, OC remained largely photostable, with recoveries of >96% on irradiation (entries 5–7). A significant decrease to approximately 88% was however observed in the presence of nano-TiO2 (entry 8). The same trend was observed in combination with BMDM (entries 9–12), although the effect of nano-TiO2 was less pronounced in this case.
Under the experimental conditions chosen, two photodegradation processes are possible, direct photolysis and TiO2 photocatalysis and these will be discussed separately.
The complete irradiation dose after 14.6 h in the solar simulator was 21 MJ/m2, which is in the range of the daily solar exposure in summer in Australia. Between October 2013 and March 2014 the average daily solar exposure in about 90% of Australia was between 20 and 28 MJ/m2, while between October 2012 and March 2013 the average daily solar exposure in the whole country was between 20 and 30 MJ/m2 [19].
3.2.1. Direct Photolysis
In the absence of TiO2, BMDM recovery was higher when irradiated in combination with OC than without, and this photoprotective nature of OC is well documented [34,35,36]. Upon continuous irradiation, triplet states of BMDM are increasingly populated via UV-absorption and subsequent intersystem crossing. Thermal deactivation processes, through the release of heat (internal conversion) or emission of light (fluorescence or phosphorescence) return the UV-filter to its ground state, thus maintaining its photoprotection. The generally low photostability of BMDM confirms that its triplet excited state is unstable. Competitive decomposition operates instead and a number of photodegradants have been identified [30,37,38,39]. In contrast, the triplet excited state of OC is significantly more stable and reverts back to its ground state via phosphorescence or internal conversion. Photodecomposition of OC can thus not compete with physical deactivation and a similar behaviour was found in solution [34,35]. In the presence of OC, the triplet state of BMDM is effectively quenched, thus returning BMDM to its ground state and elevating OC to its corresponding triplet state [40]. While the triplet energy level of BMDM has been reported (TBMDM = 59.19 ± 0.76 kcal/mol) [41,42,43], the triplet energy level of OC could not be determined directly, as OC is non- or only weakly phosphorescent. Recently, however, experimental evidence for a triplet-triplet energy transfer was provided by measuring the phosphorescence decay of the energy donor, BMDM [40].
3.2.2. TiO2 Photocatalysis
In the presence of TiO2, photocatalytically induced degradations by various ROS may compete with direct photolysis. Upon absorption of light, electrons are transferred from the valence band to the vacant conduction band of TiO2, thus generating electron-hole-pairs (eCB−/hVB+) (Equation (1)). ROS, mainly hydroxyl radicals (OH•) and superoxide radical anions (O2•−) can be formed through subsequent oxidation and reduction reactions (Equations (2) and (3)) [11,44,45]. Other oxidizing species such as hydrogen peroxide H2O2 or singlet oxygen 1O2 may also be generated [45,46]. These ROS are known to react readily with organic molecules, thus causing degradation and ultimately mineralisation [47]:
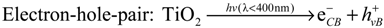
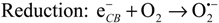
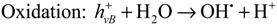
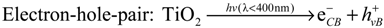
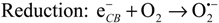
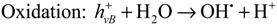
The photoreactivity of micro- and nano-TiO2 can be compared directly as they are both uncoated anatase particles differing only in their particle size. Nano-TiO2 showed a greater photoreactivity than micro-TiO2, which is attributed predominantly to its larger surface area, reduced eCB−/hVB+ recombination and faster charge transfer [48]. These small-sized particles also enable an even dispersion throughout the microemulsion, thus allowing for competitive absorption of light. ROS are subsequently generated on the surface of the TiO2 particles and induce degradation of the UV-filters. Smaller particle size and greater surface area thus result in reduced photostability [49]. In comparison, micro-sized TiO2 does not initiate any substantial photocatalytic degradation, with the larger particle size preventing light-absorption by TiO2. Instead, the UV-filters predominantly absorb light and photodegradation is dominated by direct photolysis. In the presence of micro-TiO2, the protective nature of OC on BMDM was largely maintained. Coated TiO2 showed no significant impact on the photostability. Coating is thus an effective measure to limit photodegradation by photocatalysis, although the long-term stability of these coatings has been questioned [50].
4. Conclusions
In conclusion, this study has demonstrated that nano-TiO2 induces photodegradation of the UV-filters BMDM and OC. UV-filter recovery was significantly higher in the presence of micro-TiO2 indicating that photocatalysis played only a minor role in their photodegradation. The reduction in UV-filter content and the subsequent formation of photodegradants may thus result in the photoprotection of sunscreen products containing these UV-filters being compromised. Coating is an effective measure to prevent photocatalytic processes, although ageing of these materials should be given further consideration. These findings clearly show that caution should be exercised when formulating sunscreen products containing combinations of chemical UV-filters and nano-TiO2. This is currently important since nano-TiO2 is not only becoming more commonly used in sunscreens, but also in other cosmetic products. This study clearly highlights the need for standardized photostability testing of sunscreen products as no mandatory procedures currently exist.
Acknowledgments
Jutta Kockler would like to thank the School of Pharmacy and Molecular Science at James Cook University (JCU) for a scholarship and the Graduate Research School at JCU for a Graduate Research Scheme Grant in 2012.
Author Contributions
The study design was undertaken by Beverley Glass, Jutta Kockler and Sherryl Robertson, while experimental work was performed by Jutta Kockler with the support of Sherryl Robertson. The analysis and interpretation was mainly conducted by Michael Oelgemöller, together with Beverley Glass and Jutta Kockler. The manuscript was written by Jutta Kockler, Michael Oelgemöller and Beverley Glass.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Butt, S.T.; Christensen, T. Toxicity and phototoxicity of chemical sun filters. Radiat. Prot. Dosim. 2000, 91, 283–286. [Google Scholar] [CrossRef]
- Karlsson, I.; Hillerstrom, L.; Stenfeldt, A.L.; Martensson, J.; Borje, A. Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol. 2009, 22, 1881–1892. [Google Scholar] [CrossRef]
- Guidance for Industry: Q1B Photostability Testing of New Drug Substances and Products. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073373.pdf (accessed on 20 January 2014).
- Therapeutic Goods Administration. Australian Regulatory Guidelines for Sunscreens, Version 1.0. Available online: http://www.tga.gov.au/industry/sunscreens-args.htm#.Uu7SSj2SykN (accessed on 20 January 2014).
- U.S. Department of Health and Human Services. Food and Drug Administration, Labeling and Effectiveness Testing: Sunscreen Drug Products for Over-the-Counter Human Use. Available online: http://www.gpo.gov/fdsys/pkg/FR-2011–06–17/pdf/2011–14766.pdf (accessed on 20 January 2014).
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A review of the current and future technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Yu, W.W.; Oliva, B.L.; Contreras, E.Q.; Colvin, V.L. Photochemical behavior of nanoscale TiO2 and ZnO sunscreen ingredients. J. Photochem. Photobiol. A 2013, 263, 24–33. [Google Scholar] [CrossRef]
- Kockler, J.; Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Photochemical and photocatalytic degradation of diclofenac and amoxicillin using natural and simulated sunlight. J. Sustain. Sci. Manag. 2012, 7, 23–29. [Google Scholar]
- Buchalska, M.; Kras, G.; Oszajca, M.; Lasocha, W.; Macyk, W. Singlet oxygen generation in the presence of titanium dioxide materials used as sunscreens in suntan lotions. J. Photochem. Photobiol. A 2010, 213, 158–163. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Ugazio, E.; Sapino, S.; Fenoglio, I.; Greco, G.; Fubini, B. Role of particle coating in controlling skin damage photoinduced by titania nanoparticles. Free Radic. Res. 2009, 43, 312–322. [Google Scholar] [CrossRef]
- Tiano, L.; Armeni, T.; Venditti, E.; Barucca, G.; Mincarelli, L.; Damiani, E. Modified TiO2 particles differentially affect human skin fibroblasts exposed to UVA light. Free Radic. Biol. Med. 2010, 49, 408–415. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA), Nanomaterial Case Studies: Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen. Available online: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=230972 (accessed on 31 April 2014).
- Miyoshi Kasei, I. Sunscreen Materials. Available online: http://www.miyoshikaseigroup.com/products/sunscreen-materials/ultrafine-titanium-dioxide (accessed on 31 April 2014).
- Sensient® Cosmetic Technologies. Cosmetic Ingredients, UV-Filters. Available online: http://www.sensient-cosmetics.com/pageLibre000105cf.aspx (accessed on 4 June 2014).
- European Commission Regulation No. 1223/2009 of the European Parliament and the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:EN:PDF (accessed on 23 January 2014).
- Rampaul, A.; Parkin, I.P.; Cramer, L.P. Damaging and protective properties of inorganic components of sunscreens applied to cultured human skin cells. J. Photochem. Photobiol. A 2007, 191, 138–148. [Google Scholar] [CrossRef]
- Australian Government—Bureau of Meteorology, Six-Monthly Solar Exposure for Australia. Available online: http://www.bom.gov.au/jsp/awap/solar/archive.jsp?map=solarave&colour=colour&map=solarave&year=2014&month=3&period=6month&area=nat (accessed on 31 April 2014).
- Damiani, E.; Astolfi, P.; Giesinger, J.; Ehlis, T.; Herzog, B.; Greci, L.; Baschong, W. Assessment of the photo-degradation of UV-filters and radical-induced peroxidation in cosmetic sunscreen formulations. Free Radic. Res. 2010, 44, 304–312. [Google Scholar] [CrossRef]
- Puglia, C.; Damiani, E.; Offerta, A.; Rizza, L.; Tirendi, G.G.; Tarico, M.S.; Curreri, S.; Bonina, F.; Perrotta, R.E. Evaluation of nanostructured lipid carriers (NLC) and nanoemulsions as carriers for UV-filters: Characterization, in vitro penetration and photostability studies. Eur. J. Pharm. Sci. 2014, 51, 211–217. [Google Scholar] [CrossRef]
- Seite, S.; Moyal, D.; Richard, S.; de Rigal, J.; Leveque, J.L.; Hourseau, C.; Fourtanier, A. Mexoryl (R) SX: A broad absorption UVA filter protects human skin from the effects of repeated suberythemal doses of UVA. J. Photochem. Photobiol. B 1998, 44, 69–76. [Google Scholar] [CrossRef]
- Damiani, E.; Rosati, L.; Castagna, R.; Carloni, P.; Greci, L. Changes in ultraviolet absorbance and hence in protective efficacy against lipid peroxidation of organic sunscreens after UVA irradiation. J. Photochem. Photobiol. B 2006, 82, 204–213. [Google Scholar] [CrossRef]
- Tarras-Wahlberg, N.; Stenhagen, G.; Larko, O.; Rosen, A.; Wennberg, A.M.; Wennerstrom, O. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J. Invest. Dermatol. 1999, 113, 547–553. [Google Scholar] [CrossRef]
- Gonzalez, H.; Tarras-Wahlberg, N.; Stromdahl, B.; Juzeniene, A.; Moan, J.; Larko, O.; Rosen, A.; Wennberg, A.-M. Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermatol. 2007, 7. [Google Scholar] [CrossRef]
- Maier, H.; Schauberger, G.; Brunnhofer, K.; Honigsmann, H. Change of ultraviolet absorbance of sunscreens by exposure to solar-simulated radiation. J. Invest. Dermatol. 2001, 117, 256–262. [Google Scholar] [CrossRef]
- Albertini, B.; Mezzena, M.; Passerini, N.; Rodriguez, L.; Scalia, S. Evaluation of spray congealing as technique for the preparation of highly loaded solid lipid microparticles containing the sunscreen agent, avobenzone. J. Pharm. Sci. 2009, 98, 2759–2769. [Google Scholar] [CrossRef]
- Montenegro, L.; Carbone, C.; Puglisi, G. Vehicle effects on in vitro release and skin permeation of octylmethoxycinnamate from microemulsions. Int. J. Pharm. 2011, 405, 162–168. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Emerging role of microemulsions in cosmetics. Recent Pat. Drug Deliv. Formul. 2008, 2, 275–289. [Google Scholar]
- Kockler, J.; Motti, C.A.; Robertson, S.; Oelgemöller, M.; Glass, B.D. HPLC method for the simultaneous determination of the UV-filters butyl methoxy dibenzoylmethane and octocrylene in the presence of their photodegradants. Chromatographia 2013, 76, 1721–1727. [Google Scholar] [CrossRef]
- Thomas, A.G.; Syres, K.L. Adsorption of organic molecules on rutile TiO2 and anatase TiO2 single crystal surfaces. Chem. Soc. Rev. 2012, 41, 4207–4217. [Google Scholar] [CrossRef]
- Dondi, D.; Albini, A.; Serpone, N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem. Photobiol. Sci. 2006, 5, 835–843. [Google Scholar] [CrossRef]
- Deflandre, A.; Lang, G. Photostability assessment of sunscreens—benzylidene camphor and debenzoylmethane derivatives. Int. J. Cosmet. Sci. 1988, 10, 53–62. [Google Scholar] [CrossRef]
- Bonda, G. Research pathways to photostable sunscreens. Cosmet. Toil. 2008, 123, 49–60. [Google Scholar]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter-filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochem. Photobiol. Sci. 2010, 9, 552–558. [Google Scholar] [CrossRef]
- Shaath, N.A. Ultraviolet filters. Photochem. Photobiol. Sci. 2010, 9, 464–469. [Google Scholar] [CrossRef]
- Huong, S.P.; Rocher, E.; Fourneron, J.D.; Charles, L.; Monnier, V.; Bun, H.; Andrieu, V. Photoreactivity of the sunscreen butyl methoxy dibenzoylmethane (DBM) under various experimental conditions. J. Photochem. Photobiol. A 2008, 196, 106–112. [Google Scholar] [CrossRef]
- Roscher, N.M.; Lindemann, M.K.O.; Kong, S.B.; Cho, C.G.; Jiang, P. Photodecomposition of several compounds commonly used as sunscreen agents. J. Photochem. Photobiol. A 1994, 80, 417–421. [Google Scholar] [CrossRef]
- Schwack, W.; Rudolph, T. Photochemistry of dibenzoyl methane UVA filters. Part 1. J. Photochem. Photobiol. B 1995, 28, 229–234. [Google Scholar] [CrossRef]
- Kikuchi, A.; Oguchi-Fujiyama, N.; Miyazawa, K.; Yagi, M. Triplet-triplet energy transfer from a UVA absorber butyl methoxy dibenzoylmethane to UVB absorbers. Photochem. Photobiol. 2014, 90, 511–516. [Google Scholar] [CrossRef]
- Gonzenbach, H.; Hill, T.J.; Truscott, T.G. The triplet energy levels of UVA and UVB sunscreens. J. Photochem. Photobiol. B 1992, 16, 377–379. [Google Scholar] [CrossRef]
- Kikuchi, A.; Oguchi, N.; Yagi, M. Optical and electron paramagnetic resonance studies of the excited states of 4-tert-butyl-4'-methoxydibenzoylmethane and 4-tert-butyl-4'-methoxydibenzoylpropane. J. Phys. Chem. A 2009, 113, 13492–13497. [Google Scholar] [CrossRef]
- Sayre, R.M.; Dowdy, J.C.; Gerwig, A.J.; Shields, W.J.; Lloyd, R.V. Unexpected photolysis of the sunscreen octinoxate in the presence of the sunscreen avobenzone. Photochem. Photobiol. 2005, 81, 452–456. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mills, A.; LeHunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Konaka, R.; Kasahara, E.; Dunlap, W.C.; Yamamoto, Y.; Chien, K.C.; Inoue, M. Irradiation of titanium dioxide generates both singlet oxygen and superoxide anion. Free Radic. Biol. Med. 1999, 27, 294–300. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Wahi, R.K.; Yu, W.W.; Liu, Y.P.; Mejia, M.L.; Falkner, J.C.; Nolte, W.; Colvin, V.L. Photodegradation of congo red catalyzed by nanosized TiO2. J. Mol. Catal. A 2005, 242, 48–56. [Google Scholar] [CrossRef]
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.-Y. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010, 158, 3482–3489. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).