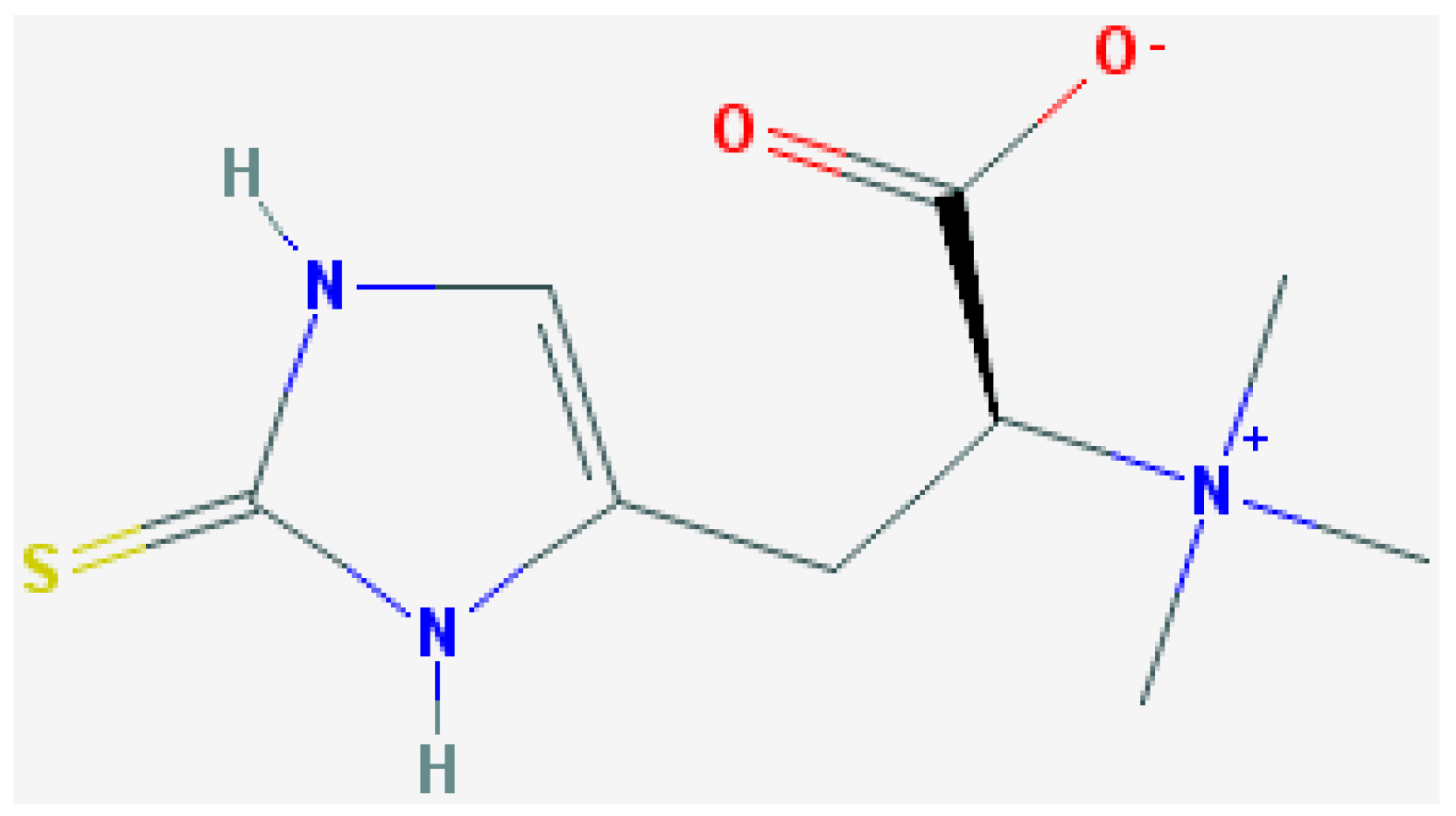
l-Ergothioneine Protects Skin Cells against UV-Induced Damage—A Preliminary Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Cell Cultures
2.2. UVB Irradiation
2.3. UVA Irradiation
2.4. Colorimetric Determination of Reduced Glutathione (GSH)
2.5. “Common Deletion” (CD) PCR
| PCR primer | Orientation | Position |
|---|---|---|
| MT1A | F | 8224-8247 |
| MT1C | F | 13176-13198 |
| MT2 | R | 13501-13477 |
3. Results and Discussion
3.1. l-Ergothioneine Increases the Reduced Glutathione (GSH) Level
| KB cells | µmol GSH/µg protein | |
|---|---|---|
| −UVB | +UVB (300 J/m2) | |
| control | 6.07 ± 0.15 | 3.03 ± 0.02 |
| 20 µM l-ergothioneine | 15.37 ± 0.13 | 4.65 ± 0.06 |
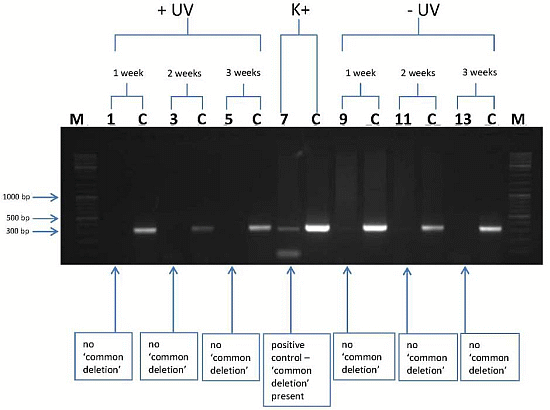
3.2. l-Rrgothioneine Shows Protective Properties against the Induction of a Photoaging-Associated mtDNA “Common Deletion”
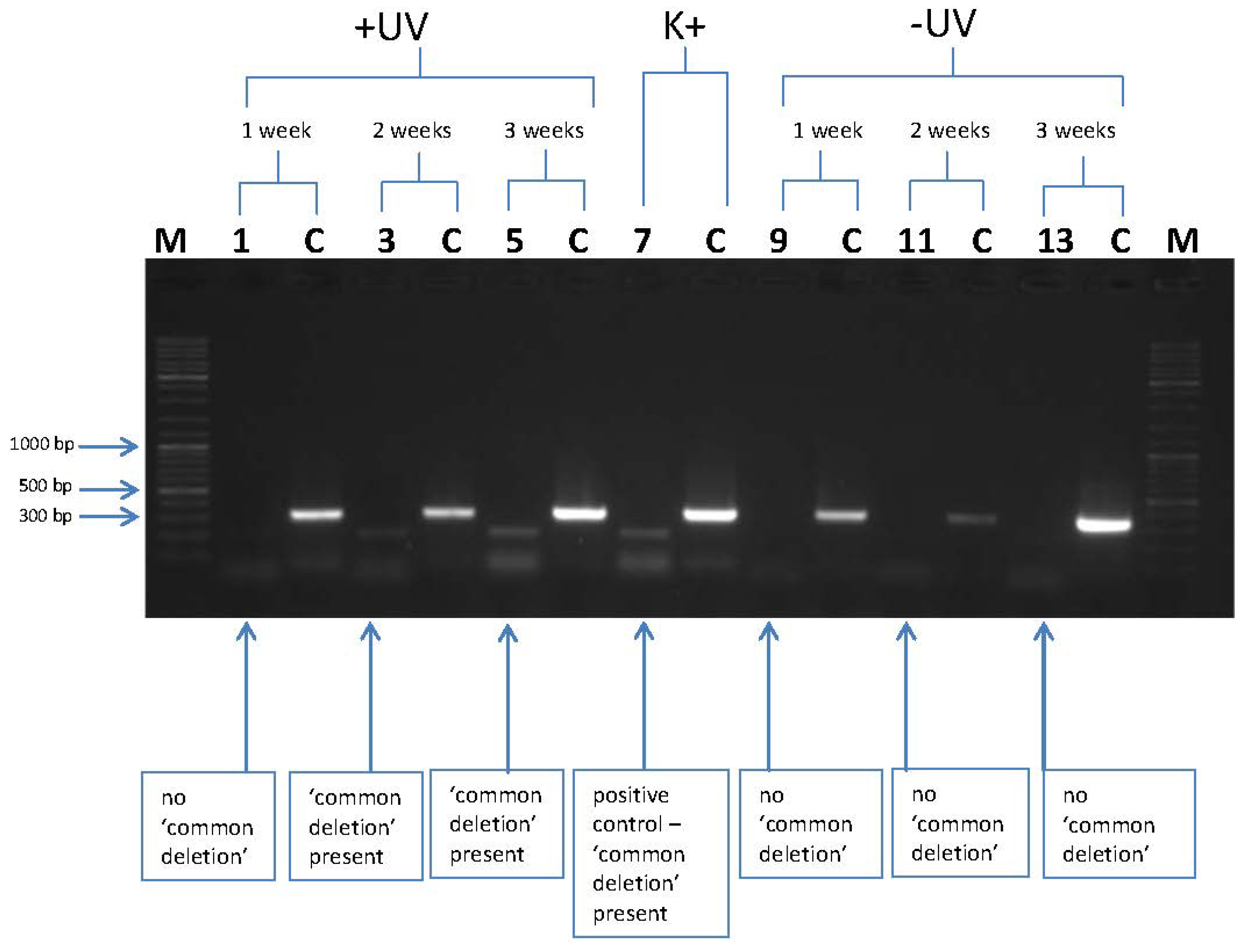
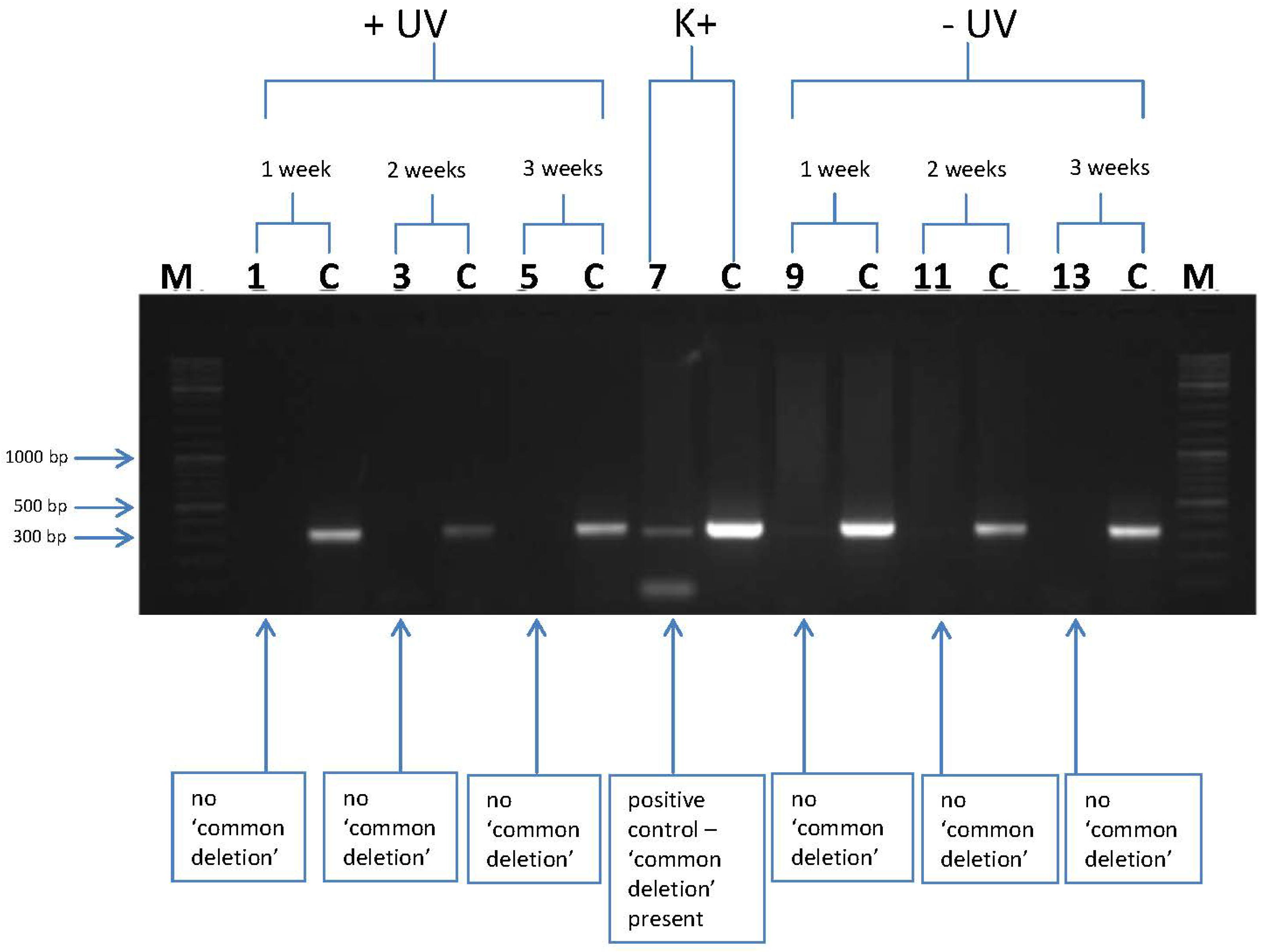
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glichrest, B.A. Photoaging. J. Invest. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006, 126, 2565–2575. [Google Scholar]
- Puizina-Ivic, N. Skin aging. Acta Derm. 2008, 17, 47–54. [Google Scholar]
- Tońska, K.; Sołyga, A.; Bartnik, E. Mitochondria and aging: Innocent bystanders or guilty parties? J. Appl. Genet. 2009, 50, 55–62. [Google Scholar] [CrossRef]
- Alexeyev, M.F.; LeDoux, S.P.; Wilson, G.L. Mitochondrial DNA and aging. Clin. Sci. 2004, 107, 355–364. [Google Scholar] [CrossRef]
- Krishnan, K.J.; Greaves, L.C.; Reeve, A.K.; Turnbull, D. The ageing mitochondrial genome. Nucleic Acids Res. 2007, 35, 7399–7405. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Tindall, M.; Turner, R.; Haldane, F.; Rees, J.L. Mitochondrial DNA deletions in human skin reflect photo rather than chronologic aging. J. Invest. Dermatol. 1998, 110, 149–152. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, H.C.; Lin, K.J.; Wei, Y.H. A specific 4977-bp deletion of mitochondrial DNA in human ageing skin. Arch. Dermatol. Res. 1994, 286, 386–390. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, H.C.; Wei, Y.H. Photoageing-associated mitochondrial DNA length mutations in human skin. Arch. Dermatol. Res. 1995, 287, 641–648. [Google Scholar] [CrossRef]
- Ray, A.J.; Turner, R.; Nikaido, O.; Rees, J.L.; Birch-Machin, M.A. The spectrum of mitochondrial DNA deletions is a ubiquitous marker of ultraviolet radiation exposure in human skin. J. Invest. Dermatol. 2000, 115, 674–679. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010, 17, 1134–1140. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta 2012, 1822, 784–793. [Google Scholar]
- Markova, N.G.; Karaman-Jurukovska, N.; Dong, K.K.; Damaghi, N.; Smiles, K.A.; Yarosh, D.B. Skin cells and tissue are capable of using l-ergothioneine as an integral component of their antioxidant defense system. Free Radic. Biol. Med. 2009, 46, 1168–1176. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilising principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Bazela, K.; Debowska, R.; Zielinska, A.; Jastrzebska-Brucka, E.; Kawczuga, D.; Eris, I. Protective role of Antioxidant Complex (AC) on human skin cells. In Proceedings of the 3rd International Conference on Oxidative Stress in Skin Medicine and Biology, Andros, Greece, 21–24 September 2006.
- Kligman, L.H. Photoaging. Manifestations, prevention, and treatment. Dermatol. Clin. 1986, 4, 517–528. [Google Scholar]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Spencer, J.P.; Mahmood, N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem. Toxicol. 1999, 37, 1043–1053. [Google Scholar] [CrossRef]
- Laurenza, I.; Colognato, R.; Migliore, L.; Del Prato, S.; Benzi, L. Modulation of palmitic acid-induced cell death by ergothioneine: Evidence of an anti-inflammatory action. Biofactors 2008, 33, 237–247. [Google Scholar] [CrossRef]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.; Di Stefano, R.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A.; et al. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef]
- Obayashi, K.; Kurihara, K.; Okano, Y.; Masaki, H.; Yarosh, D.B. l-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-α and MMP-1 expression in UV-irradiated human dermal fibroblasts. J. Cosmet. Sci. 2005, 56, 17–27. [Google Scholar]
- Rahman, I.; Gilmour, P.S.; Jimenez, L.A.; Biswas, S.K.; Antonicelli, F.; Aruoma, O.I. Ergothioneine inhibits oxidative stress- and TNF-α-induced NF-κB activation and interleukin-8 release in alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2003, 302, 860–864. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Kibitel, J.; Canning, M.T.; Smiles, K.A.; Yarosh, D.B. A comparison of the relative antioxidant potency of l-ergothioneine and idebenone. J. Cosmet. Dermatol. 2007, 6, 183–188. [Google Scholar] [CrossRef]
- Krishnan, K.J.; Reeve, A.K.; Samuels, D.C.; Chinnery, P.F.; Blackwood, J.K.; Taylor, R.W.; Wanrooij, S.; Spelbrink, J.N.; Lightowlers, R.N.; Turnbull, D.M. What causes mitochondrial DNA deletions in human cells? Nat. Genet. 2008, 40, 275–279. [Google Scholar] [CrossRef]
- Berneburg, M.; Plettenberg, H.; Medve-König, K.; Pfahlberg, A.; Gers-Barlag, H.; Gefeller, O.; Krutmann, J. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J. Invest. Dermatol. 2004, 122, 1277–1283. [Google Scholar] [CrossRef]
- Berneburg, M.; Gattermann, N.; Stege, H.; Grewe, M.; Vogelsang, K.; Ruzicka, T.; Krutmann, J. Chronically ultraviolet-exposed human skin shows a higher mutation frequency of mitochondrial DNA as compared to unexposed skin and the hematopoietic system. Photochem. Photobiol. 1997, 66, 271–275. [Google Scholar] [CrossRef]
- Koch, H.; Wittern, K.P.; Bergemann, J. In human keratinocytes the common deletion reflects donor variabilities rather than chronologic aging and can be induced by ultraviolet A radiation. J. Invest. Dermatol. 2001, 117, 892–897. [Google Scholar] [CrossRef]
- Bourgeron, T.; Chretien, D.; Rötig, A.; Munnich, A.; Rustin, P. Fate and expression of the deleted mitochondrial DNA differ between human heteroplasmic skin fibroblast and Epstein-Barr virus-transformed lymphocyte cultures. J. Biol. Chem. 1993, 26, 19369–19376. [Google Scholar]
- Wang, Y.N.; Wu, W.; Chen, H.C.; Fang, H. Genistein protects against UVB-induced senescence-like characteristics in human dermal fibroblast by p66Shc down-regulation. J. Dermatol. Sci. 2010, 58, 19–27. [Google Scholar] [CrossRef]
- Eicker, J.; Kürten, V.; Wild, S.; Riss, G.; Goralczyk, R.; Krutmann, J.; Berneburg, M. Betacarotene supplementation protects from photoaging-associated mitochondrial DNA mutation. Photochem. Photobiol. Sci. 2003, 2, 655–659. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bazela, K.; Solyga-Zurek, A.; Debowska, R.; Rogiewicz, K.; Bartnik, E.; Eris, I. l-Ergothioneine Protects Skin Cells against UV-Induced Damage—A Preliminary Study. Cosmetics 2014, 1, 51-60. https://doi.org/10.3390/cosmetics1010051
Bazela K, Solyga-Zurek A, Debowska R, Rogiewicz K, Bartnik E, Eris I. l-Ergothioneine Protects Skin Cells against UV-Induced Damage—A Preliminary Study. Cosmetics. 2014; 1(1):51-60. https://doi.org/10.3390/cosmetics1010051
Chicago/Turabian StyleBazela, Karolina, Aleksandra Solyga-Zurek, Renata Debowska, Katarzyna Rogiewicz, Ewa Bartnik, and Irena Eris. 2014. "l-Ergothioneine Protects Skin Cells against UV-Induced Damage—A Preliminary Study" Cosmetics 1, no. 1: 51-60. https://doi.org/10.3390/cosmetics1010051
APA StyleBazela, K., Solyga-Zurek, A., Debowska, R., Rogiewicz, K., Bartnik, E., & Eris, I. (2014). l-Ergothioneine Protects Skin Cells against UV-Induced Damage—A Preliminary Study. Cosmetics, 1(1), 51-60. https://doi.org/10.3390/cosmetics1010051