Safety Evaluation of Cosmetic Ingredients: In Vitro Opportunities for the Identification of Contact Allergens
Abstract
:1. Introduction
- Inappropriate immunostimulation, which may result in immune-mediated diseases, including hypersensitivity reactions and autoimmune diseases. In industrialized countries, hypersensitivity reactions represent the most frequently reported immunotoxic effects of chemicals, with prevalence in the general population of 15%–20% [2]. Contact allergy is, therefore, a common and important environmental and occupational health hazard.
2. Current in Vivo Models to Assess Hypersensitivity
3. In Vitro Assessment of Immunotoxicity
In Vitro Assessment of Contact Sensitizers
- Hazard identification: prediction of potential sensitizer (yes/no answer);
- Classification and labeling (i.e., GHS, EU-CLP (European Union regulation-Classification, Labelling and Packaging)): besides yes/no answer, some potency determination is required;
- Hazard characterization: prediction of potency of the sensitizer, i.e., non-sensitizer, weak, moderate, strong, extreme (dose-response information);
- Risk assessment: accurate evaluation of relative skin sensitizing potency to support effective risk assessment.
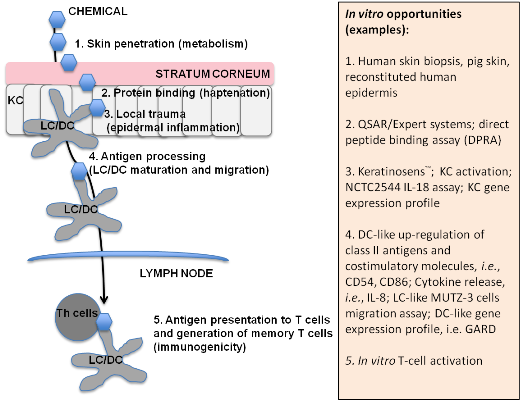
| Key passage number | In vitro opportunities | References |
|---|---|---|
| 1. Skin penetration and access to viable epidermis | Human skin biopsis, pig skin, reconstituted human epidermis | [53,54] |
| 2. Binding to macromolecules (haptenation) | QSAR/Expert systems; Peptide binding assay (DPRA); allergen-peptide/protein interaction assay | [23,28,54,55] |
| 3. Local trauma: epidermal inflammation (danger signals) | Keratinosens™; KC activation; NCTC2544 IL-18 assay; KC gene expression profile | [24,33,34,35,36,37,38,39,40,56,57] |
| 4. Antigen processing: dendritic cells maturation and migration | DC-like up-regulation of class II antigens and costimulatory molecules, i.e., CD54, CD86; Cytokine release, i.e., IL-8; LC-like MUTZ-3 cells migration assay; DC-like gene expression profile | [26,41,42,43,44,45,46,47,58,59,60,61,62,63,64] |
| 5. Antigen presentation to TH cells and memory T-cell generation (immunogenicity) | In vitro T-cell activation | [50,65,66] |
| Target cells | Name of the assay | N° of chemicals | TSF | SOP |
|---|---|---|---|---|
| Keratinocytes | NCTC2544 IL-18 assay | 33 | yes | yes |
| Human Epidermal Equivalent | 30 | yes | yes | |
| Dendritic cells | GARD | 49 | yes | yes |
| Maturation #1 (CD86, CD54, IL-8) | Stopped | - | - | |
| Maturation # 2 (DotScan) | 20 | yes | yes | |
| Migration | 12 | yes | yes | |
| T cells | Primary T-cell stimulation | 6 | yes | E |
| Others | Neutrophils-THP-1 metabolization Proteomic marker profile | 12 | yes | E |
4. Conclusions
- Applicability domains: solubility, stability, activation, cost, use of serum, etc.
- Lack of bio-availability information: real exposure at cellular level?
- Better understanding the mechanisms defining potency
- −
- Pathway analysis and marker signature identification
- −
- Quantitative relation between marker signatures and potency of a chemical
- −
- Quantitative and qualitative relation with T cell responses
- Best Integrated Testing Strategy/ies need to be identified
Conflicts of Interest
References
- Germolec, D.E. Sensitivity and predictivity in immunotoxicity testing: Immune endpoints and disease resistance. Toxicol. Lett. 2004, 149, 109–114. [Google Scholar] [CrossRef]
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.E.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef]
- Sia, I.G.; Paya, C.V. Infectious complications following renal transplantation. Surg. Clin. N. Am. 1998, 78, 95–112. [Google Scholar] [CrossRef]
- Klein, N.C.; Go, C.H.; Cunha, B.A. Infections associated with steroid use. Infect. Dis. Clin. N. Am. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Sleijffers, A.; Garssen, J.; van Loveren, H. Ultraviolet radiation, resistance to infectious diseases, and vaccination responses. Methods 2002, 28, 111–121. [Google Scholar]
- Via, T.; Descotes, J. Immunosuppressive drugs and cancer. Toxicology 2003, 185, 229–240. [Google Scholar] [CrossRef]
- Esser, C.; Jux, B. Small chemicals, bioactivation, and the immune system—A fragile balance of i-tox and benefits? Che. Biodivers. 2009, 6, 2138–2143. [Google Scholar]
- Dietert, R.R. Role of developmental immunotoxicity and immune dysfunction in chronic disease and cancer. Reprod. Toxicol. 2011, 31, 319–326. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development (OECD). Skin Sensitisation: Local Lymph Node Assay. In OECD Guidelines for the Testing of Chemicals; No. 429; OECD: Paris, France, 2010. [Google Scholar]
- Kimber, I.; Weisenberger, C. A murine local lymph node assay for the identification of contact allergens. Assay development and results of an initial validation study. Arch. Toxicol. 1989, 63, 274–282. [Google Scholar] [CrossRef]
- Basketter, D.A.; Balikie, L.; Dearman, R.J.; Kimber, I.; Ryan, C.A.; Gerberick, G.F.; Harvey, P.; Evans, P.; White, I.R.; Rycroft, R.J.G. Use of the local lymph node assay for the estimation of relative contact allergenic potency. Contact Dermat. 2000, 42, 344–348. [Google Scholar]
- Basketter, D.A.; Kimber, I. Assessing the potency of respiratory allergens: Uncertainties and challenges. Reg. Toxicol. Pharmacol. 2011, 61, 365–373. [Google Scholar] [CrossRef]
- Goebel, C.; Aeby, P.; Ade, N.; Alépée, N.; Aptula, A.; Araki, D.; Dufour, E.; Gilmour, N.; Hibatallah, J.; Keller, D.; et al. Guiding principles for the implementation of non-animal safety assessment approaches for cosmetics: Skin sensitisation. Reg. Toxicol. Pharmacol. 2012, 63, 40–52. [Google Scholar] [CrossRef]
- Luebke, R. Immunotoxicity screening and prioritization in the twenty-first Century. Toxicol. Pathol. 2012, 40, 294–299. [Google Scholar] [CrossRef]
- United Environmental Protection Agency. Available online: http://www.epa.gov/ncct/toxcas (accessed on 14 March 2014).
- Houck, K.A.; Dix, D.J.; Judson, R.S.; Kavlock, R.J.; Yang, J.; Berg, E.L. Profiling bioactivity of the ToxCast chemical library using BioMAP primary human cell systems. J. Biomol. Screen 2009, 14, 1054–1066. [Google Scholar] [CrossRef]
- Hartung, T.; Corsini, E. Immunotoxicology: Challenges in the 21st century and in vitro opportunities. ALTEX 2013, 30, 411–426. [Google Scholar] [CrossRef]
- Galbiati, V.; Mitjans, M.; Corsini, E. Present and future of in vitro immunotoxicology in drug development. J. Immunotoxicol. 2010, 7, 255–267. [Google Scholar] [CrossRef]
- Kimber, I.; Basketter, D.A.; Gerberick, G.F.; Ryan, C.A.; Dearman, R.J. Chemical allergy: Translating biology into hazard characterization. Toxicol. Sci. 2011, 120, S238–S268. [Google Scholar] [CrossRef]
- Aeby, P.; Ashikaga, T.; Bessou-Touya, S.; Schepky, A.; Gerberick, F.; Kern, P.; Marrec-Fairley, M.; Maxwell, G.; Ovigne, J.-M.; Sakaguchi, H.; et al. Identifying and characterizing chemical skin sensitizers without animal testing: Colipaʼs research and method development program. Toxicol. in Vitro 2010, 24, 1465–1473. [Google Scholar]
- McFadden, J.P.; Puangpet, P.; Basketter, D.A.; Dearman, R.J. Why does allergic contact dermatitis exist? Br. J. Dermatol. 2013, 168, 692–699. [Google Scholar] [CrossRef]
- Martin, S.F. Allergic contact dermatitis: Xenoinflammation of the skin. Curr. Opin. Immunol. 2012, 24, 720–729. [Google Scholar] [CrossRef]
- Gerberick, G.F.; Vassallo, J.D.; Foertsch, L.M.; Price, B.B.; Chaney, J.G.; Lepoittevin, J.-P. Quantification of chemical peptide reactivity for screening contact allergens: A classification tree model approach. Toxicol. Sci. 2007, 97, 417–427. [Google Scholar] [CrossRef]
- Emter, R.; Ellis, G.; Natsch, A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol. Appl. Pharmacol. 2010, 245, 281–290. [Google Scholar]
- Joint Research Centre. Available online: http://ihcp.jrc.ec.europa.eu/our_labs/eurl-ecvam/eurl-ecvam-recommendations (accessed on 14 March 2014).
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Kosaka, N.; Ito, Y.; Yoneyama, K.; Sono, S.; Itagaki, H.; Toyoda, H.; Suzuki, H. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test—Human cell line activation test (h-CLAT). Cell Biol. Toxicol. 2009, 25, 109–126. [Google Scholar] [CrossRef]
- Roggen, E.L. Application of the acquired knowledge and implementation of the Sens-it-iv toolbox for identification and classification of skin and respiratory sensitizers. Toxicol. in Vitro 2013, 27, 1122–1126. [Google Scholar]
- Gerberick, F.; Aleksic, M.; Basketter, D.; Casati, S.; Karlberg, A.-T.; Kern, P.; Kimber, L.; Lepoittevin, J.P.; Natsch, A.; Ovigne, J.M.; et al. Chemical reactivity measurement and the predictive identification of skin sensitizers. Altern. Lab. Anim. 2008, 36, 215–242. [Google Scholar]
- Barker, J.N.W.N.; Griffiths, C.E.M.; Nickoloff, B.J.; Mitra, R.S.; Dixit, V.M. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef]
- Katz, S.I.; Tamaki, K.; Sachs, D.H. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature 1979, 282, 324–326. [Google Scholar] [CrossRef]
- Bonneville, M.; Chavagnac, C.; Vocanson, M.; Rozieres, A.; Benetiere, J.; Pernet, I.; Denis, A.; Nicolas, J.-F.; Hennino, A. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J. Invest. Dermatol. 2007, 127, 1430–1435. [Google Scholar]
- Enk, A.H.; Katz, S.I. Early molecular events in the induction phase of contact sensitivity. Proc. Natl. Acad. Sci. USA 1992, 89, 1398–1402. [Google Scholar] [CrossRef]
- Corsini, E.; Primavera, A.; Marinovich, M.; Galli, C.L. Selective induction of cell-associated IL-1α in murine keratinocytes by chemical allergens. Toxicology 1998, 129, 193–200. [Google Scholar] [CrossRef]
- Van Och, F.M.; van Loveren, H.; van Wolfswinkel, J.C.; Machielsen, A.J.C.; Vandebriel, R.J. Assessment of potency of allergenic activity of low molecular weight compounds based on IL-1α and IL-18 production by a murine and human keratinocyte cell line. Toxicology 2005, 210, 95–109. [Google Scholar] [CrossRef]
- Muller, G.; Saloga, J.; Germann, T.; Bellinghausen, I.; Mohamadzadeh, M.; Knop, J.; Enk, A.H. Identification and induction of human keratinocyte-derived IL-12. J. Clin. Invest. 1994, 94, 1799–1805. [Google Scholar] [CrossRef]
- Corsini, E.; Limiroli, E.; Marinovich, M.; Galli, C.L. Selective induction of IL-12 by chemical allergens in reconstituted human epidermis. Altern. Lab. Anim. 1999, 27, 261–269. [Google Scholar]
- Corsini, E.; Mitjans, M.; Galbiati, V.; Lucchi, L.; Galli, C.L.; Marinovich, M. Use of IL-18 production in a human keratinocyte cell line to discriminate contact sensitizers from irritants and low molecular weight respiratory allergens. Toxicol. in Vitro 2009, 23, 789–796. [Google Scholar] [CrossRef]
- Galbiati, V.; Mitjans, M.; Lucchi, L.; Viviani, B.; Galli, C.L.; Marinovich, M.; Corsini, E. Further development of the NCTC 2544 IL-18 assay to identify in vitro contact allergens. Toxicol. in Vitro 2011, 25, 724–732. [Google Scholar] [CrossRef]
- Corsini, E.; Galbiati, V.; Mitjans, M.; Galli, C.L.; Marinovich, M. NCTC 2544 and IL-18 production: A tool for the identification of contact allergens. Toxicol. in Vitro 2014, 28, 13–17. [Google Scholar] [CrossRef]
- Gibbs, S.; Corsini, E.; Spiekstra, S.W.; Galbiati, V.; Fuchs, H.W.; DeGeorge, G.; Troese, M.; Patrick, H.; Deng, W.; Roggen, E. An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol. Appl. Pharmacol. 2013, 272, 529–541. [Google Scholar] [CrossRef]
- Mitjans, M.; Viviani, B.; Lucchi, L.; Galli, C.L.; Marinovich, M.; Corsini, E. Role of p38 MAPK in the selective release of IL-8 induced by chemical allergen in naive THP-1 cells. Toxicol. in Vitro 2008, 22, 386–395. [Google Scholar] [CrossRef]
- Caux, C.; Massacrier, C.; Vanbervliet, B.; Dubois, B.; van Kooten, C.; Durand, I.; Banchereau, J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994, 180, 1263–1272. [Google Scholar] [CrossRef]
- Aiba, S.; Terunuma, A.; Manome, H.; Tagami, H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur. J. Immunol. 1997, 27, 3031–3038. [Google Scholar] [CrossRef]
- Degwert, J.S.; Hoppe, U.; Kligman, L.H. In vitro model for contact sensitization: I. Stimulatory capacities of human blood-derived dendritic cells and their phenotypical alterations in the presence of contact sensitizers. Toxicol. in Vitro 1997, 11, 613–618. [Google Scholar] [CrossRef]
- Rougier, N.; Redziniak, G.; Mougin, D.; Schmitt, D.; Vincent, C. In vitro evaluation of the sensitization potential of weak contact allergens using Langerhans-like dendritic cells and autologous T-cells. Toxicology 2000, 145, 73–82. [Google Scholar] [CrossRef]
- De Smedt, A.C.A.; van den Heuvel, R.L.; Zwi Berneman, N.; Schoeters, G.E.R. Modulation of phenotype, cytokine production and stimulatory function of CD34+-derived DC by NiCl2 and SDS. Toxicol. in Vitro 2001, 15, 319–325. [Google Scholar] [CrossRef]
- Weigt, H.; Muhlradt, P.F.; Larbig, M.; Krug, N.; Braun, A. The toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-γ to reverse the Th2 skew in an in vitro allergy model. J. Immunol. 2004, 172, 6080–6086. [Google Scholar]
- Casati, S.; Aeby, P.; Basketter, D.A.; Cavani, A.; Gennari, A.; Gerberick, G.F.; Griem, P.; Hartung, T.; Kimber, I.; Lepoittevin, J.-P.; et al. Dendritic cells as a tool for the predictive identification of skin sensitisation hazard. Altern. Lab. Anim. 2005, 33, 47–62. [Google Scholar]
- Dos Santos, G.G.; Reinders, J.; Ouwehand, K.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Progress on the development of human in vitro dendritic cell based assays for assessment of the sensitizing potential of a compound. Toxicol. Appl. Pharmacol. 2009, 236, 372–382. [Google Scholar] [CrossRef]
- Martin, S.F.; Esser, P.R.; Schmucker, S.; Dietz, L.; Naisbitt, D.J.; Park, B.K.; Vocanson, M.; Nicolas, J.-F.; Keller, M.; Pichler, W.J.; et al. T-cell recognition of chemicals, protein allergens and drugs: Towards the development of in vitro assays. Cell. Mol. Life Sci. 2010, 67, 4171–4184. [Google Scholar] [CrossRef]
- Jaworska, J.; Harol, A.; Kern, P.S.; Gerberick, G.F. Integrating non-animal test information into an adaptive testing strategy—Skin sensitization proof of concept case. Altex 2011, 28, 211–225. [Google Scholar] [CrossRef]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development (OECD). Skin Absorption: In Vitro Method. In OECD Guidelines for the Testing of Chemicals; No. 428; OECD: Paris, France, 2004. [Google Scholar]
- Basketter, D.A.; Pease, C.; Kasting, G.; Kimber, I.; Casati, S.; Cronin, M.; Diembeck, W.; Gerberick, F.; Hadgraft, J.; Hartung, T.; et al. Skin sensitisation and epidermal disposition: The relevance of epidermal disposition for sensitisation hazard identification and risk assessment. Altern. Lab. Anim. 2007, 35, 137–154. [Google Scholar]
- Patlewicz, G.; Aptula, A.O.; Uriarte, E.; Roberts, D.W.; Kern, P.S.; Gerberick, G.F.; Kimber, I.; Dearman, R.J.; Ryan, C.A.; Basketter, D.A. An evaluation of selected global (Q)SARs/expert systems for the prediction of skin sensitization potential. SAR QSAR Environ. Res. 2007, 18, 515–541. [Google Scholar] [CrossRef]
- Dos Santos, G.G.; Spiekstra, S.W.; Sampat-Sardjoepersad, S.C.; Reinders, J.; Scheper, R.J.; Gibbs, S. A potential In vitro epidermal equivalent assay to determine sensitizer potency. Toxicol. in Vitro 2011, 25, 347–357. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; Pennings, J.L.; Baken, K.A.; Pronk, T.E.; Boorsma, A.; Gottschalk, R.; van Loveren, H. Keratinocyte gene expression profiles discriminate sensitizing and irritating compounds. Toxicol. Sci. 2010, 117, 81–89. [Google Scholar] [CrossRef]
- Dietz, L.; Kinzebach, S.; Ohnesorge, S.; Franke, B.; Goette, I.; Koenig-Gressel, D.; Thierse, H.-J. Proteomic allergen-peptide/protein interaction assay for the identification of human skin sensitizers. Toxicol. in Vitro 2013, 27, 1157–1162. [Google Scholar]
- Ashikaga, T.; Hoya, M.; Itagaki, H.; Katsumura, Y.; Aiba, S. Evaluation of CD86 expression and MHC calss II molecule internalization in THP-1 human monocytic cells as predictive endpoints for contact sensitizers. Toxicol. in Vitro 2002, 16, 711–716. [Google Scholar] [CrossRef]
- Azam, P.; Peiffer, J.L.; Chamousset, D.; Tissier, M.-H.; Bonnet, P.-A.; Vian, L.; Fabre, I.; Ourlin, J.-C. The cytokine-dependent MUTZ-3 cell line as an in vitro model for the screening of contact sensitizers. Toxicol. Appl. Pharmacol. 2006, 212, 14–23. [Google Scholar] [CrossRef]
- Arrighi, J.F.; Rebsamen, M.; Rousset, F.; Kindler, V.; Hauser, C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-α, and contact sensitizers. J. Immunol. 2001, 166, 3837–3845. [Google Scholar]
- Yoshida, Y.; Skaguchi, H.; Ito, Y.; Okuda, M.; Suzuki, H. Evaluation of the skin sensitization potential of chemicals using expression of co-stimulatory molecules, CD54 and CD86, on the naive THP-1 cell line. Toxicol. in Vitro 2003, 17, 221–228. [Google Scholar] [CrossRef]
- Rees, B.; Spiekstra, S.W.; Carfi, M.; Ouwehand, K.; Williams, C.A.; Corsini, E.; McLeod, J.D.; Gibbs, S. Inter-laboratory study of the in vitro dendritic cell migration assay for identification of contact allergens. Toxicol. in Vitro 2011, 25, 2124–2134. [Google Scholar]
- De Fraissinette, A.B.; Cordier, A.; Ulrich, P. Modulation of the activity of human monocyte-derived dendritic cells by chemical haptens, a metal allergen, and a staphylococcal superantigen. Toxicol. Sci. 1999, 52, 189–198. [Google Scholar]
- Gorbachev, A.V.; Fairchild, R.L. Induction and regulation of T-cell priming for contact hypersensitivity. Crit. Rev. Immunol. 2001, 21, 451–472. [Google Scholar]
- Rustemeyer, T.; de Ligter, S.; von Blomberg, B.M.; Frosch, P.J.; Scheper, R.J. Human T-lymphocyte priming in vitro by haptenated autologous dendritic cells. Clin. Exp. Immunol. 1999, 117, 209–216. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Corsini, E.; Papale, A.; Galbiati, V.; Roggen, E.L. Safety Evaluation of Cosmetic Ingredients: In Vitro Opportunities for the Identification of Contact Allergens. Cosmetics 2014, 1, 61-74. https://doi.org/10.3390/cosmetics1010061
Corsini E, Papale A, Galbiati V, Roggen EL. Safety Evaluation of Cosmetic Ingredients: In Vitro Opportunities for the Identification of Contact Allergens. Cosmetics. 2014; 1(1):61-74. https://doi.org/10.3390/cosmetics1010061
Chicago/Turabian StyleCorsini, Emanuela, Angela Papale, Valentina Galbiati, and Erwin L. Roggen. 2014. "Safety Evaluation of Cosmetic Ingredients: In Vitro Opportunities for the Identification of Contact Allergens" Cosmetics 1, no. 1: 61-74. https://doi.org/10.3390/cosmetics1010061
APA StyleCorsini, E., Papale, A., Galbiati, V., & Roggen, E. L. (2014). Safety Evaluation of Cosmetic Ingredients: In Vitro Opportunities for the Identification of Contact Allergens. Cosmetics, 1(1), 61-74. https://doi.org/10.3390/cosmetics1010061