Tropical Fruit Wastes: Physicochemical Characterization, Fatty Acid Profile and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
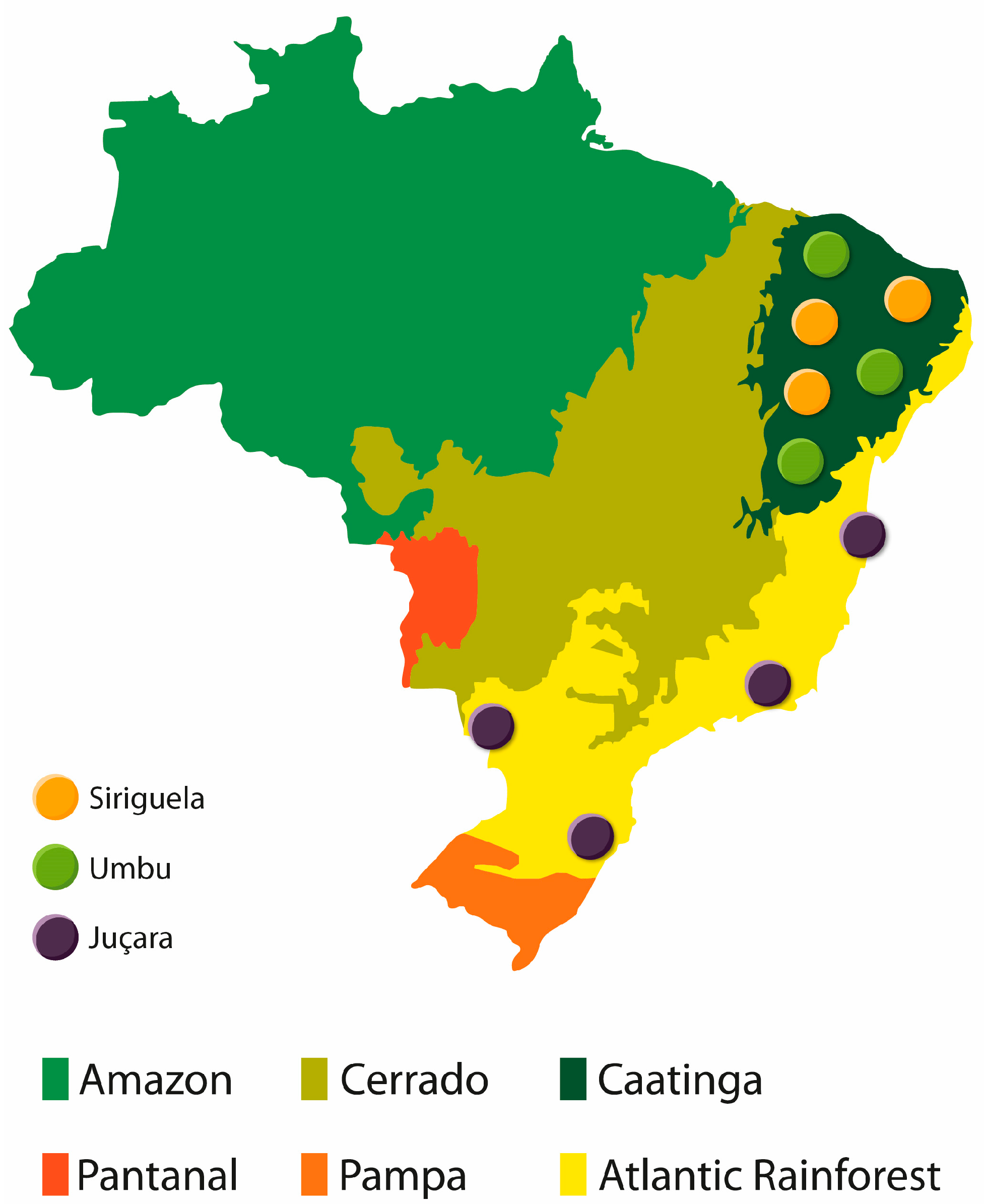
2.1. Samples
2.2. Proximate Composition and Mineral Content
2.3. Fatty Acid Profile
2.4. Extraction and Analysis of Bioactive Compounds
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition, Mineral Content, and Fatty Acid Profile
3.2. TPC and Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, S.C.; Soares-Filho, B. Opportunities of the Nagoya Protocol to Nurture the Use of Native Species in Brazil. Environ. Sci. Policy 2022, 127, 321–324. [Google Scholar] [CrossRef]
- Omena, C.M.B.; Valentim, I.B.; Guedes, G.S.; Rabelo, L.A.; Mano, C.M.; Bechara, E.J.H.; Sawaya, A.C.H.F.; Trevisan, M.T.S.; da Costa, J.G.; Ferreira, R.C.S.; et al. Antioxidant, Anti-Acetylcholinesterase and Cytotoxic Activities of Ethanol Extracts of Peel, Pulp and Seeds of Exotic Brazilian Fruits. Food Res. Int. 2012, 49, 334–344. [Google Scholar] [CrossRef]
- Ribeiro, L.O.; Viana, E.S.; Godoy, R.L.; Freitas, S.C.; Freitas, S.P.; Matta, V.M. Nutrients and bioactive compounds of pulp, peel, and seed from umbu fruit. Rural. Sci. 2019, 49, 315–324. [Google Scholar] [CrossRef]
- Ribeiro, L.O.; Freitas, S.P.; da Matta, V.M.; Jung, E.P.; Kunigami, C.N. Microencapsulation of the Extract from Euterpe edulis Co-product: An Alternative to Add Value to Fruit Agro-Chain. Waste Biomass Valor. 2021, 12, 1803–1814. [Google Scholar] [CrossRef]
- de Albuquerque, J.G.; Duarte, A.M.; da Conceição, M.L.; de Souza Aquino, J. Integral Utilization of Seriguela Fruit (Spondias purpurea L.) in the Production of Cookies. Rev. Bras. Frutic. 2016, 38, e-229. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Bonafé, E.G.; Silva, B.C.; Montanher, P.F.; Santos Júnior, O.O.; Boeing, J.S.; Visentainer, J.V. Antioxidant Capacity, Total Phenolic Content, Fatty Acids and Correlation by Principal Component Analysis of Exotic and Native Fruits from Brazil. J. Braz. Chem. Soc. 2013, 24, 797–804. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Liz, S.; Rieger, D.K.; Farah, A.C.; Vieira, F.G.; de Assis, M.A.; Di Pietro, P.F. An Update on the Biological Activities of Euterpe edulis (Juçara). Planta Med. 2018, 4, 487–499. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Costa, A.L.; Franca, A.S.; Oliveira, L.S. Chemical characterization and the bioaccessibility of bioactive compounds from seriguela (Spondias purpurea L.) pulp and by-products flours. Bioact. Carbohydr. Diet. Fibre. 2024, 31, 100404. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Rosas-Domínguez, C.; Vega-Vega, V.; González-Aguilar, G.A. Antioxidant Enrichment and Antimicrobial Protection of Fresh-Cut Fruits Using Their Own Byproducts: Looking for Integral Exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef]
- Inada, K.O.; Leite, I.B.; Martins, A.B.; Fialho, E.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Jaboticaba berry: A comprehensive review on its polyphenol composition, health effects, metabolism, and the development of food products. Food Res. Int. 2021, 147, 110518. [Google Scholar] [CrossRef]
- Selani, M.M.; Bianchini, A.; Ratnayake, W.S.; Flores, R.A.; Massarioli, A.P.; de Alencar, S.M.; Canniatti Brazaca, S.G. Physicochemical, Functional and Antioxidant Properties of Tropical Fruits Co-products. Plant Foods Hum. Nutr. 2016, 71, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.B.; Carrajola, J.F.; Gonçalves, E.C.; Martelli-Tosi, M.; Ferreira, M.S. Fruit and vegetable residues flours with different granulometry range as raw material for pectin-enriched biodegradable film preparation. Food Res. Int. 2019, 121, 412–421. [Google Scholar] [CrossRef]
- Oliveira, J.N.; Albuquerque, T.M.R.; Lima, M.S.; Andrade Wartha, E.R.S.; Monteiro, M.; Nascimento, Y.M.; Tavares, J.F.; Silva, M.S.; Souza, E.L.; Moreira, J.J. Evaluating the potential prebiotic effects of umbu-caja’ (Spondias spp.) fruit processing by-product flour on the human intestinal microbiota. Foods 2024, 210, 116764. [Google Scholar] [CrossRef]
- Sousa, W.C.; Morais, R.A.; Zuniga, A.D. Buriti (Mauritia flexuosa) shell flour: Nutritional composition, chemical profile, and antioxidant potential as a strategy for valuing waste from native Brazilian fruits. Food Res. Int. 2024, 190, 114578. [Google Scholar] [CrossRef]
- Ribeiro, L.O.; Conrado Thomaz, G.F.; de Brito, M.; de Figueiredo, N.; Przytyk Jung, E.; Norie Kunigami, C. Siriguela peels provide antioxidant compounds-rich extract by solid–liquid extraction. J. Food Process. Preserv. 2020, 44, e14719. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, X.; Zhao, K.; Chen, X.; Zhao, X.; Yang, Z.; Yin, Y.; Wakisaka, M.; Fang, W. Comparative Analysis of Pretreatment Methods for Fruit Waste Valorization in Euglena gracilis Cultivation: Impacts on Biomass, β-1,3-Glucan Production, and Photosynthetic Efficiency. Foods 2024, 13, 3439. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Maggiore, I.; Setti, L. New Biorefinery Approach for the Valorization of Fruit Processing Waste at a Local Scale: Pomegranate Pomace as Case Study. Waste Biomass Valor. 2024. [Google Scholar] [CrossRef]
- Lipiński, A.J.; Lipiński, S.; Kowalkowski, P. Utilization of post-production waste from fruit processing for energetic purposes: Analysis of Polish potential and case study. J. Mater. Cycles Waste Manag. 2018, 20, 1878–1883. [Google Scholar] [CrossRef]
- Jara Ramirez, V.N.; Ocana Gonzales, Z.I.; Lizarzaburu Aguinaga, D.A.; Munoz Ccuro, F.; Roman Perez, H.; Benites-Alfaro, E. Circular Economy: Use of Fruit Waste to Obtain Bioplastics. Chem. Eng. Trans. 2023, 100, 103–108. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Cardona Alzate, C.A.; Solarte-Toro, J.C. Orange Peel Waste as a Source of Bioactive Compounds and Valuable Products: Insights Based on Chemical Composition and Biorefining. Biomass 2024, 4, 107–131. [Google Scholar] [CrossRef]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules 2023, 28, 458. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.P.; Banerjee, A.; Murphy, E. The UN 2030 Agenda for Sustainable Development. Sustain. Dev. Goals Ser. 2022, Part F2740, 1–12. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Fatoumata, C.; Soronikpoho, S.; Souleymane, T.; Kouakou, B.; Marcellin, D.K. Centesimal composition and bioactive compounds in African mustards used as condiments in Ivory Coast. Afr. J. Food Sci. 2016, 10, 87–93. [Google Scholar] [CrossRef]
- Jung, E.P.; Ribeiro, L.O.; Kunigami, C.N.; Figueiredo, E.S.; Nascimento, F.S. Ripe banana peel flour: A Raw Material for the Food Industry. Rev. Virtual Quim. 2019, 11, 1712–1724. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Borges, G.S.C.; da Silva Campelo Borges, L.V.; Gonzaga, L.V.; Oliveira Costa, A.C.; Fett, R.; Silva, M.M. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Res. Int. 2011, 44, 2128–2133. [Google Scholar] [CrossRef]
- Hussain, T.; Kalhoro, D.H.; Yin, Y. Identification of nutritional composition and antioxidant activities of fruit peels as a potential source of nutraceuticals. Front. Nutr. 2023, 9, 1065698. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition (ISSN) Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.C.; Ribeiro, A.B.; Miranda, A.S.; Santana, R.F.; Matos, T.B.; Gomes, J.R.; Santana, J.M.; Milagres, M.P. Physicochemical composition, centesimal, and phytochemical profile of umbu seed flour (Spondias tuberosa Arruda). Food Sci. Technol. 2024, 44, e00242. [Google Scholar] [CrossRef]
- Xavier, V.L.; Feitoza, G.S.; Barbosa, J.M.L.; de Araújo, K.S.; da Silva, M.V.; Correia, M.T.S.; de Souza, M.P.; Carneiro-da-Cunha, M.G. Nutritional and technological potential of Umbu (Spondias tuberosa) processing by-product flour. An. Acad. Bras. Cienc. 2022, 94, e20200940. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.A.; Corrêa, R.C.; Barros, L.; Pereira, C.; Abreu, R.M.; Alves, M.J.; Calhelha, R.C.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Chemical composition and biological activities of Juçara (Euterpe edulis Martius) fruit by-products, a promising underexploited source of high-added value compounds. J. Funct. Foods 2019, 55, 325–332. [Google Scholar] [CrossRef]
- Weaver, C.M. Potassium and Health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine Staff. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997; ISBN 0309063507. [Google Scholar]
- Reddin, C.; Ferguson, J.; Murphy, R.; Clarke, A.; Judge, C.; Griffith, V.; Alvarez, A.; Smyth, A.; Mente, A.; Yusuf, S.; et al. Global mean potassium intake: A systematic review and Bayesian meta-analysis. Eur. J. Nutr. 2023, 62, 2027–2037. [Google Scholar] [CrossRef]
- Marín-Martínez, R.; Barber, X.; Cabrera-Vique, C.; Carbonell-Barrachina, Á.A.; Vilanova, E.; García-Hernández, V.M.; Roche, E.; García-García, E. Aluminium, nickel, cadmium and lead in candy products and assessment of daily intake by children in Spain. Food Addit. Contam. Part B 2015, 9, 66–71. [Google Scholar] [CrossRef]
- Silva, P.P.; Carmo, L.F.; Silva, G.M.; Silveira-Diniz, M.F.; Casemiro, R.C.; Spoto, M.H. Physical, chemical, and lipid composition of juçara (Euterpe edulis Mart.) pulp. Braz. J. Food Nutr. 2013, 24, 345–352. [Google Scholar]
- Schulz, M.; Borges, G.d.S.C.; Gonzaga, L.V.; Tischer Seraglio, S.K.; Olivo, I.S.; Azevedo, M.S.; Nehring, P.; de Gois, J.S.; de Almeida, T.S.; Vitali, L.; et al. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res. Int. 2015, 77, 125–131. [Google Scholar] [CrossRef]
- Riley, T.; Petersen, k.; Kris-Etherton, P. Chapter 9—Health aspects of high-oleic oils. In High Oleic Oils; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–243. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, L.A.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Sima, A.V.; Stancu, C.S. Phenolic Compounds Exerting Lipid-Regulatory, Anti-Inflammatory and Epigenetic Effects as Complementary Treatments in Cardiovascular Diseases. Biomolecules 2020, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the Chemical Composition and Occurring Antioxidants in Jabuticaba (Myrciaria jaboticaba) and Jussara (Euterpe edulis) Fruits and Their Fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
| Samples | ||||||
|---|---|---|---|---|---|---|
| FSS | FSP | FUS | FUP | FUC | FJC | |
| Lipids (%) | 0.35 ± 0.02 b | 0.88 ± 0.04 b | 1.13 ± 0.07 b | 1.08 ± 0.04 b | 0.67 ± 0.04 b | 10.63 ± 1.14 a |
| Moisture (%) | 15.70 ± 0.49 c | 21.05 ± 0.50 a | 9.43 ± 0.18 e | 14.31 ± 0.33 d | 17.69 ± 0.34 b | 9.74 ± 0.20 e |
| Ashes (%) | 2.11 ± 0.05 b,c | 4.17 ± 0.05 a | 1.09 ± 0.01 c | 3.35 ± 0.02 a,b | 2.58 ± 0.02 c | 2.07 ± 0.01 b,c |
| Proteins (%) | 24.01 ± 0.42 a,b | 17.17 ± 0.42 b | 17.68 ± 0.27 b | 25.50 ± 0.59 a,b | 25.04 ± 0.18 a,b | 30.89 ± 0.05 a |
| Carbohydrates (%) | 73.54 ± 2.63 a,b | 77.83 ± 2.77 a,b | 80.23 ± 1.74 a | 70.09 ± 3.78 b | 71.72 ± 1.07 a,b | 57.06 ± 0.56 c |
| Minerals (mg 100 g−1) | Samples | |||||
|---|---|---|---|---|---|---|
| FSS | FSP | FUS | FUP | FUC | FJC | |
| Ca | 239.83 ± 1.87 a | 76.23 ± 1.33 e | 170.37 ± 1.52 d | 216.35 ± 1.12 b | 179.01 ± 0.01 c | 168.50 ± 0.82 d |
| Cu | 27.10 ± 12.25 a | 5.10 ± 0.02 b | 6.61 ± 0.23 a,b | 8.87 ± 0.14 a,b | 1.11 ± 0.04 b | 3.72 ± 0.16 b |
| K | 1257.18 ± 27.29 b | 1403.72 ± 31.56 a | 288.91 ± 3.25 e | 1326.72 ± 24.86 a,b | 924.48 ± 22.47 c | 558.06 ± 34.17 d |
| Mg | 183.49 ± 2.19 a | 147.25 ± 5.19 a,b | 177.73 ± 19.53 a | 145.53 ± 3.14 a,b | 79.13 ± 4.18 c | 129.07 ± 11.81 b |
| Mn | 0.26 ± 0.02 f | 0.97 ± 0.01 d | 0.75 ± 0.04 e | 3.62 ± 0.01 b | 1.55 ± 0.01 c | 13.68 ± 0.05 a |
| Ni | 0.81 ± 0.21 a,b | 0.76 ± 0.00 b | 1.15 ± 0.08 a | 0.91 ± 0.15 a,b | 1.05 ± 0.01 a,b | 0.98 ± 0.01 a,b |
| Na | 6.44 ± 0.37 a | 7.57 ± 0.11 a | 2.78 ± 0.14 b | 1.49 ± 0.72 b,c | 0.88 ± 0.47 c | 2.80 ± 0.47 b |
| Fatty Acids (%) | Samples | |||||
|---|---|---|---|---|---|---|
| FSS | FSP | FUS | FUP | FUC | FJC | |
| (C12:0) Lauric acid | 1.02 ± 0.24 | - | - | - | - | - |
| (C14:0) Myristic acid | 3.11 ± 0.08 | 10.41 ± 0.22 | 0.39 ± 0.01 | 2.27 ± 0.04 | 2.88 ± 0.00 | - |
| (C16:0) Palmitic acid | 29.34 ± 1.04 | 69.31 ± 1.01 | 23.35 ± 0.09 | 42.30 ± 0.09 | 45.68 ± 0.35 | 20.19 ± 0.01 |
| (C16:1) Palmitoleic acid | - | - | - | - | - | 0.62 ± 0.01 |
| (C17:0) Margaric acid | 1.49 ± 0.06 | - | - | - | - | - |
| (C18:0) Stearic acid | 7.27 ± 0.11 | 8.95 ± 0.23 | 11.79 ± 0.08 | 7.19 ± 0.02 | 6.14 ± 0.09 | 2.51 ± 0.02 |
| (C18:1) Oleic acid | 19.42 ± 0.27 | - | 32.63 ± 0.03 | 48.24 ± 0.03 | 45.30 ± 0.26 | 61.58 ± 0.02 |
| (C18:2) Linoleic acid | 24.36 ± 0.24 | 11.33 ± 1.00 | 31.85 ± 0.03 | - | - | 15.09 ± 0.02 |
| (C18:3) Linolenic acid | 10.98 ± 0.70 | - | - | - | - | - |
| (C20:1) Gadolinic acid | 1.77 ± 0.18 | - | - | - | - | - |
| (C22:0) Behenic Acid | 1.19 ± 0.33 | - | - | - | - | - |
| Assays | Samples | |||||
|---|---|---|---|---|---|---|
| FSS | FSP | FUS | FUP | FUC | FJC | |
| TPC (mg GAE 100 g−1) | 249.11 ± 1.86 f | 1491.84 ± 6.11 b | 369.04 ± 2.48 e | 1852.81 ± 10.99 a | 1058.81 ± 14.68 c | 774.66 ± 5.44 d |
| DPPH• (µmol Trolox g−1) | 9.79 ± 0.08 f | 106.70 ± 1.80 b | 19.92 ± 0.21 e | 129.80 ± 1.02 a | 61.52 ± 0.49 c | 26.90 ± 0.42 d |
| ABTS•+ (µmol Trolox g−1) | 12.69 ± 0.20 e | 12.24 ± 0.74 e | 18.76 ± 0.36 d | 130.99 ± 2.78 a | 63.14 ± 1.01 b | 33.81 ± 1.49 c |
| FRAP (µmol Fe2+ g−1) | 39.40 ± 1.18 f | 311.51 ± 1.54 c | 82.38 ± 0.40 e | 591.10 ± 2.73 a | 391.10 ± 2.73 b | 119.78 ± 1.20 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, M.F.; de Freitas, B.P.; de Freitas, J.S.; Lage, L.S.S.; Novo, A.A.; Kunigami, C.N.; Jung, E.P.; Ribeiro, L.O. Tropical Fruit Wastes: Physicochemical Characterization, Fatty Acid Profile and Antioxidant Capacity. Resources 2025, 14, 83. https://doi.org/10.3390/resources14050083
dos Santos MF, de Freitas BP, de Freitas JS, Lage LSS, Novo AA, Kunigami CN, Jung EP, Ribeiro LO. Tropical Fruit Wastes: Physicochemical Characterization, Fatty Acid Profile and Antioxidant Capacity. Resources. 2025; 14(5):83. https://doi.org/10.3390/resources14050083
Chicago/Turabian Styledos Santos, Mariana Ferreira, Beatriz Pereira de Freitas, Jaqueline Souza de Freitas, Luane Souza Silva Lage, Alex Aguiar Novo, Claudete Norie Kunigami, Eliane Przytyk Jung, and Leilson Oliveira Ribeiro. 2025. "Tropical Fruit Wastes: Physicochemical Characterization, Fatty Acid Profile and Antioxidant Capacity" Resources 14, no. 5: 83. https://doi.org/10.3390/resources14050083
APA Styledos Santos, M. F., de Freitas, B. P., de Freitas, J. S., Lage, L. S. S., Novo, A. A., Kunigami, C. N., Jung, E. P., & Ribeiro, L. O. (2025). Tropical Fruit Wastes: Physicochemical Characterization, Fatty Acid Profile and Antioxidant Capacity. Resources, 14(5), 83. https://doi.org/10.3390/resources14050083








