High Field Strength Element (HFSE) and Rare Earth Element (REE) Enrichment in Laterite Deposit of High Background Natural Radiation Area (HBNRA) of Mamuju, West Sulawesi, Indonesia
Abstract
1. Introduction
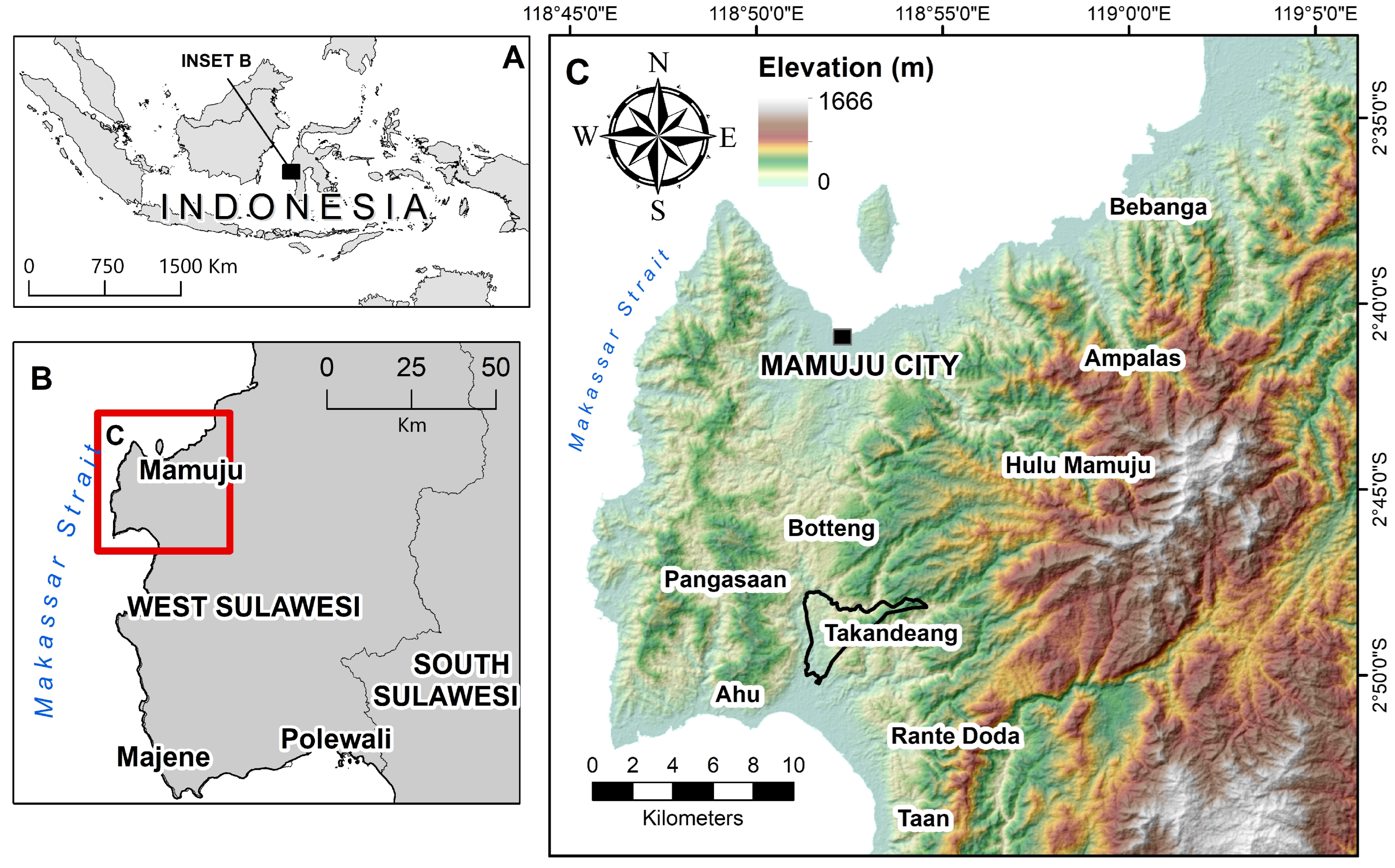
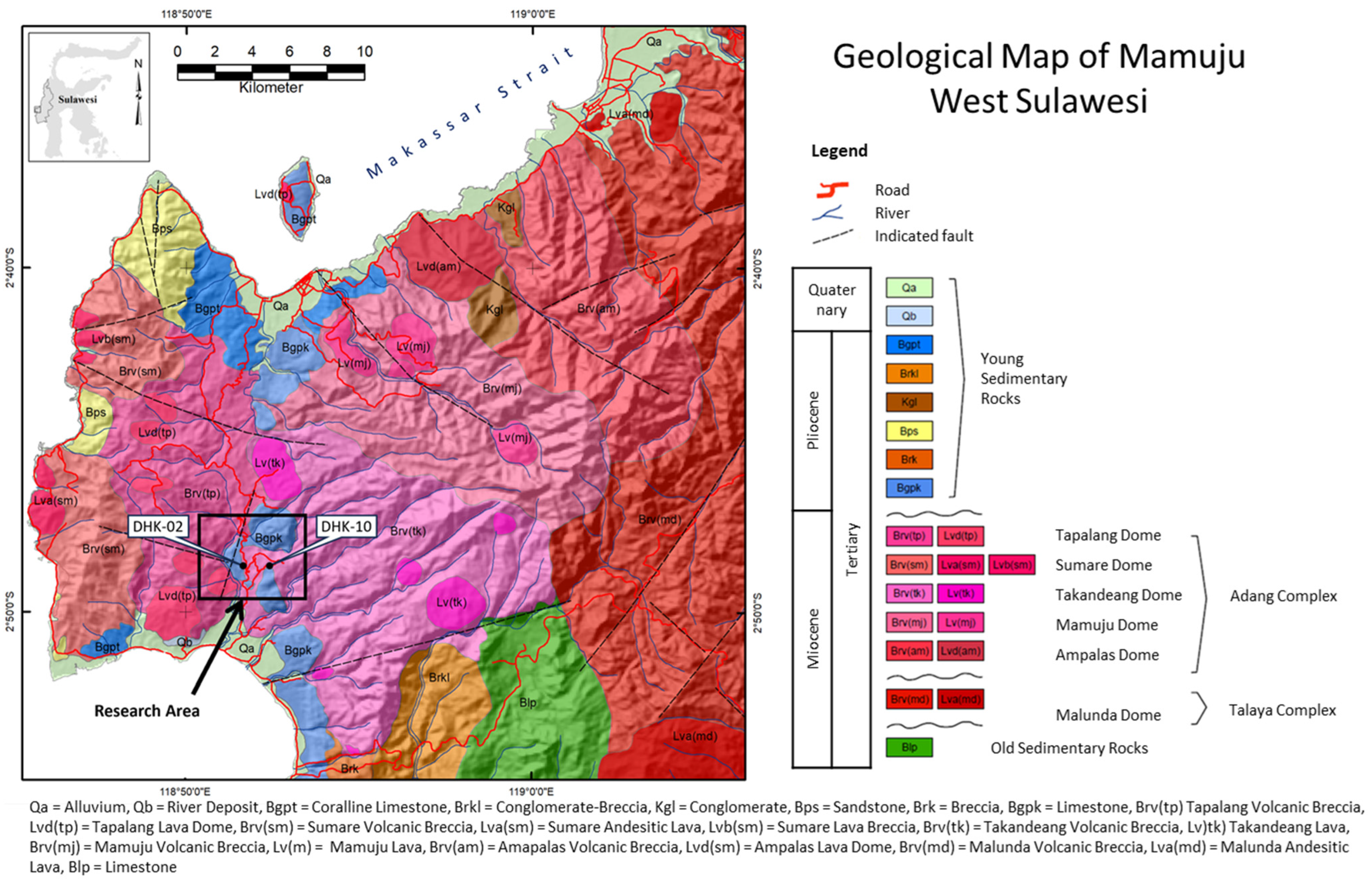
2. Geological Background
3. Materials and Methods
4. Results
4.1. Radionuclide Activity
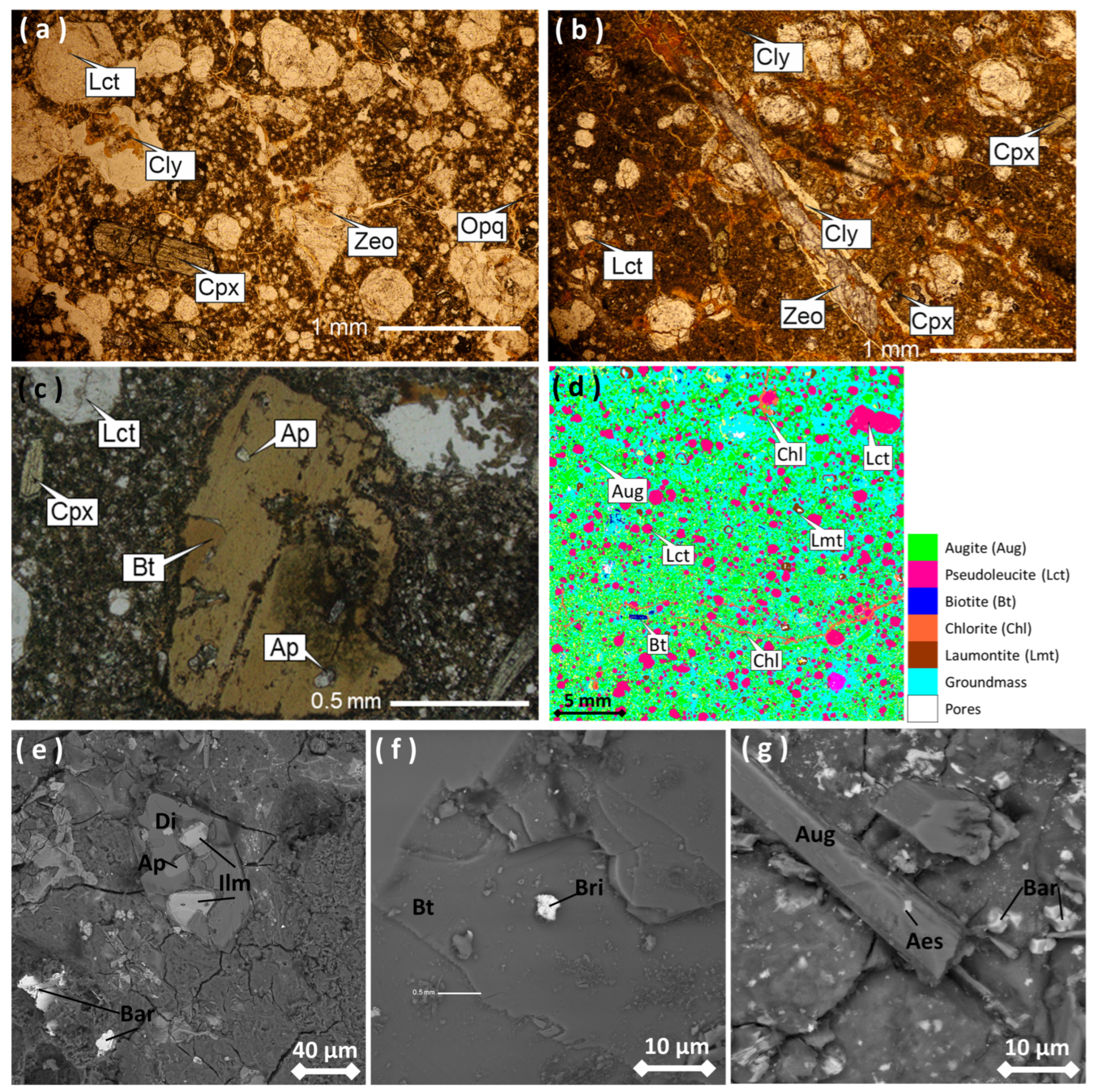
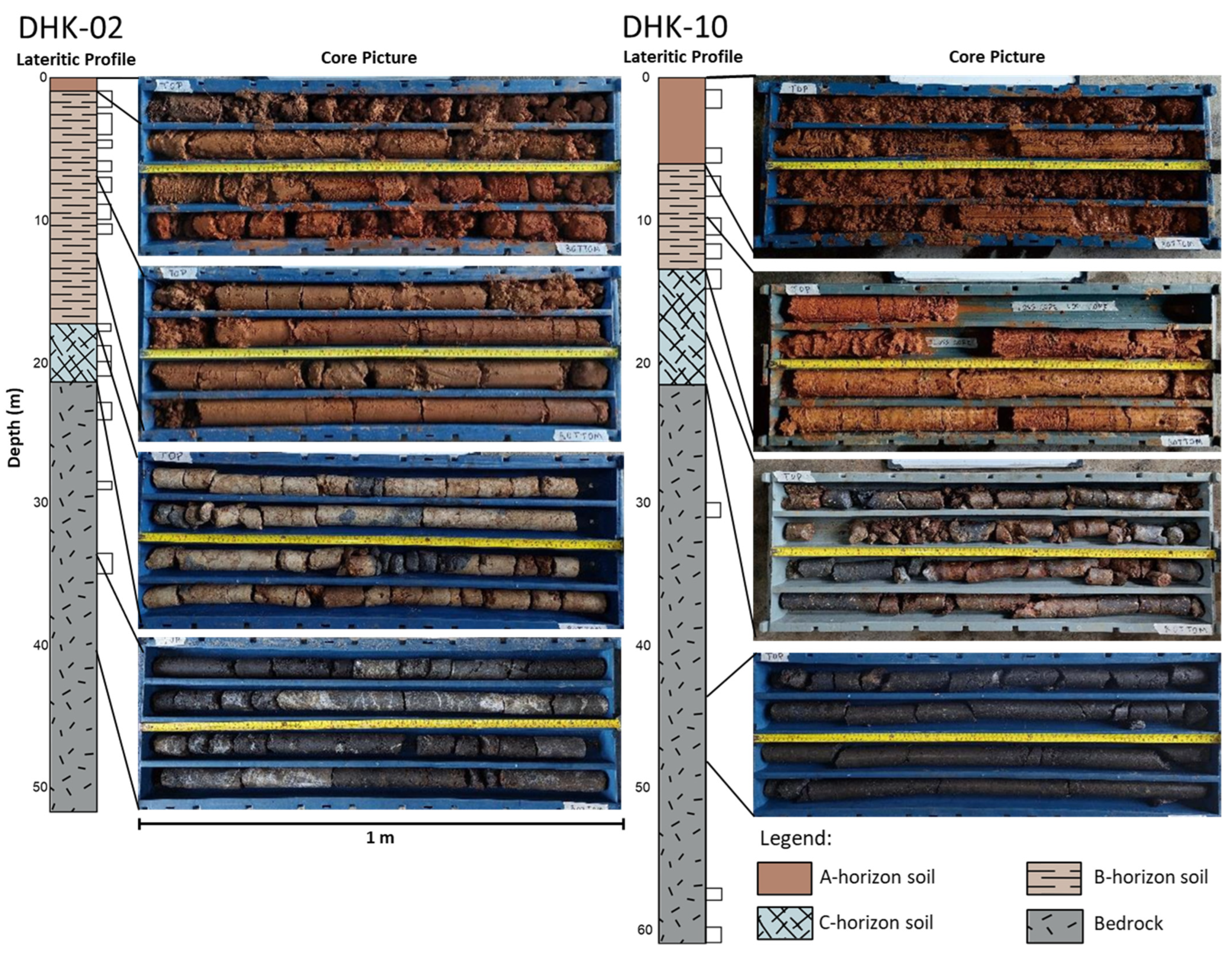
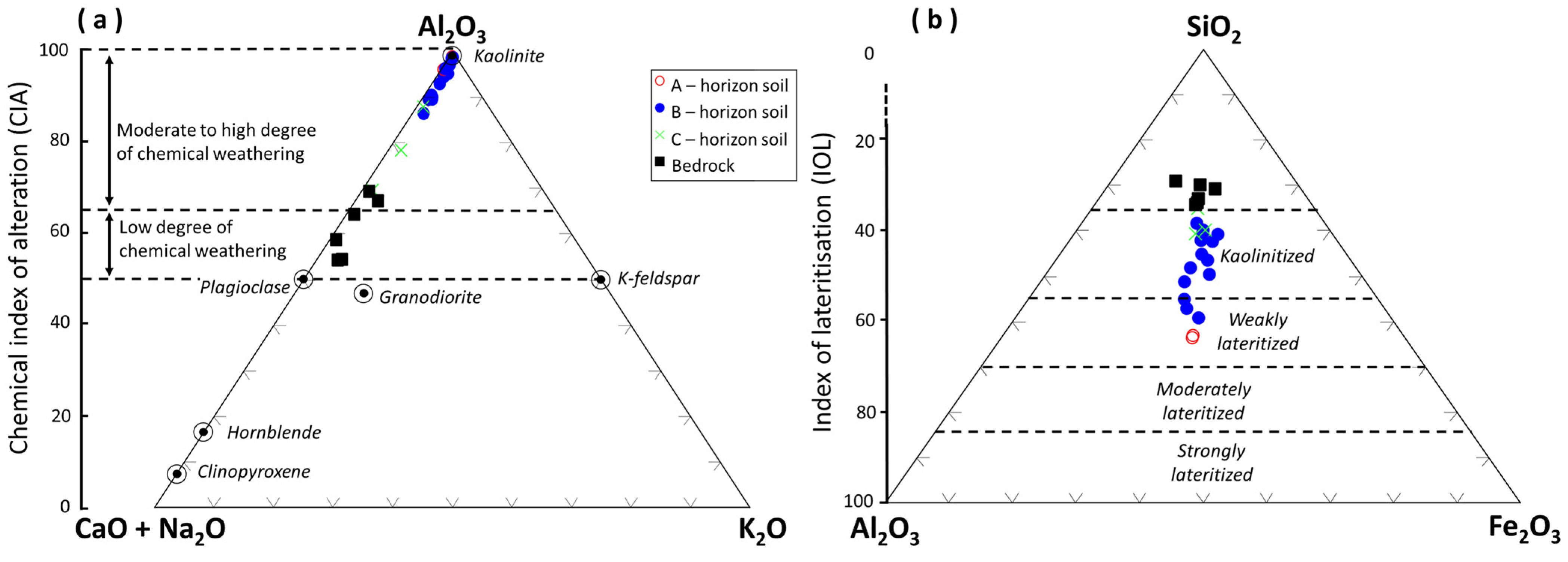
4.2. Lateritic Profile Characteristics
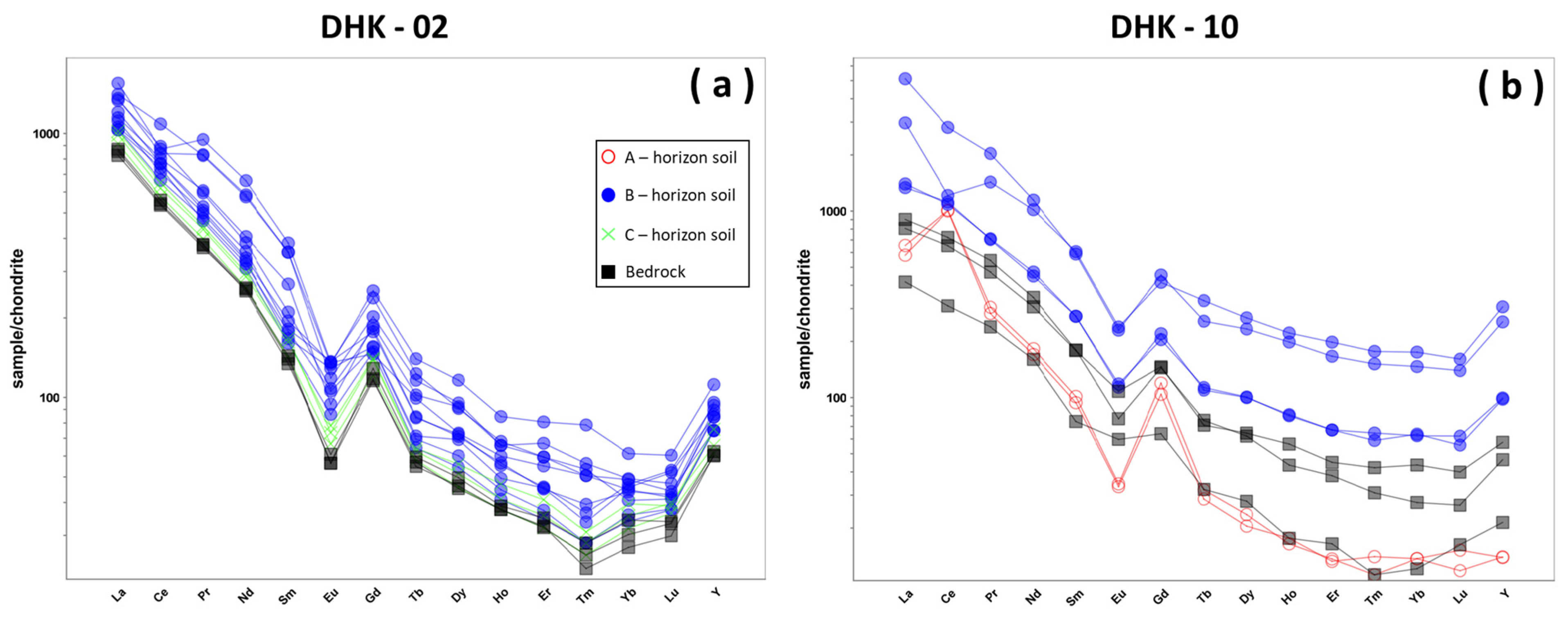
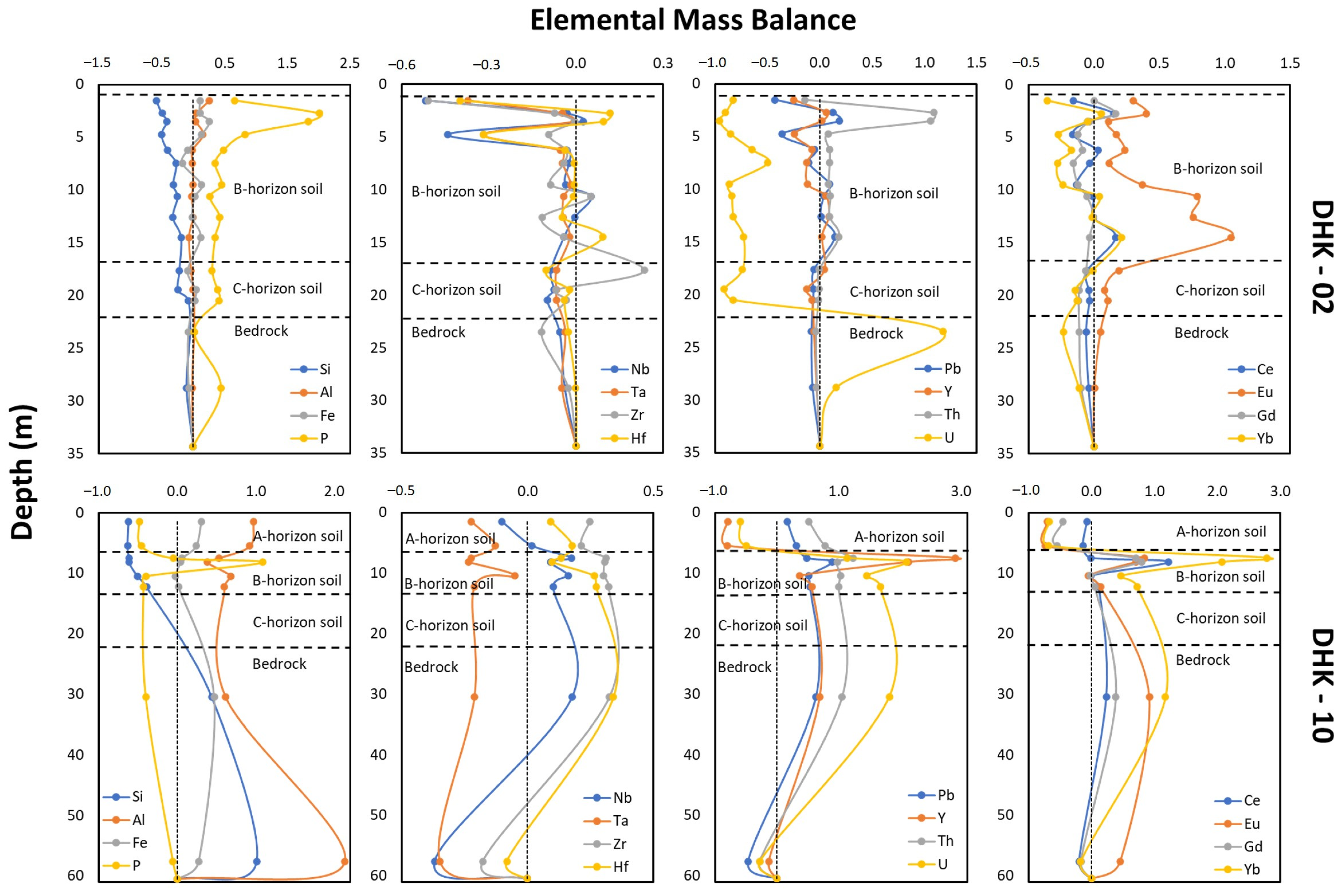
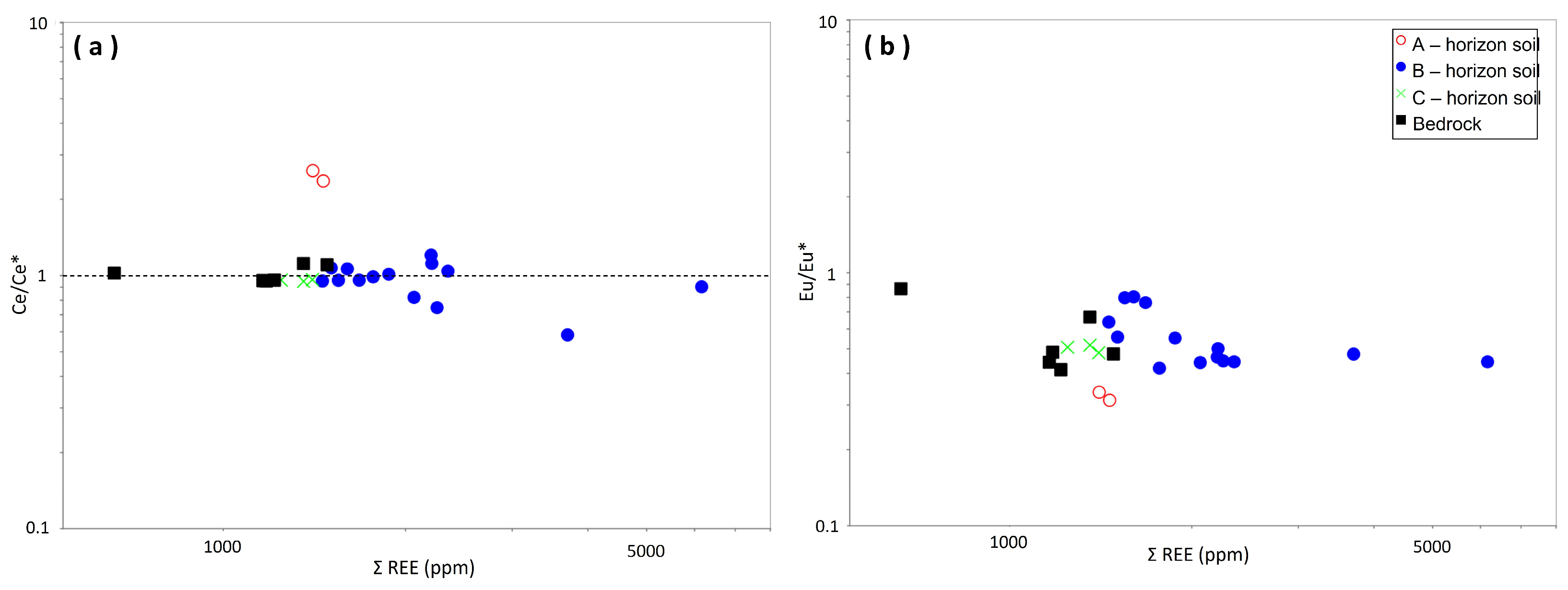
4.3. Geochemical Characteristics

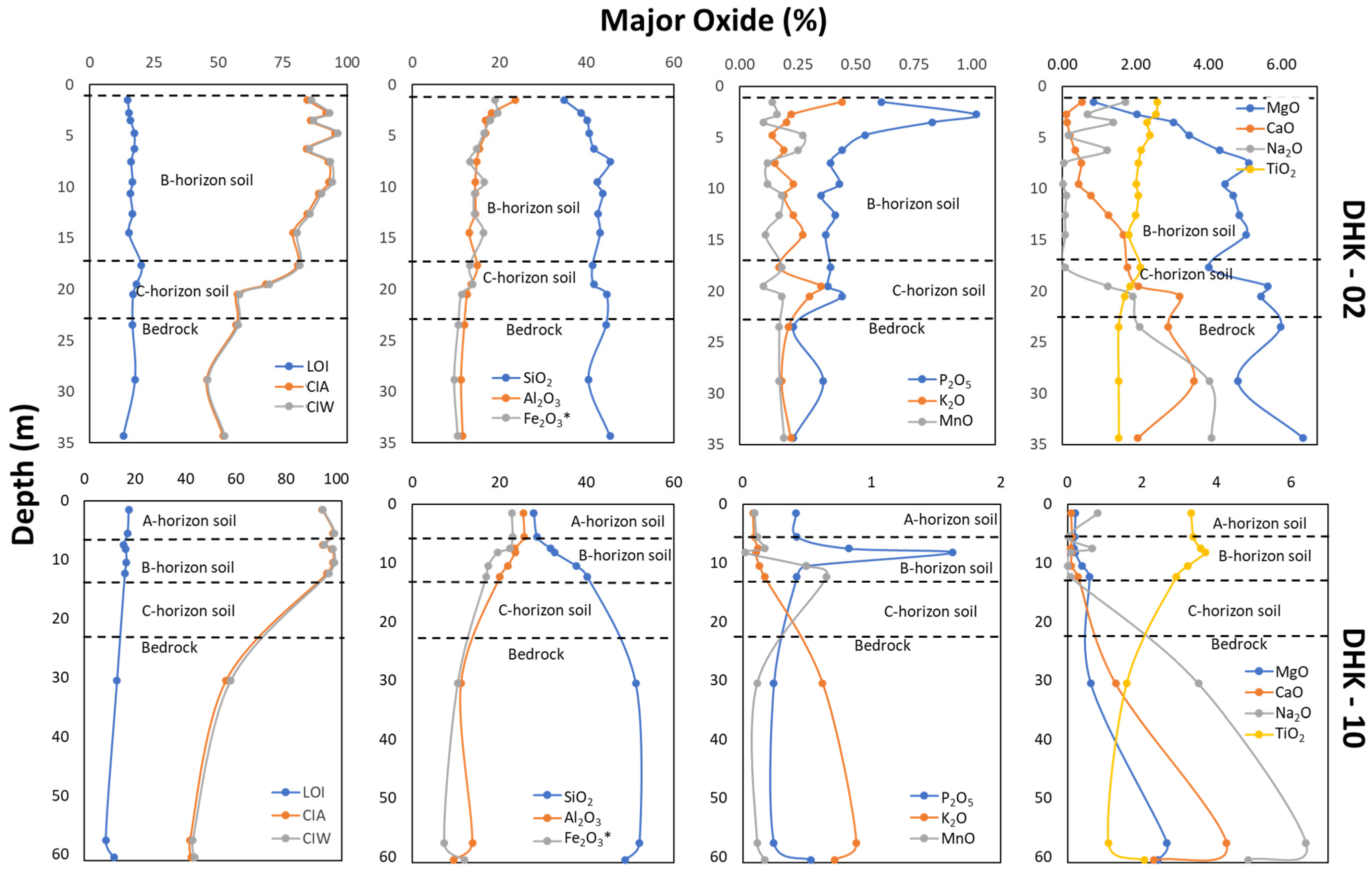
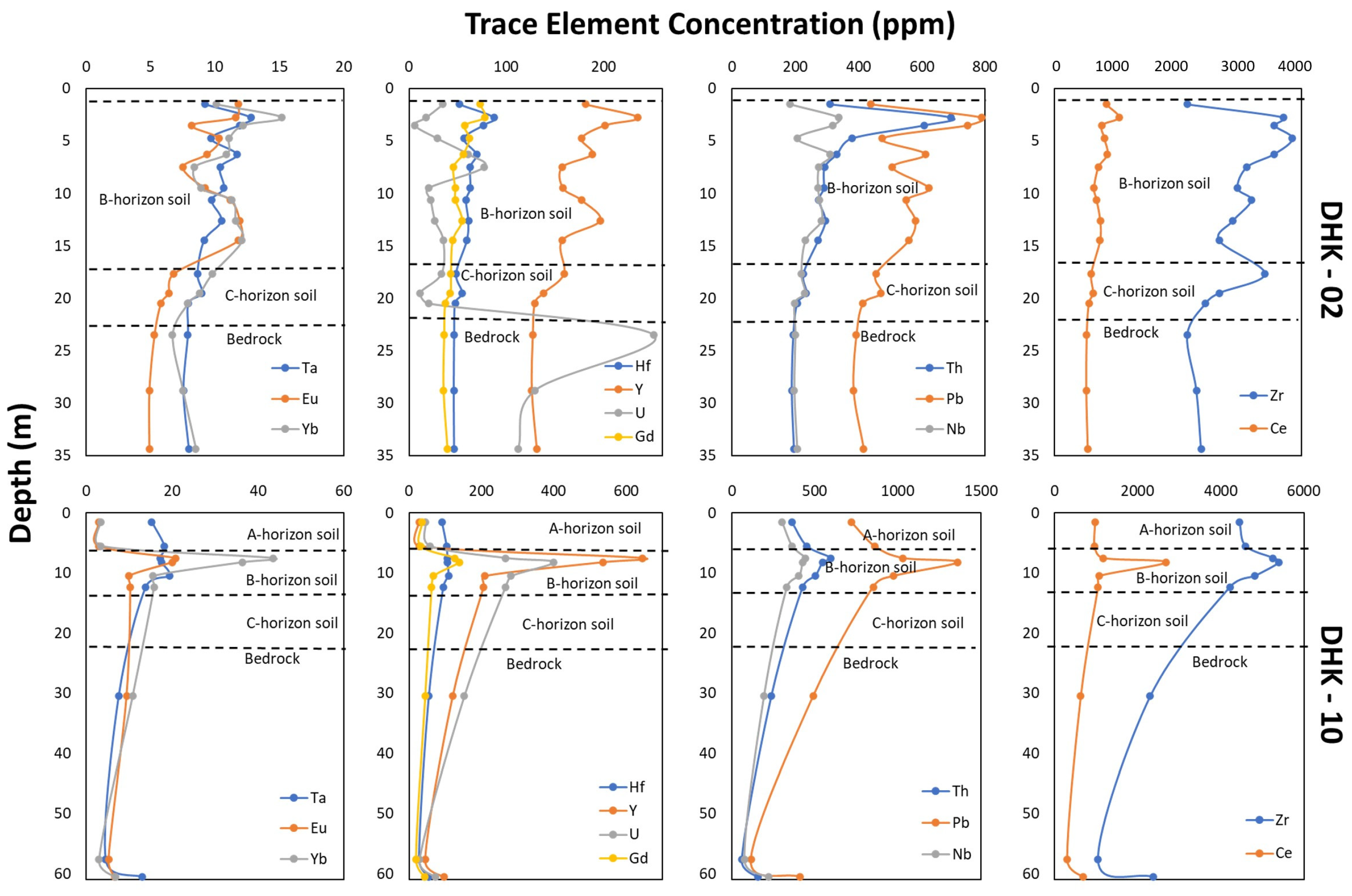
5. Discussion
5.1. Parent Rock Influences on HFSE and REE Enrichment
5.2. Lateritization Process
5.3. Enrichment of REE
5.4. Enrichment of HFSE
5.5. HFSE–REE Potential in HBNRA
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, E.; Rüsch, M. Industry 4.0 and the Current Status as Well as Future Prospects on Logistics. Comput. Ind. 2017, 89, 23–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y. Research Progress on Titanium–Niobium Micro-Alloyed High-Strength Steel. Materials 2025, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.D.; Goodenough, K.M.; Borst, A.M.; Wall, F.; Siegfried, P.R.; Deady, E.A.; Pohl, C.; Hutchison, W.; Finch, A.A.; Walter, B.F.; et al. Alkaline-Silicate REE-HFSE Systems. Econ. Geol. 2023, 118, 177–208. [Google Scholar] [CrossRef]
- Aliyu, A.S.; Ramli, A.T. The World’s High Background Natural Radiation Areas (HBNRAs) Revisited: A Broad Overview of the Dosimetric, Epidemiological and Radiobiological Issues. Radiat. Meas. 2015, 73, 51–59. [Google Scholar] [CrossRef]
- Fathabadi, N.; Salehi, A.A.; Nadda, K.; Kardan, M.R. Public Ingestion Exposure to 226Ra in Ramsar, Iran. J. Environ. Radioact. 2019, 198, 11–17. [Google Scholar] [CrossRef]
- Yang, B.; Tuo, F.; Zhou, Q.; Zhang, J.; Li, Z.; Pang, C. Dietary Exposure of Radionuclides and Heavy Metals in Adult Residents in a High Background Natural Radiation Area Using Duplicate Diet Method. Sci. Rep. 2022, 12, 16676. [Google Scholar] [CrossRef]
- Daling, L.; Chunxiang, Z.; Zujie, G.; Xian, L.; Guorong, H. Gamma-Spectrometric Measurements of Natural-Radionuclide Contents in Soil and Gamma Dose Rates in Yangjiang, PR China. Nucl. Instrum. Methods Phys. Res. A 1990, 299, 687–689. [Google Scholar] [CrossRef]
- Nugraha, E.D.; Hosoda, M.; Kusdiana; Untara; Mellawati, J.; Nurokhim; Tamakuma, Y.; Ikram, A.; Syaifudin, M.; Yamada, R.; et al. Comprehensive Exposure Assessments from the Viewpoint of Health in a Unique High Natural Background Radiation Area, Mamuju, Indonesia. Sci. Rep. 2021, 11, 14578. [Google Scholar] [CrossRef]
- Hosoda, M.; Nugraha, E.D.; Akata, N.; Yamada, R.; Tamakuma, Y.; Sasaki, M.; Kelleher, K.; Yoshinaga, S.; Suzuki, T.; Rattanapongs, C.P.; et al. A Unique High Natural Background Radiation Area–Dose Assessment and Perspectives. Sci. Total Environ. 2021, 750, 142346. [Google Scholar] [CrossRef]
- Rosianna, I.; Nugraha, E.D.; Tazoe, H.; Syaeful, H.; Muhammad, A.G.; Sukadana, I.G.; Indrastomo, F.D.; Ngadenin; Pratiwi, F.; Sumaryanto, A.; et al. Uranium Isotope Characterization in Volcanic Deposits in a High Natural Background Radiation Area, Mamuju, Indonesia. Geosciences 2023, 13, 388. [Google Scholar] [CrossRef]
- Sukadana, I.G.; Warmada, I.W.; Pratiwi, F.; Harijoko, A.; Adimedha, T.B.; Yogatama, A.W. Elemental Mapping for Characterizing of Thorium and Rare Earth Elements (REE) Bearing Minerals Using ΜXRF. Atom Indones. 2022, 48, 87–98. [Google Scholar] [CrossRef]
- Syaeful, H.; Sukadana, I.G.; Sumaryanto, A. Radiometric Mapping for Naturally Occurring Radioactive Materials (NORM) Assessment in Mamuju, West Sulawesi. Atom Indones. 2014, 40, 33–39. [Google Scholar] [CrossRef][Green Version]
- Sukadana, I.G.; Harijoko, A.; Setijadji, L.D. Tataan Tektonika Batuan Gunung Api di Komplek Adang Kabupaten Mamuju Provinsi Sulawesi Barat. Eksplorium 2015, 36, 31. [Google Scholar] [CrossRef]
- Lan, T.; Hao, L.; Lu, J.; Yin, Y.; Chen, X.; Fan, Y.; Zhao, W.; Hou, Y. Geochemical Behavior of Different Chemical Elements during Weathering of the Basalts in Changbai Mountain, Northeast China. Sustainability 2021, 13, 12796. [Google Scholar] [CrossRef]
- Cakrabuana, W.; Ciputra, R.C.; Syaeful, H. Assessing the Undiscovered Resources of Uranium and Thorium in Mamuju, West Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2021, 851, 012041. [Google Scholar] [CrossRef]
- Syaeful, H.; Muhammad, A.G.; Rachael, Y.; Pratiwi, F.; Rosianna, I.; Ngadenin, N.; Indrastomo, F.D.; Ciputra, R.C.; Sukadana, I.G. Delineation of Permissive Tract for 3-Part Quantitative Undiscovered Resources Assessment of Uranium and Thorium in Mamuju, West Sulawesi. AIP Conf. Proc. 2024, 2967, 110013. [Google Scholar]
- Alvionita, A.F.; Syafrizal, S.; Al Hakim, A.Y. Mineralogi Dan Pengayaan REE Tipe Ion-Adsorption Pada Profil Lapukan Granitoid di Sulawesi Barat; Implikasi Terhadap Eksplorasi. J. GEOSAPTA 2023, 9, 95. [Google Scholar] [CrossRef]
- Sukadana, I.G.; Warmada, I.W.; Harijoko, A.; Indrastomo, F.D.; Syaeful, H. The Application of Geostatistical Analysis on Radiometric Mapping Data to Recognized the Uranium and Thorium Anomaly in West Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 819, 012030. [Google Scholar] [CrossRef]
- Pratiwi, F.; Rachael, Y.; Syaeful, H.; Sukadana, I.G.; Indrastomo, F.D. Elemental Mapping Micro-XRF (EMMX) Analysis for Identifying Uranium and Thorium Bearing Minerals in Rantedoda Area, Mamuju, West Sulawesi. AIP Conf. Proc. 2024, 2967, 170004. [Google Scholar]
- Ritonga, R.; Maulana, A.; Tonggiroh, A. Rare Earth Elements Distribution in Weathered Volcanic Rock from Mamuju Area, West Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2021, 921, 012039. [Google Scholar] [CrossRef]
- Hall, R.; Sevastjanova, I. Australian Crust in Indonesia. Aust. J. Earth Sci. 2012, 59, 827–844. [Google Scholar] [CrossRef]
- Elburg, M.; van Leeuwen, T.; Foden, J. Muhardjo Spatial and Temporal Isotopic Domains of Contrasting Igneous Suites in Western and Northern Sulawesi, Indonesia. Chem. Geol. 2003, 199, 243–276. [Google Scholar] [CrossRef]
- Simandjuntak, T.O. Sedimentology and Tectonics of the Collision Complex in the East Arm of Sulawesi. Ph.D. Thesis, University of London, London, UK, 1986. [Google Scholar]
- Guntoro, A. The Formation of the Makassar Strait and the Separation between SE Kalimantan and SW Sulawesi. J. Asian Earth Sci. 1999, 17, 79–98. [Google Scholar] [CrossRef]
- Polvé, M.; Maury, R.C.; Bellon, H.; Rangin, C.; Priadi, B.; Yuwono, S.; Joron, J.L.; Atmadja, R.S. Magmatic Evolution of Sulawesi (Indonesia): Constraints on the Cenozoic Geodynamic History of the Sundaland Active Margin. Tectonophysics 1997, 272, 69–92. [Google Scholar] [CrossRef]
- van Leeuwen, T.M. Muhardjo Stratigraphy and Tectonic Setting of the Cretaceous and Paleogene Volcanic-Sedimentary Successions in Northwest Sulawesi, Indonesia: Implications for the Cenozoic Evolution of Western and Northern Sulawesi. J. Asian Earth Sci. 2005, 25, 481–511. [Google Scholar] [CrossRef]
- Hennig, J.; Hall, R.; Armstrong, R.A. U-Pb Zircon Geochronology of Rocks from West Central Sulawesi, Indonesia: Extension-Related Metamorphism and Magmatism during the Early Stages of Mountain Building. Gondwana Res. 2016, 32, 41–63. [Google Scholar] [CrossRef]
- Hennig, J.; Hall, R.; Forster, M.A.; Kohn, B.P.; Lister, G.S. Rapid Cooling and Exhumation as a Consequence of Extension and Crustal Thinning: Inferences from the Late Miocene to Pliocene Palu Metamorphic Complex, Sulawesi, Indonesia. Tectonophysics 2017, 712–713, 600–622. [Google Scholar] [CrossRef]
- Coffield, D.Q.; Bergman, S.C.; Garrard, R.A.; Guritno, N.; Robinson, N.M.; Talbot, J. Tectonic and Stratigraphic Evolution of the Kalosi PSC Area and Associated Development of a Tertiary Petroleum System, South Sulawesi, Indonesia. In Proceedings of the Indonesian Petroleum Association 22nd Annual Convention, Jakarta, Indonesia, 13–14 October 1993; pp. 679–706. [Google Scholar]
- Calvert, S.J.; Hall, R. Cenozoic Evolution of the Lariang and Karama Regions, North Makassar Basin Western Sulawesi, Indonesia. Pet. Geosci. 2007, 13, 353–368. [Google Scholar] [CrossRef]
- Ratman, N.; Atmawinata, S. Geological Map of The Mamuju Quadrangle, Sulawesi; Geological Research and Development Centre: Bandung, Indonesia, 1993. [Google Scholar]
- Sukadana, I.G. Magma Evolution and Radioactive Minerals Enrichment in Adang Volcanic Rocks in Mamuju, West Sulawesi. Ph.D. Thesis, Universitas Gadjah Mada, Sleman, Indonesia, 2023. [Google Scholar]
- Rosianna, I.; Nugraha, E.D.; Syaeful, H.; Putra, S.; Hosoda, M.; Akata, N.; Tokonami, S. Natural Radioactivity of Laterite and Volcanic Rock Sample for Radioactive Mineral Exploration in Mamuju, Indonesia. Geosciences 2020, 10, 376. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Saleh, I.H. Radiological and Mineralogical Investigation of Accretion and Erosion Coastal Sediments in Nile Delta Region, Egypt. J. Oceanogr. Mar. Sci. 2012, 3, 41–55. [Google Scholar] [CrossRef]
- Reguigui, N. Gamma Ray Spectrometry Practical Information; Center National des science et Technology Nuclearies: Tunis, Tunisia, 2014. [Google Scholar]
- Nesbitt, H.W. Mobility and Fractionation of Rare Earth Elements during Weathering of a Granodiorite. Nature 1979, 279, 206–210. [Google Scholar] [CrossRef]
- Jiang, K.; Qi, H.W.; Hu, R.Z. Element Mobilization and Redistribution under Extreme Tropical Weathering of Basalts from the Hainan Island, South China. J. Asian Earth Sci. 2018, 158, 80–102. [Google Scholar] [CrossRef]
- Yousefifard, M.; Ayoubi, S.; Jalalian, A.; Khademi, H.; Makkizadeh, M.A. Mass Balance of Major Elements in Relation to Weathering in Soils Developed on Igneous Rocks in a Semiarid Region, Northwestern Iran. J. Mt. Sci. 2012, 9, 41–58. [Google Scholar] [CrossRef]
- Hong, H.; Wang, J.; Wang, C.; Liu, C.; Algeo, T.J.; Zhao, L.; Zhou, L.; Zhang, D.; Fang, Q. Clay and Fe (Oxyhydr)Oxide Mineralogy in the Basalt Weathering Profile in Hainan (Southern China): Implications for Pedogenesis Process. Clays Clay Miner. 2024, 72, e8. [Google Scholar] [CrossRef]
- Lima, A.P.B.; Inda, A.V.; Zinn, Y.L.; do Nascimento, P.C. Weathering Sequence of Soils along a Basalt-Sandstone Toposequence in the Brazilian Cerrado. Geoderma 2021, 394, 115009. [Google Scholar] [CrossRef]
- Dzombak, R.M.; Sheldon, N.D. Weathering Intensity and Presence of Vegetation Are Key Controls on Soil Phosphorus Concentrations: Implications for Past and Future Terrestrial Ecosystems. Soil Syst. 2020, 4, 73. [Google Scholar] [CrossRef]
- Guagliardi, I.; Zuzolo, D.; Albanese, S.; Lima, A.; Cerino, P.; Pizzolante, A.; Thiombane, M.; De Vivo, B.; Cicchella, D. Uranium, Thorium and Potassium Insights on Campania Region (Italy) Soils: Sources Patterns Based on Compositional Data Analysis and Fractal Model. J. Geochemical Explor. 2020, 212, 106508. [Google Scholar] [CrossRef]
- Godang, S.; Idrus, A.; Fadlin, B.P.; Basuki, N.I. Saprolitization Characteristics of Rare Earth Elements in Volcanic Regolith on Drill Core #65 in Western Sulawesi, Indonesia. Asian J. Appl. Sci. 2019, 7, 435–450. [Google Scholar] [CrossRef]
- Tiomo, I.F.; Tematio, P.; Momo, M.N.; Happi, F.D.; Guimapi, N.T.; Tchaptchet, W.C. Mineralogical and Geochemical Evolution of Pre-Lateritic Soil Profiles over Schist Basement of the Lom Series (Bétaré-Oya, East Cameroon): Implication to Rock Weathering and Lithologic Constraints on Trace Elements Fractionation. J. Afr. Earth Sci. 2021, 176, 104133. [Google Scholar] [CrossRef]
- Saputro, S.P.; Godang, S.; Priadi, B.; Basuki, N.I.; Himawan, B. Geochemical Study of Al–Fe–Ti Enrichment in Rock Weathering: Implications for the Recognizing of Igneous Protolith and the Enrichment of REE in Soil Profile. Appl. Geochem. 2022, 140, 105259. [Google Scholar] [CrossRef]
- Yin, H.; Tan, N.; Liu, C.; Wang, J.; Liang, X.; Qu, M.; Feng, X.; Qiu, G.; Tan, W.; Liu, F. The Associations of Heavy Metals with Crystalline Iron Oxides in the Polluted Soils around the Mining Areas in Guangdong Province, China. Chemosphere 2016, 161, 181–189. [Google Scholar] [CrossRef]
- Patel, K.S.; Sharma, S.; Maity, J.P.; Martín-Ramos, P.; Fiket, Ž.; Bhattacharya, P.; Zhu, Y. Occurrence of Uranium, Thorium and Rare Earth Elements in the Environment: A Review. Front. Environ. Sci. 2023, 10, 1058053. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Streckeisen, A.; Zanettin, B. A Chemical Classification of Volcanic Rocks Based on the Total Alkali-Silica Diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Li, Y.H.M.; Zhao, W.W.; Zhou, M.F. Nature of Parent Rocks, Mineralization Styles and Ore Genesis of Regolith-Hosted REE Deposits in South China: An Integrated Genetic Model. J. Asian Earth Sci. 2017, 148, 65–95. [Google Scholar] [CrossRef]
- Gan, L.; Yan, B.; Liu, Y.; Gao, Y.; Yin, C.; Zhu, L.; Tan, S.; Ding, D.; Jiang, H. Geochemical and Mineralogical Characteristics of Ion-Adsorption Type REE Mineralization in the Mosuoying Granite, Panxi Area, Southwest China. Minerals 2023, 13, 1449. [Google Scholar] [CrossRef]
- Singh, B.K.; Um, W. Application of Clay Materials for Sorption of Radionuclides from Waste Solutions. Minerals 2023, 13, 239. [Google Scholar] [CrossRef]
- Norris, S. Multiple Roles of Clays in Radioactive Waste Confinement–Introduction. Geol. Soc. Spec. Publ. 2019, 482, 1–9. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Li, G.; Wen, S.; Yu, R.; Tang, C.; Feng, X.; Liu, X. Characteristics of Sandstone-Type Uranium Mineralization in the Hangjinqi Region of the Northeastern Ordos Basin: Clues from Clay Mineral Studies. Ore Geol. Rev. 2022, 141, 104642. [Google Scholar] [CrossRef]
- Grangeon, S.; Roux, C.; Lerouge, C.; Chardon, P.; Beuzeval, R.; Montavon, G.; Claret, F.; Grangeon, T. Geochemical and Mineralogical Characterization of Streams and Wetlands Downstream a Former Uranium Mine (Rophin, France). Appl. Geochem. 2023, 150, 105586. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Prediction of Some Weathering Trends of Plutonic and Volcanic Rocks Based on Thermodynamic and Kinetic Considerations. Geochim. Cosmochim. Acta 1984, 48, 1523–1534. [Google Scholar] [CrossRef]
- Lara, M.C.; Buss, H.L.; Pett-Ridge, J.C. The Effects of Lithology on Trace Element and REE Behavior during Tropical Weathering. Chem. Geol. 2018, 500, 88–102. [Google Scholar] [CrossRef]
- Yusoff, Z.M.; Ngwenya, B.T.; Parsons, I. Mobility and Fractionation of REEs during Deep Weathering of Geochemically Contrasting Granites in a Tropical Setting, Malaysia. Chem. Geol. 2013, 349–350, 71–86. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, Z. Geochemistry of Mineralization with Exchangeable REY in the Weathering Crusts of Granitic Rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Laveuf, C.; Cornu, S. A Review on the Potentiality of Rare Earth Elements to Trace Pedogenetic Processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Braun, J.J.; Muller, J.P.; Bilong, P.; Michard, A.; Guillet, B. Cerium Anomalies in Laterites. Geochim. Cosmochim. Acta. 1990, 54, 781–795. [Google Scholar] [CrossRef]
- Dinali, G.S.; Root, R.A.; Amistadi, M.K.; Chorover, J.; Lopes, G.; Guilherme, L.R.G. Rare Earth Elements (REY) Sorption on Soils of Contrasting Mineralogy and Texture. Environ. Int. 2019, 128, 279–291. [Google Scholar] [CrossRef]
- Jo, J.; Shin, D. Geochemical Characteristics of REE-Enriched Weathered Anorthosite Complex in Hadong District, South Korea. Geochem. J. 2023, 57, 13–27. [Google Scholar] [CrossRef]
- Ghosh, M.; Swain, K.K.; Verma, R. Interaction of Niobium with Iron-Oxide Colloids and the Role of Humic Acid. J. Environ. Radioact. 2017, 178–179, 101–109. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Chemical Aspects of Uranium Behavior in Soils: A Review. Eurasian Soil Sci. 2011, 44, 862–873. [Google Scholar] [CrossRef]
- Jun, T.; Jingqun, Y.; Ruan, C.; Guohua, R.; Mintao, J.; Kexian, O. Kinetics on Leaching Rare Earth from the Weathered Crust Elution-Deposited Rare Earth Ores with Ammonium Sulfate Solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, Z.; Goldfarb, R.J.; Guo, X.; Wang, L. Rare Earth Element Deposits in China. Rare Earth Crit. Elem. Ore Depos. 2016, 18, 115–136. [Google Scholar]
- Kanazawa, Y.; Kamitani, M. Rare Earth Minerals and Resources in the World. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Simandl, G.J. Geology and Market-Dependent Significance of Rare Earth Element Resources. Miner. Depos. 2014, 49, 889–904. [Google Scholar] [CrossRef]
- Omega, R.L.; Abdullah, A.R.A.; Permana, S.; Srigutomo, W.; Maulana, A.; Seno, H.; Humolungo, I.; Nasyidiyah, F.I.; Zulfahmi; Rejeki, F.; et al. Examining External Dose Rates in Mamuju Regency, Indonesia: A Personal Radiation Dosimetry Approach. J. Eng. Technol. Sci. 2023, 55, 681–688. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Ault, T.; Krahn, S.; Croff, A. Radiological Impacts and Regulation of Rare Earth Elements in Non-Nuclear Energy Production. Energies 2015, 8, 2066–2081. [Google Scholar] [CrossRef]


| Sample Code | Radionuclide Activity (Bq/kg) | ||
|---|---|---|---|
| Ra-226 | Th-232 | K-40 | |
| TKD-02 | 2766.14 ± 61.31 | 1134.20 ± 24.81 | 75.93 ± 4.09 |
| TKD-01 | 2883.85 ± 63.95 | 1529.18 ± 33.35 | 105.19 ± 4.77 |
| Rantedunia | 502.75 ± 11.31 | 1236.54 ± 26.90 | 78.19 ± 2.91 |
| Palada | 729.54 ± 16.32 | 1136.75 ± 24.78 | 79.74 ± 3.08 |
| No | Drill Hole | Horizon | Depth (m) | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MnO (%) | MgO (%) | CaO (%) | Na2O (%) | K2O (%) | TiO2 (%) | P2O5 (%) | LOI | Total Oxide (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DHK-02 | B-horizon soil | 1.5 | 34.87 | 23.61 | 18.94 | 0.14 | 0.85 | 0.53 | 1.72 | 0.44 | 2.61 | 0.61 | 14.87 | 99.19 |
| 2 | 2.75 | 38.78 | 18.26 | 19.69 | 0.16 | 2.04 | 0.11 | 0.69 | 0.22 | 2.56 | 1.02 | 15.36 | 98.89 | ||
| 3 | 3.5 | 40.17 | 16.86 | 17.83 | 0.10 | 3.05 | 0.13 | 1.40 | 0.20 | 2.32 | 0.83 | 15.92 | 98.81 | ||
| 4 | 4.75 | 40.66 | 16.85 | 16.44 | 0.27 | 3.48 | 0.21 | 0.16 | 0.14 | 2.39 | 0.54 | 17.36 | 98.50 | ||
| 5 | 6.25 | 41.75 | 15.36 | 14.82 | 0.25 | 4.32 | 0.35 | 1.22 | 0.19 | 2.16 | 0.44 | 17.25 | 98.11 | ||
| 6 | 7.5 | 45.41 | 14.81 | 13.25 | 0.12 | 5.12 | 0.52 | 0.04 | 0.15 | 2.09 | 0.39 | 16.17 | 98.07 | ||
| 7 | 9.5 | 42.53 | 14.48 | 16.57 | 0.12 | 4.46 | 0.45 | 0.02 | 0.23 | 2.02 | 0.43 | 16.69 | 98.00 | ||
| 8 | 10.65 | 43.75 | 14.44 | 14.36 | 0.18 | 4.69 | 0.77 | 0.10 | 0.19 | 2.08 | 0.35 | 15.80 | 96.71 | ||
| 9 | 12.6 | 42.64 | 14.47 | 14.31 | 0.17 | 4.86 | 1.26 | 0.07 | 0.23 | 2.01 | 0.41 | 16.65 | 97.08 | ||
| 10 | 14.5 | 43.09 | 13.10 | 16.38 | 0.11 | 5.03 | 1.67 | 0.08 | 0.27 | 1.82 | 0.37 | 15.26 | 97.18 | ||
| 11 | C-horizon soil | 17.65 | 41.34 | 14.94 | 13.19 | 0.18 | 4.02 | 1.78 | 0.07 | 0.17 | 2.14 | 0.39 | 20.14 | 98.36 | |
| 12 | 19.5 | 41.63 | 13.50 | 13.90 | 0.10 | 5.64 | 2.09 | 1.24 | 0.35 | 1.85 | 0.38 | 18.07 | 98.75 | ||
| 13 | 20.5 | 44.59 | 12.58 | 11.34 | 0.18 | 5.45 | 3.21 | 1.94 | 0.30 | 1.71 | 0.44 | 16.84 | 98.58 | ||
| 14 | Bedrock | 23.5 | 44.46 | 11.93 | 10.62 | 0.17 | 5.99 | 2.90 | 2.12 | 0.21 | 1.54 | 0.23 | 16.66 | 96.83 | |
| 15 | 28.8 | 40.42 | 11.20 | 9.68 | 0.17 | 4.81 | 3.60 | 4.03 | 0.18 | 1.54 | 0.36 | 17.60 | 93.59 | ||
| 16 | 34.35 | 45.41 | 11.59 | 10.50 | 0.19 | 6.61 | 2.07 | 4.09 | 0.22 | 1.55 | 0.23 | 13.09 | 95.55 | ||
| 17 | DHK-10 | A-horizon soil | 1.5 | 27.90 | 25.56 | 22.87 | 0.09 | 0.21 | 0.10 | 0.80 | 0.08 | 3.32 | 0.41 | 17.52 | 98.86 |
| 18 | 5.5 | 28.67 | 25.65 | 23.08 | 0.11 | 0.19 | 0.12 | 0.02 | 0.09 | 3.38 | 0.42 | 17.08 | 98.81 | ||
| 19 | B-horizon soil | 7.5 | 31.79 | 23.54 | 22.36 | 0.17 | 0.16 | 0.08 | 0.66 | 0.12 | 3.58 | 0.82 | 15.38 | 98.66 | |
| 20 | 8.2 | 32.64 | 23.63 | 19.63 | 0.02 | 0.20 | 0.12 | 0.08 | 0.10 | 3.70 | 1.63 | 16.32 | 98.07 | ||
| 21 | 10.5 | 37.63 | 21.96 | 17.36 | 0.49 | 0.38 | 0.10 | 0.01 | 0.13 | 3.23 | 0.49 | 16.68 | 98.46 | ||
| 22 | 12.3 | 40.19 | 20.15 | 16.98 | 0.65 | 0.58 | 0.28 | 0.07 | 0.17 | 2.92 | 0.42 | 16.03 | 98.44 | ||
| 23 | Bedrock | 30.5 | 51.31 | 11.23 | 10.45 | 0.11 | 0.62 | 1.29 | 3.51 | 0.62 | 1.58 | 0.24 | 12.89 | 93.85 | |
| 24 | 57.6 | 52.17 | 13.79 | 7.37 | 0.11 | 2.67 | 4.26 | 6.40 | 0.88 | 1.09 | 0.24 | 8.37 | 97.35 | ||
| 25 | 60.5 | 48.82 | 9.47 | 12.04 | 0.17 | 2.43 | 2.31 | 4.85 | 0.71 | 2.06 | 0.53 | 11.76 | 95.15 |
| No | Drillhole | Horizon | Depth (m) | Zr (ppm) | Hf (ppm) | Nb (ppm) | Pb (ppm) | Ta (ppm) | Th (ppm) | U (ppm) | Y (ppm) | Ce (ppm) | Dy (ppm) | Er (ppm) | Eu (ppm) | Gd (ppm) | Ho (ppm) | La (ppm) | Lu (ppm) | Nd (ppm) | Pr (ppm) | Sm (ppm) | Tb (ppm) | Tm (ppm) | Yb (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DHK-02 | B | 1.50 | 2146 | 51 | 184 | 438 | 9.2 | 310 | 34.9 | 181 | 835 | 34.6 | 14.8 | 11.8 | 72.9 | 5.8 | 569 | 1.5 | 471 | 130 | 88.8 | 7.1 | 1.9 | 10.1 |
| 2 | 2.75 | 3701 | 87 | 337 | 788 | 12.8 | 692 | 17.8 | 235 | 1040 | 44.3 | 20.1 | 11.6 | 77.6 | 7.2 | 516 | 2.3 | 409 | 113 | 81.9 | 8.1 | 2.8 | 15.2 | ||
| 3 | 3.50 | 3553 | 76 | 318 | 744 | 11.9 | 608 | 5.9 | 201 | 768 | 35.1 | 16.7 | 8.2 | 57.4 | 5.6 | 421 | 1.8 | 289 | 83 | 62.1 | 5.9 | 2.0 | 12.2 | ||
| 4 | 4.75 | 3849 | 56 | 206 | 475 | 9.7 | 379 | 28.9 | 177 | 805 | 36.3 | 14.8 | 10.3 | 61.9 | 5.6 | 498 | 1.6 | 416 | 114 | 82.1 | 6.7 | 1.8 | 11.1 | ||
| 5 | 6.25 | 3553 | 69 | 310 | 611 | 11.7 | 332 | 60.6 | 188 | 855 | 27.1 | 11.2 | 9.4 | 55.7 | 4.8 | 490 | 1.6 | 274 | 812 | 48.6 | 4.9 | 1.4 | 10.9 | ||
| 6 | 7.50 | 3109 | 63 | 275 | 507 | 10.4 | 293 | 77.0 | 157 | 708 | 20.7 | 8.6 | 7.5 | 45.6 | 3.5 | 379 | 1.4 | 219 | 64 | 37.1 | 3.7 | 1.0 | 8.4 | ||
| 7 | 9.50 | 2961 | 63 | 272 | 621 | 10.7 | 290 | 19.9 | 158 | 637 | 22.9 | 9.3 | 9.2 | 47.3 | 3.8 | 379 | 1.4 | 228 | 65 | 41.0 | 4.0 | 1.0 | 8.9 | ||
| 8 | 10.65 | 3183 | 58 | 277 | 551 | 9.7 | 275 | 22.3 | 177 | 680 | 26.4 | 11.3 | 11.2 | 47.7 | 4.2 | 409 | 2.0 | 234 | 69 | 38.6 | 4.1 | 1.2 | 11.3 | ||
| 9 | 12.60 | 2887 | 61 | 283 | 579 | 10.5 | 295 | 26.6 | 196 | 737 | 27.6 | 11.4 | 11.9 | 54.3 | 4.7 | 442 | 2.0 | 254 | 72 | 41.9 | 4.9 | 1.3 | 11.6 | ||
| 10 | 14.50 | 2665 | 59 | 232 | 558 | 9.2 | 272 | 35.2 | 157 | 735 | 27.9 | 13.7 | 11.8 | 44.7 | 5.1 | 388 | 1.7 | 240 | 70 | 45.0 | 5.8 | 1.8 | 12.1 | ||
| 11 | C | 17.65 | 3405 | 48 | 219 | 455 | 8.6 | 226 | 33.4 | 159 | 593 | 21.3 | 10.2 | 6.8 | 43.0 | 4.0 | 362 | 1.5 | 204 | 59 | 37.5 | 3.7 | 1.1 | 9.8 | |
| 12 | 19.50 | 2665 | 54 | 230 | 471 | 9.0 | 235 | 11.2 | 138 | 623 | 19.6 | 8.9 | 6.4 | 42.4 | 3.5 | 374 | 1.5 | 211 | 61 | 38.7 | 3.6 | 1.0 | 8.8 | ||
| 13 | 20.50 | 2443 | 47 | 198 | 414 | 7.9 | 206 | 20.1 | 129 | 553 | 17.4 | 8.0 | 5.8 | 37.1 | 3.2 | 336 | 1.4 | 185 | 55 | 32.9 | 3.4 | 0.9 | 7.9 | ||
| 14 | Bedrock | 23.50 | 2146 | 46 | 200 | 393 | 8.0 | 194 | 251.0 | 127 | 519 | 17.6 | 8.1 | 5.3 | 36.0 | 3.2 | 314 | 1.1 | 180 | 52 | 31.0 | 3.2 | 0.8 | 6.7 | |
| 15 | 28.80 | 2294 | 46 | 197 | 384 | 7.5 | 189 | 129.0 | 126 | 512 | 17.2 | 8.0 | 4.9 | 35.4 | 3.2 | 303 | 1.3 | 183 | 51 | 32.3 | 3.3 | 0.9 | 7.5 | ||
| 16 | 34.35 | 2368 | 46 | 206 | 416 | 8.0 | 196 | 112.0 | 131 | 535 | 18.9 | 8.7 | 4.9 | 39.4 | 3.3 | 320 | 1.3 | 185 | 52 | 33.2 | 3.5 | 1.0 | 8.5 | ||
| 17 | DHK-10 | A | 1.50 | 4441 | 91 | 301 | 719 | 15.3 | 361 | 44.5 | 29 | 968 | 9.0 | 3.4 | 3.0 | 36.6 | 1.4 | 240 | 0.6 | 130 | 42 | 23.4 | 1.9 | 0.4 | 3.4 |
| 18 | 5.50 | 4589 | 104 | 362 | 859 | 18.2 | 451 | 58.0 | 29 | 958 | 7.8 | 3.3 | 2.9 | 31.9 | 1.5 | 213 | 0.5 | 121 | 39 | 21.7 | 1.7 | 0.5 | 3.4 | ||
| 19 | B | 7.50 | 5256 | 106 | 443 | 1030 | 17.2 | 597 | 267.0 | 644 | 1160 | 102.0 | 49.4 | 20.8 | 127.0 | 18.9 | 1090 | 6.1 | 723 | 196 | 140.0 | 19.2 | 6.3 | 43.5 | |
| 20 | 8.20 | 5404 | 106 | 425 | 1360 | 17.5 | 547 | 399.0 | 535 | 2686 | 88.9 | 41.5 | 20.0 | 139.0 | 16.9 | 1880 | 5.3 | 814 | 279 | 136.0 | 14.9 | 5.4 | 36.4 | ||
| 21 | 10.50 | 4811 | 109 | 404 | 974 | 19.4 | 502 | 282.0 | 209 | 1070 | 38.1 | 16.7 | 9.9 | 67.4 | 6.9 | 490 | 2.4 | 319 | 96 | 63.1 | 6.4 | 2.3 | 15.5 | ||
| 22 | 12.30 | 4219 | 95 | 331 | 851 | 13.9 | 427 | 266.0 | 206 | 1040 | 38.4 | 16.7 | 10.3 | 62.6 | 6.8 | 513 | 2.1 | 335 | 97 | 63.0 | 6.6 | 2.1 | 15.8 | ||
| 23 | Bedrock | 30.50 | 2294 | 54 | 192 | 490 | 7.6 | 238 | 152.0 | 121 | 624 | 24.6 | 11.2 | 9.4 | 44.8 | 4.8 | 296 | 1.5 | 218 | 65 | 41.3 | 4.1 | 1.5 | 10.8 | |
| 24 | 57.60 | 1036 | 27 | 74 | 116 | 4.5 | 61 | 28.2 | 45 | 297 | 10.6 | 4.1 | 5.2 | 19.6 | 1.5 | 153 | 0.6 | 114 | 33 | 17.2 | 1.9 | 0.4 | 3.0 | ||
| 25 | 60.50 | 2368 | 55 | 223 | 410 | 13.1 | 158 | 73.5 | 98 | 691 | 23.7 | 9.5 | 6.7 | 44.2 | 3.7 | 331 | 1.0 | 245 | 75 | 41.6 | 4.3 | 1.1 | 6.8 |
| B-Horizon Soil | Zr | Ti | Hf | Nb | Ta | Y | Ce | Eu | Gd | Yb |
|---|---|---|---|---|---|---|---|---|---|---|
| Th | 0.67 | 0.61 | 0.75 | 0.69 | 0.66 | 0.56 | 0.47 | 0.40 | 0.62 | 0.58 |
| U | 0.85 | 0.86 | 0.86 | 0.78 | 0.87 | 0.70 | 0.79 | 0.64 | 0.74 | 0.76 |
| Bedrock | Zr | Ti | Hf | Nb | Ta | Y | Ce | Eu | Gd | Yb |
| Th | 0.88 | 0.35 | 0.83 | 0.82 | 0.33 | 0.93 | 0.73 | 0.46 | 0.85 | 0.95 |
| U | 0.49 | 0.05 | 0.42 | 0.52 | 0.04 | 0.73 | 0.30 | 0.08 | 0.39 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukadana, I.G.; Sulaeman; Syaeful, H.; Indrastomo, F.D.; Adimedha, T.B.; Ciputra, R.C.; Pratiwi, F.; Mustika, D.; Sumaryanto, A.; Burhannudinnur, M.; et al. High Field Strength Element (HFSE) and Rare Earth Element (REE) Enrichment in Laterite Deposit of High Background Natural Radiation Area (HBNRA) of Mamuju, West Sulawesi, Indonesia. Resources 2025, 14, 84. https://doi.org/10.3390/resources14050084
Sukadana IG, Sulaeman, Syaeful H, Indrastomo FD, Adimedha TB, Ciputra RC, Pratiwi F, Mustika D, Sumaryanto A, Burhannudinnur M, et al. High Field Strength Element (HFSE) and Rare Earth Element (REE) Enrichment in Laterite Deposit of High Background Natural Radiation Area (HBNRA) of Mamuju, West Sulawesi, Indonesia. Resources. 2025; 14(5):84. https://doi.org/10.3390/resources14050084
Chicago/Turabian StyleSukadana, I Gde, Sulaeman, Heri Syaeful, Frederikus Dian Indrastomo, Tyto Baskara Adimedha, Roni Cahya Ciputra, Fadiah Pratiwi, Deni Mustika, Agus Sumaryanto, Muhammad Burhannudinnur, and et al. 2025. "High Field Strength Element (HFSE) and Rare Earth Element (REE) Enrichment in Laterite Deposit of High Background Natural Radiation Area (HBNRA) of Mamuju, West Sulawesi, Indonesia" Resources 14, no. 5: 84. https://doi.org/10.3390/resources14050084
APA StyleSukadana, I. G., Sulaeman, Syaeful, H., Indrastomo, F. D., Adimedha, T. B., Ciputra, R. C., Pratiwi, F., Mustika, D., Sumaryanto, A., Burhannudinnur, M., Rijanti, R. A. P., Santosa, P., & Widodo, S. (2025). High Field Strength Element (HFSE) and Rare Earth Element (REE) Enrichment in Laterite Deposit of High Background Natural Radiation Area (HBNRA) of Mamuju, West Sulawesi, Indonesia. Resources, 14(5), 84. https://doi.org/10.3390/resources14050084













