Abstract
Considerable amounts of agro-industrial by-products are discarded every year. Moreover, these represent an interesting source of phenolics, cellulose and lignin, in addition to useful compounds such as antioxidants. However, these compounds may be affected by external factors such as genotype, locality and productive season, increasing or decreasing the antioxidant potential of by-products. In this study, hazelnut (Corylus avellana L.) and walnut (Juglans regia L.) nutshells were investigated for their fiber content and antioxidant capacity as valorized by-products in this industry. The determination of oxygen radical absorbance capacity (ORAC), total phenolic content (TPC) and color difference was performed using hazelnut and walnut shells collected from orchards located in Southern Chile during three consecutive seasons (2020/21, 2021/22 and 2022/23). The ORAC in nutshells showed the highest values in both species for the season 2020/21 (3217 and 4663 µmol TE g DW−1 for hazelnut and walnut), whereas the variability in consecutive seasons was lower for hazelnut than for walnut. The TPC in hazelnut shells was positively correlated with L* (R: 0.883) and ΔE (r = 0.924) during the 2020/21 season and with L* for 2022/23 (r = 0.907). On the other hand, the ORAC was negatively correlated with L* (r = 0.922) in 2021/22. In addition, the morphological and structural features of both nutshells examined by scavenging electron microscopy (VP-SEM) and confocal scavenging laser microscopy (CSLM) revealed differential tissue distribution and accumulation patterns of both cellulose and lignin. In addition, photo-colorimetric values were determined for both shells and corresponding seasons, and non-significant differences were found for both shells and among seasons. Finally, our results provide new insights into the physicochemical characteristics of these two types of nutshells as valorized by-products, considering their antioxidant properties as residual materials derived from this agroindustry.
1. Introduction
The development of society worldwide has led to the growing demand to maximize natural resources in a sustainable way; thus, the use of waste-derived by-products from the agri-food industry has become a key aspect in terms of the circular economy [1]. In this sense, during the last few decades, certain nut crops such as walnut (Juglans regia L.) and hazelnut (Corylus avellana L.) and their shells as the main by-products from this industry have generated significant interest [2]. In production terms, the nut industry plays an important role in the global agricultural economy [3]. Global nut production has grown over the past decade, with 7.7 million tons (t) in the 2023/24 season. Walnuts and hazelnuts represent around 48 and 15.5%, respectively, which resulted in a production of 4.9 million t in the previous season (2023/24) [3,4,5,6]. In the case of hazelnuts, the annual world production exceeds 1.2 million t and Chile has become the main hazelnut producer in the southern hemisphere, with an off-season nut supply of 65,646.73 t in 2023 [6,7]. About 90% of hazelnuts produced in Chile are marketed without shells (unshelled) [7], whereas for walnuts, the exportation volume of both peeled and shelled nuts was approximately 191,972 t for the year 2023, with an estimated increase to 214,344 in 2025 [8]. This nut tree represents the main species utilized in the nut market in Chile and the world, with a global production of over 3.7 million t, experiencing an increase of 23% in the last ten years [6,7]. In fact, Chilean walnut exportation reached around 160,000 t and USD 474.8 M in 2023, with walnuts in shells being the main exported product in terms of value, and shipments totaling 97,371 t in 2023 [8]. Moreover, around 50% of exports correspond to shelled walnuts, while the shells from the other 50% remain in the country of origin [7,8]. The weight value (w/w) of the shell of both types of nuts is between 50 and 60% of the yield in relation to the kernel (40–50%) [9,10]. Thus, a huge amount of annual by-product biomass is available for its potential use [2]. Currently, as a cheap raw material, nutshell biomass is mainly used as a low-value-added heat energy source for heaters [11,12], without considering their high phenolic and lignin content. In fact, there are limited studies on nutshell use in industrial applications [2]. The utilization of hazelnut shells for the extraction of phenolic compounds has been explored with the purpose of obtaining naturally valued additives. Contini et al. (2012) [13] reported that phenolic compounds in shells processed at different roasting temperatures and times were about 2.7 mg g−1 DW for cultivars Tonda Gentile Romana, Tonda di Giffoni, Tonda Gentile delle Langhe and Tombul. Furthermore, Manterola-Barroso et al. (2022) [9] reported total phenolic content (TPC) and oxygen radical absorbance capacity (ORAC) mean values of 180 mg GAE (gallic acid equivalent) g−1 DW and 2119.7 μmol TE g−1 DW in Tonda di Giffoni shell samples, respectively, demonstrating their significant antioxidant potential. In terms of TPC and antioxidant potential, 31.79 mg GAE g−1 DW and TEAC (Trolox equivalent antioxidant capacity) values around 18.86 mg TE g−1 DW, respectively, were reported for walnut shell methanol extracts by Queirós et al. (2020) [14]. In fact, there are limited studies on its use in industrial applications [9]. Polyphenolic compounds found in plants play a variety of roles, such as color determination, antimicrobial and antifungal action and antioxidant protection against free radicals [15,16]. Moreover, there is a close relationship between antioxidant compounds such as polyphenols, phenolic acids and anthocyanidins with the color of fruits such as berries [17]. Therefore, it would be of great interest to determine whether there is a link between color and antioxidant parameters in the shells of species such as hazelnut and walnut. However, there are limited studies in which a relationship between both parameters in nutshells has been highlighted. However, Jensen et al. (2008) [17] proposed a method of prediction of wine color from phenolic profiles in red grapes, demonstrating the strong relationship between grape anthocyanins and wine color (r = 0.961) and the importance of anthocyanins, phenols and flavanols in the color intensity of young wines. In this sense, colorimeters have been extensively used in the food industry due to their cheap and simple measurements of food color [18].
On the other hand, in relation to their structural conformation, these shells are composed of hemicelluloses (25–30%), cellulose (26–32%), lignin (40–43%) and extractives (3.3–4%) [19]. Lignin is composed of phenolic polymers endowed with potent antioxidant properties that are finding increasing applications in several fields [20]. In relation to walnut shells, Queirós et al. (2020) [14] reported the chemical composition of walnut shell, comprising 10.6% total extractives, 29.9% lignin and 49.7% polysaccharides; Domingos et al. (2022) [21] reported a composition of 35% lignin and 55.2% holocellulose (30.4 and 24.9% as α-cellulose and hemicelluloses, respectively). Today, with a global campaign pursuing a circular economy and a sustainable atmosphere, nut industrial waste material could offer a new industrial re-incorporation route; nut industrial waste has potential applications that could help in CO2 emission reduction [22]. This study contributes by increasing our knowledge about the potential of nut agroindustry by-products for sustainable uses. Therefore, the aim of this work was to evaluate the potential of hazelnut and walnut shells from Southern Chile agroindustry as a source of antioxidant compounds with ORAC antioxidant capacity effect in order to reveal their association with color and microstructure as new insights for these by-products’ valorization.
2. Materials and Methods
2.1. Sample Collection and Processing
During the 2020/21, 2021/22 and 2022/23 productive seasons, hazelnut (cv. Tonda di Giffoni) and walnut (cv. Franquette) were collected from commercial orchards located in Radal (39°10′06″ S, 72°19′11″ W; Altitude: 168 m.a.s.l.) and “Campo Experimental Maquehue” (38°50′28.5″ S, 72°41′40.4″ W; Altitude: 200 m.a.s.l.), respectively, in La Araucanía region, Chile. The meteorological regime showed an expected behavior according to the evaluated area (mostly temperate localities) where precipitation shows higher volumes and frequencies during June and July for three studied seasons. Maximum temperatures (30.9 °C) occurred during the 2022/23 season, while minimum temperatures (−7 °C) were measured during May of the 2021/22 season (Figure 1). In both locations, the nuts harvest was performed from late March to the beginning of April with two Cifarelli V1200 vacuum backpacks (Cifarelli, Voghera, Italy). Then, the collected nuts were gently transported to the Laboratory of Plant Physiology and Nutrition in Fruits Crops (BIOREN-UFRO) and weighted in an I-Thermo G163L thermo balance (Bel Engineering, Monza MB, Italy) in order to determinate field humidity. Afterwards, the samples were washed with deionized water and prepared for the stabilization process at 40 °C for 72 h in a forced-air oven (Heratherm OGS100, Thermo Scientific, Waltham, MA, USA; Memmert UF-55 Büchenbach, Germany) to be stabilized at 6% of humidity and stored at room temperature (20 °C) until further analysis. Samples were subjected to a mechanical peeling process using a traditional nutcracker (IDS, Santiago, Chile). For total phenolic content (TPC) and oxygen radical absorbance capacity (ORAC) determinations, nutshells were milled and sieved (<1 mm) in an ultra-centrifugal mill ZM 200 (Retsch, Haan, Germany), obtaining a powder.
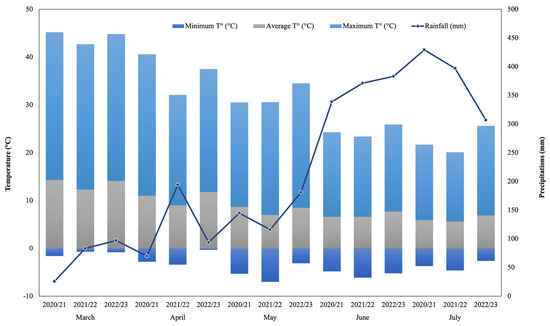
Figure 1.
Weather parameters among studied seasons in Freire, La Araucanía region, Chile, which includes the two evaluated localities: Campo Experimental Maquehue (38°50′28.5″ S, 72°41′40.4″ W; altitude: 200 m.a.s.l.) and Radal (39°10′06″ S, 72°19′11″ W; altitude: 168 m.a.s.l.). Data were provided by Agrometeorología INIA (https://agrometeorologia.cl) (accessed on 14 May 2025).
Finally, whole nutshells were used in order to evaluate color and microstructural properties.
2.2. Extraction of Phenolic Compounds
Extraction was performed according to methods described by Yuan et al. (2018) and Manterola-Barroso et al. (2022) [9,23]. Shell powder (5 g), sieved at 35 ASTM), was suspended on 20 mL aqueous methanol (70% v/v), and the samples were sonicated (Elma S10H, Singen, Germany) at 37 khz for 30 min in iced water (4 °C). Later, the samples were centrifuged (Eppendorf, 5804, Hamburg, Germany) at 8000× g (4 °C) for 10 min and then shaken at 200 rpm (15 °C) in darkness for 18 h (Orbital Shaker ZHWY-100B Zhicheng, Shanghai, China). After this procedure, the solution was re-centrifuged at 8000× g for 10 min. Finally, the samples were filtered through a 0.45 µm pore size (BIOFIL Syringe Driven, Guangzhou, China) and stored at −20 °C until analysis. The pellet was re-suspended for a re-extraction under identical conditions (only 6 h), resulting in a total extract volume of 40 mL. The extracts were used for biochemical determinations (TPC and ORAC).
2.3. Total Phenolic Content Determination
The total phenolic content was quantified by Folin–Ciocalteu methodology performed by Singleton and Rossi (1965) [24] with minor modifications, using Na2CO3 (Sodium carbonate) (20% w/v) (Merck KGaA, Darmstadt, Germany), and the above-mentioned extracts were diluted (1:10 v/v). The analysis was performed by a TECAN Infinite® 200 PRO NanoQuant multi-plate reader (Männedorf, Switzerland) at a wavelength of 765 nm. The results were expressed as mg of gallic acid equivalents (GAE) g−1 of dry weight (DW) following a gallic acid calibration curve between 0 and 750 µg mL−1.
2.4. Oxygen Radical Absorbance Capacity (ORAC)
In order to determine the antioxidant capacity of hazelnut and walnut shells, the “Methodology for the determination of ORAC antioxidant capacity in the pericarp of European hazelnut (Corylus Avellana L.) fruits” was used (ownership of the Universidad de La Frontera, registered in the Chilean Department of Intellectual Rights (DDI) (Nº 2021-A-8614)), and it was adapted for walnut samples. The ORAC was analyzed in a multi-mode reader (Synergy H1 Hybrid, Biotek, Winooski, VT, USA) with five replicates. The working solutions used for the determination were AAPH (2,2′-Azobis (2-methylpropionamidine) dihydrochloride) (153 mM) (Sigma-Aldrich, St. Louis, MO, USA) and 70 nM sodium fluorescein (Sigma-Aldrich, St. Louis, MO, USA). After the preliminary tests, the extracts were diluted 1:1000 and a Trolox calibration curve (0 to 300 µM) was applied. The analyses were carried out using the Gen5™ V 2.0 software. Measures of 25 μL of each sample, standard or blank, were loaded in 96-well black TPC micro-plates, filling the external wells with 250 μL of distilled H2O; then, the loaded plate was inserted into the multi-plate reader. Then, injector 1 immediately dispensed 150 μL of fluorescein (70 nM, followed by an orbital shaking for 10 s at maximum intensity and incubated for 15 min). Subsequently, 25 μL of AAPH was injected through injector 2, followed by an orbital shaking for 50 s at maximum intensity. Subsequently, kinetic readings were generated every 60 s for a total of 2.5 h.
Finally, the raw data were interpreted using the following equations in view to calculate the area under the curve (AUC).
where:
- R1 = Fluorescence reading at the initiation of the reaction.
- Rn = Last measurement.
2.5. Color Determination
The superficial color was determined by photo-colorimetry using a handheld colorimeter model CR400, Minolta Co (Tokyo, Japan). Color functions were calculated for the CIELAB or CIE (Commission Internationale d’Eclairage) (1976) as L*a*b* uniform color space based on a modified methodology described by Lixia et al. (2013) [25]. Measurements were performed using a Petri plate containing enough powder to reach a height of 1 cm (20 g approximately of each nutshell powder sample); white paper had previously been placed under the plates. Five replicates were measured (four cardinal points and the central one of each plate). Subsequently, mathematical differences (Delta E or ΔE) between the parameters (L*, a* and *b) of each sample were calculated considering the differences (Δ) between the first seasons and the last one and among all the calculated color parameters, according to the following equation:
where:
∆E = [∆L*2 + ∆a*2 + ∆b*2] ½
- ΔL* = Difference between light and dark (+brighter − darker).
- Δa* = Difference between red and green (+red − green).
- Δb* = Difference between yellow and blue (+yellow − blue).
- ΔE = Total color difference between the L*, a* and b* parameters.
2.6. Nutshell Microstructural Analysis
For scanning electron microscopy and confocal laser scanning microscopy, whole nutshell samples were used (n = 9 for each species).
2.6.1. Scanning Electron Microscopy (VP-SEM)
The nutshell samples were fractured to avoid sealing in order to prevent the visualization of the microstructure. Subsequently, the samples were fixed in place using double-sided carbon tape. The used instrument corresponds to a VP-SEM Electron Microscope (Hitachi SU3500, Tokyo, Japan) that works in variable pressures; therefore, it is not necessary to dry the samples at critical point or metallize them with Au/C/Au-Pd. All samples were visualized at 15 KeV, with 40 Pa pressure; the distances depended on the height of the sample, fractionated at approximately 10 mm. Additionally, the used detector was a BSE (backscatter) chemical contrast detector. Images were acquired and analyzed with a Hitachi V 3.4 software controller. These data are provided in the labels for each image.
2.6.2. Confocal Laser Scanning Microscopy (CLSM)
To obtain the relative contents, organization and apparent distribution of cellulose and lignin in nutshell samples, a fluorescence assay was performed. Transversal sections of the nutshells measuring approximately 0.3 × 0.3 mm in size were prepared. The samples were stained with safranin 0.1% for lignin (λ excitation/emission, 546/590 nm), or congo red 1% for cellulose (λ excitation/emission, 633/700 nm); both were incubated for 30 min at 25 °C in the dark room. Subsequently, the nutshell samples were washed with deionized water three times to remove the excess dye. The samples were mounted on plates (from Fluodish), and were visualized at 20× magnification using a confocal laser scanning microscope, FV1000 Olympus (Hamburg, Germany). Images were captured at every 2.5 μm for a total of 50 μm and Fv10 Ver 2.0c software was used to analyze the images and quantify the relative fluorescence intensity.
2.7. Experimental Design and Statistical Analyses
The experimental design was a factorial, completely randomized design where the factor was the season. All analyses were performed with five biological replicates per species per season, and the data were submitted to a normality test (Kruskall–Wallis). A one-way analysis of variance (ANOVA) was performed to determine the differences among the seasons. A Tukey post hoc test was implemented to establish mean differences at 5%. A Pearson’s correlation test was performed to determine the linear relationship between the analyzed dependent variables. All the statistical analyses were carried out with the free software R©, V 3.6.1 (R Development Core Team 2008).
3. Results
In this study, the properties of walnut and hazelnut shells were assessed during three seasons (2020/21, 2021/22 and 2022/23) with the aim of determining whether they can be used as beneficial by-products of nut processing instead of being treated as waste material.
3.1. Total Phenolic Content
The analyses of total phenolics content (TPC) showed overall similar amounts between hazelnut and walnut shells (in gallic acid equivalents). The TPC of hazelnut samples had a mean of 0.42 mg GAE g−1 in 2020/21 season, similarly to values obtained in the following season (2021/22), around 0.44 mg GAE g−1 (Figure 2). Moreover, in the last productive season (2022/23), the determined values were quite similar (mean 0.45 mg GAE g−1). Thus, the values obtained for the three evaluated seasons did not show significant differences among themselves. Moreover, the TPC of walnut shells was found to be similar to that of hazelnut, although it showed greater seasonal variability. In fact, TPC values in walnut shells in 2020/21 were lower than those in the 2021/22 season; the same was observed for the 2022/23 season, in which the TPC values increased significantly compared to the previous season. Trends showed a 10.5% increase from 2020/21 to 2021/22, whereas, for 2022/23, an 11% increase was determined (ranges from 0.36 to 0.51 mg GAE g−1 DW) (Figure 2).

Figure 2.
Total phenolic content (µg GAE g−1 DW) and ORAC (µmol TE g−1 DW) in hazelnut (Tonda di Giffoni) and walnut (Franquette) shell samples from La Araucanía region orchards. Bars represent the average of five replicates ± S.E. Different letters indicate statistical differences (p ≤ 0.05) between three different productive seasons.
3.2. Oxygen Radical Absorbance Capacity
To determinate the antioxidant capacity of hazelnut and walnut shells, the ORAC method was used. Overall, ORAC values were higher for walnut extracts than for hazelnut extracts; this was true across the three investigated seasons. For hazelnut, the mean ORAC values varied slightly between the seasons (3217, 3282 and 3100 µmol TE g−1 DW for 2020/21, 2021/22 and 2022/23 seasons, respectively) (Figure 2). These values of antioxidant capacity were also a bit higher than those obtained by Manterola-Barroso et al. (2022) [9], who reported a mean of 2119 µmol TE g−1 DW in control trees from Southern Chilean hazelnut shell extracts. Regarding walnut shells, the values ranged between 4600 and 5500 µmol TE g−1 DW; the highest ORAC values were obtained in 2021/22 and 2022/23.
3.3. Nutshell Color
The relationship between certain antioxidant compounds in fresh fruits and their nature of origin (e.g., color parameters and certain pigments) is well known, however, limited studies are available regarding this relationship in nuts. In our study, luminosity (L*) parameters were not significantly changed in hazelnut or walnut shells over the three evaluated seasons (2020/21, 2021/22 and 2022/23). Nevertheless, in hazelnut shells, the parameters a* and b* showed significant increases (p ≤ 0.05) of 6.7 and 8.4%, respectively, for the 2022/23 season in relation to the two previous ones. Therefore, hazelnut shells exhibited a chromatic parameter that was simultaneously more red (a*) and more yellow (b*); however, the lightness parameter (L*) was not changed (Table 1). In walnut shell samples, L* was significantly increased, by 19.4 and 20.9% for the 2021/22 and 2022/23 seasons, respectively. In relation to a* and b*, these parameters increased progressively from the first to the last evaluated season, increasing by 13% on average for a* and 6.6% on average for b* from 2020/21 to 2022/23. Consequently, in chromatic terms, the shells exhibited an increase in chromatic luminosity and a slight trend towards red and yellow. The aforementioned parameters can be explained in a better way by understanding the chroma changes that occurred through the calculation of ΔE (Table 1). Finally, ΔE was determined for each evaluated season and for each species (hazelnut and walnut). In this regard, ΔE had an increasing trend from the first to the third season in both species, with more significant increases occurring in walnut (Table 1).

Table 1.
Colorimetric parameters (L*, a*, b* and ΔE) obtained for hazelnut and walnut shell samples in relation to three productive seasons. ΔE corresponds to the difference between colorimetric parameters. Values represent the average of five replicates ± S.E. Different low letters indicate statistical differences (p ≤ 0.05) between the three seasons.
3.4. Pearson’s Correlation Between Antioxidants and Color Parameters
Figure 3 and Figure 4 show the Pearson correlation coefficients in relation to all the variables evaluated in each production season for hazelnut shell samples. Strong significant correlations (p ≤ 0.05) were found for the 2020/21 season between TPC and L* (CIE L*a*b* brightness parameter), and between TPC and ΔE (colorimetric change parameter), as well as for ΔE with L* and with b*. For the 2021/22 season, strong significant correlations were determined; these were mostly negative, as in the case of AC and a* with L*, leading to the identification of a possible relationship between a* and other types of antioxidants that are present in European hazelnut shells. In relation to the color differences, ΔE resulted in a positive correlation with b*, showing a color change that is possibly related to a more yellow chroma. Regarding the last season evaluated (2022/23), no significant correlations were found among the evaluated variables. Significant correlations between ΔE, L* and b* were found for walnut shells (Figure 4) during the 2020/21 season, indicating a relationship between the chroma difference with a higher lightness (L*) and possibly toward a more yellowish chroma. Similarly, during the 2021/22 season, a perfect positive correlation (1.00) was determined between ΔE and L*, indicating a change in the trend of chromas towards higher luminosities. In relation to the 2022/23 season, no significant correlations were found between any of the response variables evaluated in walnut shells.
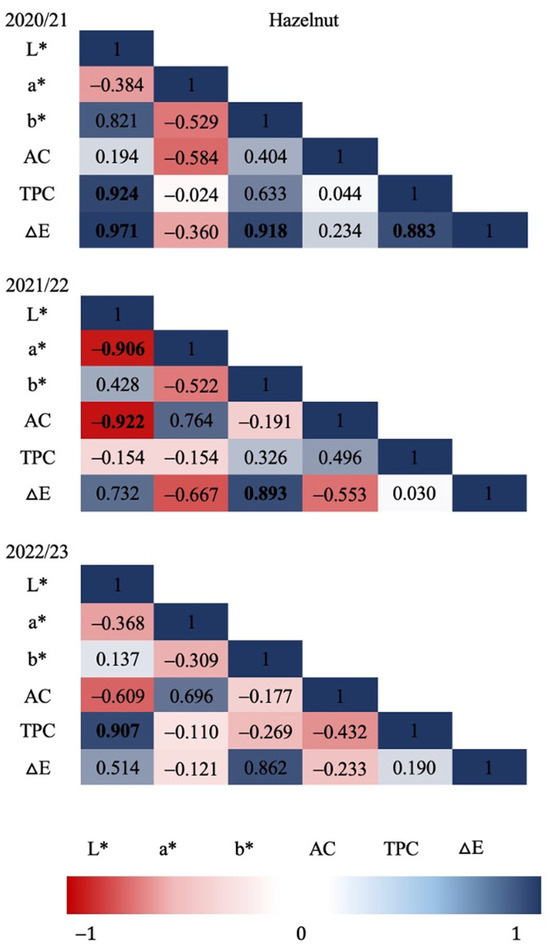
Figure 3.
Hazelnut shell: Pearson’s correlation coefficient matrix among seasons and all variables. Correlation was calculated for three productive seasons (2020/21, 2021/22 and 2022/23). Pearson’s coefficients that are significant at p ≤ 0.05 are indicated by bold numbers. Positive and negative correlations are distinguished by blue and red colors, respectively.
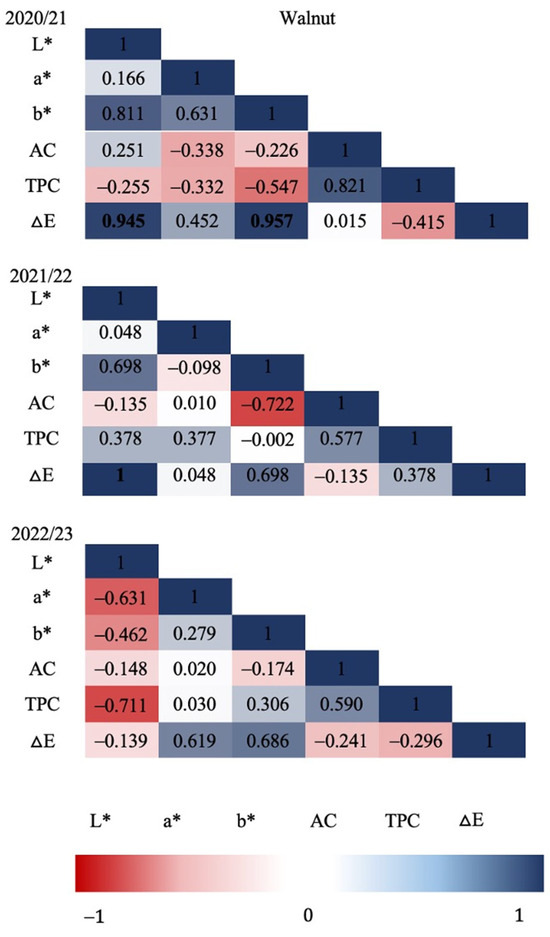
Figure 4.
Walnut shell: Pearson’s correlation coefficient matrix among seasons and all variables. Correlation was calculated for three productive seasons (2020/21, 2021/22 and 2022/23). Pearson’s coefficients that are significant at p ≤ 0.05 are indicated by bold numbers. Positive and negative correlations are distinguished by blue and red colors, respectively.
3.5. Microstructure
3.5.1. Scanning Electron Microscopy (VP-SEM) of Nutshell Profiles
In Figure 5A, six different cellular layers are denoted; these comprise the total profile of the hazelnut shell and represent different types of vegetal tissues. The observed thickness values of the layers ranged between 179 and 175 μm for the first two and were around 354 μm for the central ones. However, those closest to the core ranged between 168 and 119 μm in thickness. Conversely, the walnut shell profile was composed of only three cell layers with similar thicknesses to those closest to the exterior (between 154 and 237 μm). Nevertheless, the layer closest to the nucleus was much thinner, measuring around 55 μm. Each of the walnut layers was thicker than the hazelnut shells and had a higher porosity. The endocarp of the walnuts consists of stone cells, most with very thick cell walls; however, towards the interior, the cell walls become thinner and have relatively large pits (Figure 5D). Also, a zone of flattened parenchyma cells can be observed inside the endocarp (Figure 5E).
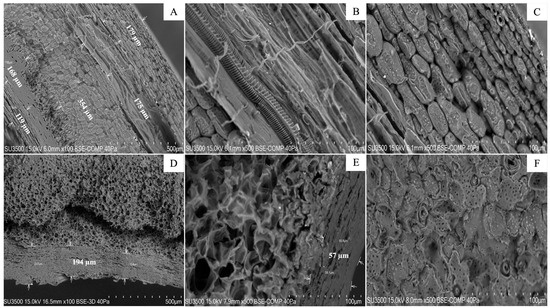
Figure 5.
Scanning electron microscopy (VP-SEM) visualization of nutshell profiles (hazelnut (A–C) and walnut (D–F)). Images are zoomed to 500 µm (A,D) and 100 µm (B,C,F). The arrows point to different cell layers in each sample. Analyses were carried out at the Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera, Temuco, Chile.
3.5.2. Lignin and Cellulose Accumulation Patterns in Nutshell Profiles Obtained by Confocal Laser Scanning Microscopy (CLSM)
In addition to morphological observations, confocal microscopy provided insight into the composition of the shells in terms of lignin and cellulose distribution and accumulation. Comparing the images of SEM (Figure 5) and lignin and cellulose staining (Figure 6A,D,G), it was visible that the parenchyma cells in the hazelnut shell samples contain huge amounts of cellulose; meanwhile, the vascular bundles and cell walls were the main points of lignin accumulation. An overlap between vascular and parenchyma tissues and the formation of three-dimensional shapes could explain the distribution of the different fluorescence signals observed.
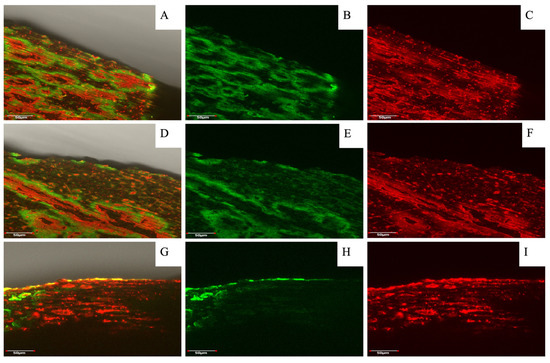
Figure 6.
Hazelnut shell profiles after dyeing and fluorescence visualization assay using laser confocal microscopy. Figures (A,D,G) correspond to a MERGE (overlap of both dying solutions) of nutshell profiles in relation to three seasons (2020/21, 2021/22 and 2022/23, respectively); meanwhile, figures (B,C,E,F,H,I) correspond to the same picture with separate dyeing. All figures are zoomed in to 50 µm. The staining solutions used were “red congo” (red) for cellulose and “safranin” (green) for lignin. Analyses were carried out at the Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera.
In relation to the effect of the three evaluated seasons on the relative fluorescence of lignin and cellulose in hazelnut shells, there was a slight decrease in lignin fluorescence and a greater decrease in cellulose fluorescence for the 2022/23 season compared to the two previous seasons; in contrast, the fluorescence in walnut shells had a fairly similar relative fluorescence of lignin and cellulose in the 2020/21 and 2022/23 seasons. However, in the 2021/22 season, there was a higher fluorescence of lignin (green) and a lower fluorescence of celluloses (red). In a comparison among the different seasons, the accumulation of lignin was the lowest in the 2022/23 season. Nevertheless, hazelnut and walnut shells’ fluorescence intensity changes among the three evaluated seasons were not significant.
In relation to the walnut shell profile, a different order and distribution of lignin and cellulose was observed compared to hazelnut (Figure 7). In the walnut shell, both compounds seemed to co-localize. It is probable that the surface layers contain major relative concentrations of lignin, and the deeper layers contain cellulose that visually appears to be sparse; alternatively, this is effect is a result of the confocal microscopy measurement, which better shows the surface layers, while the deeper ones do not manifest correctly.
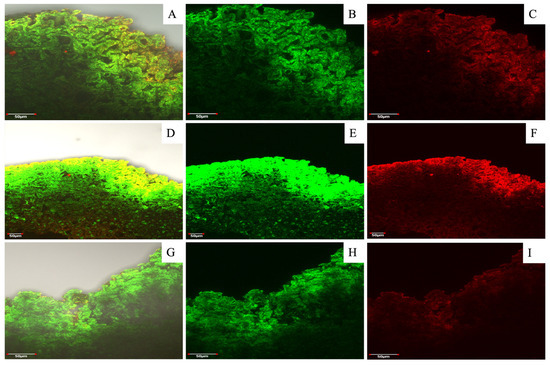
Figure 7.
Walnut shell profiles after dyeing and fluorescence visualization assay using laser confocal microscopy. Figures (A,D,G) correspond to a MERGE (overlap of both dying solutions) of nutshell profiles in relation to three seasons (2020/21, 2021/22 and 2022/23, respectively); meanwhile, figures (B,C,E,F,H,I) correspond to the same picture with separate dyeing. All figures were zoomed in to 50 µm. The staining solutions used were “red congo” (red) for cellulose and “safranin” (green) for lignin. Analyses were carried out at the Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera, Temuco, Chile.
4. Discussion
The present work reveals the potential of the valorization of useful phytochemical features from agro-food by-products (nutshells) which could be subsequently used in construction materials design and development.
4.1. Total Phenolic Content and Antioxidant Capacity
The obtained results are within the ranges of the data reported by Contini et al. [13], who estimated the content of phenolic compounds in the shells to be around 2.7 mg GAE g−1 DW for hazelnut cultivars Tonda Gentile Romana, Tonda di Giffoni, Tonda Gentile delle Langhe, and Tombul. They are also aligned with the data reported by Manterola-Barroso et al. [9] (between 100 and 280 µg GAE g−1 DW). Moreover, values as high as around 1938 µg GAE g−1 have been reported for hazelnut shell extracts by Esposito et al. [26]. The walnut TPC values in this study were found to be in the range reported for these nutshells by Queirós et al. (2019) [14], who reported TPC values of around 31.79 mg GAE g−1 for walnut shells; similarly, Jalili et al. [27] reported values ranging from 13.4 to 30.1 mg GAE g−1 for seven cultivars of the same species planted in Iran. The content of phenolics can be determined by the genotype, time of harvest and abiotic and biotic factors of the nut [2]. In fact, Manterola-Barroso et al. [9] and Meriño-Gergichevich et al. [10] demonstrated that the location and the agronomical management of hazelnuts significantly affected the TPC content and other antioxidant features in the hazelnuts’ shells. Moreover, it is important to monitor the parameters of interest in time course due to variability in environmental factors, as shown in this study.
Comparing the data obtained to those of other nutshells investigated in the literature, we can see that Prakash et al. (2018) [28] reported on the total phenolic content in cashew nut (Anacardium occidentale) shell cake, finding that it ranges from 32.97 to 35.82 mg GAE g−1. These results reflect significantly higher values than those determined in this study. Moreover, they proposed an extraction method (comprising 360 min extraction time, shaking, gentle heating conditions and methanol extraction solution) which is quite similar to the approach used in the present investigation. Conversely, Chandrasekara and Shahidi et al. [29] studied the effect of roasting on the phenolic content of cashew nut testa; they found ranges in total soluble phenolic content between 269 to 347 GAE mg g−1 for the defatted meal of commercial cashew nut (unknown genotype) testa extracts. Moreover, the increase in antioxidant capacity in walnuts was found to be correlated with increases in their total phenolic content.
Although TPC values were similar between walnut and hazelnut shells, higher ORAC in walnut indicates that either the composition of phenolic compounds or the presence of other low-molecular-weight antioxidants (e.g., phenolic acids, flavanols, anthocyanins and proanthocyanins) contribute to the high antioxidant activity of those shells. These results, together with the few variations that have been found between production seasons, highlight a great stability in the antioxidant capacity of shells from both nuts; the antioxidant capacity did not increase by more than 20% and did not decrease over time by more than 2%. To our knowledge, published data regarding ORAC antioxidant capacities in nutshell samples or extracts are scarce or not available. The difference between TPC and ORAC values indicates a need to evaluate the antioxidant properties of shells on different levels to be able to recommend their most suitable use.
ORAC values were higher for walnut than for hazelnut extracts in all three studied seasons. Moreover, these values are also a little higher than those that were presented by Manterola-Barroso et al. [9], who reported a mean of 2119 µmol TE g−1 DW in hazelnut trees (Tonda di Giffoni) planted in four localities of Southern Chile with different environmental conditions. The TPC was found to be similar between hazelnut and walnut shells; the difference that was identified between the TPC and ORAC values highlights a need to identify the phenolic compounds that are involved in hazelnut and walnut shells. To our knowledge, published data regarding ORAC antioxidant capacities in hazelnut and walnut samples or extracts are scarce. Therefore, in relation to other nutshells, Cardullo et al. [30] reported ORAC values of around 1340 µmol TE g−1 DW for Italian pistachio (Pistacia vera cv. Bronte) shell extract. Meanwhile, the ORAC values obtained by Chandrasekara and Shahidi et al. [29] showed values of around 74 mmol of TE g−1 for defatted meal. These values were obtained through measuring the ORAC of the phenolic extract, which explains the higher quantity of ORAC activity data. Moreover, they identified a very strong positive relationship between ORAC and TPC (R2 = 0.977; p < 0.001) and between TPC and ORAC; in fact, the identified correlation was an inspiration for this study of hazelnut and walnut shells.
On the other hand, Griffin and Dean et al. [31] studied the nutritional and antioxidant compositions of skin-on cashew nuts. They reported ORAC values of around 42 μg TE g−1. The increase in this value with respect to that obtained with skin-off cashew nut samples (85.8% lower) demonstrates the high antioxidant load that is present in the testa and consequently in the nut shells.
Finally, Chirinos et al. [32] reported ORAC maximum values for Sacha inchi (Plukenetia volubilis L.) shell samples of 192.6 μmol TE g−1 using an extraction solution of ethanol/acetone/water/acetic acid (40/40/10/1, v/v/v/v). Therefore, their findings reaffirm the efficacy of methanolic extracts in the extraction of antioxidant compounds.
4.2. Relationship Between Antioxidants and Nutshell Color
In the case of hazelnut shells, a relationship between these parameters was found, although further studies are needed to facilitate an understanding of the relationship between certain types of antioxidant compounds and colorimetric parameters; here, compounds such as anthocyanins are probably involved in the coloring process of hazelnut shells [17,33]. Suriano et al. (2021) [33] observed a significant difference (around 300%) among TPC values in diverse maize genotypes, ranging from 1359 ± 34.4 μg CE (catechin equivalents) g−1 DW for a yellow genotype to 4047 ± 26.8 μg CE g−1 DW for a purple maize genotype (VA1245w × Morado). On the other hand, CIE Lab parameters were correlated by principal component analysis (PCA), resulting in a high correlation between phenolic compounds (flavanols and anthocyanidins) and VA1245w × Morado maize. This correlation, related to the results of our research, allows us to find a relationship between these phenolic compounds and certain darker color patterns linked to L* and b* parameters. On the other hand, Jensen et al. [17] have demonstrated the strong relationship between grape anthocyanins and total wine color (r = 0.96) and the importance of anthocyanins, total phenols and flavanols in the color intensity of young wines; these factors are actually widely studied in fruits. Nevertheless, reports on color assignment in nutshells are scarce and, to our knowledge, there are no reports on that of hazelnut or walnut shell samples. No correlations were found between colorimetric and antioxidant parameters in walnut shells (for all studied seasons). Although, for hazelnut shells, interesting positive correlations were found between L* and TPC in two of the evaluated seasons; this finding allows us to explore a potential link between phenolic compounds and the luminosity color parameter (L*). This is in agreement with the findings of other studies [17,33], which have reported on the participation of these compounds in the browning processes of fruits. In addition, this allows us to investigate the interactions and reactions that occur among different antioxidants, leading to the conformation and/or change of coloration parameters in relation to the function of time.
4.3. Nutshell Microstructure (VP-SEM and CLSM)
In relation to the physical and morphological features of the studied hazelnut and walnut shells, the physical structure of the hazelnut shells comprised six different cellular layers composing the total profile of the nutshell, representing different types of tissues. The walnut shell profile was composed of only three cell layers, each of them thicker than the hazelnut ones and, structurally, they had higher porosity. In hazelnut shell samples, parenchyma cells may contain huge amounts of cellulose; meanwhile, the vascular bundles have major lignin contents and they possibly overlap in three-dimensional shapes. This would explain the distribution of the observed fluorescence signals. Making a connection between the above findings and the fluorescence assays, it is possible to explain the overlapping of fluorescence by visualizing the images corresponding to MERGE; here, the fluorescence intensity that corresponds to lignin can be observed on the surface, possibly indicating an over-positioning of the tissues that are rich in this compound over tissues related to cellulose presence. In this sense, the walnut shell profile presents an aspect that is quite similar to that obtained by Nicolás-Bermúdez et al. [34], who reported on the morphological and micromechanical characterization of Mexican pecan nut (Carya illinoinensis) shells. The thick, porous layers of the walnut shell have high potential for applications in both the scientific community and in industry; this has been shown for pecan nut shells, which are used for activated carbon and exhibit an excellent performance as bio-adsorbent materials for heavy metal ions such as Zn2+, Cd2+, Ni2+ and Cu2+ [35,36]. Other authors have analyzed the microstructure of commercial walnut shells and have been able to identify two layers of tissues (dense tissue and porous one) in the walnut shell profile (approximately 1 cm) [37,38]. These findings are quite similar to those reported in this study, except for the third layer that was considered to be the surface in this case. Moreover, Cortat et al. [39] investigated macadamia shell powder using SEM before milling and after being sieved with a 35 ASTM (American Society for Testing Material). They found particles with rough surfaces, some irregular-shaped flake morphologies and high porosities (Figure 5), justified by the lignocellulosic material. Regarding other nutshells evaluated, Andrade et al. [40] used SEM to assess the surfaces of samples of pecan nut shells planted in Brazil (unknown genotype). They reported nutshell regions with pores, whereas others showed an amorphous material, probably with high hemicellulose accumulation. Presenting results that are consistent with those presented for hazelnut and walnut shells, Sonego et al. (2020 and 2021) [41,42] reported hierarchical levels of organization in Brazilian nut (Bertholletia excelsa) shells. In addition, these authors found microstructural features that are similar to those reported in our study for the studied walnuts, which were related to the distribution of cell layers in the nutshell. In relation to possible further applications of some nutshells in the function of their microstructures, pecan nutshell has been reported for potential applications as a lignocellulosic fiber for polymeric composites development [40] and as bio-absorbent of certain compounds [35,36]. With ecofriendly purposes in biocomposite manufacturing, commercial Indian pistachio shell samples were analyzed using SEM; researchers found particles with a rough surface and some irregular-shaped flake morphology, adding to their high porosity [43]; these results are concomitant with our findings for the surfaces of hazelnut shells during three seasons (Figure 5).
Finally, the results reported here agree on certain common characteristics among several types of nut shells (pecan nut, cashew nut, macadamia nut, hazelnut and walnut), such as their highly porous microstructure and their cellular layers comprising irregular and agglomerated shapes; these features can be explained by the high amount of structural and hard components in the shells, such as lignocellulosic compounds.
This study provides insights into the antioxidant features, colorimetric parameters, morphologies and structures of hazelnut and walnut shells from La Araucanía region through three productive seasons; this research was motivated by their potential valorization as a residual materials of agro-industrial processes. The chemical composition of the nutshells showed high cellulose and lignin contents as well as rich phenolic contents and antioxidant capacities. This suggests a potential application of these by-products in biotechnological processes. Further investigations into the hemicelluloses, lignin fractionation and their concentrations in these nutshells will support their applications in circular bio-economical pathways.
5. Conclusions
Through observing the effects of seasonal changes on the antioxidant capacity and total phenolic content of nutshells, it was possible to establish certain differences between the evaluated seasons. For walnut shells, we observed a tendency for increases in both TPC and ORAC throughout the evaluated seasons. The assessment of nuts from three consecutive seasons allowed us to determine the high antioxidant potential of the by-products of hazelnuts and their nutshells; based on the stability of the features investigated (as was the case for hazelnuts) and even their increases (as was the case for walnuts) across three consecutive years, we have been able to present results highlighting their applicability as by-products.
In both hazelnuts and walnuts, the color change parameter (ΔE) showed a significant progressive upward increase (3 and 8%, respectively) according to the chronology of the studied seasons (2020/21 to 2022/23); meanwhile, a* and b* showed a significant inclination towards reddish and yellowish colorations in the nutshells of both nuts.
The relative fluorescence of lignin and cellulose in hazelnut and walnut shells did not show significant differences across the seasons evaluated, indicating an equal distribution of these compounds across different productive seasons and showing complex overlapping of layers that are rich in lignocellulosic compounds (3D shapes). In terms of the microstructure in both shells, the number of cell layers was determined (six and three cellular layers, respectively) and the cell types were described (mostly structural parenchyma); meanwhile, the high porosity of walnut shells was observed. However, more studies are needed to identify the relationship between the nutshells’ microstructures and antioxidant compounds in order to assess their potential in future applications of walnut and hazelnut shells for industrial purposes.
Finally, the present work revealed the potential of the valorization of useful phytochemical features from agro-food by-products (nutshells); these could be used as new alternative materials for the design and development of construction materials.
Author Contributions
Conceptualization, C.M.-B. and C.M.-G.; methodology, C.M.-B., C.M.-G., K.G.S., E.S. and D.P.-C.; formal analysis, C.M.-B., K.G.S. and C.M.-G.; writing—original draft, C.M.-B., C.M.-G. and F.M.; investigation, C.M.-B., K.G.S., D.P.-C. and C.M.-G.; visualization and project administration, C.M.-B. and C.M.-G.; funding acquisition, C.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Fondef VIU20P0027 and Fondecyt 11160762, projects from the Agencia Nacional de Investigación y Desarrollo (ANID) and CORFO-16PTECFS-66647 from Corporación de Fomento de la Producción (CORFO). DIUFRO DI22-0045 and partially by DI22-2001 from Dirección de Investigación. The authors thank the Doctoral Program in Science of Natural Resources and to the Scientific and Technological Bioresource Nucleus (BIOREN-UFRO) from Universidad de La Frontera for their technical support. F.M. was supported by grant KOROLID, CZ.02.1.01/0.0/0.0/15_003/0000336 and the Czech Academy of Sciences, RVO 60077344.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank Scientific and Technological Bioresource Nucleus (BIOREN-UFRO) and its professional staff for providing us with help and knowledge about specific equipment and processes for this research. Moreover, the authors extend their thanks to the following organizations: Frutícola Agrichile S.A.; Doctoral Program in Science of Natural Resources of the Universidad de La Frontera; VIU20P0027, CORFO 16PTECFS-66647, Fondecyt 11160762, DIUFRO DI22-0045 and DI22-2001; TD24-0006 fundings, projects and their professional formation units. Also, to the Laboratory of Physiology and Plant Nutrition for Fruit Trees and its wonderful scientific and professional staff for their confidence and for making this original article possible.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflicts of interest.
References
- Frazzini, S.; Zuorro, A.; Panseri, S.; Pavlovic, R.; Sgoifo Rossi, C.A.; Rossi, L. Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli. Sustainability 2023, 15, 3268. [Google Scholar] [CrossRef]
- Manterola-Barroso, C.; Padilla-Contreras, D.; Ondrasek, G.; Horvatinec, J.; Gavilán-Cuicui, G.; Meriño-Gergichevich, C. Hazelnut and Walnut Nutshell Features, as Emerging Added Value By-Products of the Nut Industry: A Review. Plants 2024, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- USDA. Tree Nuts: World Markets and Trade. 2022. Available online: https://usda.library.cornell.edu/concern/publications/tm70mv16z?locale=en (accessed on 3 November 2022).
- Nuts Dried Fruits Statistical Yearbook. International Nut and Dried Fruit Council. 2023. Available online: https://inc.nutfruit.org/technical-projects/ (accessed on 25 April 2023).
- Du, F.; Tan, T. Recent Studies in Mechanical Properties of Selected Hard Shelled Seeds: A Review. JOM 2021, 73, 1723–1735. [Google Scholar] [CrossRef]
- FAOSTAT. 2023. Agriculture Data. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 April 2025).
- Agrichile—Ferrero Hazelnut Company. Available online: https://www.ferrerohazelnutcompany.com/agrichile/es/avellano-europeo (accessed on 12 June 2024).
- ChileNut, Reportes de Embarques. 2023. Available online: https://www.chilenut.cl/our-harvest/ (accessed on 26 December 2023).
- Manterola-Barroso, C.; Godoy, K.; Alarcón, D.; Padilla, D.; Meriño-Gergichevich, C. Antioxidants in Shell and Nut Yield Components after Ca, Mg and K Preharvest Spraying on Hazelnut Plantations in Southern Chile. Plants 2022, 11, 3536. [Google Scholar] [CrossRef]
- Meriño-Gergichevich, C.; Luengo-Escobar, A.; Alarcón, D.; Reyes-Díaz, M.; Ondrasek, G.; Morina, F.; Ogass, K. Combined spraying of boron and zinc during fruit set and premature stage improves yield and fruit quality of European hazelnut cv. Tonda di Giffoni. Front. Plant Sci. 2021, 12, 984. [Google Scholar] [CrossRef]
- Demirkaya, E.; Dal, O.; Yüksel, A. Liquefaction of waste hazelnut shell by using sub-and supercritical solvents as a reaction medium. J. Supercrit. Fluids 2019, 150, 11–20. [Google Scholar] [CrossRef]
- Rivas, S.; Moure, A.; Parajó, J.C. Pre-treatment of hazelnut shells as a key strategy for the solubilization and valorization of hemicelluloses into bioactive compounds. Agronomy 2020, 10, 760. [Google Scholar] [CrossRef]
- Contini, M.; Baccelloni, S.; Frangipane, M.T.; Merendino, N.; Massantini, R. Increasing espresso coffee brew antioxidant capacity using phenolic extract recovered from hazelnut skin waste. J. Funct. Foods 2012, 4, 137–146. [Google Scholar] [CrossRef]
- Queirós, C.S.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefin 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Crozier, A.; Yokota, T.; Jaganath, I.B.; Marks, S.; Saltmarsh, M.; Clifford, M.N. Secondary metabolites in fruits, vegetables, beverages and other plant-based dietary components. In Plant Secondary Metabolites; Blackwell Publishing: Oxford, UK, 2007; pp. 208–302. [Google Scholar]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Jensen, J.S.; Demiray, S.; Egebo, M.; Meyer, A.S. Prediction of wine color attributes from the phenolic profiles of red grapes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Kara, Ş.; Erçelebi, E.A. Thermal degradation kinetics of anthocyanins and visual color of Urmu mulberry (Morus nigra L.). J. Food Eng. 2013, 116, 541–547. [Google Scholar] [CrossRef]
- Barbu, M.C.; Reh, R.; Çavdar, A.D. Non-Wood Lignocellulosic Composites in Materials Science and Engineering: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2017; pp. 947–977. [Google Scholar]
- Argenziano, R.; Moccia, F.; Esposito, R.; D’Errico, G.; Panzella, L.; Napolitano, A. Recovery of lignin with potent antioxidant properties from shells of edible nuts by a green ball milling/deep eutectic solvent (des) based protocol. Antioxidants 2022, 11, 1860. [Google Scholar] [CrossRef]
- Domingos, I.; Ferreira, J.; Cruz-Lopes, L.P.; Esteves, B. Liquefaction and chemical composition of walnut shells. Open Agric. 2022, 7, 249–256. [Google Scholar] [CrossRef]
- Baran, Y.; Gökçe, H.S.; Durmaz, M. Physical and mechanical properties of cement containing regional hazelnut shell ash wastes. J. Clean. Prod. 2020, 259, 120965. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Lixia, H.; Bo, L.; Shaojin, W. Kinetics of color degradation of chestnut kernel during thermal treatment and storage. Int. J. Agric. Biol. Eng. 2013, 8, 106–115. [Google Scholar]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) shells extract: Phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef]
- Jalili, A.; Heydari, R.; Sadeghzade, A.; Alipour, S. Reducing power and radical scavenging activities of phenolic extracts from Juglans regia hulls and shells. Afr. J. Biotechnol. 2012, 11, 9040–9047. [Google Scholar]
- Prakash, A.; Vadivel, V.; Banu, S.F.; Nithyanand, P.; Lalitha, C.; Brindha, P. Evaluation of antioxidant and antimicrobial properties of solvent extracts of agro-food by-products (cashew nut shell, coconut shell and groundnut hull). Agric. Nat. Resour. 2018, 52, 451–459. [Google Scholar] [CrossRef]
- Chandrasekara, N.; Shahidi, F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Griffin, L.E.; Dean, L.L. Nutrient composition of raw, dry-roasted, and skin-on cashew Nuts. J. Food Res. 2017, 6, 13–28. [Google Scholar] [CrossRef]
- Chirinos, R.; Necochea, O.; Pedreschi, R.; Campos, D. Sacha inchi (Plukenetia volubilis L.) shell: An alternative source of phenolic compounds and antioxidants. Int. J. Food Sci. Technol. 2016, 51, 986–993. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT Food Sci. Technol. 2021, 144, 111257. [Google Scholar] [CrossRef]
- Nicolás-Bermúdez, J.; Arzate-Vázquez, I.; Chanona-Pérez, J.J.; Méndez-Méndez, J.V.; Rodríguez-Castro, G.A.; Martínez-Gutiérrez, H. Morphological and micromechanical characterization of calcium oxalate (CaOx) crystals embedded in the pecan nutshell (Carya illinoinensis). Plant. Physiol. Biochem. 2018, 132, 566–570. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Struct. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Zazycki, M.A.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J. Clean. Prod. 2018, 171, 57–65. [Google Scholar] [CrossRef]
- Antreich, S.J.; Xiao, N.; Huss, J.C.; Horbelt, N.; Eder, M.; Weinkamer, R.; Gierlinger, N. The puzzle of the walnut shell: A novel cell type with interlocked packing. J. Adv. Sci. 2019, 6, 1900644. [Google Scholar] [CrossRef]
- Xiao, N.; Felhofer, M.; Antreich, S.J.; Huss, J.C.; Mayer, K.; Singh, A.; Bock, P.; Gierlinger, N. Twist and lock: Nutshell structures for high strength and energy absorption. R. Soc. Open Sci. 2021, 8, 210399. [Google Scholar] [CrossRef] [PubMed]
- Cortat, L.O.; Zanini, N.C.; Barbosa, R.F.; de Souza, A.G.; Rosa, D.S.; Mulinari, D.R. A sustainable perspective for macadamia nutshell residues revalorization by green composites development. J. Polym. Environ. 2021, 29, 3210–3226. [Google Scholar] [CrossRef]
- De Prá Andrade, M.; Piazza, D.; Poletto, M. Pecan nutshell: Morphological, chemical and thermal characterization. J. Mater. Res. Technol. 2021, 13, 2229–2238. [Google Scholar] [CrossRef]
- Sonego, M.; Fleck, C.; Pessan, L.A. Hierarchical levels of organization of the Brazil nut mesocarp. Sci. Rep. 2020, 10, 6786. [Google Scholar] [CrossRef]
- Sonego, M.; Madia, M.; Eder, M.; Fleck, C.; Pessan, L.A. Microstructural features influencing the mechanical performance of the Brazil nut (Bertholletia excelsa) mesocarp. J. Mech. Behav. Biomed. 2021, 116, 104306. [Google Scholar] [CrossRef]
- Balasundar, P.; Narayanasamy, P.; Senthil, S.; Al-Dhabi, N.A.; Prithivirajan, R.; Kumar, T.; Ramkumar Bhat, K.S. Physico-chemical study of pistachio (Pistacia vera) nutshell particles as a bio-filler for eco-friendly composites. Mater. Res. Express 2019, 6, 105339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).