3.1. Physicochemical Analysis of the Components of the Soil Substitutes
The chemical properties of the CCPs and organic materials used for the elaboration of the soil substitutes are presented in
Table 2. Among all the investigated components, two organic materials, CL and GC, contained much higher amounts of organic carbon (426.1 and 173.8 g/kg, respectively) and nitrogen (8.4 and 15.6 g/kg, respectively), with relatively low concentrations of mineral oxides, except for 461.3 g/kg of SiO
2 in GC. The high content of mineral oxides such as SiO
2, Al
2O
3, and CaO in CCPs was typical of fly ash from lignite combustion and suggested the presence of calcium-based compounds. A higher amount of Fe
2O
3 in mineral samples (90.3 g/kg for ST and 90.1 g/kg for SG), compared to the organic samples (31.4 g/kg for CL and 18.1 g/kg for GC), showed the mineralogical nature of CCPs. The typical mineral composition of ST and SG samples was reflected in the higher concentrations of MgO and K
2O. Moreover, a higher content of P
2O
5 (6.3 mg/kg) in GC was likely due to the organic nature of the sample.
The analysis revealed that the total sulfur (St) concentration varied from 2.6 g/kg in the GC to 49.3 g/kg in the SG. The reason for the greater amount of St in the CCPs was the amount of gypsum generated in the flue gas desulfurization process.
Moreover, the chemical analysis showed that the concentrations of trace elements in CCPs were greater than those in organic materials. However, the highest amounts of Zn (624 mg/L) and Pb (108 mg/L) were observed in the GC. The highest concentration of molybdenum in the components was observed in CL and ST, whereas the highest amount of As (39 mg/L) was detected in the ST sample. Notably, high concentrations of toxic metals in soils may reduce sprouting and biomass yields and have harmful effects on plant growth [
31].
3.2. Preparation and Analysis of the Soil Substitutes
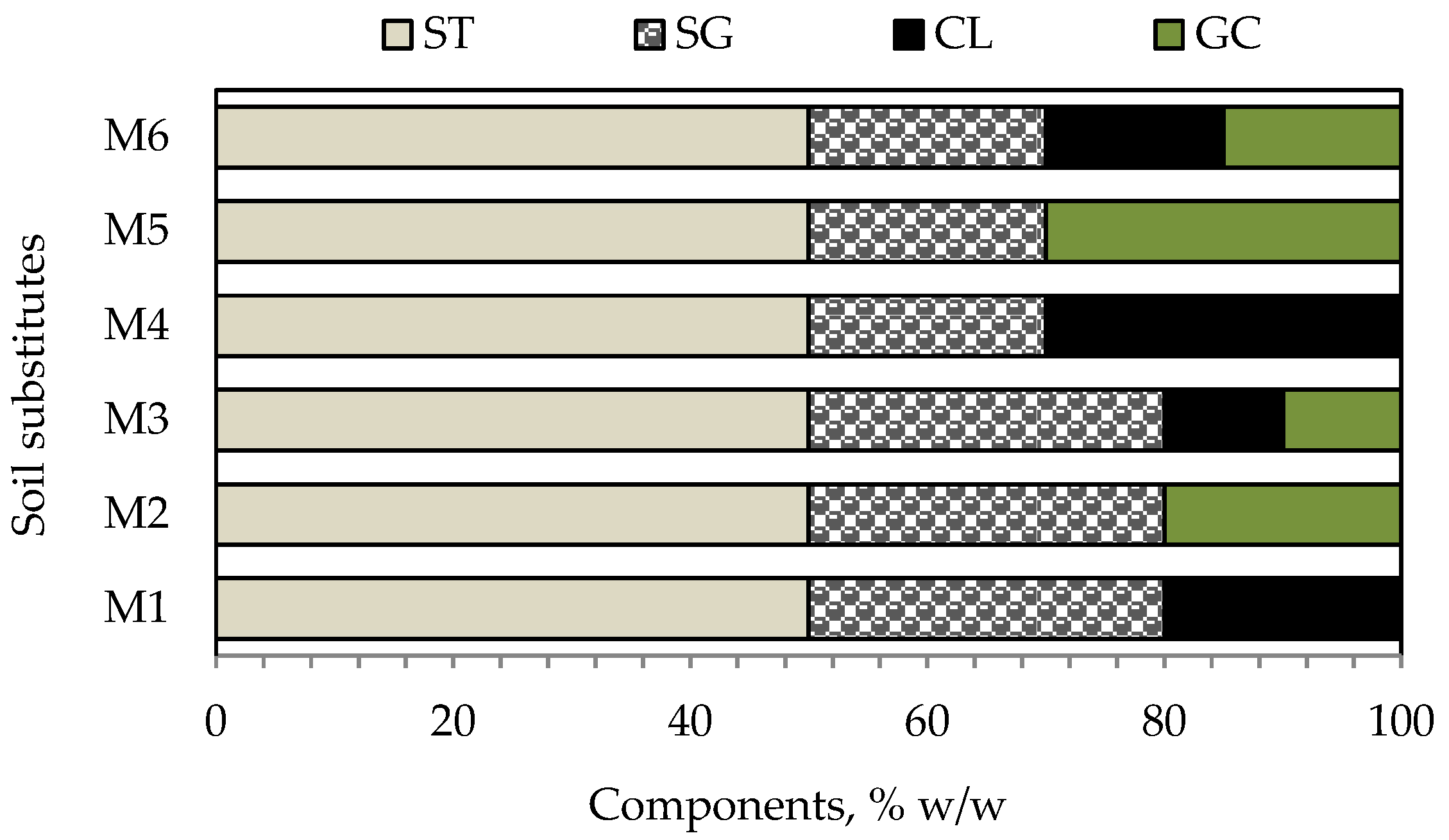
The soil substitute samples were prepared via the weight method based on the results of the physicochemical analysis of the CCPs and organic materials. A list of the component materials and their percentage ranges for the preparation soil substitutes (M1-M6) is presented in
Figure 3.
The main component of each mixture was the ST used to prevent subsidence in the post-mining area, with a 50% w/w content. The amount of SG ranged from 20 to 30% w/w, whereas the percentages of CL and GC were between 0 and 30% w/w. The purpose of this blending process was to ensure that the soil had appropriate parameters, including low salinity, a balanced content of essential nutrients, a low concentration of toxic metals, and an optimal structure.
The results of the trace elements analysis in soil substitutes, along with their permissible thresholds for limit, warning, and critical concentrations in soils according to the Official Journal of the Republic of Slovenia [
32,
33] and their contents in soil substitutes are presented in
Table 3.
According to the regulations in Slovenia [
32], the permissible thresholds for dangerous substances are classified into three categories: (i) the limit value indicates the soil load where living conditions for plants and animals are ensured and groundwater quality and soil fertility do not deteriorate. The effects or impacts on human health or the environment are still acceptable at this value; (ii) the warning value is the density of an individual hazardous substance in the soil, indicating a probability of harmful effects or impacts on human health or the environment in particular land use; (iii) the critical value determines the concentrations at which, due to harmful effects or impacts on humans and the environment, contaminated soil is not suitable for producing plants intended for human or animal consumption.This study demonstrates that the concentrations of arsenic (>20 mg/kg) and molybdenum (>10 mg/kg) in soil substitutes exceeded their respective limit values. Additionally, a warning concentration of cadmium (>2 mg/kg) was detected in samples M2, M5, and M6. However, it is essential to highlight that soil substitutes should be applied only in areas degraded by industry, with no plans to carry out agricultural activities. In that case, the use of mixtures that slightly exceed the limit values for the biological reclamation of post-mining areas is safe for the environment. It poses an acceptable risk to human and animal health, as well as to plant growth.
3.3. Analysis of Water Leakage from the Soil Substitutes
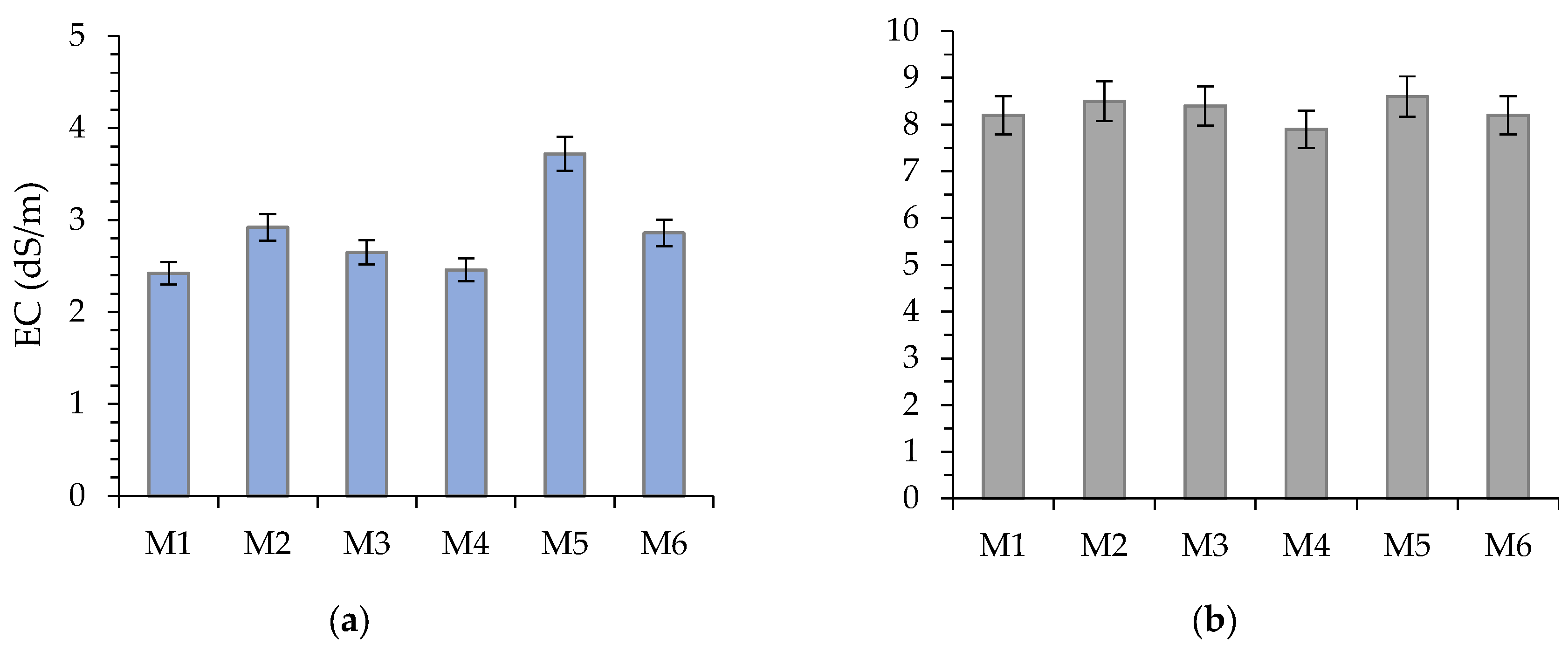
The measured parameters for the soil substitute leachates (M1-M6) are shown in
Figure 4.
According to the general classification [
34], the measured salinity of soil substitutes M1-M6, expressed as electrical conductivity, can be classified as slightly alkaline soils (2.0–4.0 dS/m). In this approach, the sprouting and biomass growth of sensitive plants, such as Sinapis alba, may be restricted. However, the literature data [
31] reported that most crops prefer growing in EC between 2.0 and 3.5 dS/m. The highest EC (3.72 dS/m) measured in sample M5 may restrict water availability to plants, as increased soil salinity induces osmotic stress. In response to such conditions, plants may reduce their growth rate or change their root structure to increase water absorption [
35].
Based on the general interpretation of the pH value [
36], the leachates of the soil substitutes were moderately alkaline (pH 7.9–8.4) and strongly alkaline (pH 8.5–9.0). The solubility of metals in aqueous soil solutions depends on their pH value. Many studies have concluded that the solubility of Fe, Cu, Zn, Mn, and Co decreases in alkaline solutions [
37]. At pH levels above 7, a reduced bioavailability of Al and Mn is observed due to the formation of insoluble hydroxy salts such as Al(OH)
3 and Mn(OH)
2, respectively. Conversely, higher pH values increase the mobility of molybdenum and arsenic in the form of anions MoO
42− and AsO
43−, which are weakly adsorbed by alkaline soils [
38]. More stable and less mobile alkaline conditions lead to the formation of insoluble hydroxyl complexes or compounds with organic substances [
39]. The chemical parameters of soil substitute leachates, including the concentrations of nutrients and trace elements, are listed in
Table 4.
Sample M5 is characterized by the highest EC (3.72 dS/m), which corresponds with the highest concentrations of K
+ (447 mg/L), Cl
− (144 mg/L), and SO
42− ions (1760 mg/kg). More K
+ (228 mg/L) and Cl
─ ions (77 mg/L) were detected in sample M2, corresponding to a salinity of 2.92 dS/m. The highest contents of N
t (29 mg/L) and DOC (49 mg/L) were observed in M5, whereas the concentrations of other nutrients, such as Ca
2+ (591–654 mg/L), Mg
2+ (19.9–42.6 mg/kg), and Na
+ (50.1–65.3 mg/kg), were comparable for all the samples. Dissolved organic carbon is a parameter that indicates the presence of organic nutrients in the soil–water solution. It acts as a primary energy source for soil microorganisms, thereby supporting biogeochemical cycles and enhancing nutrient availability to plants. Elevated DOC concentrations can influence metal mobilization by forming organic complexes that increase their solubility [
40].
The use of CCPs as soil components, mainly fly ash, can lead to an increase in soluble ions such as Ca
2+, Na
+, Cl
−, and SO
42+ in the soil–water mixture, which are responsible for soil salinity [
19]. When present at high concentrations, sulfate and chloride ions may hinder proper plant growth and development. Excessive amounts of SO
42− and Cl
− ions increase the concentrations of H
+ ions in soils, leading to their acidification. However, since chlorides and sulfates do not possess significant buffering capabilities, their influence on soil pH is typically short-lived and limited. The concentrations of toxic metals such as Cr, Cd, Cu, Hg, Ni, and Pb were below the detection limit, whereas the highest content of trace elements was recorded for Mo (0.58–0.86 mg/L).The levels of nutrients (Ca, Mg, P, K, N, and Na) in all leachates indicate that these amounts may be sufficient to support plant growth. According to the literature, the primary nutrients N, P, and K are responsible for biomass build-up, the development of plant root systems, and internal water management [
41,
42,
43]. Nevertheless, a relatively high concentration of K may cause strong soil salinity and reduce green biomass production.
3.4. Composition of Water from the Velenje Coal Mine
The quality of irrigation water and its detrimental effects on soil properties were assessed via several parameters, such as EC, SAR, RSC, and the content of boron (B), and classified into four risk criteria according to the Standard Guidelines for Irrigation Water [
44] as presented in
Table 5.
The results suggest that the risk of irrigation with PVM water is low, according to the EC, SAR, and B concentrations. However, the calculated value of the RSC (3.02 meq/L) indicates that the risk of applying water for irrigation is medium or even high. Based on this classification (
Table 1), waters containing more than 2.5 meq/L of RSC are considered unsuitable for irrigation [
28]. However, a literature study revealed that to neutralize the RSC of irrigation water to over 2.5 meq/L, the addition of acid or gypsum to the soil structure is recommended [
45]. A study carried out by Kumar et al. [
46] indicated that the application of gypsum to soil with a high RCS water index decreases the pH of the soil, resulting in an increased crop yield. The pH decreased due to the removal of sodium carbonate and bicarbonate after the gypsum treatment.
The compositions of the water samples from the PVM and the recommended quality standards for irrigation water, adapted from the Food and Agriculture Organization of the United Nations Standards [
47], are presented in
Table 6.
The EC and TDS of treated mine water were 0.869 dS/m and 666 mg/L, respectively, which classified the water as slightly to moderately suitable for irrigation, according to the guideline interpretations [
47]. The EC and pH values indicate the neutral and nonsaline characteristics of the PVM water, with negligible toxicity effects on plant growth. However, for water used for long-term irrigation, values of EC lower than 0.7 dS/m are needed to avoid soil salinization [
48]. The results demonstrated that the concentrations of cations and anions responsible for the salinity were below the threshold values. Additionally, the presence of sodium and chloride ions indicated that the irrigation water could be used without restrictions. The salinity of PVM water is caused mainly by SO
42− ions, which are less harmful than chloride and sodium ions to plants in terms of osmotic stress. As a result of osmotic stress, plants have a reduced ability to take up water through their roots, resulting in dry and wilting leaves and overall weakening. However, research on osmotic stress in an indicator plant (
Zea mays) has shown that sulfate ions (SO
42−) can act to buffer osmotic stress to some extent, meaning that they can help reduce its adverse effects on plants [
49].
The calculated SAR value (2.091 meq/L) is the relative percentage of sodium to Ca
2+ and Mg
2+ ions in water. High Na
+ concentrations in irrigation water can also affect calcium and potassium deficiencies in soils with low contents of these nutrients. In general, high levels of SAR in water for irrigation can reduce soil stability, decrease the rate of water infiltration, and increase the risk of sodium accumulation to toxic levels in plants [
50]. According to the classification of water irrigation by the sodium absorption ratio (
Table 1), the PVM water may be categorized as excellent (SAR < 10) [
28].
The results for the bicarbonate (HCO
3─) ion content classified the PVM water’s irrigation quality as slight to moderate. Higher concentrations of HCO
32─ ions in the presence of dissolved Ca
2+ or Mg
2+ ions can lead to the formation of insoluble calcium carbonate (CaCO
3) and magnesium carbonate (MgCO
3), the precipitation of which increases the risk of sodium hazards affecting plant growth [
51].
A comparison of the concentrations of the biogenic forms revealed that the contents of nutrient ions (P-PO
43−, N-NH
4+, and N-NO
3─) were within the range suitable for irrigation, whereas the amounts of N-NO
3─ (<5 mg/L) and boron (<0.7 mg/L) suggest that the water for irrigation can be used with no restrictions. The concentration of K
+ ions was 3.93 mg/L, which was above the usual range for irrigation water (<2 mg/L). However, the water quality guidelines [
47] do not indicate limit values for the potassium content. The reason is that potassium is one of the essential nutrients for plant growth, and the excessive content of K
+ ions has a much less negative effect on the physical properties of soils than Na
+ ions have. According to the literature data, the harmful impact of the potassium content in irrigation water was estimated to be approximately one-third of the sodium concentration [
52]. In conclusion, an antagonistic interaction exists between potassium and sodium contents, and the K
+/Na
+ ratio in saline soils is positively correlated with biomass weight and nutrient accumulation [
53].
The water suitability of irrigation water is also assessed by the concentration of trace elements, excessive amounts of which can lead to reduced plant growth. According to water quality guidelines [
47], the toxicity limits of trace elements for long and short-term irrigation, and the results of the analysis of PVM water are presented in
Table 7.
The concentrations of metals varied in the following order: Fe > Al > Zn > B > Li > Mn > Mo > As > Se > Cu > V > Cr. They did not exceed the permissible thresholds for irrigation water for long-term use. The exception was the content of molybdenum (0.013 mg/L), which slightly exceeded the threshold for long-term use. The availability of molybdenum to plants in the soil solution is determined by increasing the soil pH and the contents of available phosphorus and organic carbon [
54]. In acidic soils (pH < 5.5), molybdenum is rarely available for plant roots but increases in alkalinity at a pH in the range between 7.5 and 8.5 pH > 7.5 [
55]. As shown in
Table 6, the PVM water is slightly alkaline (pH = 7.6). Considering that the pH of soil substitutes ranges between 7.9 and 8.5 (
Table 4), the mobility of molybdenum can decrease after irrigation [
56]. Moreover, it should be taken into account, that in European countries, including Slovenia, there is a possibility of acid rain (acid deposition) from regional air pollution caused by sulfur and nitrogen emissions. Typical pH values of occurring acid rain may range from 3.5 to 5.0 [
57]. For this reason, the risk of the intensive bioavailability of Mo in soil substitute solutions will be decreased. Results showed that the concentrations of dissolved metals such as Be, Cd, Co, Hg, Ni, and Pb in PVM water were below the detection limit; therefore, the risk of the dispersion of these elements in the soil can be considered very low. However, long-term irrigation with PVM water, due to its low alkalinity, may affect the availability of heavy metals, which show elevated concentrations in the soil substrates (As and Cd). The reduction in soil substrate pH as a result of mine water irrigation can also increase the bioavailability of cadmium and decrease the bioavailability of arsenic [
58] This effect may be intensified in the presence of acid rain.
3.5. Phytotoxicity Tests of the Soil Substitutes
The experimental data indicated various levels of phytotoxicity of the tested soil substitutes. The soils saturated with distilled water presented slightly greater root growth than the samples saturated with PVM water. The average root length for the soil substituted with distilled water ranged from 17.27 to 41.10 mm, whereas the measured root length for the soils treated with PVM water ranged from 13.84 to 40.16 mm. High toxicity was observed for the soil substitute M5, which contained 30% w/w of green compost. In contrast, the most effective soil substitutes were M1 and M4, which contained 20 to 30% w/w of coal lignite, i.e., 38.65 and 41.10 mm for distilled water and 36.20 and 40.16 mm for PVM water, respectively.
The analysis of variance (ANOVA) between the groups of soil substitutes revealed a significant difference for
S. alba early growth, both for samples irrigated with distilled water (
p < 0.001; R
2 = 0.94, R
2adj = 0.86; F = 22.16) and those treated with PVM water (
p < 0.001; R
2 = 0.95, R
2adj = 0.93; F = 43.25). The results of the
S. alba root length and the significance of the difference between the groups are presented in
Figure 5.
The phytotoxic effects of
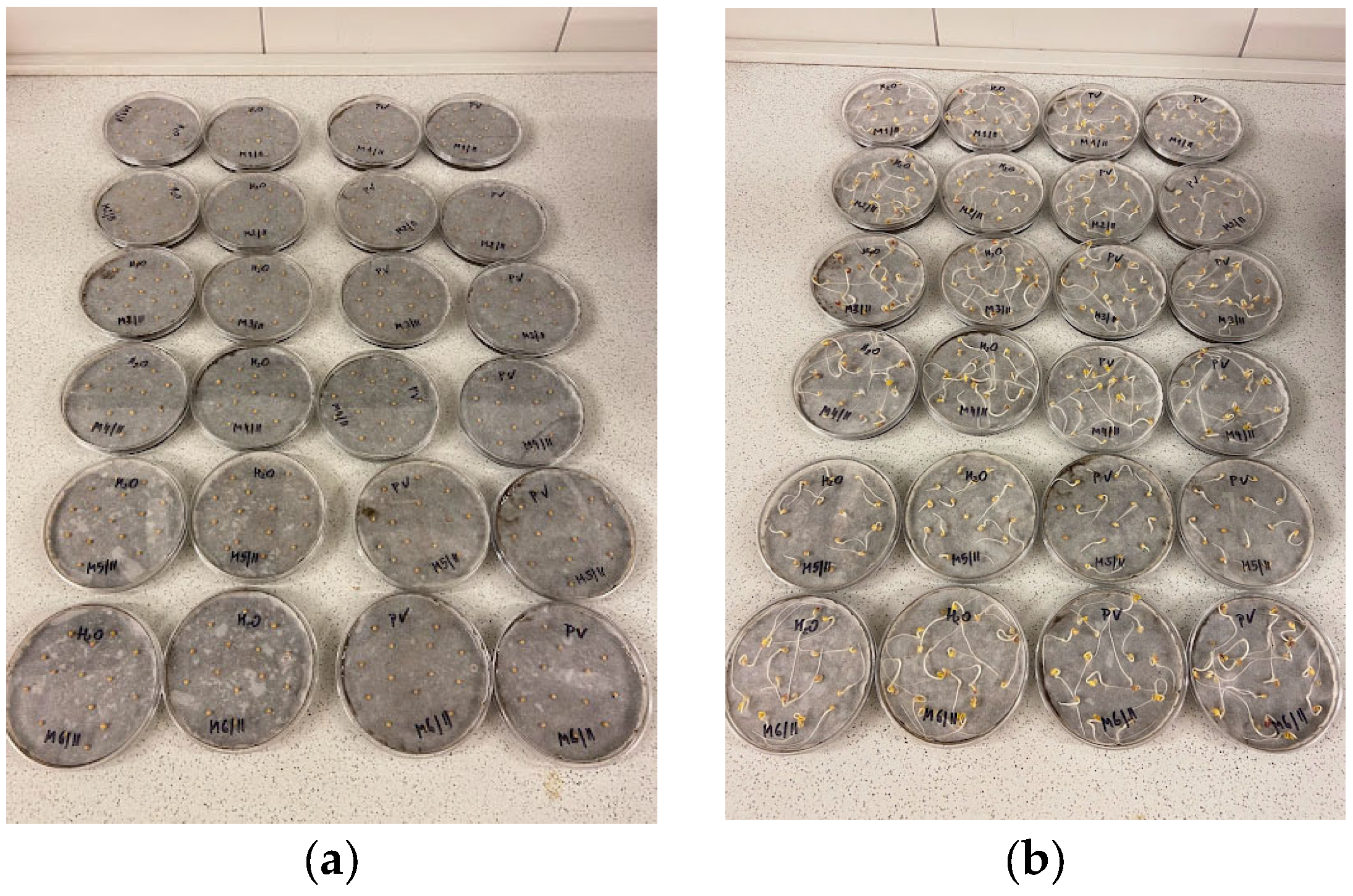
Sinapis alba root growth on Petri dishes are presented in
Figure 6.
These results indicate that the pH and EC of soil substitutes can have an inhibitory effect on the early growth of
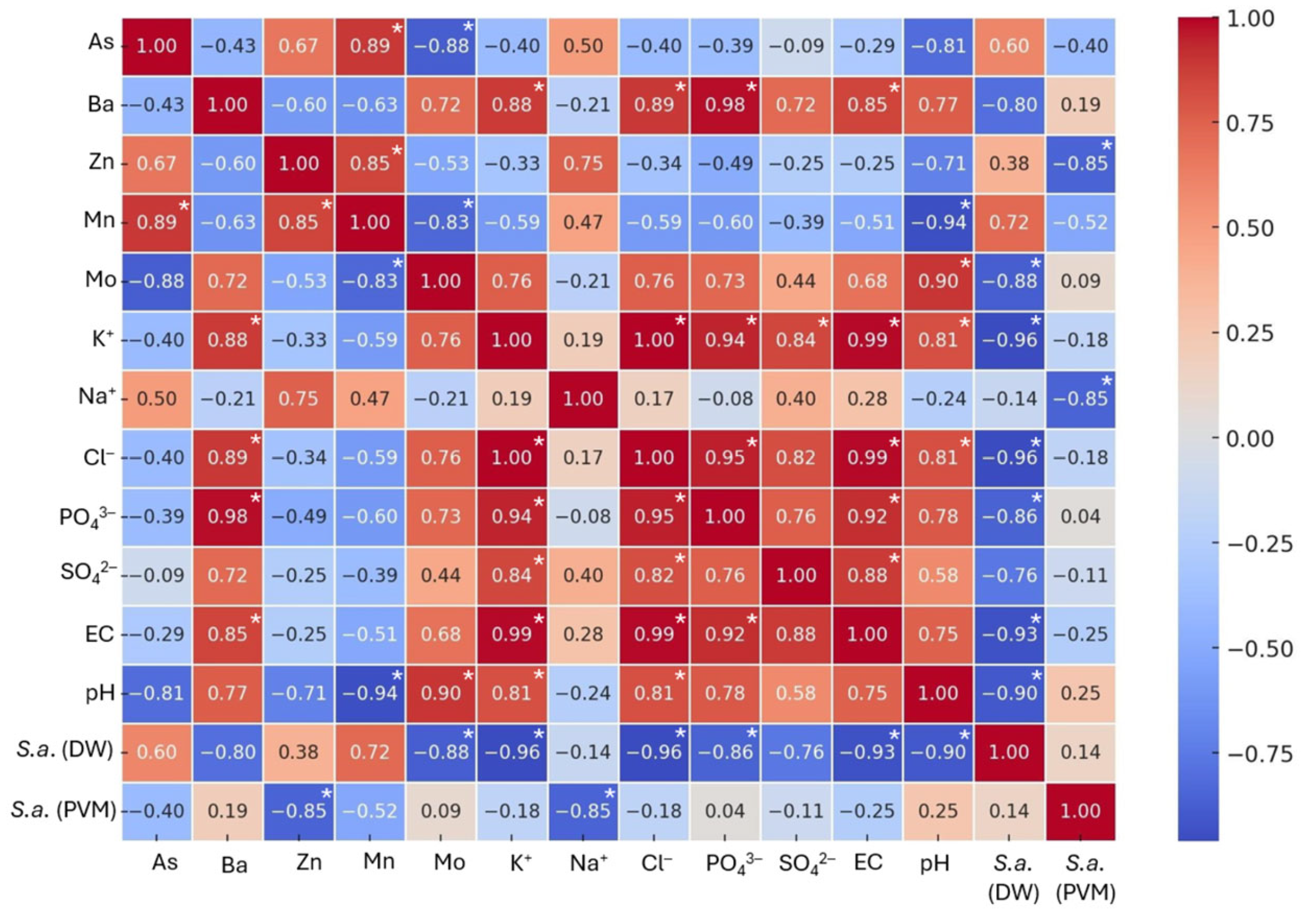
Sinapis alba, indicating the length of the seedling root. The results of the Pearson analysis of the physicochemical parameters of the soil substitutes saturated with distilled water and the phytotoxicity parameters of white mustard are presented in
Figure 7.
The analysis of the samples irrigated with distilled water revealed a significant negative correlation between the length of the roots of
Sinapis alba and the pH of the soil substitutes (r = −0.90). A significant negative correlation was detected between the results of the phytotoxicity tests and the salinity parameters, i.e., EC (r = −0.93), K
+ (r = −0.96), Cl
− (r = −0.96), and PO
43− (r = −0.86). The results of this study confirmed that an increased salt level in the soil limits seed germination and reduces the osmotic activity of plants, thereby preventing them from transporting water and nutrients to their roots [
59].
The Pearson analysis indicated that the increased content of Mo (r = −0.88) in the soil–water mixtures led to a decrease in
Sinapis alba root growth. A negative but non-significant correlation was also observed for plant growth and the contents of Ba (r = −0.80) and SO
42− (r = −0.76). The availability of Mo (r = 0.90) for plant roots in soil solutions increases with an increasing soil pH (r = 0.90), as verified by the literature data [
55,
56]. The opposite effect was observed between pH values and other trace elements, i.e., Mn (r = −0.94), Zn (r = −0.74), and As (r = −0.81). The availability of some plant nutrients is strongly affected by soil pH and has been widely confirmed by other studies [
37,
60].
The analysis of soil substitutes irrigated with PVM water revealed that the most significant and negative correlation with the early growth of
S. alba was observed for the concentration of Na
+ ions (r = −0.85). A strong negative significant correlation was also detected between
S. alba root growth (
S.a. PVM) and the content of Zn (r = −0.85). However, the literature results suggest that white mustard growth on zinc-contaminated soils is likely due to the accumulation of this metal in plant stems, which supports its potential for the phytoremediation of soils with high contents of Zn [
61]. In contrast to soil substitutes watered with distilled water, samples with mine water (
S.a. PVM) presented a weak and insignificant correlation between EC (r = −0.25) and K
+ and Cl
─ ions (r = −0.18). This means that PVM water may reduce the negative impact of the increased salinity of soil substitutes on the early growth of
Sinapis alba. The reason summarized in
Table 6 may be the greater abundance of Ca
2+ (2.337 mEq/L) and Mg
2+ (1.875 mEq/L) ions in PVM water compared to Cl
− ions (0.310 mEq/L).
Both calcium and magnesium are essential nutrients for plants, playing critical roles in plant growth and being required in relatively large amounts [
62]. Calcium in the soil improves its structure, increasing permeability and aeration. It is crucial for the formation of cell walls, the proper functioning of cell membranes, and cell division processes [
63]. However, magnesium is a key component of chlorophyll and is vital for photosynthesis [
64]. The high content of these ions in PVM water for irrigation can be beneficial to the proper growth of plants; however, excessive levels of these nutrients can lead to more alkaline soil, which reduces the uptake of other important nutrients. The contents of calcium and magnesium carbonates (CaCO
3 and MgCO
3) in soils can increase their pH to 7.8 [
36]. Therefore, monitoring and maintaining appropriate levels of calcium and magnesium in the soil are important to ensure optimal conditions for plant growth and ensure the applicability of the phytoremediation process [
65,
66].
3.6. Pot Test
The results for the shoot length and biomass growth of
Sinapis alba in the pot test with soil substitutes treated with distilled water or mine water (PVM) are presented in
Table 8.
The phytotoxicity test revealed little difference between the pots watered with distilled water and those treated with PVM mine water after seven days of growth. The average length of the shoots in the pots with distilled water ranged from 30.14 mm (M2) to 34.45 mm (M1), whereas that of the samples with PVM water ranged from 25.5 mm (M3) to 35.0 mm (M1). The highest mean shoot length was obtained for the soil substituted with 20% w/w of coal lignite.
The average weight of the fresh biomass of Sinapis alba on the tested soil substitutes was comparable and ranged between 1.27 and 1.39 g (pots with distilled water) and between 1.11 and 1.19 g (pots with PVM water). Only two soil substitutes, watered with PVM water (M2 and M3), presented lower biomass weights, 0.99 and 0.78 g, respectively. A lower germination of Sinapis alba in this treatment was also observed (78% for M2 and 58% for M3).
One-way ANOVA for the soil samples irrigated with PVM water revealed significant differences in shoot elongation (
p = 0.0387) and biomass weight (
p = 0.0038). Significantly less biomass was obtained on soil substrate M3. The shoot length (
p = 0.5035) and biomass weight (
p = 0.1130) did not differ significantly among the soil substrates treated with distilled water. In addition, no plant necrosis or other changes were observed, indicating a negative impact of the soil substitute and irrigation with mine water on plant growth. The images of the growth of the
Sinapis alba samples at the end of the experiment during the pot test are presented in
Figure 8.
Research on mine water quality and its environmental impact has been carried out worldwide. For example, the use of mine water containing a large amount of sulfide compounds for the irrigation of crops was investigated in South Africa [
67]. These results showed that gypsiferous waters do not seem to have any deleterious effects on the soil environment.
Another study in southern India reported that the quality of water collected from lignite mine channels was suitable for both domestic use and irrigation. The analysis revealed very minor concentrations of trace elements, and the calculated values of SAR and RSC revealed that the mine water samples fell under the excellent category [
68]. However, studies carried out in Portugal indicated that the samples of water from coal mines were unsuitable for land irrigation due to high concentrations of iron, manganese, bicarbonates, magnesium, and potassium [
29]. Considering the results from the physicochemical analysis and phytotoxicity tests, it is feasible to conclude that the application of mine water from the PVM for irrigation is possible in degraded areas.
The results of our study suggest that producing soil mixtures from CCP amendment with lignite can be a crucial component of a long-term mine management strategy and a cost-effective method for rehabilitating and maintaining post-mining land.
However, to assess the long-term irrigation of biologically reclaimed areas, further research is needed.