Abstract
The genus Artemisia L. (tribe Anthemidea), belonging to the family Asteraceae, has a rich diversity of essential oil-bearing species distributed throughout the Western Himalayan (WH) and Trans-Himalayan (TH) regions of India. The present study evaluated the essential oils of the eighty-one accessions representing 40 Artemisia spp. from India’s WH and TH regions for their essential oil yield, chemical composition, and variability among and within the species. The essential oil yield ranged between 0.02% and 1.65%. One hundred fifty-five major compounds identified by GC-MS technique accounted for 81–100% of the total oil composition in the studied accessions. 1,8-cineole, thujone, camphor, artemisia ketone, borneol, and caryophyllene were present in most of the studied Artemisia accessions. Results of PCA indicated that the first two components contributed 42.31% of total variation and showed a significant positive correlation with thujone, camphor, 1,8-cineole, caryophyllene oxide, caryophyllene, borneol, artemisia ketone, and p-cymene. Based on the chemical composition of essential oil, different accessions were grouped into two major clusters and subdivided into several subgroups. The study has identified many new chemotypes of Artemisia spp. with industrial potential that had not been studied before in this region. Based on the results, new agro-technologies may be developed using Artemisia spp. of commercial interest.
1. Introduction
Artemisia L. is the largest genus of the tribe Anthemidea, belonging to the Asteraceae family. It comprises over 500 species, mainly dwelling in the northern temperate region of Asia, Europe, and North America [1,2]. Forty-eight species have been reported from India, with the majority of them distributed in the Western Himalayan (WH) and Trans-Himalayan (TH) regions [3,4]. Artemisia is an aromatically and medicinally important genus that contains secondary metabolites in the aerial parts with fragrant monoterpenes and sesquiterpenes. Various Artemisia spp. are used to treat ailments such as malaria, cancer, hepatitis, and infectious diseases caused by bacteria, fungi, viruses, and COVID-19 [5,6,7]. Essential oils of various Artemisia spp. have shown promising pharmacological activities, viz., antidiabetic [8], anti-inflammatory [9,10], antimicrobial [11], and anticancer [12,13]. The systematics of the genus Artemisia is very complex. Due to variations in chromosome number, interspecific hybridization, occurrence of polyploidy, and similar morphology under different environmental conditions, identifying Artemisia spp. is challenging [14,15,16].
The essential oil of aerial parts of the Artemisia spp. bears many commercially important chemicals like capillene, β-caryophyllene, artemisia ketone, α-thujone, 1,8-cineole, limonene, camphor, camphene, and α-pinene [17]. Different chemotypes possess characteristic smells and bioactivities that indicate their use in the pharmaceutical and aroma industries. Many previous studies have shown significant variations in the composition and properties of the essential oils of Artemisia spp. from the different altitudinal gradients [15,17,18]. The main ingredient in Chinese, French, and Indian Artemisia essential oils is reported to be artemisia ketone. The most explored species of Artemisia is Artemisia annua. The primary components in the essential oils isolated from North American A. annua were artemisia ketone (35.70–68.00%) and 1,8-cineole (22.80–31.50%) [19]. Several chemotypes of Artemisia have been reported from different locations in India. Capillene (58.38%) was reported from the essential oil of Indian Artemisia dracunculus [17]. Three major chemotypes were reported for A. dracunculus collected from the Kashmir and Himachal Pradesh states of India [17,20]. A chemotype of A. nilagirica var. septentrionalis rich in artemisia ketone was reported from the foothills of the western Himalayas of India [21]. Recently, six chemotypes have been described for A. annua, such as artemisia ketone, camphor, β-cubebene, 1,8-cineole, α-pinene, and β-selinene [7,22].
Essential oil yield and chemistry are affected by various extrinsic and intrinsic factors. In addition to chemotypic and genetic factors, essential oils are influenced by the developmental stage [23], altitudinal effect [15], harvesting stages [24], and seasonal variation [21]. Plant diversity has been greatly aided by the evolution of chemical complexity [25]. The essential oil chemical fingerprint may help assess quality and aid in identifying complex species. Despite the availability of huge natural resources of Artemisia spp. across the Indian Himalayan region, only a few species have been explored so far. It is crucial to assess the chemical makeup of essential oils of different Artemisia spp. from various geographical locations of the WH and TH regions of India. The evaluation of Artemisia germplasm from the region can help us understand this valuable natural resource for the development of novel products from their essential oils and aromatic compounds. Therefore, the present study aims to evaluate the essential oils of the eighty-one accessions representing 40 Artemisia spp. from India’s WH and TH regions for their essential oil yield, chemical composition, and variability among and within the species.
2. Materials and Methods
2.1. Study Area and Collection of Plant Material
Eighty-one accessions of 40 Artemisia spp. were collected from different geographical locations from the Western Himalayan (WH) and Trans-Himalayan (TH)regions of India, covering an altitudinal range from 322 m to 5046 m above mean sea level (m asl) (Figure 1, Table 1). There was no requirement for the collection permit to collect studied Artemisia spp. accessions from their natural habitat. Most of the studied Artemisia spp. had global distribution except for A. amygdalina, A. austrohimalayaensis, A. banihalensis, A. dubia, and A. filiformilobulata which were endemic to the region. The study area comprises the two states of India, viz., Uttarakhand and Himachal Pradesh, as well as two Indian Union territories, Jammu and Kashmir and Ladakh. The study area covers a wide range of topography, from hot plains to hilly and mountainous valleys characterized by tropical to temperate and alpine environments. The collected Artemisia spp. accessions were identified by using regional floras, eFloras, research publications on the Artemisia spp. of the study area [3,4,26,27,28,29], online Kew Herbarium and Janaki Ammal Herbarium (RRLH) at the Council of Scientific and Industrial Research (CSIR-IIIM), Jammu, Union Territory of Jammu and Kashmir, India. Botanical names and synonyms were authenticated through Kew’s ’Plants of the World’ database (www.plantsoftheworldonline.org, accessed on 22 December 2022). Voucher specimens were prepared and deposited in the internationally recognized Janaki Ammal Herbarium (RRLH).
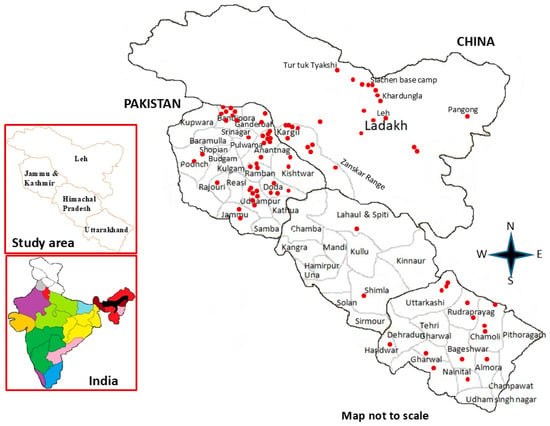
Figure 1.
Map of the study area showing collection sites (red dots) of studied Artemisia accessions.

Table 1.
Details of the studied accessions of Artemisia spp. from WH and TH regions of India.
2.2. Chemicals Used
All chemicals used in the present study were of analytical grade.
2.3. Extraction of the Essential Oils
Aerial parts of 81 accessions representing 40 Artemisia spp. were collected from their natural habitat. All the collected plant samples were strictly subjected to uniform processing/drying conditions. After a daylong collection, collected aerial parts were partially dried in the shade overnight at the camping site or in the room and then wrapped in paper bags. To avoid mold growth on samples and essential oil loss, plant samples were immediately brought to the laboratory and further dried for seven days under uniform controlled laboratory conditions with room temperature 28.0 ± 3.0 °C and relative humidity between 45% and 55%. Dried aerial parts were weighed and subjected to hydro-distillation using a Clevenger-type apparatus for 4 h. Obtained essential oils were dried over anhydrous sodium sulfate and stored in air-tight glass vials at −20 °C till further use. The essential oil yield was calculated as volume/weight (%), i.e., volume (ml) of essential oil obtained per dry weight (g) of plant material used, and the color variation was noted down.
2.4. Analysis of the Essential Oils and Compound Identification
Gas chromatography with mass spectrometry (GC-MS) was performed using Agilent 7890-USA (sourced from J&W Scientific, Santa Clara, United States) equipped with a 5975 triple-axis detector and DB 5MS column (30 m × 0.250 mm diameter × 0.25 μm thickness). The oven temperature program was as follows: 60 °C (2 min hold), ramp to 120 °C at 30 °C/min (2 min hold), then ramp to 300 °C at 5 °C/min (2 min hold). A 1 μL sample was injected with a 100:1 split ratio. The injection temperature was 280 °C, and the ion source was set to 70 eV. Helium (99.9995% purity) was used as the carrier gas at a flow rate of 1 mL/min. Mass spectra were acquired in full scan mode (m/z 35–780). Component identification was performed by comparing experimental retention indices (RIs) with literature values [30] and matching mass spectra against the NIST17 library, using retention time and molecular ion mass (m/z) as additional criteria [31].
2.5. Data Analysis
Multivariate statistical techniques were used to analyze the relative percentage content of the essential oil components. To determine the usefulness of identified essential oil components in reflecting chemotaxonomic relationships in the studied accessions, 48 compounds detected in the essential oil samples with concentrations greater than 10% of the total were used for the statistical analysis. The dendrogram was created using Ward’s method for hierarchical cluster analysis (HCA). PAST Version 4.01 was used to perform the Principal Components Analysis (PCA) and the cluster analysis.
3. Results and Discussion
3.1. Essential Oil Yield and Color
In the present study, efforts were made to collect the maximum number of accessions of Artemisia spp. to cover as much of the natural range of distribution of Artemisia from the WH and TH regions of India. A total of 81 accessions of 40 Artemisia spp. (38 identified and two unidentified) were collected from the diverse habitats of the WH and TH regions of India. In many earlier studies, researchers have reported many mono-, sesquiterpenoids, and phenolic aromatic compounds from Artemisia spp. that are commercially used in the cosmetics and aroma industries. Due to the presence of various compounds, essential oils possess a wide range of biological properties [32]. For instance, artemisinin is a well-recognized and effective antimalarial compound isolated from A. annua [33]. The yield and color of the essential oils of different Artemisia spp. accessions showed variations at inter and intra-specific levels.
Artemisia vestita had the highest essential oil yield (1.65%), followed by A. scoparia (1.44%), while A. biennis and one unidentified species collected from Gurez valley had the lowest essential oil yield of 0.03% and 0.02%, respectively. Haider et al. [34] and Nin et al. [35] also found essential oil yield variation within the accessions of some Artemisia spp. collected from different locations. In the present study, we observed that the essential oil composition varied among the species and within the studied species, which may be due to variations in environmental conditions and other factors of the study area.
The colors of the essential oils of the studied Artemisia accessions ranged from yellow, red, green, blue, white, and pale-olive to olive-yellow, but most commonly, yellow shades were observed. Color shades varied even in the same species collected from different altitudes, making the oil color a noteworthy character (Supplementary Figure S1). It was reported in previous studies that the essential oil of some Artemisia spp. was yellow, varying from greenish-yellow, yellow, and pale yellow to yellowish brown [7,36,37,38,39]. Table 2 shows the oil yield and color variations among the studied accessions of Artemisia spp. from the WH and TH regions of India. However, our results showed some unique colors of essential oils, like crimson blue in A. myriantha and A. roxburghiana and very dusky red in A. absinthium, also reported earlier by Msaada et al. and Zhou et al. [40,41].

Table 2.
The yield (v/w%) and color of essential oils of collected accessions of Artemisia spp.
3.2. Chemical Composition of Essential Oils
Gas chromatography with mass spectrometry (GC-MS) was used to determine the composition of the extracted essential oils of 81 accessions of Artemisia spp. The GC-MS analysis of essential oils allowed the detection of 421 chemical constituents in the whole collection, and out of these, 155 compounds that have an average concentration of more than 2% in any of the studied accessions were identified by comparison of relative retention indices of the peak with the MS library of standard essential oils. The identified essential oil constituents represented 81–100% of the essential oils. One fifty-five major compounds at an average concentration greater than 2% of the total essential oil were used for statistical analysis (Table 3). Details of major compounds (>10% in at least one accession) detected in the essential oil of the studied Artemisia accessions from the WH and TH regions of India are given in Supplementary Table S1.

Table 3.
The chemical variability of major compounds (>2%) in the essential oils of the studied Artemisia accessions from the WH and TH regions of India.
The essential oils of A. vulgaris accessions contained santolina triene, 1,8-cineole, artemisia ketone, thujone, pinocarveol, borneol, caryophyllene oxide, β-eudesmol, and camphor (Table 3). Large variations were observed in the quantities of essential oil compounds of seven accessions of A. vulgaris collected from the different locations that suggested the distinctive chemotypes (Table 3). Accessions Av1, Av2, Av3, Av4, Av5, Av6 and Av7 were characterized by the presence 2-thujene, 2,3-bornanediol, α-cadinol, β-eudesmene, 2,6-dimethyl-3,5-heptadien-2-ol, torreyol, cubenol, agarospirol, myrtenol, 3-carene, curcumene, limonene, β-sesquiphellandrene, and γ-eudesmol (Table 3). The principal components in A. maritima accessions (Am8, Am9, and Am10) essential oils were 1,8-cineole, chrysanthenone, myrtenol, camphor, and verbenyl acetate. Accessions of A. absinthium, Aab11, Aab12, and Aab13 were found to be rich in verbenyl acetate with concentrations of 80.67%, 74.91%, and 62.87%, respectively. Cis-verbenone (5.13%) was observed only in the Aab11 accession. However, trans-thujone (54.70%) was found to be the main component in Algerian A. absinthium essential oil, as reported in a previous study [42]. The main constituents in A. annua accessions were thujone (52.84%) and terpinolene (6.77%) in Aa15, and camphor (39.97%), caryophyllene (12.86%), germacrene (10.25%), caryophyllene oxide (4.14%), and γ-himachalene (5.02%) in Aa14.
The A. nilagirica accessions (An16, An17, and An18) had 1,8-cineole, thujone, borneol, β-eudesmol, and camphor as the major components of essential oil. Accession An17 contained cis-verbenone (9.58%) and could be classified as a distinct chemotype. The major components in A. cashemirica (Ac19) accession were 1,8-cineole (22.68%), caryophyllene oxide (19.72%) and camphor (20.45%). The essential oil of A. japonica had caryophyllene oxide as the major compound in both the accessions Aj20 (27.46%) and Aj21 (25.27%). Artemisia vestita accessions (Ave22, Ave23, and Ave24) were found to be rich in borneol, camphor, chrysanthenone, 2,6-dimethyl-3,5-heptadien-2-ol, bornyl acetate, sabinen, and trans-2,7-dimethyl-4,6-octadien-2-ol (Table 3). The A. scoparia accessions (As25, As26, and As27) had high amounts of limonene, caryophyllene, p-cymene, and spathulenol,1,8-cineole, caryophyllene oxide, and thujone.
Essential oils of accessions Ai28, Ai29, and Ai30 belonging to A. indica were found to be rich in β-eudesmol, caryophyllene oxide, borneol, germacrene, and curcumene. Further, A. incisa (Ain31) accession collected from Kashmir contained davanone (10.34%), β-cubebene (12.26%), caryophyllene oxide (10.67%), δ-cadinene (5.19%), widdrol (4.84%), and α-cadinol (4.16%). Artemisia laciniata accessions were represented by distinct chemotypes (Table 3). Accessions Ala32 contained camphor (28.50%), thujone (15.78%) and 1,8-cineole (7.66%), accession Ala33 contained verbenyl acetate (22.36%), artemisia ketone (17.56%) and camphor (17.10%), and accession Ala34 contained Furan,3-(4,8-dimethyl-3,7-nonadienyl)- (21.62%) and 4,4-diethyl-3-methylene-1-oxetan-2-one (20.07%). Accession Asi37 was found to be rich in camazulene (45.53%) and linalyl iso-valerate (17.17%). Accessions Asi35 and Asi36 had thujone, camphor, 1,8-cineole, and endoborneol as major constituents.
The essential oil of A. amygdalina (Aam38), the only species Indigenous to Kashmir Himalaya, contained α-bisabolol (21.67%), α-cadinol (8.32%), thujone (6.40%) and aromadendrene oxide-(2) (2.16%). Artemisia verlotiorum (Aver40) had myrtenol (23.68%), camphor (10.12%), borneol (9.29%), sabinen (7.73%), juniper camphor (4.86%) and 4-terpenol (4.80%) as the main components. The A. moorcroftiana accessions could be classified into two distinct chemotypes. Accession Amo41 was characterized by the presence of thujone (55.74%), which was higher than all the investigated accessions of Artemisia, whereas accession Amo42 contained a maximum amount of cis-p-mentha-2,8-dien-1-ol (48.14%).
Accessions of A. persica collected from the Trans Himalayan region could be identified as chemotype of α-terpinene (Ap43; 44.18%), 2-carene (Ap44; 27.91%), and piperitone oxide (Ap45; 6.35%). Further, accessions Asa46 and Asa47, belonging to A. salsoloides from Trans Himalaya, were rich in 1,8-cineole, thujone, camphor, and chrysanthenone. Davanone and bornylene were the two chemotypes identified from the A. gmelinii accessions (Agm48 and Agm49). Other compounds found in A. gmelinii accessions were 1,8-cineole, thujone, 4,4-diethyl-3-methylene-1-oxetan-2-one, and 4-terpineol.
The main constituents in the essential oils of A. tournefortiana (Ato50) accession were 1,8-cineole (26.57%), thujone (22.70%), and camphor (14.97%), while the same constituents were found in A. biennis (Abi51, and Abi52) accessions in higher concentration. Accession Abi52 had 1,8-cineole (43.57%), camphor (27.70%), and β-pinene (10.05%), whereas caryophyllene oxide (8.21%) and artemisia ketone (4.66%) were found in the Ato50 accession. Not much variation was observed in the accessions of A. minor (Ami53, Ami54) collected from the Trans Himalayan region of Ladakh. They had thujone, camphor, p-cymene, and 4-terpineol as the major components in the essential oil.
Artemisia macrocephala (Ama55) accession was characterized by the presence of 2-isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydronaphthalene (61.09%). Essential oils of A. rutifolia accessions (Ar56, Ar57) were rich in verbenyl acetate, β-eudesmol, artemisia ketone, and ledene oxide-(I). A total of three accessions of A. stechmanniana were investigated. Verbenyl acetate (44.49%), chrysanthenone (9.12%), and myrtenol (21.10%) were the major constituents in Aste58, Aste59, and Aste60 accessions, respectively. Terpinolene was reported only in the Ad61 (3.85%) and Ad62 (11.95%) accessions belonging to A. desertorum that are restricted to the cold desert region of Ladakh. Further, A. stracheyi (Ast63) from the high altitude of Changla Pass in Ladakh was found to be rich in spathulenol (22.35%), 1,8-cineole (18.87%), camphor (11.78%), and thujone (11.24%).
The Abr64 accession of A. brevifolia was a distinct chemotype having the highest amount of 4-terpineol (26.06%), whereas the Abr65 accession had camphor (36.14%) and 1,8-cineole (13.29%). The major components in A. dubia (Adu66) essential oil were thujone (9.89%), 1,8-cineole (8.42%), and caryophyllene oxide (8.14%). The main components of A. myriantha accessions were β-eudesmol (36.53%) in Amy67 and artemisia ketone (19.33%) in Amy68. Artemisia banihalensis (Ab69) accession had borneol (12.06%), germacrene (10.64%), and 4-terpineol (10.51%) as major components of essential oil.
Artemisia gmelinii accessions (Ag70 and Ag71) were rich in 1,8-cineole, caryophyllene oxide, thujone, and camphor. Naphthalene,2-ethenyl (11.49%) was found only in accession Ag71 only. Essential oil of A. austrohimalayaensis (Aau72) accession contained β-cubebene (5.62%) and elixene (5.13%), which were specific to this species only. A total of 31 compounds were detected in A. austrohimalayaensis oil. of which thujone, camphor, and 1,8-cineole were the major compounds. Artemisia filiformilobulata accessions, indigenous to Gangotri (Af73) and Mana (Af74) regions of Uttarakhand, had thujone, 1,8-cineole, and camphor as the major constituents. In addition, some other unique compounds like δ-cadinene (7.27%), cubenol (5.97%), and torreyol (3.51%) were also observed in accession Af73. An unidentified Artemisia species accession Au75 had epicubenol (11.24%), davanone (6.89%), and epiglobulol (6.53%) as major constituents. Accessions Aw76 belonging to A. wallichiana (species name now considered as a synonym of A. roxburghiana as per the world flora of online plant) was found rich in β-eudesmol (18.62%), cis-β-terpineol, (8.46%), and 1,8-cineole (6.44%).
However, A. roxburghiana accessions Ar77 and Ar78 were dominated by caryophyllene oxide (46.69%) and α-eudesmol (61.05%). They can be recognized as two distinct chemotypes. Germacrene (13.93%) was only found in Ar78, whereas cis-β-terpineol (8.46%), β-sesquiphellandrene (8.85%), and bergamotol, Z-α-trans (5.14%) were only found in the essential oil of Ar79 accession. β-eudesmol (33.05%), santolina triene (17.10%), 3-cyclohexene-1-carboxaldehyde,1,3,4-trimethyl (13.16%), and isothujol (5.74%) were the main compounds in A. roxburghiana var. grata accession Arg80. Essential of A. capillaris accession Ac81 had caryophyllene oxide (27.03%), caryophyllene (15.09%), thujone (10.36%), and 1,8-cineole (9.52%) as major constituents. Pictures and essential oil chemical composition for the unidentified Artemesia accessions (Aun39, Au75) are given in Supplementary Figure S2 and Supplementary Table S2.
The data revealed that 1,8-cineole, thujone, camphor, artemisia ketone, borneol, and caryophyllene were present in the essential oils of most of the Artemisia accessions. Most of the Artemisia accessions were characterized by unique compounds that were not present in other accessions. These compounds could be used as markers to delimit these complex Artemisia spp. (Table 3). The constituents that were present in the higher concentration were verbenyl acetate in A. absinthium, α-eudesmol and caryophyllene oxide in A. roxburghiana, 1,8-cineole in A. biennis, thujone in A. annua and camazulene in A. sieversiana. The present findings indicated that essential oils of some of the studied Artemisia spp. could be considered a good source of santolina triene, artemisia ketone, camphor, 1,8-cineole, davanone, and caryophyllene oxide, which are known to have extensive use in pharmaceutical industries (Table 3). Due to the presence of these compounds in the essential oils of Artemisia, many of these species have been reported to have antimicrobial, antifungal, and anti-inflammatory activities [43,44].
Essential oil chemical composition is well known to depend on various intrinsic and extrinsic factors, climatic conditions and geographical location, collection time, drying, and distillation mode [45]. Despite widespread distribution and the availability of many Artemisia spp., the essential oil composition studies of this genus are minimal. Previous studies described a few chemotypes [17,20]. Some researchers from different parts of the world have studied the essential oil composition of some Artemisia spp. They have reported significant variation in the components of Artemisia essential oils and identified different chemotypes of Artemisia spp. Sati et al. [44] reported thujone (36.35%), β-thujone (9.37%), germacrene (6.32%), and 4-terpineol (6.31%) as major constituents of A. nilagirica. In another study, nerolidol, camphor, and α-thujones were identified as major constituents of A. absinthium essential oils [9,46,47]. High concentrations of borneol, 1,8-cineole, p-cymene, and sabinen have been reported in A. amygdalina [48]. Camphor, 1,8-cineole, camphene, spathulenol, and germacrene were reported to be present in aerial parts of A. annua [49,50]. Satyal et al. [51] identified chrysanthenone, coumarin, and camphor as the major components in A. dubia essential oil.
Artemisia ketone, cis-chrysanthenyl acetate, 1,8-cineole, and pinocarvone were major compounds found in A. gmelinii essential oil [5]. Davanone, β-pinene, and germacrene have been reported as major components of A. indica essential oil [52], whereas linalool, spathulenol, germacrene, and β-elemene have been reported from A. japonica essential oil [37,53]. Weyerstahl et al. [54] reported cis-chrysanthenyl acetate silphiperfol-5-en-3-ol A, presilphiperfolan-9α-ol, artedouglasia oxide, and α-zingiberene as main compounds in the essential oil of A. laciniata. Thujone, 3-thujanone, and cineole were the major constituents of A. macrocephala essential oil [55]. Artemisia maritima was reported to be rich in α-thujone and β-thujone [5,56]. Germacrene, β-eudesmol, chrysanthenone, 1,8-cineole, and β-pinene oxide were found to be the major constituents of A. myriantha essential oil [57]. In A. nilagirica, the major components were α-thujone, β-thujone, 4-terpineol, borneol, linalool, isopulegyl acetate, and sabinene [18]. The main components identified in the essential oil of A. parviflora were β-caryophyllene, germacrene, camphor, artemisia ketone, 1,8-cineole, α-copaene, artemisia alcohol, terpinene-4-ol, caryophyllene oxide, α-pinene, and sabinyl acetate [50]. Nikbakht et al. [13] and Sadeghpour et al. [58] reported β-thujone, davanone, cis-chrysanthenyl acetate, limonene, α-pinene, davanone, ether isomer, and thujene as the main components in A. persica essential oil.
The main compounds in A. salsoloides essential oil were reported to be 2,4-Penta diynylbenzene, β-trans-ocimene, sabinene, and 2,5-etheno(4,2,2)propella-3,7,9-triene [59]. Artemisia roxburghiana essential oil had artemisia alcohol, β-eudesmol, cis-sabinene hydrate, caryophyllene oxide, 1,8-cineole, terpinen-4-ol, borneol, eucarvone, α-thujone, and cis-chrysanthenyl acetate as main constituents [5]. Sharopov and Setzer [60] found β-thujone and α-thujone in A. rutifolia essential oil, while nerolidol, α-thujone, β-thujone, cineole, terpin-4-ol, and p-cymene were the major constituents in A. santolinifolia essential oil [61]. Singh et al. [62] studied A. scoparia and found β-myrcene, β-ocimene, limonene, and γ-terpinene as the main compounds in its essential oil. In A. sieversiana, 1,8-cineole, geranyl butyrate, borneol, and camphor were the main constituents of essential oil [63]. Kazemi et al. [64] analyzed A. tournefortiana essential oil and found trans-thujone, sabinene, and β-pinene as the major constituents. Further, Carnat et al. [65] studied the essential oil of A. verlotiorum and identified 1,8-cineole, β-caryophyllene, β-thujone, germacrene, and caryophyllene oxide as its major constituents. Artemisia ketone, α-phellandrene, artemisia alcohol, β-caryophyllene, and 1,8-cineole were identified as the main constituents in A. vestita oil [66]. Haider et al. [67] investigated the essential oil of A. vulgaris and reported camphor, isoborneol, 1,8-cineole, and α-thujone as the main components. In A. wallichiana sabinene, germacrene and vulgarone were major constituents of essential oil [68].
Artemisia ketone is mentioned as a chemotype of A. nilagirica var. septentrionalis [21]. A series of chemotypes have been identified for A. annua, such as artemisia ketone, camphor, β-cubebene, 1,8-cineole, α-pinene, and β-selinene [7]. Further present investigation revealed the presence of new essential oil components/chemotypes of the Artemisia resources, which were not described before from the study area. There is no literature available on the essential oil composition of A. austrohimalayana, A. banihalensis, A. macrocephala, A. desertorum, A. filiformilobulata, A. roxburghiana var. grata, A. stracheyi, and A. cashemirica. These species have been studied for the first time. The detailed essential oil composition of these accessions is given in Supplementary Table S2. Previous studies have revealed that crude extracts, single compounds, and essential oils of A. annua, A. macrocephala, A. persica, A. absinthium, A. sacrorum, A. sieversiana, and A. amygdalina exhibited a broad spectrum of biological activities, including antimicrobial, antimalarial, antioxidant, anti-inflammatory, antidiabetic, and anticancer [8,9,55,69,70,71,72,73,74,75,76]. They have significant applications in the pharmaceutical industry.
3.3. Chemical Principal Components Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
The first two principal components showed the highest variation. PC1 explained 28.32% of the total variation and had a positive correlation with thujone, camphor, 1,8-cineole, caryophyllene oxide, caryophyllene, borneol, artemisia ketone, and p-cymene. The PC2 showed 16.80% of the total variation and had a high negative correlation with verbenyl acetate and β-eudesmol.
Since PC1 and PC2 possessed a significant share in chemical constituents, scatter plots for the first two components were made to evaluate phytochemical distance (Figure 2a,b). According to the PCA, the studied accessions were divided into four groups. As shown in Figure 2, the presence and amounts of some essential oil compounds helped identify groups. For example, the accessions Ad62, As25, Ar77, and Av22, characterized by the camphor, limonene, caryophyllene oxide, caryophyllene, and borneol, were situated in the low right quadrant of the plot. The accessions Ap44, Ap43, Aa15, and Ag49, characterized by thujone, 1,8-cineole, verbenyl acetate, spathulenol, and p-cymene, were in the top right quadrant of the plot.
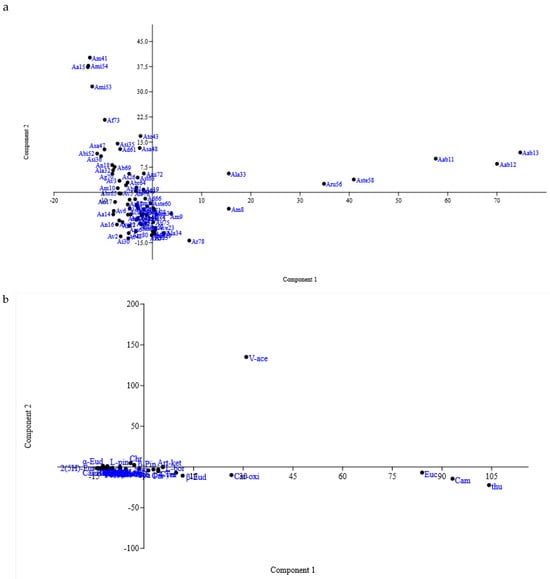
Figure 2.
(a) PCA score plot for the main variation in essential oil composition among Artemisia spp. accessions. (b) Loading plot for essential oil components explaining 42.31% of the variation on PC1 and PC2 axes.
A dendrogram constructed from the statistical analysis of the identified compounds of each accession is shown in Figure 3. Essential oil composition showed notable differences among the studied Artemisia spp. accessions. The dendrogram of 81 accessions consisted of two major clusters named A and B. Accessions with relatively high amounts of verbenyl acetate were grouped in Cluster B. These accessions were Aru 56 (38.66%), Aste58 (44.49%), Aab11 (62.87%), Aab12 (74.87%) and Aab13 (80.67%). The results indicate that the accessions of A. absinthium (Aab11, Aab12, and Aab13) fall in the same clusters due to similar major compounds.
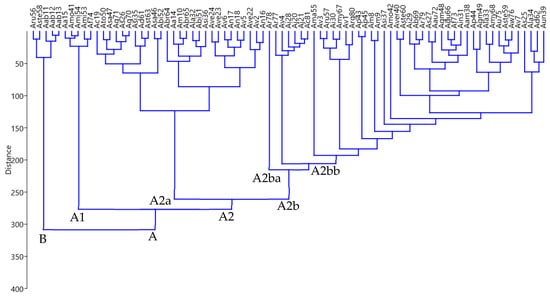
Figure 3.
Dendrogram generated by cluster analysis of the essential oil composition of eighty-one Artemisia accessions using the HCA by Ward’s method.
Cluster A contained the accessions of 35 Artemisia spp. and was divided into two further sub-groups, A1 and A2. Sub-group A1 contained accessions characterized by a considerable percentage of thujone. These accessions were Aa15 (52.84%), Amo41 (55.74%), Ami54 (51.96%), Ami53 (45.33%), and Af74 (35.54%). Sub-group A2 was further segregated into A2a and A2b sub-groups. Sub-group A2a comprised accessions of 16 Artemisia spp., and most of the accessions of these species fall in this group due to similar constituents. Group A2b formed two sub-groups, A2ba and A2bb. Sub-group A2ba contained only one accession, Ar78, which was characterized by the presence of α-eudesmol (61.05%). Many of the species in the dendrogram are segregated due to the presence of some unique compounds specific to a particular species or accession.
The essential oils or particular chemical components of many of these species have the potential for the development of antidiabetic, anti-inflammatory, anticancer, antioxidant, antimicrobial, and antiparasitic medicines/products [8,9,55,69,70,71,72,73,74,75,76]. The data on essential oil profiles of Artemisia spp. generated in the present study will provide the baseline information about chemical diversity in the genus Artemisia from the WH and TH regions of India. The important components in the essential oils of the studied Artemisia spp. such as davanone, 1,8-cineole, camphor, and artemisia ketone, have high industrial value. Reporting of such components in the essential oils of the studied Artemisia spp. offer great opportunities to expand such resources to meet the market supplies of specific essential oils or single molecules. Further, by exploiting these resources, we can decrease the pressure from other plant species. For instance, some species of Eucalyptus, a highly water-requiring tree species, are mainly over-exploited for the production of 1,8-cineole, which leads to adverse environmental impact. Similarly, the current trend is to overexploit wild populations of many important plant species for essential oils, which leads to biodiversity loss and disturbances. Most of the studied Artemisia spp. grow as weeds in the wasteland, and they do not require much water and nutrients to grow. Some of the studied Artemesia spp. and their identified chemotypes can be targeted to develop new high-value varieties that could be used for the production of specific essential oils or their components at an industrial scale. That will help us to reduce pressure on other species and provide us with an alternative sustainable resource, which can also be promoted for cultivation in the region. The cultivation of some of these Artemisia spp. will be helpful in mitigating the demand for specific essential oils or important components, which will lead to a sustainable essential oil industry.
The current study’s findings made it abundantly evident that there was significant chemical variation among the essential oils of the studied eighty-one accessions representing 40 Artemisia spp. from India’s WH and TH regions. Accessions of different and even the same species differed considerably in essential oil yield and chemotypic characteristics. Identifying major components in the Artemisia essential oils in the present study has provided a chemical fingerprint for the authentication and identification of these complex species. This information will serve as a practical tool to resolve taxonomic ambiguities in these species. Most of these species have not been earlier investigated in this region to such a large extent. Therefore, information on essential oil characterization is of great commercial significance and helpful in developing new varieties of Artemisia germplasm from this region for flavor, fragrance, aroma, and pharmaceutical industries.
4. Conclusions
Since most of the Artemisia spp. was reported to be growing in natural conditions in mostly dry areas on wastelands, their cultivation can be promoted in these areas. It would help to bring unutilized barren wastelands into use and provide an alternate source of income to the people. For example, some products from Artemisia spp. collected from the Trans-Himalayan region of Ladakh, where vegetation is scarce, could be developed to provide a major source of income to the poor and unemployed people of the region. Agrotechnologies for all the reported species except A. annua and A. pallens have not been developed. There is a need to develop new cultivars, varieties, and agro-technologies for the economically important Artemisia spp. to promote their cultivation. We hope that the information generated in the present study will help researchers plan future studies and initiate new research on these under-explored/underutilized Artemisia spp., which have a lot of potential for product development. Further, the essential oils of many of these species can be used pharmacologically. Therefore, this study offers great opportunities for inhabitants of the region to cultivate these species and meet the industrial supply of specific essential oils or compounds. Our study will contribute to the knowledge of the chemical diversity of Artemisia spp., which may enhance our ability to utilize Indian Artemisia resources for industrial use. Further breeding programs and research on Artemisia evolution may benefit from our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/resources14030042/s1, Table S1. Major compounds (>10% in at least one accession) detected in the essential oil of the studied Artemisia accessions from the WH and TH regions of India; Table S2. Essential oil chemical composition of A. austrohimalayana, A. banihalensis, A. macrocephala, A. desertorum, A. filiformilobulata, A. roxburghiana var. grata, A. stracheyi, A. cashemirica, and two unidentified Artemisia spp. accessions; Figure S1. Color variation in the essential oils of collected Artemisia spp. Accessions. Note: Aca81 Picture is not included due to the meager amount of oil obtained; Figure S2. Unidentified Artemisia spp. (A) Accession Aun39, (B) Accession Au75.
Author Contributions
Conceptualization, S.G. and B.K.; methodology, S.G. and B.K.; software, B.K.; validation, S.G. and B.K.; formal analysis, B.K. and I.A.W.; investigation, B.K., J.F.L. and I.A.W.; resources, S.G.; data curation, B.K.; writing—original draft preparation, B.K.; writing—review and editing, S.G. and K.S.; visualization, B.K.; supervision, S.G.; project administration, S.G. and K.S.; funding acquisition, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the Director of IIIM Jammu for providing the necessary facilities to carry out the work.
Conflicts of Interest
Authors declare no competing or financial interest.
References
- Bremer, K.; Humphries, C. Generic monograph of the Asteraceae-Anthemideae. Bull. Nat. Hist. Mus. Lond. 1993, 23, 71–177. [Google Scholar]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Kaul, M.K.; Bakshi, S.K. Studies on the genus Artemisia L. in North-west Himalaya with particular reference to Kashmir. Folia Geobot. Phytotax 1984, 19, 299–316. [Google Scholar] [CrossRef]
- BSI—Botanical Survey of India. Checklist of Plants of India. 2016. Available online: www.bsi.gov.in (accessed on 16 June 2022).
- Pandey, V.; Verma, R.S.; Chauhan, A.; Tiwari, R. Compositional characteristics of the volatile oils of three Artemisia spp. from Western Himalaya. J. Essent. Oil Res. 2015, 27, 107–114. [Google Scholar] [CrossRef]
- Walia, S.; Rana, A.; Singh, A.; Sharma, M.; Reddy, S.G.E.; Kumar, R. Influence of harvesting time on essential oil content, chemical composition and pesticidal activity of Artemisia maritima growing wild in the cold desert region of western Himalayas. J. Essent. Oil-Bear. Plants 2019, 22, 396–407. [Google Scholar] [CrossRef]
- Hong, M.; Kim, M.; Jang, H.; Bo, S.; Deepa, P.; Sowndhararajan, K.; Kim, S. Multivariate Analysis of Essential Oil Composition of Artemisia annua L. Collected from Different Locations in Korea. Molecules 2023, 28, 1131. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, K.; Ganai, B.A.; Akbar, S.; Mubashir, K.; Dar, S.A.; Dar, M.Y.; Tantry, M.A. Antidiabetic activity of Artemisia amygdalina Decne in streptozotocin induced diabetic rats. Bio. Med. Res. Int. 2014, 2014, 185676. [Google Scholar]
- Hadi, A.; Hossein, N.; Shirin, P.; Najmeh, N.; Abolfazl, M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 237–244. [Google Scholar]
- Singh, A.P.; Duggal, S. Anti-inflammatory activities of aqueous ethanolic extract of aerial part of Artemisia vulgaris Linn. in Sprague Dawley rats. In Herbal Drugs as Therapeutic Agents; CRC Press: Boca Raton, FL, USA, 2015; pp. 37–41. [Google Scholar]
- Khan, S.; Ali, A.; Ahmad, S.; Abdin, M.Z. Affordable and rapid HPTLC method for the simultaneous analysis of artemisinin and its metabolite artemisinic acid in Artemisia annua L. Biomed. Chrom. 2015, 29, 1594–1603. [Google Scholar] [CrossRef]
- Cha, J.D.; Moon, S.E.; Kim, H.Y.; Cha, I.H.; Lee, K.Y. Essential oil of Artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. J. Food Sci. 2009, 74, 75–81. [Google Scholar] [CrossRef]
- Nikbakht, M.R.; Sharifi, S.; Emami, S.A.; Khodaie, L. Chemical composition and anti proliferative activity of Artemisia persica Boiss. and Artemisia turcomanica Gand. essential oils. Res. Pharm. Sci. 2014, 9, 155–163. [Google Scholar] [PubMed]
- Hayat, M.Q.; Khan, M.A.; Ashraf, M.; Jabeen, S. Ethnobotany of the Genus Artemisia L. (Asteraceae) in Pakistan. Ethnobot. Res. Appl. 2009, 7, 147–162. [Google Scholar] [CrossRef]
- Haider, F.; Kumar, N.; Banerjee, S.; Naqvi, A.A.; Bagchi, G.D. Effect of Altitude on the Essential Oil Constituents of Artemisia roxburghiana Besser var. purpurascens (Jacq.) Hook. J. Essent. Oil Res. 2009, 2, 303–304. [Google Scholar] [CrossRef]
- Tantray, Y.R.; Singhal, V.K.; Gupta, R.C. Deciphering the meiotic behaviour in species of genus Artemisia from Cold Deserts of Ladakh (Jammu & Kashmir). Flora 2020, 262, 151520. [Google Scholar]
- Chauhan, R.S.; Kitchlu, S.; Ram, G.; Kaul, M.K.; Tava, A. Chemical composition of capillene chemotype of Artemisia dracunculus L. from North-West Himalaya, India. Ind. Crops Prod. 2010, 31, 546–549. [Google Scholar] [CrossRef]
- Badoni, R.; Semwal, D.K.; Rawat, U. Altitudinal variation in the volatile constituents of Artemisia nilagirica. Inter. J. Essent. Oil Ther. 2009, 3, 66–68. [Google Scholar]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential oil of Artemisia annua L.: An extraordinary component with numerous antimicrobial properties. Evid.-Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Verma, M.K.; Anand, R.; Chisti, A.M.; Kitchlu, S.; Chandra, S.; Shawl, A.S.; Khajuria, R.K. Essential Oil Composition of Artemisia dracunculus L. (Tarragon) Growing in Kashmir-India. J. Essent. Oil-Bear. Plants 2010, 13, 331–335. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S. Seasonal Variation in Essential Oil Composition of Artemisia nilagirica var. septentrionalis from Foot Hills of Western Himalaya. Rec. Nat. Prod. 2014, 8, 281–285. [Google Scholar]
- Demirbolat, I.; Ulusoy, S.; Inal, E.; Kartal, M. Variation in essential oil composition and biological activities of Artemisia annua L. (sweet wormwood) at different growth periods. J. Essent. Oil-Bear. Plants 2023, 26, 1008–1017. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Tabatabaei, S.M.F.; Hadian, J.; Ebrahimi, S.N.; Sonboli, A. Phenological variation of the essential oil of Artemisia scoparia Waldst. et Kit from Iran. J. Essent. Oil Res. 2007, 19, 326–329. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sani, T.A.; Ameri, A.A.; Imani, M.; Golmakani, E.; Kamali, H. Seasonal variation in the chemical composition, antioxidant activity, and total phenolic content of Artemisia absinthium essential oils. Pharmacogn. Res. 2014, 7, 329–334. [Google Scholar]
- Boachon, B.; Buell, C.R.; Crisovan, E.; Dudareva, N.; Garcia, N.; Godden, G.; Henry, L.; Kamileen, M.O.; Kates, H.R.; Kilgore, M.B.; et al. Phylogenomic mining of the mints reveals multiple mechanisms contributing to the evolution of chemical diversity in Lamiaceae. Mol. Plant 2018, 11, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kachroo, P. Forest Flora of Srinagar and Plants of Neighborhood; Bishen Singh Mahendra Pal Singh: Dehradun, India, 1976. [Google Scholar]
- Kachroo, P.; Bansilal, S.; Dhar, U. Flora of Ladakh: An Ecological and Taxonomical Appraisal; International Book Distributors: Dehradun, India, 1977. [Google Scholar]
- Chowdhery, H.J.; Wadhwa, B.M. Flora of Himachal Pradesh; Botanical Survey of India Publication: Delhi, India, 1984; Volume 1. [Google Scholar]
- eFloras. Missouri Botanical Garden, St. Louis, MO, USA and Harvard University Herbaria, Cambridge, MA, USA. 2022. Available online: http://www.efloras.org/ (accessed on 2 December 2022).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017. [Google Scholar]
- Stein, S.E. Mass Spectral Database and Software, version 3.02; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2005.
- Hong, M.J.; Kim, M.J.; Kim, S. Biological activities of sweet wormwood (Artemisia annua L.). Korean J. Weed Turf. Sci. 2021, 10, 243–263. (In Korean) [Google Scholar]
- Goel, D.; Singh, V.; Ali, M.; Mallavarupu, G.R.; Kumar, S. Essential oils of petal, leaf and stem of the antimalarial plant Artemisia annua. J. Nat. Med. 2007, 61, 187–191. [Google Scholar] [CrossRef]
- Haider, F.; Kumar, N.; Naqvi, A.A.; Bagchi, G.D. Oil constituents of Artemisia nilagirica var. septentrionalis growing at different altitudes. Nat. Prod. Commun. 2010, 5, 1959–1960. [Google Scholar]
- Nin, S.; Arfaioli, P.; Bosetto, M. Quantitative determination of some essential oil components of selected Artemisia absinthium plants. J. Essent. Oil Res. 1995, 7, 271–277. [Google Scholar] [CrossRef]
- Dob, T.; Chelghoum, C. Chemical composition of the essential oil of Artemisia judiaca L. from Algeria. Flav. Frag. J. 2006, 21, 343–347. [Google Scholar] [CrossRef]
- Rashmi, T.R.; Francis, M.S.; Murali, S. Essential oil composition of Artemisia japonica Thunb.from Kerala. J. Pharmacogn. Phytochem. 2014, 3, 160–163. [Google Scholar]
- Hussein, S.A.; Hussein, M.S.; Tkachenko, K.G.; Nmoko, M.; Mudau, F.N. Essential oil composition of Artemisia vulgaris grown in Egypt. Int. J. Pharma. Sci. 2016, 8, 120–123. [Google Scholar]
- Bedini, S.F.; Cosci, G.F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasites Vectors 2017, 10, 80. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef]
- Zhou, J.; Zou, K.; Zhang, W.; Guo, S.; Liu, H.; Sun, J.; Li, J.; Huang, D.; Wu, Y.; Du, S.; et al. Efficacy of compounds isolated from the essential oil of Artemisia lavandulaefolia in control of the cigarette beetle Lasioderma serricorne. Molecules 2018, 23, 343. [Google Scholar] [CrossRef] [PubMed]
- Benmimoune, S.; Tigrine, C.; Mouas, Y.; Kameli, A. Chemical profiling and assessment of biological activities of wild Artemisia absinthium L. essential oil from Algeria. J. Essent. Oil-Bear. Plants 2023, 26, 1410–1423. [Google Scholar] [CrossRef]
- Krestev, T.M.; Jovanovic, B.; Jovic, J.; Ilic, B.; Miladinovic, D.; Matejic, J.; Rajkovic, J.; Dorđevic, L.; Cvetkovic, V.; Zlatkovic, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar]
- Sati, S.C.; Sati, N.; Ahluwalia, V.; Walia, S.; Sati, O.P. Chemical composition and antifungal activity of Artemisia nilagirica essential oil growing in northern hilly areas of India. Nat. Prod. Res. 2013, 27, 45–48. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Derwich, E.; Zineb, B.; Abdellatif, B. Chemical compositions and insecticidal activity of essential oils of three plants Artemisia spp: Artemisia herba-alba, Artemisia absinthium and Artemisia pontica (Morocco). Electron. J. Environ. Agric. Food Chem. 2009, 8, 1202–1211. [Google Scholar]
- Dhen, N.; Majdoub, O.; Souguir, S. Chemical composition and fumigant toxicity of Artemisia absinthium essential oil against Rhyzoperthadominica and Spodoptera littoralis. Tunisian. J. Pl. Protect. 2014, 9, 57–65. [Google Scholar]
- Rather, M.A.; Sofi, S.N.; Dar, B.A.; Ganai, B.A.; Masood, A.; Qurishi, M.A.; Shawl, A.S. Comparative GC-FID and GC-MS analysis of the leaf and stem essential oil constituents of Artemisia amygdalina Decne from Kashmir. J. Pharm. Res. 2011, 4, 1637–1639. [Google Scholar]
- Verdian-Rizi, M.R.; Sadat-Ebrahimi, E.; Hadjiakhoondi, A.; Fazeli, M.R.; Hamedani, M.P. Chemical composition and antimicrobial activity of Artemisia annua L. essential oil from Iran. J. Med. Plants 2008, 1, 58–62. [Google Scholar]
- Rana, V.S.; Juyal, J.P.; Blazquez, M.A.; Bodakhe, S.H. Essential oil composition of Artemisia parviflora aerial parts. Flavour Fragr. J. 2003, 18, 342–344. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Kafle, A.; Pokharel, S.K.; Lamichhane, B.; Dosoky, N.S.; Moriarity, D.M.; Setzer, W.N. Bioactivities of volatile components from Nepalese Artemisia species. Nat. Prod. Commun. 2012, 7, 1651–1658. [Google Scholar] [CrossRef]
- Haider, S.Z.; Mohan, M.; Andola, H.C. Constituents of Artemisia indica Willd. from Uttarakhand Himalaya: A source of davanone. Pharmacog. Res. 2014, 6, 257–259. [Google Scholar]
- Pradeep, D.P.; Meenu Krishnan, V.G.; Lubaina, A.S.; Krishnan, R.; Murugan, K. Comparison of essential oils composition and antioxidant activities of Artemisia japonica Thunb. and A. nilagirica (Clarke) Pamp. Indo Am. J. Pharmaceut. Res. 2014, 4, 832–837. [Google Scholar]
- Weyerstahl, P.; Marschall, H.; Schröder, M.; Wahlburg, H.C.; Kaul, V.K. The sesquiterpene fraction of the essential oil of Artemisia laciniata Willd. Flavour Frag. J. 1997, 12, 315–325. [Google Scholar] [CrossRef]
- Ali, N.; Shah, I.; Shah, S.W.A.; Ahmed, G.; Shoaib, M.; Junaid, M.; Ali, W.; Ahmed, Z. Antioxidant and relaxant activity of fractions of crude methanol extract and essential oil of Artemisia macrocephala Jacquem. BMC Complement. Altern. Med. 2013, 13, 96. [Google Scholar] [CrossRef]
- Mathela, C.S.; Kharkwal, H.; Shah, G.C. Essential oil composition of some Himalayan Artemisia species. J. Essent. Oil Res. 1994, 6, 345–348. [Google Scholar] [CrossRef]
- Shah, G.S.; Mathela, C.S. Investigation on Himalayan Artemisia species VI: Essential oil constituents of Artemisia myriantha Wall. ex Bess. var. pleiocephala (Pamp.) Ling. J. Essent. Oil Res. 2006, 18, 633–634. [Google Scholar] [CrossRef]
- Sadeghpour, O.; Asghari, G.; Ardekani, M.R. Composition of essential oil of Artemisia persica Boiss. from Iran. Iran J. Pharm. Res. 2004, 3, 65–67. [Google Scholar]
- Wani, H.; Shah, S.A.; Banday, J.A. Chemical composition and antioxidant activity of the leaf essential oil of Artemisia salsoloides growing wild in Kashmir Himalayas. Elixir J. 2014, 71, 24581–24583. [Google Scholar]
- Sharopov, F.S.; Setzer, W.N. The essential oil of Artemisia scoparia from Tajikistan is dominated by phenyldiacetylenes. Nat. Prod. Commun. 2011, 6, 119–122. [Google Scholar] [CrossRef]
- Shatar, S.; Dung, N.X.; Karashawa, D. Essential oil composition of some Mongolian Artemisia species. J. Essent. Oil-Bear. Plants 2003, 6, 203–206. [Google Scholar] [CrossRef]
- Singh, H.P.; Mittal, S.; Daizy, S.K.; Batish, R.; Kohli, R.K. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chem. 2009, 114, 642–645. [Google Scholar] [CrossRef]
- Liu, Z.L.; Liu, Q.R.; Chu, S.S.; Jiang, G.H. Insecticidal activity and chemical composition of the essential oils of Artemisia lavandulaefolia and Artemisia sieversiana from China. Chem. Biodivers. 2010, 7, 2040–2045. [Google Scholar] [CrossRef]
- Kazemi, M.; Mozaffarian, V.; Rustaiyan, A.; Larijanic, K.; Ahmadi, M.A. Constituents of Artemisia tournefortiana Rchb. essential oil from Iran. J. Essent. Oil-Bear. Plants 2010, 13, 185–190. [Google Scholar] [CrossRef]
- Carnat, A.P.; Chalchat, J.C.; Fraisse, D.; Lamaison, J.L. Chemical composition of the essential oil of Artemisia verlotiorum Lamotte growing in Auvergne (France). J. Essent. Oil Res. 2001, 13, 336–339. [Google Scholar] [CrossRef]
- Chowdhury, A.R. GC/MS studies of volatiles from Artemisia vestita aerial parts. J. Essent. Oil-Bear. Plants 2003, 6, 210–213. [Google Scholar] [CrossRef]
- Haider, F.; Dwivedia, P.D.; Naqvia, A.A.; Bagchi, G.D. Essential oil composition of Artemisia vulgaris harvested at different growth periods under Indo-Gangetic plain conditions. J. Essent. Oil Res. 2003, 15, 376–378. [Google Scholar] [CrossRef]
- Shah, G.S.; Mathela, C.S. Essential oil constituent of Artemisia wallichiana Bess. J. Essent. Oil Res. 2006, 18, 377–378. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, G.N.; Miao, R.D.; Chen, N.Y.; Wang, Q. Studies on the chemical constituents of Artemisia sieversiana and their anticancer activities. J. Lanzhou Univ. Nat. Sci. Ed. 2004, 40, 68–71. [Google Scholar]
- Lone, S.H.; Bhat, K.A.; Naseer, S.; Rather, R.A.; Khuroo, M.A.; Tasduq, S.A. Isolation, cytotoxicity evaluation and HPLC-quantification of the chemical constituents from Artemisia amygdalina Decne. J. Chromatogr. B 2013, 940, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ahmadvand, H.; Amiri, H.; Dalvand, H.; Bagheri, S.; Dehnoo, M.G.; Moghadam, S.; Moradi, F.H. Chemical composition and antioxidant properties of Lorestan province Artemisia persica. J. Chem. Pharm. Res. 2015, 7, 16–22. [Google Scholar]
- Desiree, C.K.R.; René, F.K.P.; Jonas, K.; Bibiane, D.T.; Roger, S.M.; Lazare, K. Antibacterial and antifungal activity of the essential oil extracted by hydro-distillation from Artemisia annua grown in West-Cameroon. Brit. J. Pharmacol. Med. 2013, 4, 89–94. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, J.J.; Li, Z.H. Ethanol extract of Artemisia sieversiana exhibits anticancer effects and induces apoptosis through a mitochondrial pathway involving DNA damage in COLO-205 colon carcinoma cells. Bangladesh J. Pharmacol. 2015, 10, 518–523. [Google Scholar] [CrossRef]
- Khan, M.; Ganai, B.A.; Ghazanfar, K.; Akbar, S.; Malik, A.H.; Masood, A. Evaluation of Artemisia amygdalina D. for anti-inflammatory and immunomodulatory potential. ISRN Inflammation 2013, 2013, 483646. [Google Scholar] [CrossRef]
- Daradka, H.M.; Abas, M.M.; Mohammad, M.A.M.; Jaffar, M.M. Antidiabetic effect of Artemisia absinthium extracts on alloxan-induced diabetic rats. Comp. Clin. Path. 2014, 23, 1733–1742. [Google Scholar] [CrossRef]
- Choi, N.Y.; Kang, S.Y.; Kim, K.J. Artemisia princeps inhibits biofilm formation and virulence-factor expression of antibiotic-resistant bacteria. BioMed Res. Inter. 2015, 2015, 239519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).