Thermodynamics and Electrochemistry of the Interaction of Sphalerite with Iron (II)-Bearing Compounds in Relation to Flotation
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure of Experimental Studies of Precipitates Formation in Alkaline Solutions
2.2. A Technique to Measure the Electrochemical Potential of Sphalerite Electrode
2.3. Procedure of Sphalerite Flotation
3. Results and Discussion
3.1. Thermodynamics of the Interaction of Sphalerite with Iron (II)-Bearing Ions
3.2. Experimental Study of Precipiates Formation during the Hydrolysis of Iron and Zinc Sulfates in Alkaline Solutions
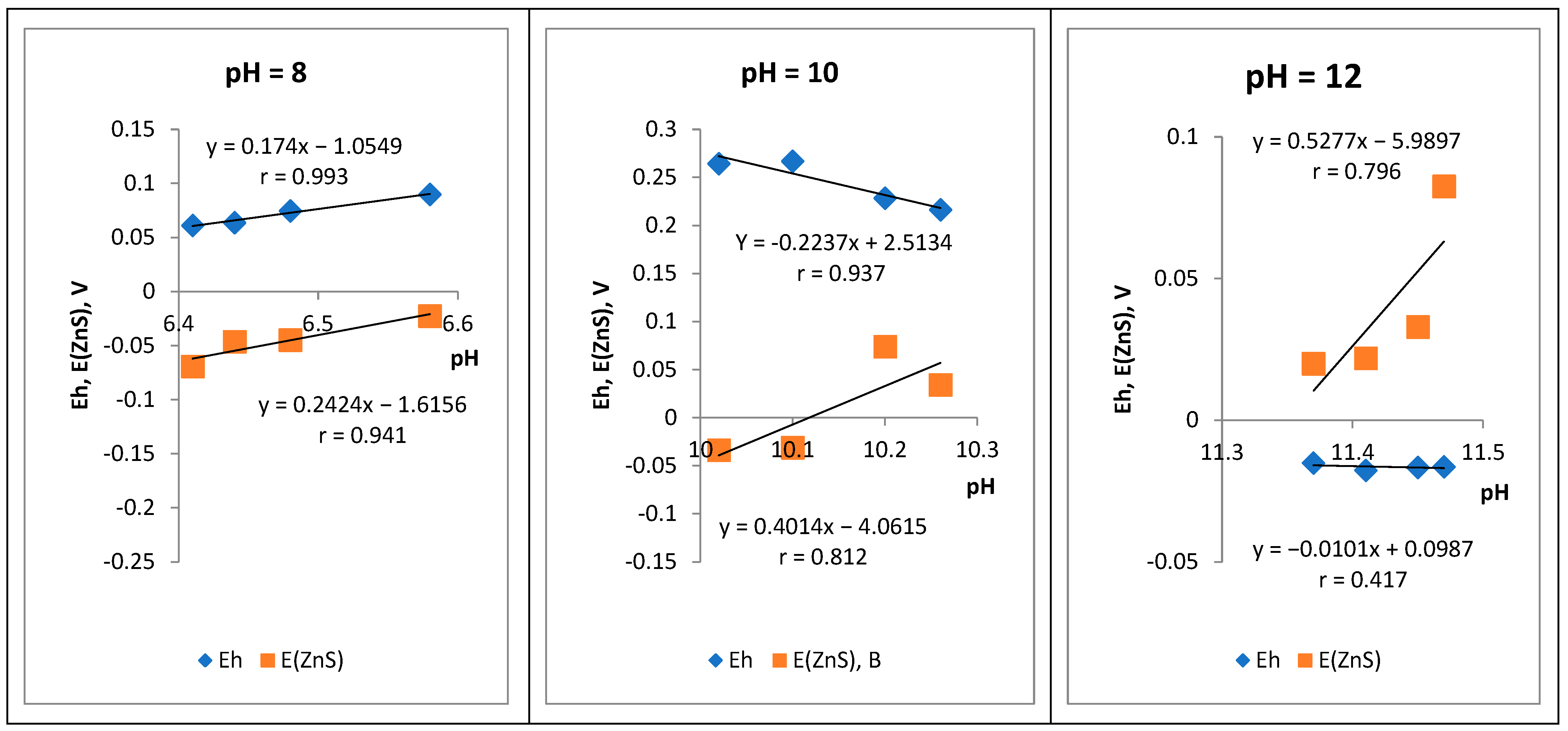
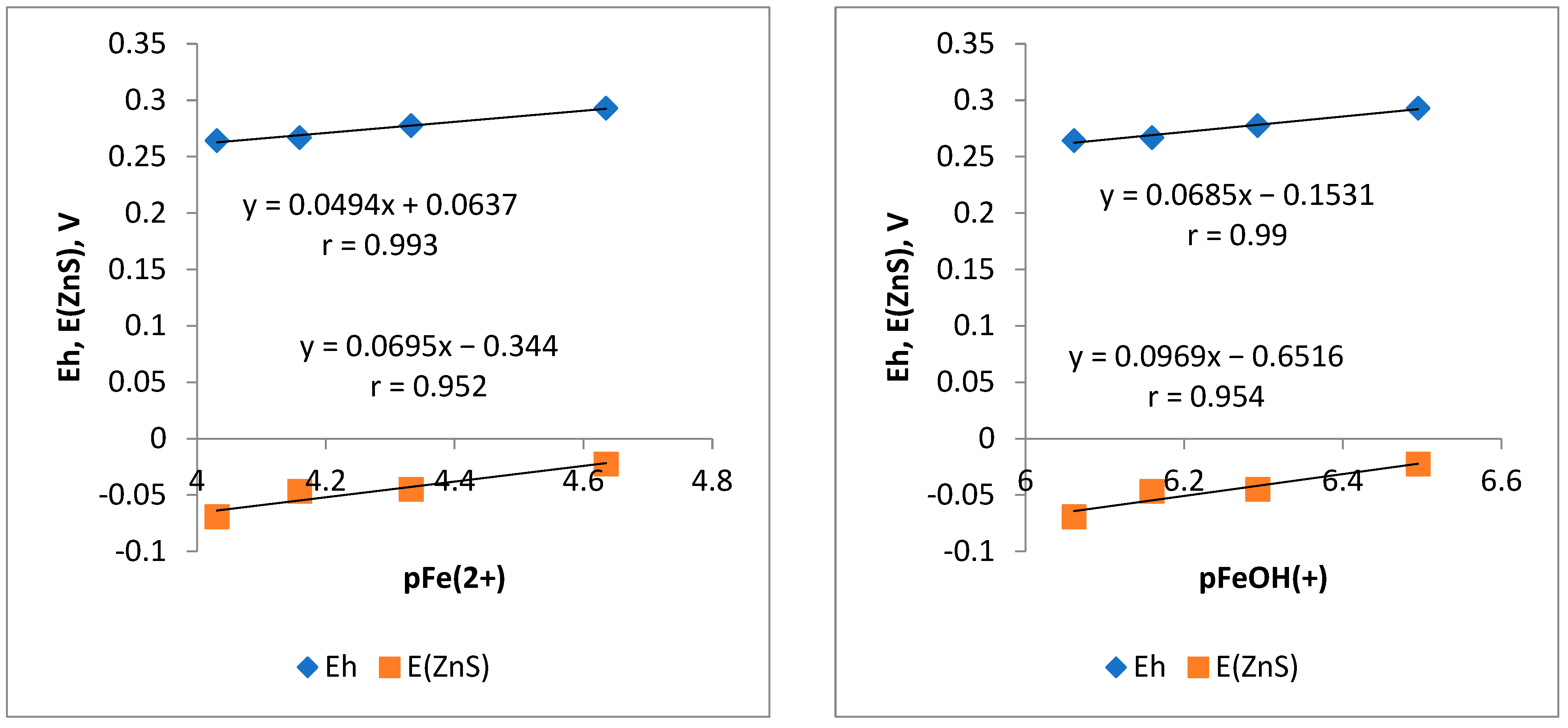
3.3. Electrochemistry of the Sphalerite Electrode in Iron (II) Sulfate Solutions at Different pH Values
3.4. Flotation of Sphalerite Treated with Iron Sulphate Solutions by Thiol Collectors
4. Conclusions
- The results of thermodynamic calculations revealed the possibility of interaction of the sphalerite surface with the products of iron (II) hydrolysis. The iron (II) sulfide was considered as the most probable product which was formed on the sphalerite surface.
- The effect of the concentration of hydroxide ions on the precipitation of iron (II) hydroxides was experimentally studied. At the initial pH = 8 and at all the studied concentrations of iron (II) sulfate in the working solutions, the precipitation process was not observed. However, an increase in the pH of the solutions up to 8 and 12 led to the formation of iron (II) hydroxides.
- The potentiometric studies established that the Fe2+ and FeOH+ cations are potential-determining in weak alkaline solutions.
- In conclusion, the flotation tests demonstrated the effect of the dose of iron (II) sulfate and pH on the flotation recovery of sphalerite with thiol collectors. Interestingly, an activation effect of iron sulfate on sphalerite flotation was observed at pH = 12. This resulted in an increase in the flotation recovery of sphalerite at low dosages of iron sulfate in suspension.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavolová, H.; Čulková, K.; Šimková, Z.; Seňová, A.; Kudelas, D. Contribution of Mining Industry in Chosen EU Countries to the Sustainability Issues. Sustainability 2022, 14, 4177. [Google Scholar] [CrossRef]
- Tomazinakis, S.; Valakas, G.; Gaki, A.; Damigos, D.; Adam, K. The Importance and Challenges of Sustainable Development for the Raw Materials Sector: The Views of Key Stakeholders in Three ESEE Countries. Sustainability 2022, 14, 3933. [Google Scholar] [CrossRef]
- Mambwe, P.; Shengo, M.; Kidyanyama, T.; Muchez, P.; Chabu, M. Geometallurgy of Cobalt Black Ores in the Katanga Copperbelt (Ruashi Cu-Co Deposit): A New Proposal for Enhancing Cobalt Recovery. Minerals 2022, 12, 295. [Google Scholar] [CrossRef]
- Litvinenko, V. Advancement of geomechanics and geodynamics at the mineral ore mining and un-derground space development. Geomech. Geodyn. Rock Masses 2018, 1, 3–16. [Google Scholar]
- Rybak, J.; Khayrutdinov, M.M.; Kuziev, D.A.; Kongar-Syuryun, C.B.; Babyr, N.V. Prediction of the geomechanical state of the rock mass when mining salt deposits with stowing. J. Min. Inst. 2022, 253, 61–70. [Google Scholar] [CrossRef]
- Hau, L.V. Determination of Parameters of the Underground Inclined Coal Seam Mining in Quang Ninh Basin Under Protected Objects on the Surface. J. Min. Inst. 2017, 226, 412–419. [Google Scholar] [CrossRef]
- Khayrutdinov, M.M.; Kongar-Syuryun, C.B.; Khayrutdinov, A.M.; Tyulyaeva, Y.S. Improving safety when extracting water-soluble ores by optimizing the parameters of the backfill mass. Bezop. Tr. V Promyshlennosti 2021, 2021, 53–59. [Google Scholar] [CrossRef]
- Earl, C.; Hussain, I.H.; Cook, S.; Cheeseman, C.R. Environmental Sustainability and Supply Resilience of Cobalt. Sustainability 2022, 14, 4124. [Google Scholar] [CrossRef]
- Kongar-Syuryun, C.; Tyulyaeva, Y.; Khairutdinov, A.; Kowalik, T. Industrial waste in concrete mixtures for construction of underground structures and minerals extraction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 869, 032004. [Google Scholar] [CrossRef]
- Tcvetkov, P. Climate Policy Imbalance in the Energy Sector: Time to Focus on the Value of CO2 Utilization. Energies 2021, 14, 411. [Google Scholar] [CrossRef]
- Dvoynikov, M.V.; Budovskaya, M.E. Development of a hydrocarbon completion system for wells with low bottomhole temperatures for conditions of oil and gas fields in Eastern Siberia. J. Min. Inst. 2022, 253, 12–22. [Google Scholar] [CrossRef]
- Khayrutdinov, A.; Paleev, I.; Artemov, S. Replacement of traditional components of the backfill mixture with man-made waste. IOP Conf. Ser. Earth Environ. Sci. 2021, 942, 012005. [Google Scholar] [CrossRef]
- Buslaev, G.; Tsvetkov, P.; Lavrik, A.; Kunshin, A.; Loseva, E.; Sidorov, D. Ensuring the Sustainability of Arctic Industrial Facilities under Conditions of Global Climate Change. Resources 2021, 10, 128. [Google Scholar] [CrossRef]
- Herbut, A.; Khairutdinov, M.M.; Kongar-Syuryun, C.; Rybak, J. The surface wave attenuation as the effect of vibratory compaction of building embankments. IOP Conf. Ser. Earth Environ. Sci. 2019, 362, 012131. [Google Scholar] [CrossRef]
- Mardashov, D.V. Development of blocking compositions with a bridging agent for oil well killing in conditions of abnormally low formation pressure and carbonate reservoir rocks. J. Min. Inst. 2021, 251, 667–677. [Google Scholar] [CrossRef]
- Raupov, I.R.; Burkhanov, R.N.; Lutfullin, A.A.; Maksyutin, A.V.; Lebedev, A.B.; Safiullina, E.U. Experience in the Application of Hydrocarbon Optical Studies in Oil Field Development. Energies 2022, 15, 3626. [Google Scholar] [CrossRef]
- Mardashov, D.V.; Limanov, M.N. Improving the efficiency of oil well killing at the fields of the volga-ural oil and gas province with abnormally low reservoir pressure. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2022, 333, 185–194. [Google Scholar] [CrossRef]
- Abramov, A.A. Tekhnologiya Obogashcheniya rud Tsvetnykh Metallov; Nedra: Moscow, Russia, 1983; p. 271. [Google Scholar]
- Herrera-Urbina, R.; Hanson, J.S.; Harris, G.H.; Fuerstenau, D.W. Principles and practice of sulphide mineral flotation. In Sulphide Deposits—Their Origin and Processing; Springer: Dordrecht, The Netherlands, 1990; pp. 87–101. [Google Scholar] [CrossRef]
- Napier-Munn, T.; Wills, B.A. Wills’ Mineral Processing Technology; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2005; pp. 246–266. [Google Scholar] [CrossRef]
- Sehlotho, N.; Sindane, Z.; Bryson, M.; Lindvelt, L. Flowsheet development for selective Cu-Pb-Zn recovery at Rosh Pinah concentrator. Miner. Eng. 2018, 122, 10–16. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Ravishankar, S.A. Flotation reagents—A critical overview from an industry perspective. In Froth Flotation: A Century of Innovation; Fuerstenau, M.C., Jameson, G.J., Yoon, R., Eds.; Society of Mining, Metallurgy, and Exploration, Inc. (SME): Littleton, CO, USA, 2007; pp. 375–413. [Google Scholar]
- Marion, C.; Jordens, A.; Li, R.; Rudolph, M.; Waters, K. An evaluation of hydroxamate collectors for malachite flotation. Sep. Purif. Technol. 2017, 183, 258–269. [Google Scholar] [CrossRef]
- Goryachev, B.E.; Nikolaev, A.A. Principles of kinetic “ion” modeling of adsorptive collector layer at the surface of nonferrous heavy metal sulfides. J. Min. Sci. 2013, 49, 499–506. [Google Scholar] [CrossRef]
- Zuev, B.Y. Methodology of modeling nonlinear geomechanical processes in blocky and layered rock masses on models made of equivalent materials. J. Min. Inst. 2021, 250, 542–552. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, A.A.; Thu, S.; Goryachev, B.E. Upon bubble-mineral attachment kinetics with Sphalerite under the conditions of application of Thiol collectors and mixtures of these collectors. Obogashchenie Rud 2016, 5, 14–18. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Luo, D.; Zeng, Y. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation. Miner. Eng. 2018, 121, 31–38. [Google Scholar] [CrossRef]
- Yang, B.; Tong, X.; Lan, Z.; Cui, Y.; Xie, X. Influence of the interaction between sphalerite and pyrite on the copper activation of sphalerite. Minerals 2018, 8, 16. [Google Scholar] [CrossRef]
- Abramov, A.A. Fundamentals for Optimization of Cyanide Processes in Flotation. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 15–29. Available online: https://www.911metallurgist.com/blog/wp-content/uploads/2015/12/Fundamentals-for-optimization-of-cyanide-processes-in-flotation.pdf (accessed on 15 November 2022).
- Gerson, A.R.; Lange, A.G.; Prince, K.E.; Smart, R.S.C. The mechanism of copper activation of sphalerite. Appl. Surf. Sci. 1999, 137, 207–223. [Google Scholar] [CrossRef]
- El-Shall, H.E.; Elgillani, D.A.; Abdel-Khalek, N.A. Role of zinc sulfate in depression of lead-activated sphalerite. Int. J. Miner. Process. 2000, 58, 67–75. [Google Scholar] [CrossRef]
- Finkelstein, N.; Allison, S. The chemistry of activation, deactivation and depression in the flotation of zinc sulfide: A review. Flotation 1976, 1, 414–457. [Google Scholar]
- Goryachev, B.E.; Linoo, N.; Nikolaev, A.A.; Polyakova, Y.N. Peculiarities of influence of copper, zinc and iron cations on flotability of pyrite of one of copper-zinc ural deposits. Tsvetnye Met. 2015, 1, 12–17. [Google Scholar]
- Strizhnev, K.V.; Gumerov, R.R.; Alekseev, Y.V.; Sagirova, L.R.; Suleimanov, A.G.; Zuevskly, I.A. Destructed guar gel effect on the mechanism of formation of calcite deposits in the high-pressure conduits of southern section of the priobskoye field. Neft. Khozyaystvo Oil Ind. 2019, 12, 56–58. [Google Scholar]
- Kotova, O.B.; Ustyugov, V.A.; Sun, S.; Ponaryadov, A.V. Mullite production: Phase transformations of kaolinite, thermodynamics of the process. J. Min. Inst. 2022, 254, 129–135. [Google Scholar] [CrossRef]
- Lyashenko, V.I.; Golik, V.I.; Klyuev, R.V. Evaluation of efficiency and environmental impact (on subsoil and groundwater) of underground block leaching (UBL) of metals from ores. Min. Sci. Technol. (Russ. Fed.) 2022, 7, 5–17. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the influence of marine residual fuel composition on sedimentation due to incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Beloglazov, I.; Islamov, S.; Ong, M.C. Exploring of the incompatibility of marine residual fuel: A case study using machine learning methods. Energies 2021, 14, 8422. [Google Scholar] [CrossRef]

| Reactions | ΔG0, kcal | lgKp | ΔG, (pH = 10) kcal | |
|---|---|---|---|---|
| No | Equation | |||
| A | ZnS + Fe2+ + 2OH− = FeS + Zn(OH)20 | −6.22 | 4.5581 | +5.42 |
| B | ZnS + Fe(OH)3− = FeS + Zn2+ + 3OH− | −0.47 | 0.3507 | −17.44 |
| C | ZnS + Fe(OH)3− = FeS + ZnOH+ + 2OH− | −6.40 | 4.6884 | −17.36 |
| D | ZnS + Fe(OH)3− = FeS + Zn(OH)20 + OH− | −15.89 | 11.6475 | −17.45 |
| E | ZnS + Fe(OH)42− = FeS + Zn2+ + 4OH− | −2.48 | 1.8181 | −17.93 |
| F | ZnS + Fe(OH)42− = FeS + ZnOH+ + 3OH− | −8.40 | 6.1590 | −17.85 |
| G | ZnS + Fe(OH)42− = FeS + Zn(OH)20 + 2OH− | −17.89 | 13.1181 | −17.93 |
| pH of the Working Solutions | Consumption of Iron (II) Sulfate or Zinc Sulfate, g/t | Initial Concentration of FeSO4/ZnSO4, mol/L | pH of Working Solution after Mixing | Oxidation-Reduction Potential (ORP) of Working Solution, mV | The Presence of Precipitates Fe(OH)2/ Zn(OH)2 (+),(−) * | ||
|---|---|---|---|---|---|---|---|
| CaO/ FeSO4 | CaO/ ZnSO4 | CaO/ FeSO4 | CaO/ ZnSO4 | ||||
| 8 | 0 | 0 | 7.76 | 7.30 | 264 | 244 | |
| 20 | 9.40·10−6/8.87·10−6 | 7.76/7.1 | 7.30/7.25 | 264/61.2 | 244/243 | (−)/(+) | |
| 100 | 4.70·10−5/ 4.44·10−5 | 7.70/6.43 | 7.35/7.18 | 264/68.3 | 224.1/246.5 | (−)/(+) | |
| 200 | 9.40·10−5/ 8.87·10−5 | 7.65/6.42 | 7.4/7.22 | 206/60.3 | 256.7/240.3 | (−)/(+) | |
| 400 | 1.88·10−4/ 1.77·10−4 | 7.32/6.36 | 7.57/7.2 | 178/48.8 | 245.1/152.4 | (−)/(+) | |
| 800 | 3.76·10−4/ 3.55·10−4 | 7.16/6.31 | 7.54/7.23 | 114/52.1 | 193.4/232.1 | (−)/(+) | |
| 10 | 0 | 0 | 9.86 | 9.65 | 79 | 100 | |
| 20 | 9.40·10−6/8.87·10−6 | 9.61/ 9.61 | 9.68/ 9.32 | 81/84 | 108/116 | (−)/(+) | |
| 100 | 4.70·10−5/ 4.44·10−5 | 9.88/ 9.98 | 9.82/ 9.37 | 84/94 | 104/119 | (+)/(+) | |
| 200 | 9.40·10−5/ 8.87·10−5 | 9.67/ 7.53 | 9.78/ 8.43 | 66/49 | 108/146 | (+)/(+) | |
| 400 | 1.88·10−4/ 1.77·10−4 | 9.64/ 6.19 | 9.81/ 8.14 | 151/57 | 105/157 | (+)/(+) | |
| 800 | 3.76·10−4/ 3.55·10−4 | 9.70/ 6.13 | 9.83/ 8.01 | 72/21 | 105/161 | (+)/(+) | |
| 12 | 0 | 0 | 11.65 | 11.85 | 15 | 3 | |
| 20 | 9.40·10−6/8.87·10−6 | 11.77/ 11.77 | 11.86/ 11.84 | −3/−4 | 3/3 | (−)/(−) | |
| 100 | 4.70·10−5/ 4.44·10−5 | 11.83/ 11.57 | 11.87/ 11.83 | −6/6 | 3/4 | (+)/(−) | |
| 200 | 9.40·10−5/ 8.87·10−5 | 11.79/ 11.67 | 11.88/ 11.81 | −2/1 | 3/5 | (+)/(−) | |
| 400 | 1.88·10−4/ 1.77·10−4 | 11.86/ 11.69 | 11.87/ 11.75 | −5/−1 | 3/7 | (+)/(+) | |
| 800 | 3.76·10−4/ 3.55·10−4 | 11.85/ 11.48 | 11.86/ 11.60 | −6/−11 | 4/13 | (+)/(+) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ya, K.Z.; Goryachev, B.; Adigamov, A.; Nurgalieva, K.; Narozhnyy, I. Thermodynamics and Electrochemistry of the Interaction of Sphalerite with Iron (II)-Bearing Compounds in Relation to Flotation. Resources 2022, 11, 108. https://doi.org/10.3390/resources11120108
Ya KZ, Goryachev B, Adigamov A, Nurgalieva K, Narozhnyy I. Thermodynamics and Electrochemistry of the Interaction of Sphalerite with Iron (II)-Bearing Compounds in Relation to Flotation. Resources. 2022; 11(12):108. https://doi.org/10.3390/resources11120108
Chicago/Turabian StyleYa, Kyaw Zay, Boris Goryachev, Arkadiy Adigamov, Karina Nurgalieva, and Igor Narozhnyy. 2022. "Thermodynamics and Electrochemistry of the Interaction of Sphalerite with Iron (II)-Bearing Compounds in Relation to Flotation" Resources 11, no. 12: 108. https://doi.org/10.3390/resources11120108
APA StyleYa, K. Z., Goryachev, B., Adigamov, A., Nurgalieva, K., & Narozhnyy, I. (2022). Thermodynamics and Electrochemistry of the Interaction of Sphalerite with Iron (II)-Bearing Compounds in Relation to Flotation. Resources, 11(12), 108. https://doi.org/10.3390/resources11120108









