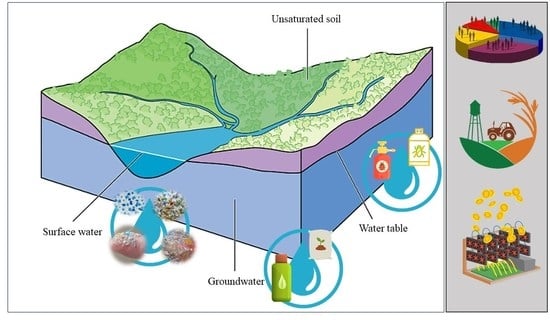
An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers
Abstract
1. Introduction
2. Environmental Vulnerabilities and Primary Emerging Pollutants in Karst Systems
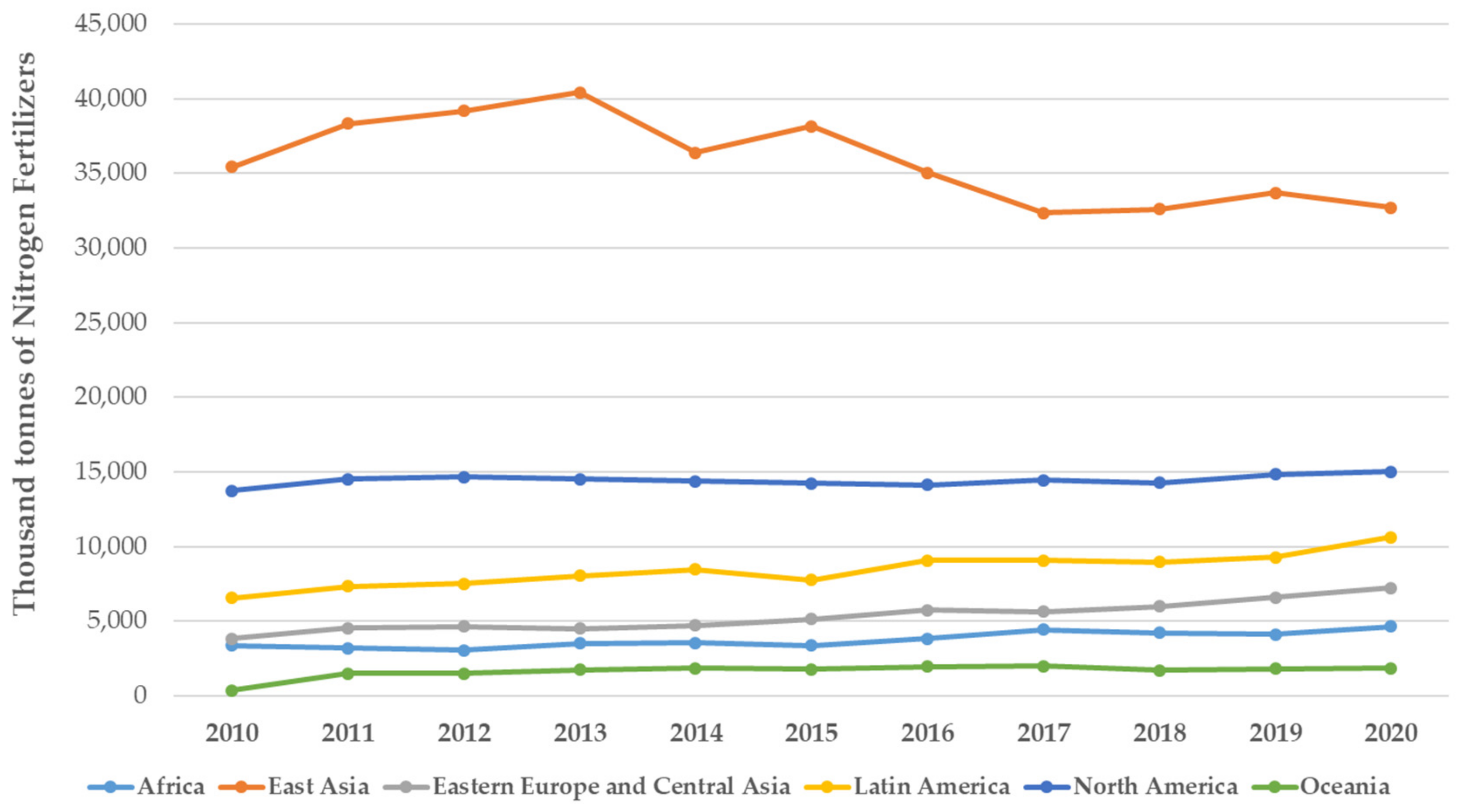
2.1. Fertilisers
2.2. Plant Protection Products
Glyphosate in Karst Systems
2.3. PFASs
2.4. PPCPs
2.5. Microplastics
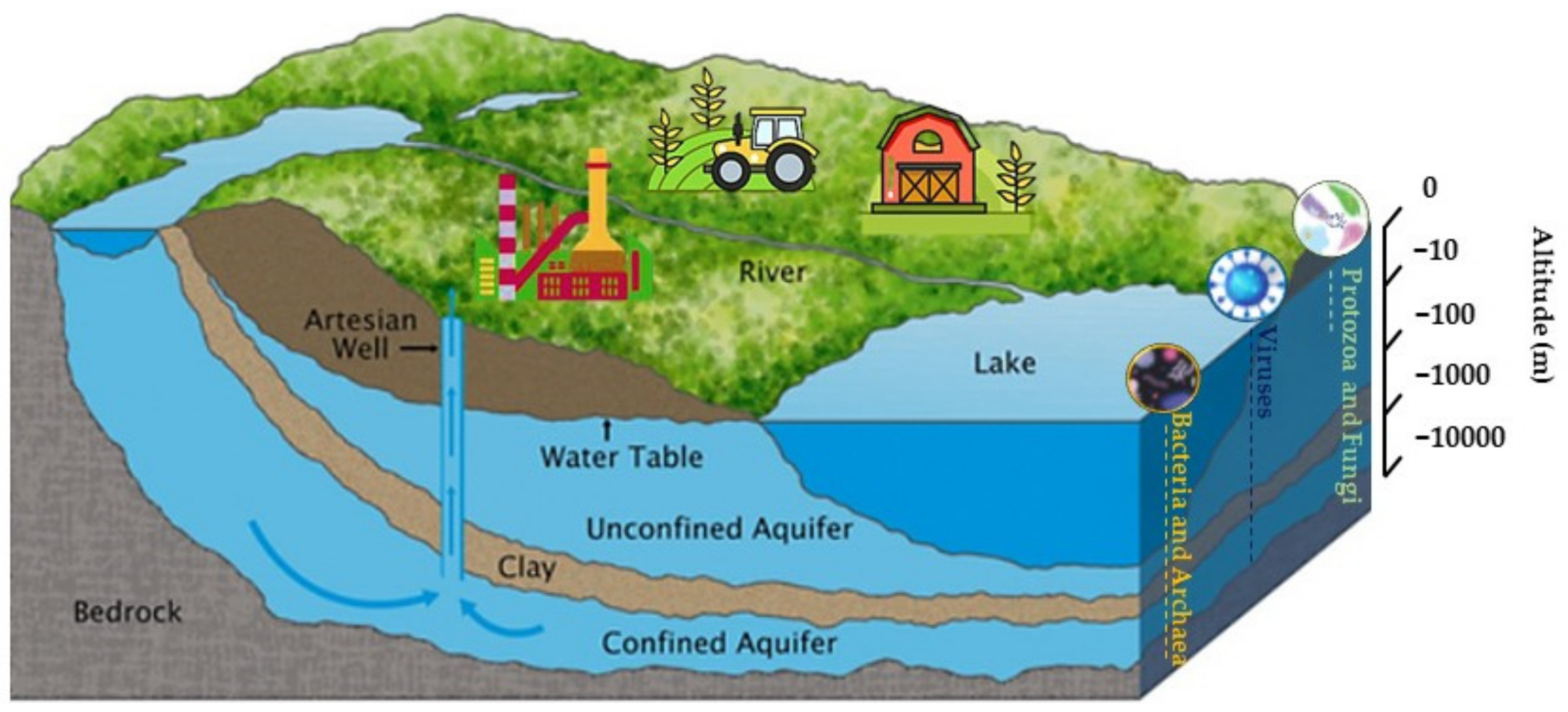
3. Alterations of Microbial Biodiversity and Transport of Pathogens through Karst Systems
4. Influences of the Temporal Dynamic of Karst Systems
5. Perspectives of Monitoring and Control Strategies of Karst Systems
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalhor, K.; Ghasemizadeh, R.; Rajic, L.; Alshawabkeh, A. Assessment of groundwater quality and remediation in karst aquifers: A review. Groundw. Sustain. Dev. 2019, 8, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology-Derek Ford; Paul, D., Ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic Contamination in Karst Groundwater Systems. Ground Water 2019, 57, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.H.; Moore, C.H. Carbonate Reservoirs: Porosity Evolution and Diagenesis in a Sequence Stratigraphic Framework; Elsevier: Amsterdam, The Netherlands, 2001; p. 444. [Google Scholar]
- Mylroie, J. Biospheleologists; Gunn, J., Ed.; Routledge: London, UK, 2004. [Google Scholar]
- Vadillo, I.; Ojeda, L. Carbonate aquifers threatened by contamination of hazardous anthropic activities: Challenges. Curr. Opin. Environ. Sci. Health 2022, 26, 100336. [Google Scholar] [CrossRef]
- Smart, P.L. Geomorphology and hydrology of karst terrains. WB WHITE Publisher Oxford University Press 1988 £35.00 (464 pp) ISBN 0 19 504444 4. J. Quat. Sci. 1989, 4, 186–187. [Google Scholar] [CrossRef]
- Hershey, O.S.; Kallmeyer, J.; Wallace, A.; Barton, M.D.; Barton, H.A. High microbial diversity despite extremely low biomass in a deep karst aquifer. Front. Microbiol. 2018, 9, 2823. [Google Scholar] [CrossRef] [PubMed]
- Selak, A.; Reberski, J.L.; Klobučar, G.; Grčić, I. Ecotoxicological aspects related to the occurrence of emerging contaminants in the Dinaric karst aquifer of Jadro and Žrnovnica springs. Sci. Total Environ. 2022, 825, 153827. [Google Scholar] [CrossRef] [PubMed]
- Beynen, P.E. Karst Management; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Civita, M. The prediction and prevention of the risk of groundwater pollution at regional level through the Vulnerability Cards. In Proceedings of the Inquinamento Delle Acque Sotterranee: Previsione e Prevenzione, Mantova, Italy, 11 March 1987; pp. 9–18. [Google Scholar]
- Aller, L.; Bennett, T.; Lehr, J.H.; Petty, R.J. Drastic: A Standardized System for Evaluating Ground Water Pollution Potential Using Hydrogeologic Settings; EPA: Washington, DC, USA, 1987. [Google Scholar]
- Lasagna, M.; De Luca, D.A.; Franchino, E. Intrinsic groundwater vulnerability assessment: Issues, comparison of different methodologies and correlation with nitrate concentrations in NW Italy. Environ. Earth Sci. 2018, 77, 277. [Google Scholar] [CrossRef]
- Cotecchia, V. Portrait of a Coastal Karst Aquifer: The City of Bari. AQUA Mundi 2010, 1, 187–196. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2020, 92, 473–505. [Google Scholar] [CrossRef]
- Massarelli, C.; Campanale, C.; Uricchio, V.F. A handy open-source application based on computer vision and machine learning algorithms to count and classify microplastics. Water 2021, 13, 2104. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical overview of methodologies for sampling and analysis of microplastics in riverine environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Tartari, G.; Bergna, G.; Lietti, M.; Rizzo, A.; Lazzari, F.B.C. Inquinanti Emergenti; Lombardy Energy Cleantech Cluster: Milano, Italy. 2020. Available online: https://www.snpambiente.it/wp-content/uploads/2020/10/REPORT_INQUINANTI-EMERGENTI-compresso.pdf (accessed on 1 July 2022).
- Campanale, C.; Massarelli, C.; Bagnuolo, G.; Savino, I.; Uricchio, V.F. The problem of microplastics and regulatory strategies in Italy. Handb. Environ. Chem. 2019, 112, 255–276. [Google Scholar]
- Ren, K.; Pan, X.; Yuan, D.; Zeng, J.; Liang, J.; Peng, C. Nitrate sources and nitrogen dynamics in a karst aquifer with mixed nitrogen inputs (Southwest China): Revealed by multiple stable isotopic and hydro-chemical proxies. Water Res. 2022, 210, 118000. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, C.; Losacco, D.; Tumolo, M.; Campanale, C.; Uricchio, V.F.; Costa-Pereira, F.; Miller, A.Z.; Tomás Jiménez-Morillo, N. Protection of Water Resources from Agriculture Pollution: An Integrated Methodological Approach for the Nitrates Directive 91–676-EEC Implementation. Int. J. Environ. Res. Public Health 2021, 18, 13323. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Khalil, M.S.; Ghorab, M.A.; Khalil, M.S.; Ghorab, M.A. Environmental Pollution by Heavy Metals in the Aquatic Ecosystems of Egypt Pesticide Influence on environmenta, biodiversity and human health View project Monitoring lakes water pollution View project Open Acc J of Toxicol Environmental Pollution by Heavy Metals in the Aquatic Ecosystems of Egypt. Open Acc. J. Toxicol. 2018, 3, 555603. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Kotzias, D.; Spartà, C. VOCs and water pollution. In Chemistry and Analysis of Volatile Organic Compounds in the Environment; Springer: Dordrecht, The Netherlands, 1993; pp. 175–201. [Google Scholar] [CrossRef]
- Roy, J.W.; Grapentine, L.; Bickerton, G. Ecological effects from groundwater contaminated by volatile organic compounds on an urban stream’s benthic ecosystem. Limnologica 2018, 68, 115–129. [Google Scholar] [CrossRef]
- Tsai, W.T. An overview of health hazards of volatile organic compounds regulated as indoor air pollutants. Rev. Environ. Health 2019, 34, 81–89. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC-Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, G.M.; Long, S.M.; Jones, O.A.H. What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere 2020, 258, 127340. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhu, R.; Cai, Y.; Xu, N.; Yap, P.S.; Zhang, Y.; He, Y.; Zhang, Y. Environmental fate and impacts of microplastics in aquatic ecosystems: A review. RSC Adv. 2021, 11, 15762–15784. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental Impacts of Microplastics and Nanoplastics: A Current Overview. Front. Microbiol. 2021, 12, 3728. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Campanale, C.; Massarelli, C.; Uricchio, V.F.; Volta, P. Do freshwater fish eat microplastics? A review with a focus on effects on fish health and predictive traits of mps ingestion. Water 2021, 13, 2214. [Google Scholar] [CrossRef]
- Shiklomanov, L.A. World Freshwater Resources. In Water in Crisis A Guide to World’s Freshwater Resources; Gleick, P.H., Ed.; Scientific Research Publishing; Oxford University Press: New York, NY, USA, 1993; pp. 13–24. Available online: https://www.scirp.org/(S(czeh2tfqyw2orz553k1w0r45))/reference/ReferencesPapers.aspx?ReferenceID=2254269 (accessed on 8 September 2022).
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wakida, F.T.; Lerner, D.N. Non-agricultural sources of groundwater nitrate: A review and case study. Water Res. 2005, 39, 3–16. [Google Scholar] [CrossRef]
- Fetter, C.W. Applied Hydrogeology, 4th ed.; Jiazhe Liu-Academia.edu (PDF); Prentice Hall: Hoboken, NJ, USA, 2000. Available online: https://www.academia.edu/37164391/C_W_Fetter_Applied_Hydrogeology_4th_Edition_2000_Prentice_Hall_ (accessed on 8 September 2022).
- El Alfy, M.; Faraj, T. Spatial distribution and health risk assessment for groundwater contamination from intensive pesticide use in arid areas. Environ. Geochem. Health 2017, 39, 231–253. [Google Scholar] [CrossRef]
- Sagasta, J.M.; Zadeh, S.M.; Turral, H. More People, More Food, Worse Water?—A Global Review of Water Pollution from Agriculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Losacco, D.; Tumolo, M.; Cotugno, P.; Leone, N.; Massarelli, C.; Convertini, S.; Tursi, A.; Uricchio, V.F.; Ancona, V. Use of Biochar to Improve the Sustainable Crop Production of Cauliflower (Brassica oleracea L.). Plants 2022, 11, 1182. [Google Scholar] [CrossRef]
- Ji, X.; Xie, R.; Hao, Y.; Lu, J. Quantitative identification of nitrate pollution sources and uncertainty analysis based on dual isotope approach in an agricultural watershed. Environ. Pollut. 2017, 229, 586–594. [Google Scholar] [CrossRef]
- Perrin, A.S.; Probst, A.; Probst, J.L. Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: Implications for weathering CO2 uptake at regional and global scales. Geochim. Cosmochim. Acta 2008, 72, 3105–3123. [Google Scholar] [CrossRef]
- Yue, F.-J.; Li, S.-L.; Zhong, J.; Liu, J.; Yue, F.-J.; Zhong, J.; Liu, J.; Li, S.-L. Evaluation of Factors Driving Seasonal Nitrate Variations in Surface and Underground Systems of a Karst Catchment. Vadose Zo. J. 2018, 17, 170071. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Wu, X.; Chang, L.; Ham, B.; Song, L.; Groves, C. Nitrate sources and biogeochemical processes in karst underground rivers impacted by different anthropogenic input characteristics. Environ. Pollut. 2020, 265, 114835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Zhao, T.; Sun, W.; Yang, Z. Identifying nitrate sources and transformations in Taizi River Basin, Northeast China. Environ. Sci. Pollut. Res. Int. 2017, 24, 20759–20769. [Google Scholar] [CrossRef]
- Conde-Costas, C.; Gómez-Gómez, F. Assessment of Nitrate Contamination of the Upper Aquifer in the Manati-Vega Baja Area, Puerto Rico Assessment of Nitrate Contamination of the Upper Aquifer in the Manati-Vega Baja Area, Puerto Rico. USGS Water-Resour. Investig. Rep. 1999, 99, 50. [Google Scholar] [CrossRef]
- Pan, L.; Dai, J.; Wu, Z.; Wan, Z.; Zhang, Z.; Han, J.; Li, Z.; Xie, X.; Xu, B. Spatio-Temporal Dynamics of Riverine Nitrogen and Phosphorus at Different Catchment Scales in Huixian Karst Wetland, Southwest China. Water 2020, 12, 2924. [Google Scholar] [CrossRef]
- Oliver, D.M.; Zheng, Y.; Naylor, L.A.; Murtagh, M.; Waldron, S.; Peng, T. How does smallholder farming practice and environmental awareness vary across village communities in the karst terrain of southwest China? Agric. Ecosyst. Environ. 2020, 288, 106715. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, M.; Jin, M.; Huang, X.; Zhang, Z.; Kang, F. Identifying the source and transformation of riverine nitrates in a karst watershed, North China: Comprehensive use of major ions, multiple isotopes and a Bayesian model. J. Contam. Hydrol. 2022, 246, 103957. [Google Scholar] [CrossRef]
- Matiatos, I. Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: A case study of Asopos basin (Central Greece). Sci. Total Environ. 2016, 541, 802–814. [Google Scholar] [CrossRef]
- Mayer, B.; Boyer, E.W.; Goodale, C.; Jaworski, N.A.; Van Breemen, N.; Howarth, R.W.; Seitzinger, S.; Billen, G.; Lajtha, K.; Nadelhoffer, K.; et al. Sources of nitrate in rivers draining sixteen watersheds in the northeastern US: Isotopic constraints. Biogeochemistry 2002, 57, 171–197. [Google Scholar] [CrossRef]
- Padilla, I.Y.; Vesper, D.J. Fate, Transport, and Exposure of Emerging and Legacy Contaminants in Karst Systems: State of Knowledge and Uncertainty; Springer: Berlin/Heidelberg, Germany, 2018; pp. 33–49. [Google Scholar]
- Hartmann, A.; Jasechko, S.; Gleeson, T.; Wada, Y.; Andreo, B.; Barbera, J.A.; Brielmann, H.; Bouchaou, L.; Charlier, J.B.; Darling, W.G.; et al. Risk of groundwater contamination widely underestimated because of fast flow into aquifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2024492118. [Google Scholar] [CrossRef] [PubMed]
- Close, M.E.; Rosen, M.R.; Smith, V.R. Fate and transport of nitrates and pesticides in New Zealand’s aquifers. In Groundwaters of New Zealand; New Zealand Hydrological Society: Wellington, New Zealand, 2001; pp. 185–220. [Google Scholar]
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2022, 127, 234–250. [Google Scholar] [CrossRef]
- Funari, E.; Donati, L.; Sandroni, D.; Vighi, M. Pesticide Levels in Groundwater: Value and Limitations of Monitoring. In Pesticide Risk in Groundwater; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–44. [Google Scholar]
- Kolpin, D.W.; Schnoebelen, D.J.; Thurman, E.M. Degradates provide insight to spatial and temporal trends of herbicides in ground water. Ground Water 2004, 42, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.E.; Manamsa, K.; Talbot, J.C.; Crane, E.J. Emerging Contaminants in Groundwater; British Geological Survey: Nottingham, UK, 2011.
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- ISPRA; Paris, P.; Esposito, D.; Maschio, G.; Presicce, D.P.; Ursino, S.; Citro, L.; Pace, E.; Romoli, I.D. Sostenibilità Ambientale Dell’uso dei Pesticidi Il Bacino del Fiume Po ISPRA; Superiore per la Protezione e la Ricerca Ambientale: Roma, Italy, 2017.
- Jeffrey, M.G.; Todd, A.; Hall, L., Jr.; Alan, J.H.; Ronald, J.K.; Richards, R.P.; Keith, R.S.; Williams, W.M.V. Atrazine in North American Surface Waters: A Probabilistic Aquatic Ecological Risk Assessment da; Florida, U., Ed.; SETAC Press: Pensacola, FL, USA, 2005. [Google Scholar]
- Salahshoor, Z.; Van Ho, K.; Hsu, S.Y.; Lin, C.H.; de Cortalezzi, M.F. Detection of Atrazine and its metabolites by photonic molecularly imprinted polymers in aqueous solutions. Chem. Eng. J. Adv. 2022, 12, 100368. [Google Scholar] [CrossRef]
- Schleder, A.A.; Vargas, L.M.P.; Hansel, F.A.; Froehner, S.; Palagano, L.T.; da Filho, E.F.R. Avaliação da ocorrência de NO3−, coliformes e atrazina em um aquífero cárstico, Colombo, PR. Rev. Bras. Recur. Hidr. 2017, 22, 2318. [Google Scholar] [CrossRef]
- Iker, B.C.; Kambesis, P.; Oehrle, S.A.; Groves, C.; Barton, H.A. Microbial Atrazine Breakdown in a Karst Groundwater System and Its Effect on Ecosystem Energetics. J. Environ. Qual. 2010, 39, 509–518. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere 2021, 283, 131447. [Google Scholar] [CrossRef]
- Campanale, C.; Triozzi, M.; Massarelli, C.; Uricchio, V.F. Development of a UHPLC-MS/MS method to enhance the detection of Glyphosate, AMPA and Glufosinate at sub-microgram / L levels in water samples. J. Chromatogr. A 2022, 1672, 463028. [Google Scholar] [CrossRef]
- Carretta, L.; Masin, R.; Zanin, G. Review of studies analysing glyphosate and aminomethylphosphonic acid (AMPA) occurrence in groundwater. Environ. Rev. 2022, 30, 88–109. [Google Scholar] [CrossRef]
- Cederlund, H. Environmental fate of glyphosate used on Swedish railways—Results from environmental monitoring conducted between 2007–2010 and 2015–2019. Sci. Total Environ. 2022, 811, 152361. [Google Scholar] [CrossRef] [PubMed]
- Van Stempvoort, D.R.; Spoelstra, J.; Senger, N.D.; Brown, S.J.; Post, R.; Struger, J. Glyphosate residues in rural groundwater, Nottawasaga River Watershed, Ontario, Canada. Pest Manag. Sci. 2016, 72, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Gurson, A.P.; Ozbay, I.; Ozbay, B.; Akyol, G.; Akyol, N.H. Mobility of 2,4-Dichlorophenoxyacetic Acid, Glyphosate, and Metribuzine Herbicides in Terra Rossa-Amended Soil: Multiple Approaches with Experimental and Mathematical Modeling Studies. Water. Air. Soil Pollut. 2019, 230, 220. [Google Scholar] [CrossRef]
- Ozbay, B.; Akyol, N.H.; Akyol, G.; Ozbay, I. Sorption and desorption behaviours of 2,4-D and glyphosate in calcareous soil from Antalya, Turkey. Water Environ. J. 2018, 32, 141–148. [Google Scholar] [CrossRef]
- Mahler, B.; Musgrove, M. Emerging contaminants in groundwater, karst, and the Edwards (Balcones Fault Zone) Aquifer. In The Edwards Aquifer: The Past, Present, and Future of a Vital Water Resource; Geoscience: Boca Raton, FL USA, 2019; pp. 239–251. [Google Scholar]
- Eggen, T.; Moeder, M.; Arukwe, A. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci. Total Environ. 2010, 408, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- Eschauzier, C.; Raat, K.J.; Stuyfzand, P.J.; De Voogt, P. Perfluorinated alkylated acids in groundwater and drinking water: Identification, origin and mobility. Sci. Total Environ. 2013, 458–460, 477–485. [Google Scholar] [CrossRef]
- Schaider, L.A.; Rudel, R.A.; Ackerman, J.M.; Dunagan, S.C.; Brody, J.G. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2014, 468–469, 384–393. [Google Scholar] [CrossRef]
- Schultz, M.M.; Barofsky, D.F.; Field, J.A. Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. Environ. Sci. Technol. 2004, 38, 1828–1835. [Google Scholar] [CrossRef]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015, 503–504, 32–47. [Google Scholar] [CrossRef]
- Kuroda, K.; Murakami, M.; Oguma, K.; Takada, H.; Takizawa, S. Investigating sources and pathways of perfluoroalkyl acids (PFAAs) in aquifers in Tokyo using multiple tracers. Sci. Total Environ. 2014, 488–489, 51–60. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Comero, S.; Contini, S.; Schwesig, D.; Werres, F.; Balsaa, P.; Gans, O.; Weiss, S.; Blaha, L.; et al. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 2010, 44, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Plumlee, M.H.; Larabee, J.; Reinhard, M. Perfluorochemicals in water reuse. Chemosphere 2008, 72, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Post, G.B.; Louis, J.B.; Lippincott, R.L.; Procopio, N.A. Occurrence of perfluorinated compounds in raw water from New Jersey public drinking water systems. Environ. Sci. Technol. 2013, 47, 13266–13275. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, E.; Madden, C.; Szabo, D.; Coggan, T.L.; Clarke, B.; Currell, M. Contamination of groundwater with per- and polyfluoroalkyl substances (PFAS) from legacy landfills in an urban re-development precinct. Environ. Pollut. 2019, 248, 101–113. [Google Scholar] [CrossRef]
- Silori, R.; Shrivastava, V.; Singh, A.; Sharma, P.; Aouad, M.; Mahlknecht, J.; Kumar, M. Global groundwater vulnerability for Pharmaceutical and Personal care products (PPCPs): The scenario of second decade of 21st century. J. Environ. Manage. 2022, 320, 115703. [Google Scholar] [CrossRef]
- Sharma, K.; Thakur, I.S.; Kaushik, G. Occurrence and distribution of pharmaceutical compounds and their environmental impacts: A review. Bioresour. Technol. Rep. 2021, 16, 100841. [Google Scholar] [CrossRef]
- Dodgen, L.K.; Kelly, W.R.; Panno, S.V.; Taylor, S.J.; Armstrong, D.L.; Wiles, K.N.; Zhang, Y.; Zheng, W. Characterizing pharmaceutical, personal care product, and hormone contamination in a karst aquifer of southwestern Illinois, USA, using water quality and stream flow parameters. Sci. Total Environ. 2017, 578, 281–289. [Google Scholar] [CrossRef]
- Huang, C.; Jin, B.; Han, M.; Yu, Y.; Zhang, G.; Arp, H.P.H. The distribution of persistent, mobile and toxic (PMT) pharmaceuticals and personal care products monitored across Chinese water resources. J. Hazard. Mater. Lett. 2021, 2, 100026. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Yang, Q.; Jiang, L.; Li, G. Occurrence and distribution of Pharmaceuticals and Personal Care Products (PPCPs) in wastewater related riverbank groundwater. Sci. Total Environ. 2022, 821, 153372. [Google Scholar] [CrossRef]
- Al-Rajab, A.J.; Al Bratty, M.; Hakami, O.; Alhazmi, H.A.; Sharma, M.; Reddy, D.N. Investigation of the presence of pharmaceuticals and personal care products (Ppcps) in groundwater of Jazan area, Saudi Arabia. Trop. J. Pharm. Res. 2018, 17, 2061–2066. [Google Scholar] [CrossRef]
- Ślósarczyk, K.; Jakóbczyk-Karpierz, S.; Różkowski, J.; Witkowski, A.J. Occurrence of pharmaceuticals and personal care products in the water environment of Poland: A review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; De Sousa, J.M.I., Ed.; Marine Project Officer; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017. [Google Scholar]
- Li, W.; Wufuer, R.; Duo, J.; Wang, S.; Luo, Y.; Zhang, D.; Pan, X. Microplastics in agricultural soils: Extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total Environ. 2020, 749, 141420. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Campanale, C.; Dierkes, G.; Massarelli, C.; Bagnuolo, G.; Uricchio, V.F. A Relevant Screening of Organic Contaminants Present on Freshwater and Pre-Production Microplastics. Toxics 2020, 8, 100. [Google Scholar] [CrossRef]
- Chia, R.W.; Lee, J.Y.; Kim, H.; Jang, J. Microplastic pollution in soil and groundwater: A review. Environ. Chem. Lett. 2021 196 2021, 19, 4211–4224. [Google Scholar] [CrossRef]
- Khant, N.A.; Kim, H. Review of Current Issues and Management Strategies of Microplastics in Groundwater Environments. Water 2022, 14, 1020. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the soil-groundwater environment: Aging, migration, and co-transport of contaminants—A critical review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Savino, I.; Campanale, C.; Trotti, P.; Massarelli, C.; Corriero, G.; Uricchio, V.F. Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles. Polymer 2022, 14, 1958. [Google Scholar] [CrossRef]
- Ryu, H.S.; Moon, J.; Kim, H.; Lee, J.Y. Modeling and Parametric Simulation of Microplastic Transport in Groundwater Environments. Appl. Sci. 2021, 11, 7189. [Google Scholar] [CrossRef]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Wang, Y.; Zhang, H.; Guo, F.; Li, A.; Zhang, S.; Liu, S.; Liu, T. High abundance of microplastics in groundwater in Jiaodong Peninsula, China. Sci. Total Environ. 2022, 839, 156318. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, A.; Abbasi, S.; Peely, A.B.; Mowla, D.; Ghanbarian, M.A.; Oleszczuk, P.; Turner, A. Distribution and transport of microplastics in groundwater (Shiraz aquifer, southwest Iran). Water Res. 2022, 220, 118622. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, N.; Goldscheider, N. Experimental field evidence for transport of microplastic tracers over large distances in an alluvial aquifer. J. Hazard. Mater. 2021, 408, 124844. [Google Scholar] [CrossRef]
- Balestra, V.; Bellopede, R. Microplastic pollution in show cave sediments: First evidence and detection technique. Environ. Pollut. 2022, 292, 118261. [Google Scholar] [CrossRef]
- Bharath, K.M.; Natesan, U.; Vaikunth, R.; Kumar, R.P.; Ruthra, R.; Srinivasalu, S. Spatial distribution of microplastic concentration around landfill sites and its potential risk on groundwater. Chemosphere 2021, 277, 130263. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Jeyanthi Kumari, V. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Zheng, Y.; Yang, Y.; Zhang, Y.; Gao, B. Microplastic pollution in soils and groundwater: Characteristics, analytical methods and impacts. Chem. Eng. J. 2021, 425, 131870. [Google Scholar] [CrossRef]
- Viaroli, S.; Lancia, M.; Re, V. Microplastics contamination of groundwater: Current evidence and future perspectives. A review. Sci. Total Environ. 2022, 824, 153851. [Google Scholar] [CrossRef]
- Ghiorse, W.C.; Wilson, J.T. Microbial Ecology of the Terrestrial Subsurface. Adv. Appl. Microbiol. 1988, 33, 107–172. [Google Scholar] [CrossRef]
- Herrick, J.B.; Madsen, E.L.; Batt, C.A.; Ghiorse, W.C. Polymerase chain reaction amplification of naphthalene-catabolic and 16S rRNA gene sequences from indigenous sediment bacteria. Appl. Environ. Microbiol. 1993, 59, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tumolo, M.; Volpe, A.; Leone, N.; Cotugno, P.; De Paola, D.; Losacco, D.; Locaputo, V.; de Pinto, M.C.; Uricchio, V.F.; Ancona, V. Enhanced Natural Attenuation of Groundwater Cr(VI) Pollution Using Electron Donors: Yeast Extract vs. Polyhydroxybutyrate. Int. J. Environ. Res. Public Health 2022, 19, 9622. [Google Scholar] [CrossRef] [PubMed]
- Groundwater Microbiology—The Groundwater Project. Available online: https://gw-project.org/books/groundwater-microbiology/ (accessed on 12 September 2022).
- Bonte, M.; van Breukelen, B.M.; Stuyfzand, P.J. Temperature-induced impacts on groundwater quality and arsenic mobility in anoxic aquifer sediments used for both drinking water and shallow geothermal energy production. Water Res. 2013, 47, 5088–5100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Stefan, H.G. Shallow groundwater temperature response to climate change and urbanization. J. Hydrol. 2009, 375, 601–612. [Google Scholar] [CrossRef]
- Amend, J.P.; Teske, A. Expanding frontiers in deep subsurface microbiology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 131–155. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Stumm, W., Morgan, J.J., Eds.; Wiley: Hoboken, NJ, USA. Available online: https://books.google.it/books?hl=it&lr=&id=NLV_yfulgkQC&oi=fnd&pg=PT14&ots=cL2X3tf5FI&sig=8Jj35DrbRYZefoDNzYpQXswAylA&redir_esc=y#v=onepage&q&f=false (accessed on 12 September 2022).
- Bethke, C.M.; Sanford, R.A.; Kirk, M.F.; Jin, Q.; Flynn, T.M. The thermodynamic ladder in geomicrobiology. Am. J. Sci. 2011, 311, 183–210. [Google Scholar] [CrossRef]
- Paul, A.; Stösser, T.R.; Zehl, A.; Zwirnmann, E.; Vogt, R.D.; Steinberg, C.E.W. Nature and abundance of organic radicals in natural organic matter: Effect of pH and irradiation. Environ. Sci. Technol. 2006, 40, 5897–5903. [Google Scholar] [CrossRef]
- Leprince, F.; Quiquampoix, H. Extracellular enzyme activity in soil: Effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus Hebeloma cylindrosporum. Eur. J. Soil Sci. 1996, 47, 511–522. [Google Scholar] [CrossRef]
- Horikoshi, K. Alkaliphiles: Some Applications of Their Products for Biotechnology. Microbiol. Mol. Biol. Rev. 1999, 63, 735–750. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.M.L.; Edwards, B.A.; Ferris, F.G. Long Range Correlation in Redox Potential Fluctuations Signals Energetic Efficiency of Bacterial Fe(II) Oxidation. Sci. Rep. 2019, 9, 4018. [Google Scholar] [CrossRef] [PubMed]
- Liebensteiner, M.G.; Tsesmetzis, N.; Stams, A.J.M.; Lomans, B.P. Microbial redox processes in deep subsurface environments and the potential application of (per)chlorate in oil reservoirs. Front. Microbiol. 2014, 5, 428. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zuo, R.; Wang, J.S.; Li, Q.; Du, C.; Liu, X.; Chen, M. Response of the redox species and indigenous microbial community to seasonal groundwater fluctuation from a typical riverbank filtration site in Northeast China. Ecol. Eng. 2021, 159, 106099. [Google Scholar] [CrossRef]
- Losacco, D.; Ancona, V.; De Paola, D.; Tumolo, M.; Massarelli, C.; Gatto, A.; Uricchio, V.F. Development of ecological strategies for the recovery of the main nitrogen agricultural pollutants: A review on environmental sustainability in agroecosystems. Sustainability 2021, 13, 7163. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Van Breukelen, B.M.; Braster, M.; Lin, B.; Van Verseveld, H.W. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Appl. Environ. Microbiol. 2001, 67, 4619–4629. [Google Scholar] [CrossRef]
- Fluorescence of Dissolved Organic Matter as a Natural Tracer of Ground Water-ProQuest. Available online: https://www.proquest.com/docview/236850756?pq-origsite=gscholar&fromopenview=true (accessed on 12 September 2022).
- Cho, J.C.; Kim, S.J. Increase in bacterial community diversity in subsurface aquifers receiving livestock wastewater input. Appl. Environ. Microbiol. 2000, 66, 956–965. [Google Scholar] [CrossRef][Green Version]
- Buckerfield, S.J.; Waldron, S.; Quilliam, R.S.; Naylor, L.A.; Li, S.; Oliver, D.M. How can we improve understanding of faecal indicator dynamics in karst systems under changing climatic, population, and land use stressors?—Research opportunities in SW China. Sci. Total Environ. 2019, 646, 438–447. [Google Scholar] [CrossRef]
- Future Research Needs Involving Pathogens in Groundwater. Available online: https://www.cabdirect.org/cabdirect/abstract/20173258488 (accessed on 3 November 2022).
- Karst Groundwater Contamination and Public Health; Springer: Berlin/Heidelberg, Germany, 2018. [CrossRef]
- Worthington, S.R.H.; Smart, C.C. Contamination bactérienne transitoire d’un aquifère à double-porosité à Walkerton, Ontario, Canada. Hydrogeol. J. 2017, 25, 1003–1016. [Google Scholar] [CrossRef]
- White, W.B. Contaminant Transport in Karst Aquifers: Systematics and Mechanisms; Springer: Berlin/Heidelberg, Germany, 2018; pp. 55–81. [Google Scholar] [CrossRef]
- Mahler, B.J.; Personné, J.C.; Lods, G.F.; Drogue, C. Transport of free and particulate-associated bacteria in karst. J. Hydrol. 2000, 238, 179–193. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Wang, Z.; Zhu, X.; Ning, K. High biodiversity and distinct assembly patterns of microbial communities in groundwater compared with surface water. Sci. Total Environ. 2022, 834, 155345. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Raza, S.H.A.; Yang, B.; Sun, Y.; Wang, G.; Sun, W.; Qian, A.; Wang, C.; Kang, Y.; Shan, X. Aeromonas veronii Infection in Commercial Freshwater Fish: A Potential Threat to Public Health. Animals 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Tekedar, H.C.; Kumru, S.; Blom, J.; Perkins, A.D.; Griffin, M.J.; Abdelhamed, H.; Karsi, A.; Lawrence, M.L. Comparative genomics of Aeromonas veronii: Identification of a pathotype impacting aquaculture globally. PLoS ONE 2019, 14, e0221018. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Pembroke, J.T. Brevundimonas spp: Emerging global opportunistic pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Porporato, A. Common hydrologic and biogeochemical controls along the soil-stream continuum. Hydrol. Process. 2011, 25, 1355–1360. [Google Scholar] [CrossRef]
- Ouedraogo, I.; Girard, A.; Vanclooster, M.; Jonard, F. Modelling the temporal dynamics of groundwater pollution risks at the African scale. Water 2020, 12, 1406. [Google Scholar] [CrossRef]
- Hartmann, A.; Mudarra, M.; Andreo, B.; Marín, A.; Wagener, T.; Lange, J. Modeling spatiotemporal impacts of hydroclimatic extremes on groundwater recharge at a Mediterranean karst aquifer. Water Resour. Res. 2014, 50, 6507–6521. [Google Scholar] [CrossRef]
- Opsahl, S.P.; Musgrove, M.; Slattery, R.N. New insights into nitrate dynamics in a karst groundwater system gained from in situ high-frequency optical sensor measurements. J. Hydrol. 2017, 546, 179–188. [Google Scholar] [CrossRef]
- Ravbar, N.; Petrič, M.; Blatnik, M.; Švara, A. A multi-methodological approach to create improved indicators for the adequate karst water source protection. Ecol. Indic. 2021, 126, 107693. [Google Scholar] [CrossRef]
- Maas, B.; Peterson, E.W.; Honings, J.; Oberhelman, A.; Oware, P.; Rusthoven, I.; Watson, A. Differentiation of Surface Water and Groundwater in a Karst System Using Anthropogenic Signatures. Geoscience 2019, 9, 148. [Google Scholar] [CrossRef]
- Vucinic, L.; O’Connell, D.; Teixeira, R.; Coxon, C.; Gill, L. Flow Cytometry and Fecal Indicator Bacteria Analyses for Fingerprinting Microbial Pollution in Karst Aquifer Systems. Water Resour. Res. 2022, 58, e2021WR029840. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez, M.; Martínez-Salvador, C.; Liedl, R.; Stefan, C.; Pacheco, J. First application of the Integrated Karst Aquifer Vulnerability (IKAV) method-potential and actual vulnerability in Yucatán, Mexico. Nat. Hazards Earth Syst. Sci. 2022, 22, 1591–1608. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, F.; Liu, W.; Bu, J.; Hu, G.; Xie, S.; Yao, H.; Zhou, H.; Qi, S.; Huang, H. Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 11589. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, H.; Oliver, D.M.; Dai, Y.; Li, H.; Shi, Y.; Long, H.; Xiong, K.; Zhao, Z. Decoding river pollution trends and their landscape determinants in an ecologically fragile karst basin using a machine learning model. Environ. Res. 2022, 214, 113843. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.I.; Martín Rodríguez, J.F.; Barberá, J.A.; Fernández-Ortega, J.; Mudarra, M.; Sánchez, D.; Andreo, B. Groundwater vulnerability to pollution in karst aquifers, considering key challenges and considerations: Application to the Ubrique springs in southern Spain. Hydrogeol. J. 2021, 29, 379–396. [Google Scholar] [CrossRef]
- Jakeman, A.J.; Barreteau, O.; Hunt, R.J.; Rinaudo, J.D.; Ross, A.; Arshad, M.; Hamilton, S. Integrated Groundwater Management: An Overview of Concepts and Challenges; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–20. [Google Scholar] [CrossRef]
- Vižintin, G.; Ravbar, N.; Janež, J.; Koren, E.; Janež, N.; Zini, L.; Treu, F.; Petrič, M. Integration of models of various types of aquifers for water quality management in the transboundary area of the Soča/Isonzo river basin (Slovenia/Italy). Sci. Total Environ. 2018, 619–620, 1214–1225. [Google Scholar] [CrossRef]
- Kaçaroǧlu, F. Review of Groundwater Pollution and Protection in Karst Areas. Water Air Soil Pollut. 1999, 113, 337–356. [Google Scholar] [CrossRef]
| Contaminant Type | Source of Contamination into Aquifers | Effects on Aquatic Ecosystems | Effects on Human Health |
|---|---|---|---|
| Nutrients, e.g., (NO3−, PO43−) | Old septic systems, landfills, leaks from cracks in sewer pipelines, acid mining waters, fertilisers used in agriculture, untreated industrial wastewater and urban sewage. | Eutrophication and hypoxia [20]. | Methemoglobinemia. [21]. |
| Pharmaceutical and Personal Care Products (PPCPs), e.g., Antibiotics, Anti-inflammatories, Lipid regulators, Psychiatric drugs, Stimulants, Insect Repellants and Sunscreen agents | Wastewater and contaminated surface water, landfills, septic systems and sewer leakages. |
|
|
| Metals | Industrial activities and urban waste, urban surface runoff containing a high concentration of metals go through karst aquifers via sinkholes and conduit networks, natural leaching from rocks and soils within karst media and can be introduced with acidic deposition. |
|
|
| Volatile Organic Compounds (VOC) e.g., (e.g., trichloroethylene), fuel oxygenates (e.g., MTBE, ETBE), and by-products produced by chlorination during water treatment (e.g., chloroform) | Industrial activities, improper management of landfills, accidental spills, unidentified waste disposals, or residential septic systems. |
|
|
| Plant Protection Products (PPPs) | Point and non-point sources including runoff waters from agricultural and urban areas, deposition from the atmosphere, pesticide manufacturing plants, mixing-and-loading facilities, spills, wastewater recharge facilities (wells or basins), waste disposal sites and sewage treatment plants. |
|
|
| Per- and polyfluoroalkyl substances (PFASs) | Wastewater treatment plants and resulting biosolids, domestic wastewater, landfills, fire training/fire response sites, industrial sites. |
|
|
| Pathogens, e.g., viruses and bacteria and protozoa | Agricultural runoff, animal manure, compost, wastewater and sanitation systems; sources are intimately related to inadequate or absent sewage facilities and leaking from sewer pipes and septic tanks. |
|
|
| Micro- and nanoplastics, e.g., microbeads, pellets, microfibres, fragments | Wastewater, fragmentation of large plastic litter, and atmospheric deposition. |
|
|
| Type of the Paper | Aim of the Work | Type of Aquifer/Depth | Country | Matrix Investigated | MPs Abundance (Mean) | Year of Publication | Ref. |
|---|---|---|---|---|---|---|---|
| Research Article | To provide modelling and simulations for a clear understanding of the transport phenomena of MPs | Saturated porous medium/NA. | NA. | NA. | NA. | 2021 | [100] Ryu et al., 2021 |
| To analyse microplastics in groundwater sampled from an alluvial sedimentary aquifer, using properly constructed monitoring bores that preclude atmospheric deposition as a major source of MPs | Alluvial sedimentary aquifer/10 to 25 m | Victoria, Australia | Water | 38 microplastics/L | 2022 | [101] Samandra et al., 2022 | |
| To investigate the occurrence of microplastics in groundwater sampled from five sites in Jiaodong Peninsula, China. | NA./4 to 8 m | The Jiaodong Peninsula, China | Water | 2103 microplastics/L | 2022 | [102] Mu et al., 2022 | |
| To investigate the presence of MPs in ten well samples obtained from an alluvial aquifer in a semi-arid region following filtration, digestion and inspection under a binocular microscope. | Alluvial aquifer with Quaternary deposits and surrounding karstic limestone/NA. | Shiraz, Iran | Water | 0.48 microplastics/L | 2022 | [103] Esfandiari et al., 2022 | |
| To simulate the transport of MP tracer particles compared to the solute conservative tracer uranine in a shallow alluvial aquifer over distances from 3.1 to 200 m using a natural gradient tracer test. | Shallow alluvial aquifer consisting of permeable sands and gravels/1.5 to 3 m | Upper Rhine Valley, Germany | NA. | NA. | 2021 | [104] Goeppert et al., 2021 | |
| To investigate the sediments of a show cave in Italy, developing a methodology based on a cave-adapted version of the methods used in several studies to detect MPs from sediments of different environments and with various laboratory tests. | Karst aquifer/NA. | Piedmont, Italy | Sediment | 1600 microplastics/kg 4390 microplastics/kg dry | 2022 | [105] Balestra et al., 2022 | |
| To identify, characterise and quantify MPs in groundwater samples around Perungudi and Kodungaiyur municipal solid waste dumpsites in South India. | NA./3 to 34.48 m | Perungudi and Kodungaiyur, South India | Water | 2 to 80 microplastics/L | 2021 | [106] Bharath et al., 2021 | |
| To collect groundwater samples in mid-November under low-flow conditions from eight springs and three shallow (<65 m) wells to investigate MPs. | Karst aquifer/NA. | Illinois, USA | Water | 15.2 microplastics/L | 2019 | [3] Panno et al., 2019 | |
| To research MPs in groundwater and surface water from coastal south India (Tamil Nadu state) and to evaluate the heavy metal adsorption capacities of different polymers | NA./2–5 m | Tamil Nadu, South India | Water | 4.2 microplastics/L | 2021 | [107] Selvam et al., 2021 | |
| Review Article | References | [96,97,98,108,109] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanale, C.; Losacco, D.; Triozzi, M.; Massarelli, C.; Uricchio, V.F. An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers. Resources 2022, 11, 105. https://doi.org/10.3390/resources11110105
Campanale C, Losacco D, Triozzi M, Massarelli C, Uricchio VF. An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers. Resources. 2022; 11(11):105. https://doi.org/10.3390/resources11110105
Chicago/Turabian StyleCampanale, Claudia, Daniela Losacco, Mariangela Triozzi, Carmine Massarelli, and Vito Felice Uricchio. 2022. "An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers" Resources 11, no. 11: 105. https://doi.org/10.3390/resources11110105
APA StyleCampanale, C., Losacco, D., Triozzi, M., Massarelli, C., & Uricchio, V. F. (2022). An Overall Perspective for the Study of Emerging Contaminants in Karst Aquifers. Resources, 11(11), 105. https://doi.org/10.3390/resources11110105