Modeling Excitable Cells with Memristors
Abstract
1. Introduction
2. Chay Neuron Model of an Excitable Cell
3. Pinched Hysteresis Fingerprints of the Ion Channel Memristor
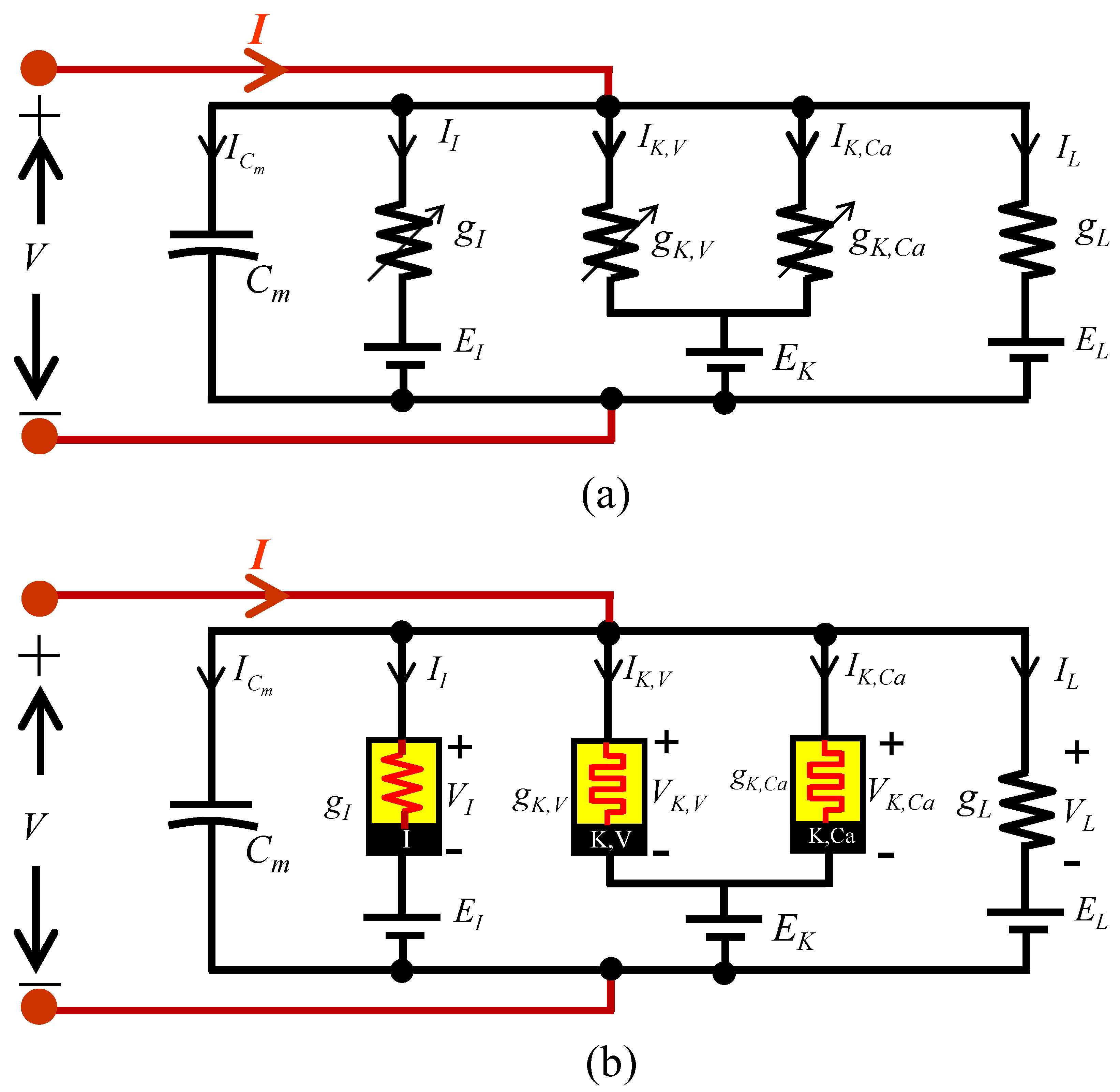
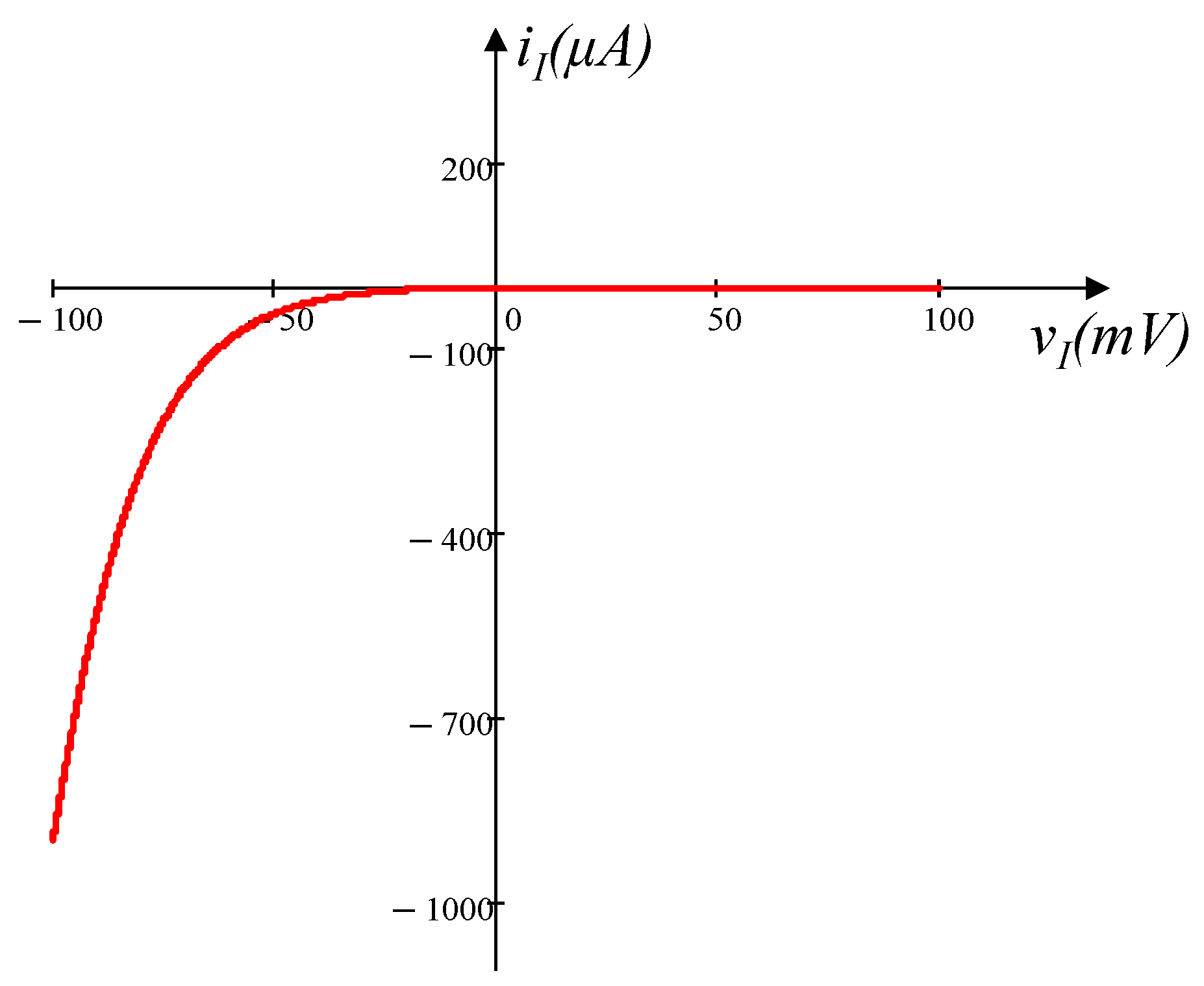
3.1. Voltage-Sensitive Mixed Ion channel Nonlinear Resistor
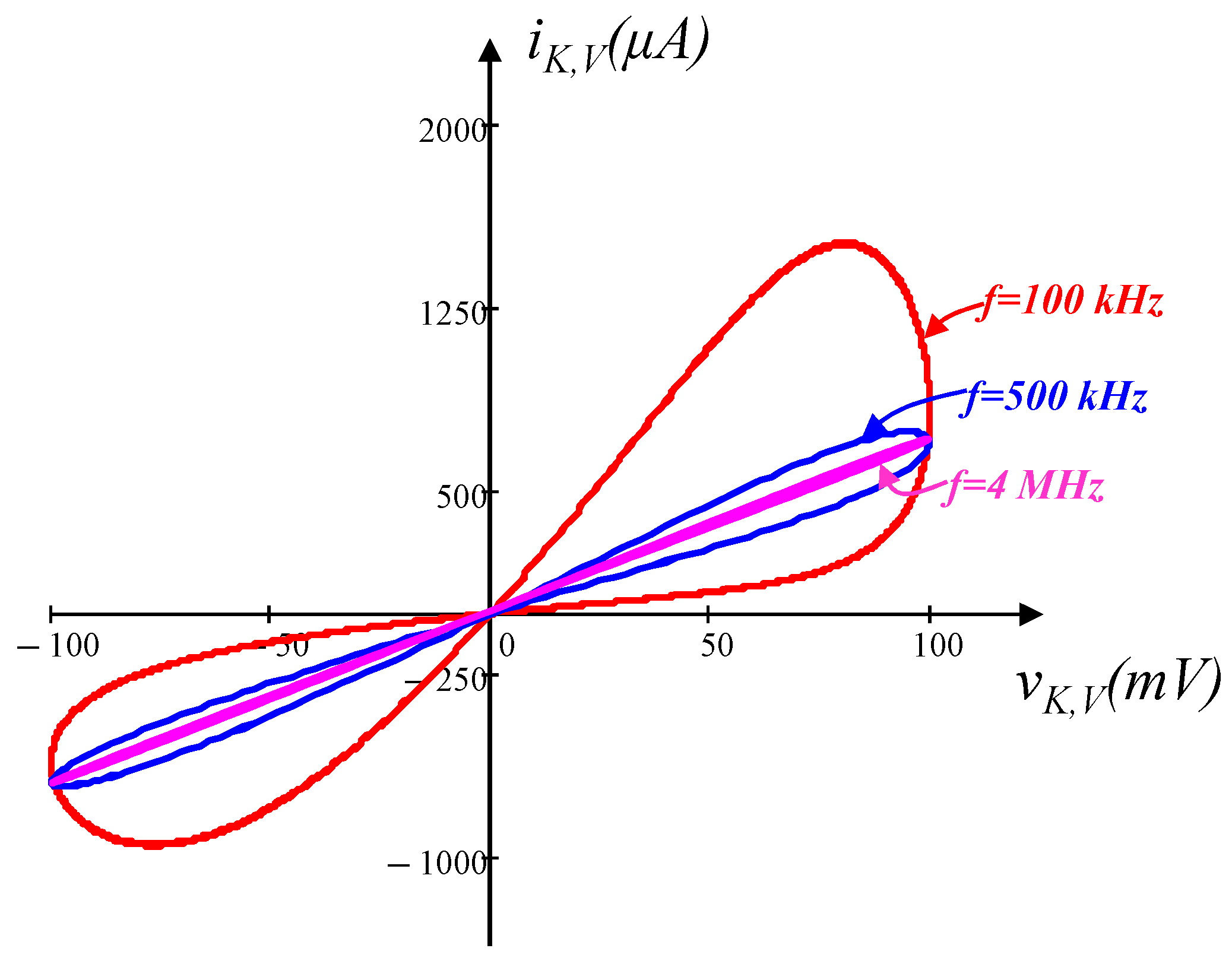
3.2. Voltage-Sensitive Potassium Ion Channel Memristor
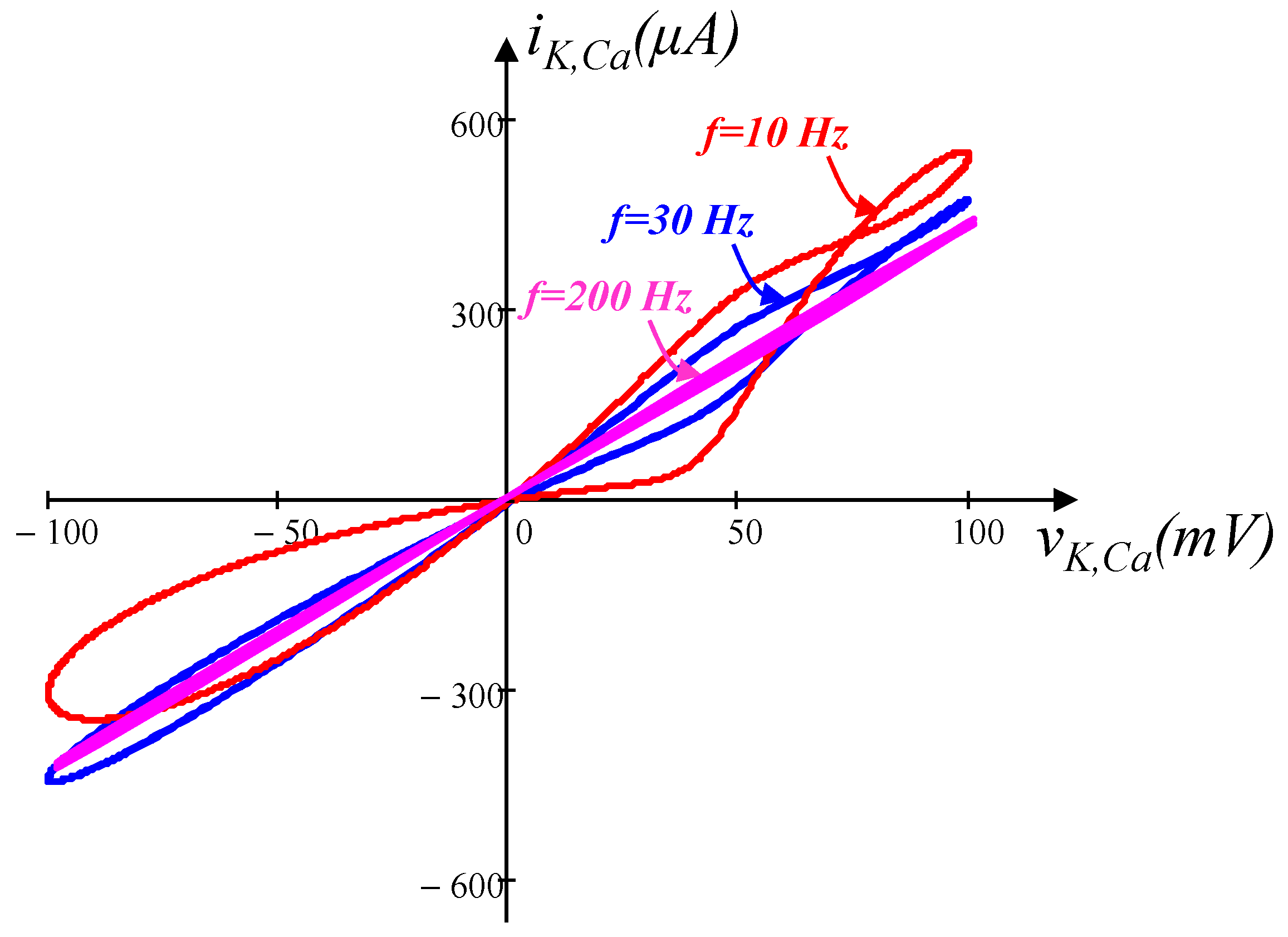
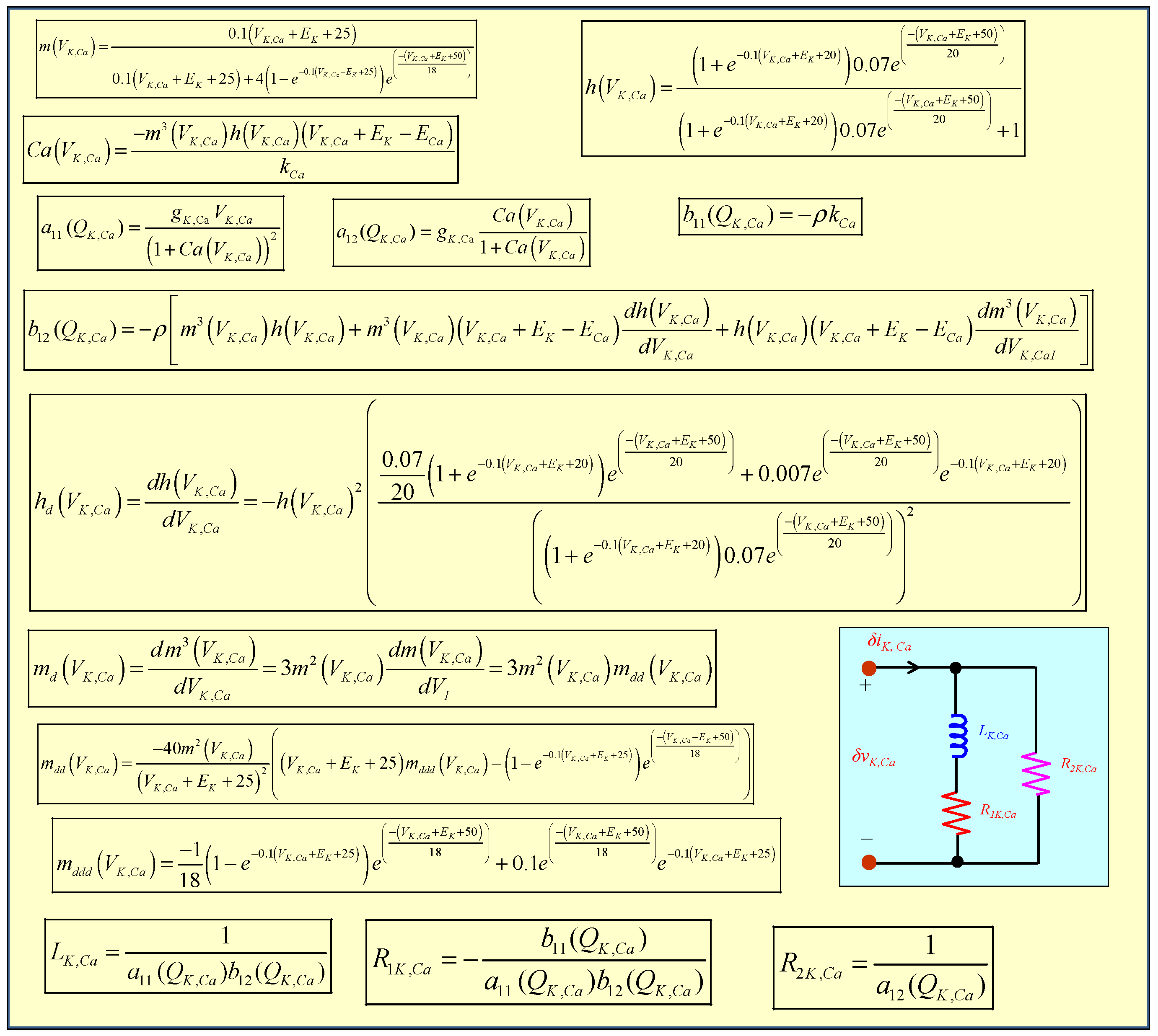
3.3. Calcium-Sensitive Potassium Ion Channel Memristor
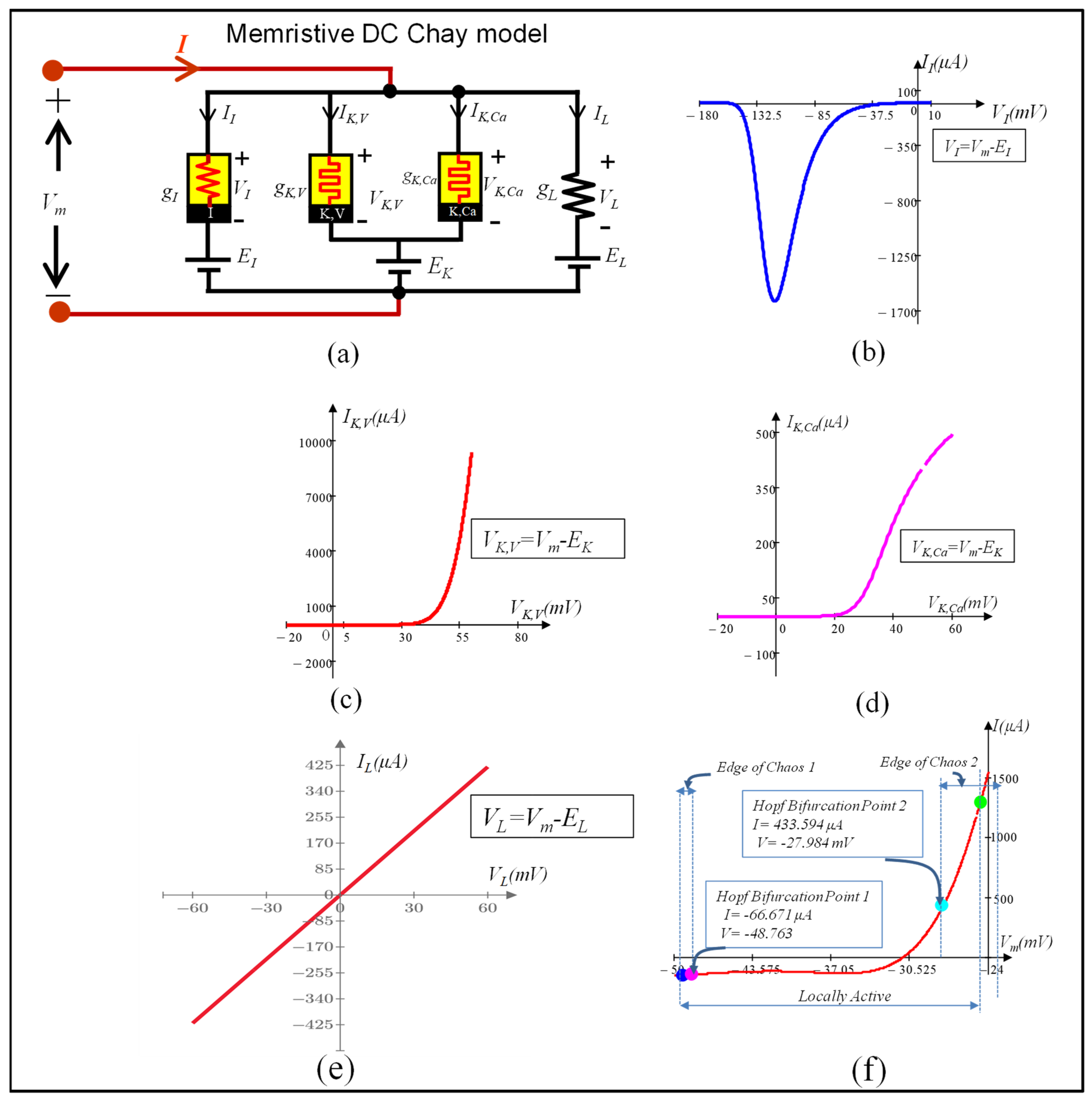
4. DC Analysis of the Memristive Chay Model of an Excitable Cell
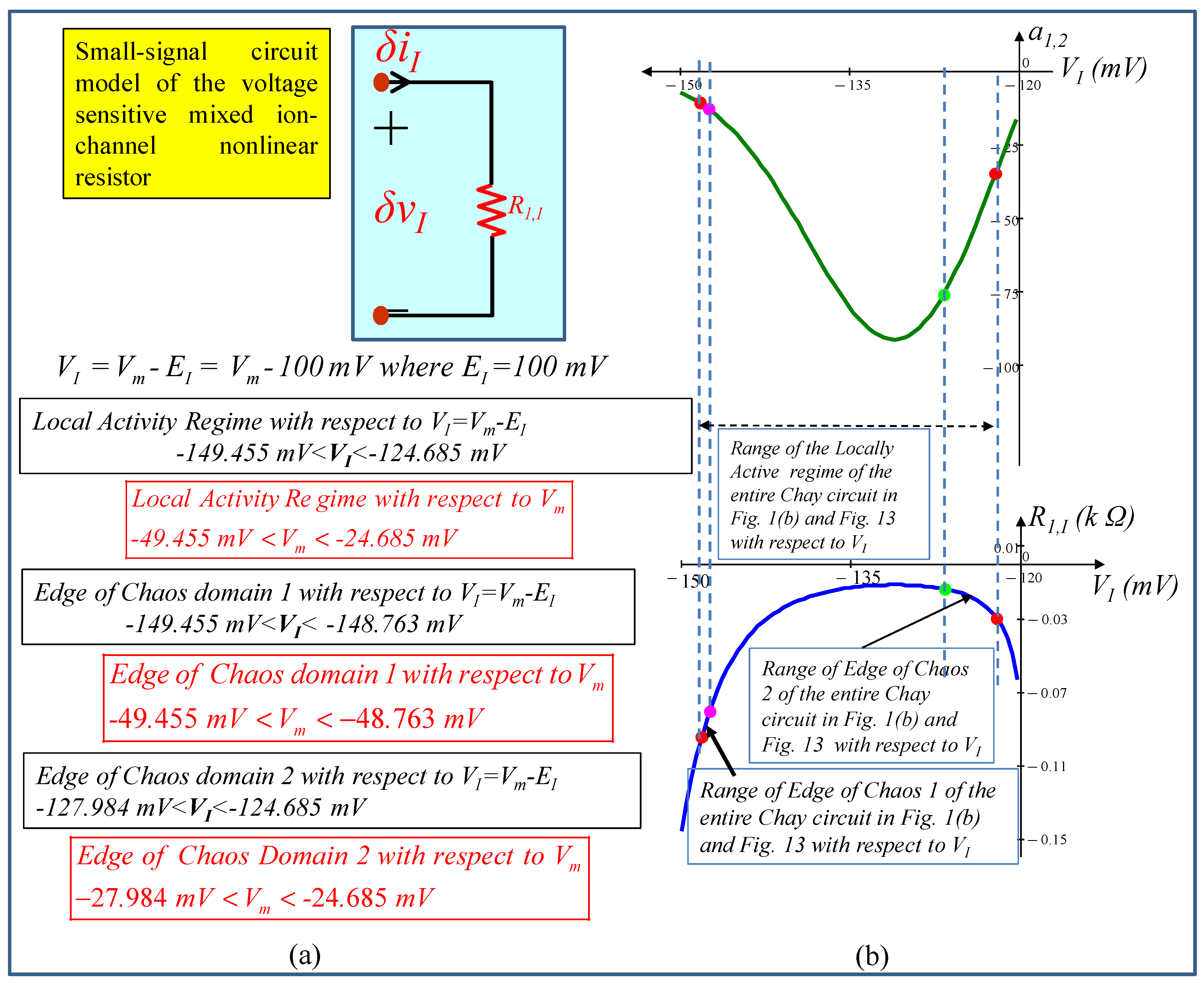
5. Small-Signal Circuit Model
5.1. Small-Signal Circuit Model of the Mixed Ion Channel Nonlinear Resistor
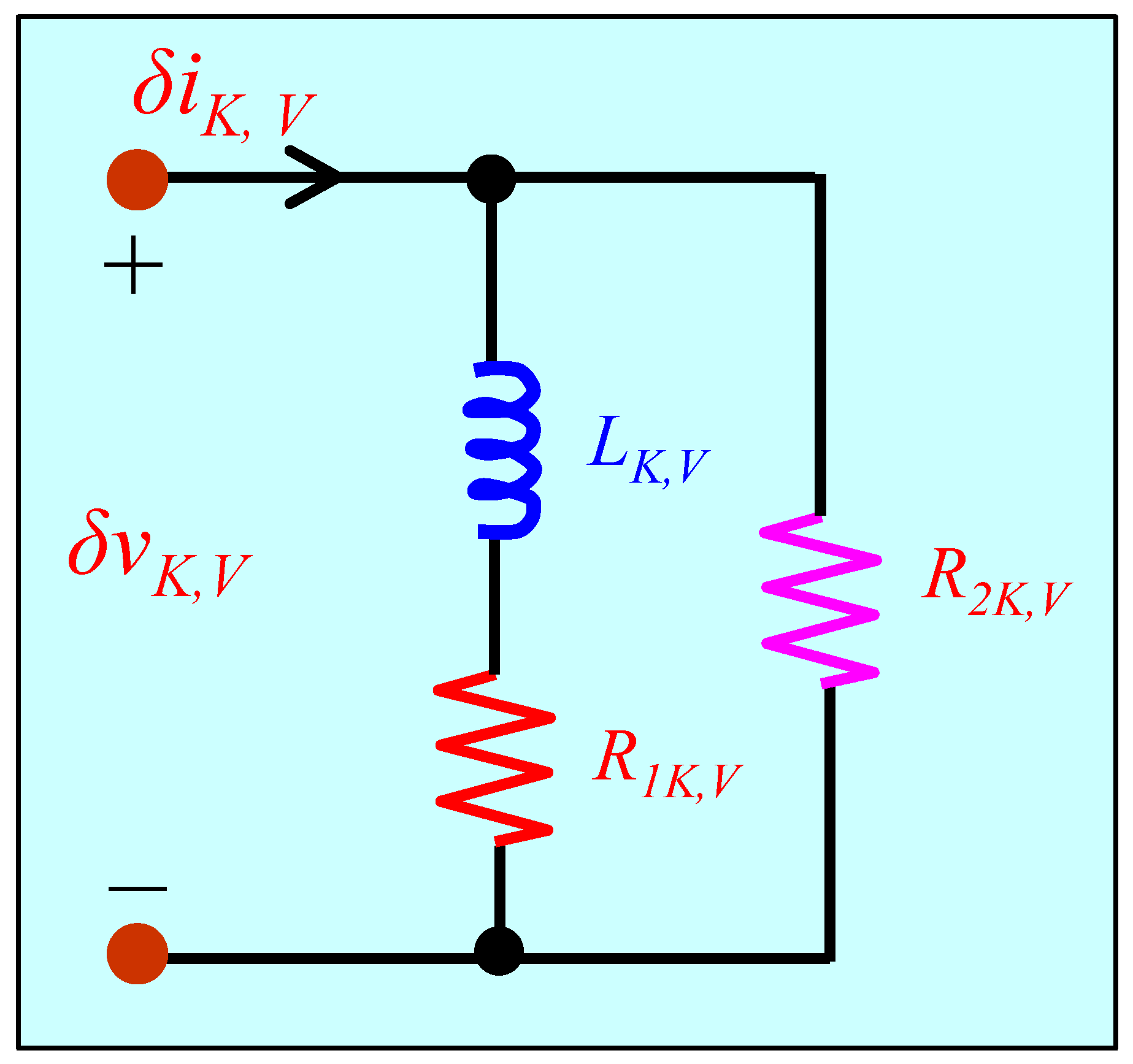
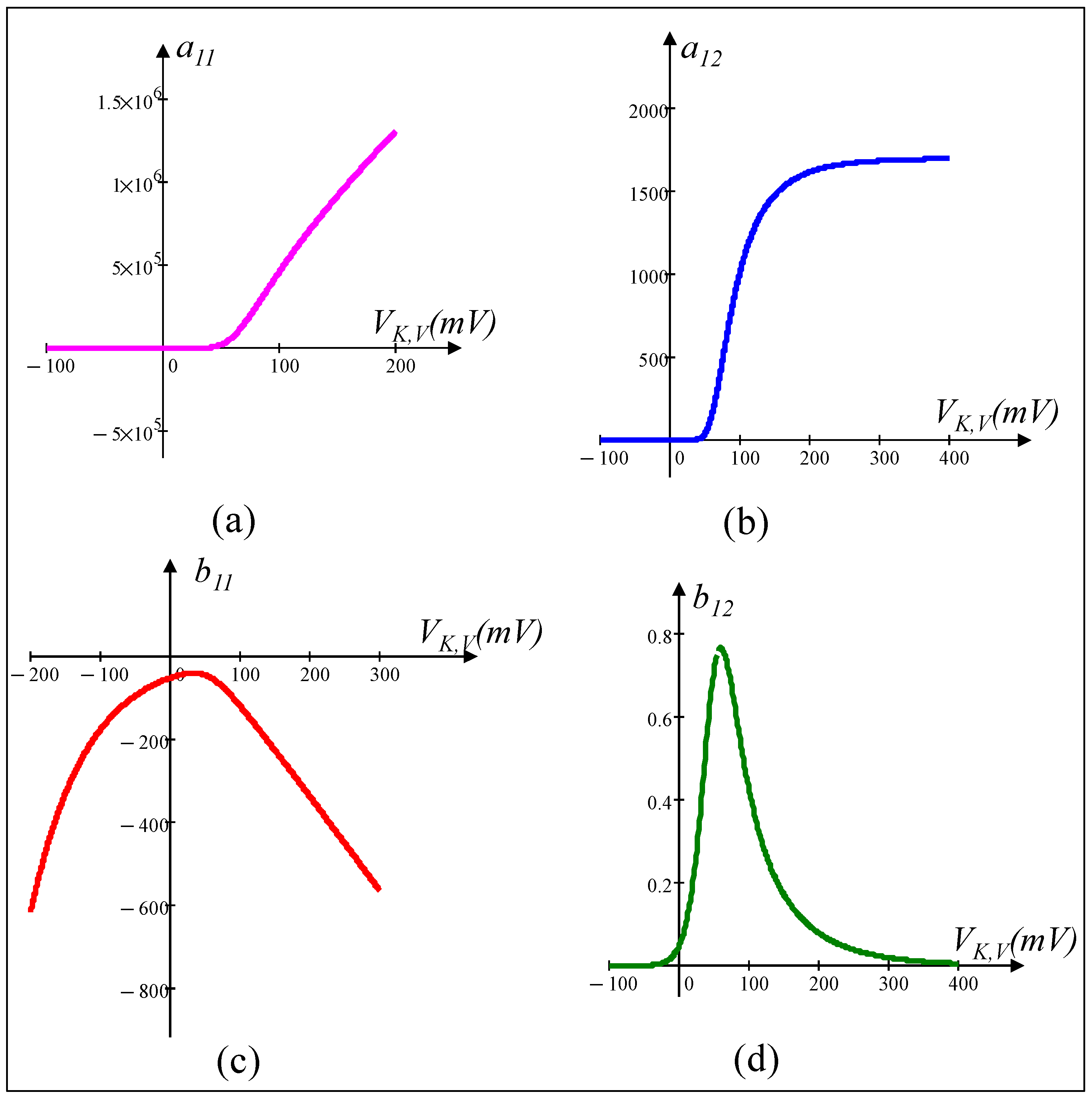
5.2. Small-Signal Circuit Model of the Voltage-Sensitive Potassium Ion Channel Memristor
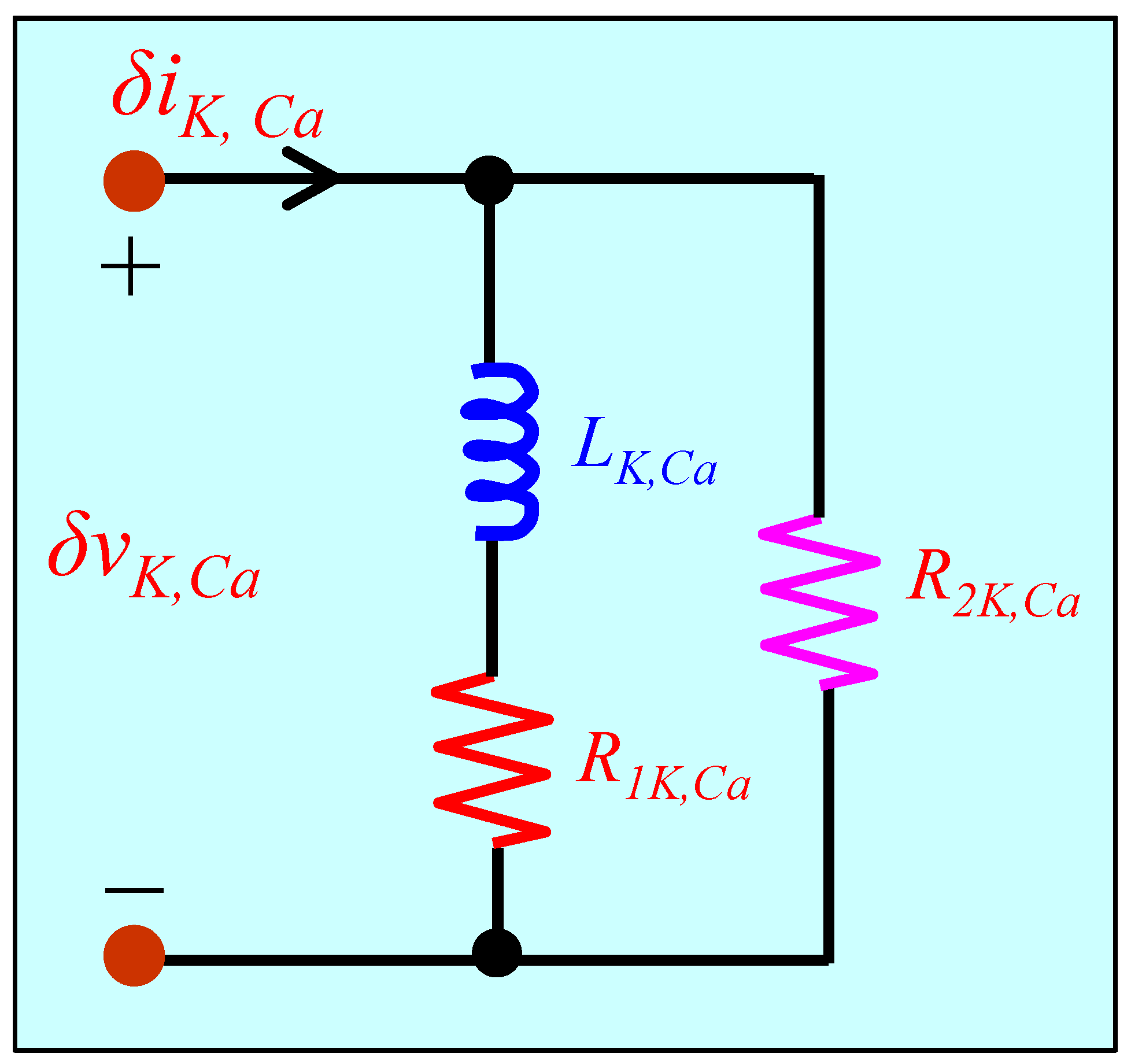
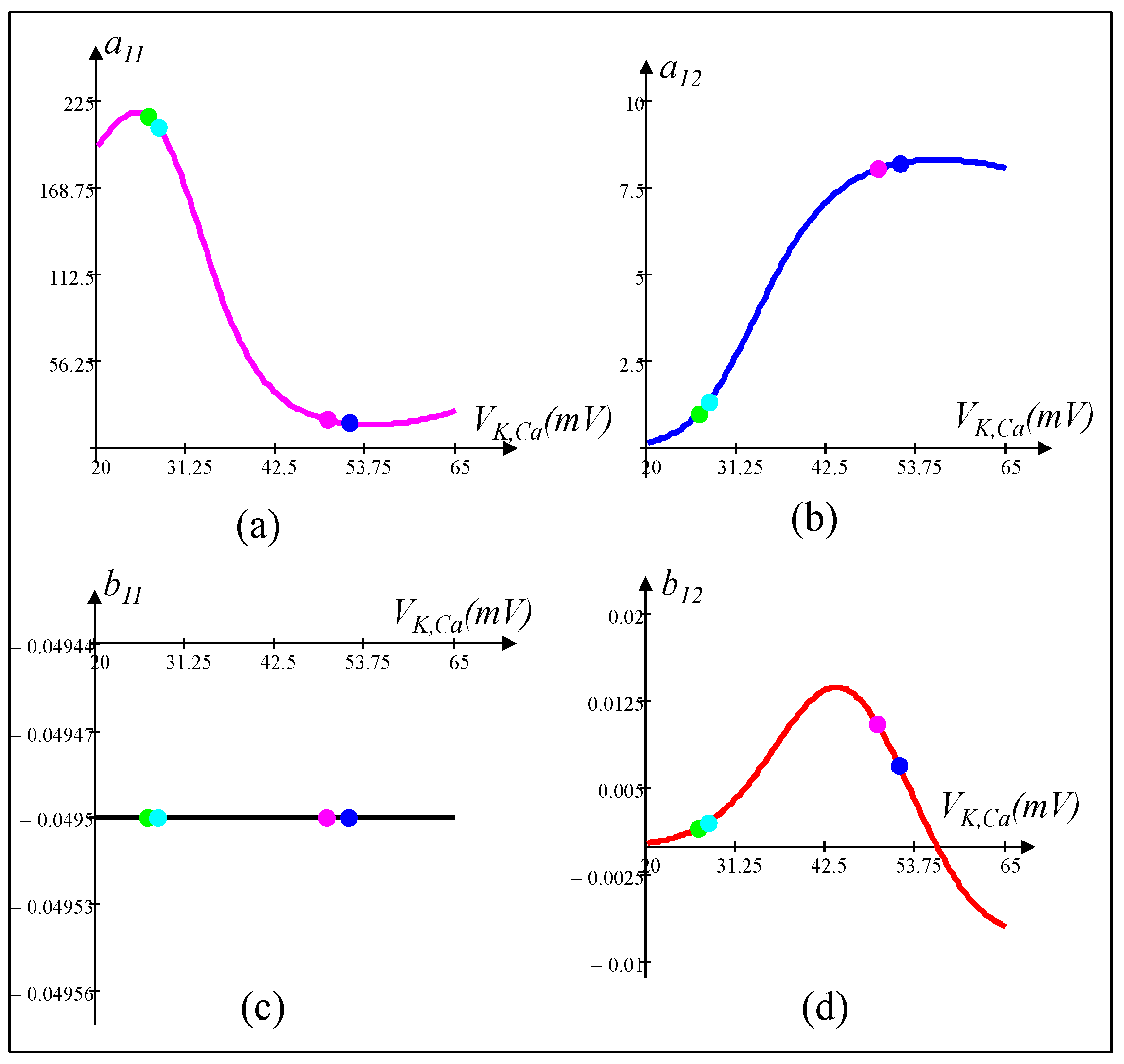
5.3. Small-Signal Circuit Model of the Calcium-Sensitive Potassium Ion Channel Memristor
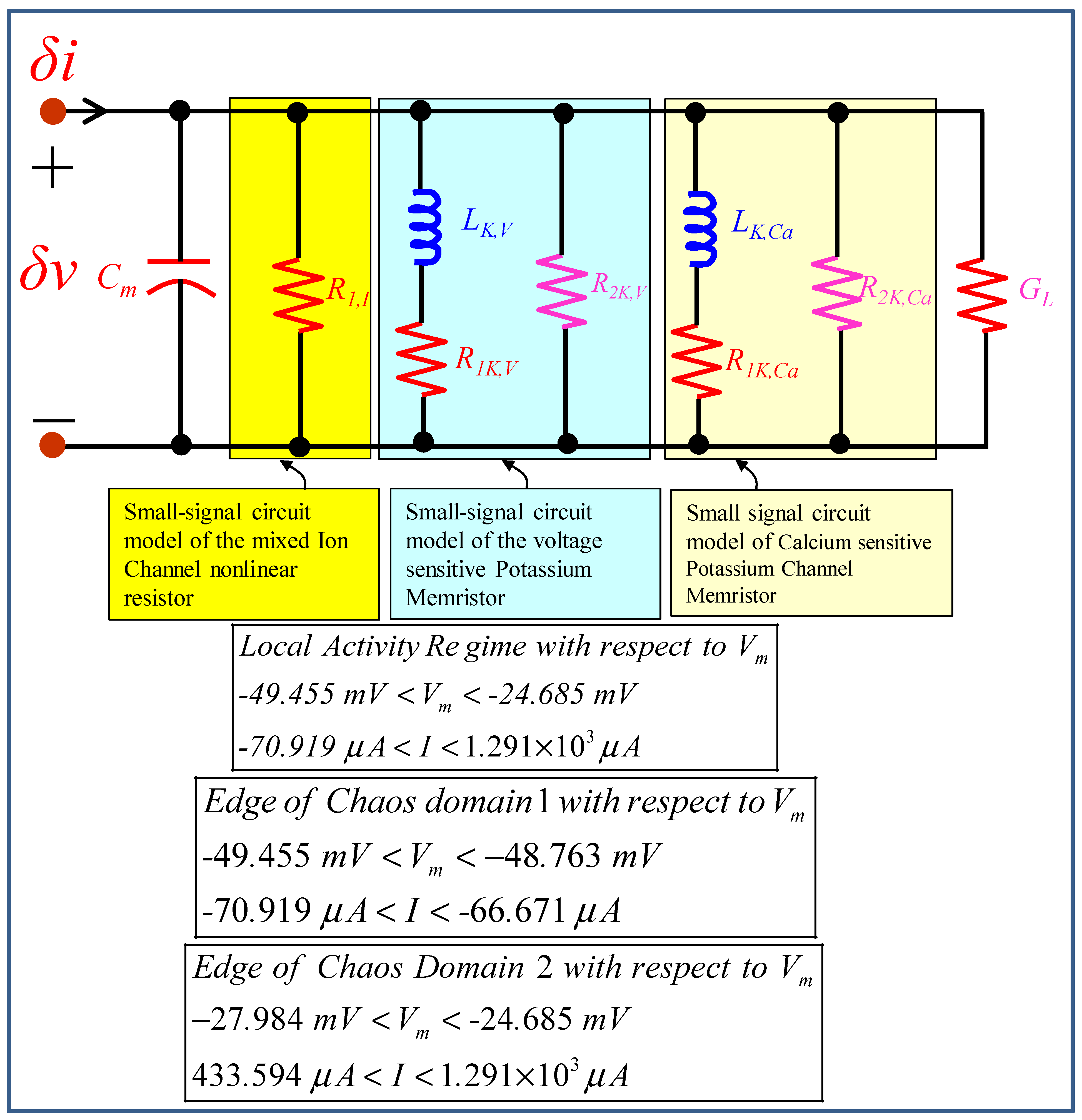
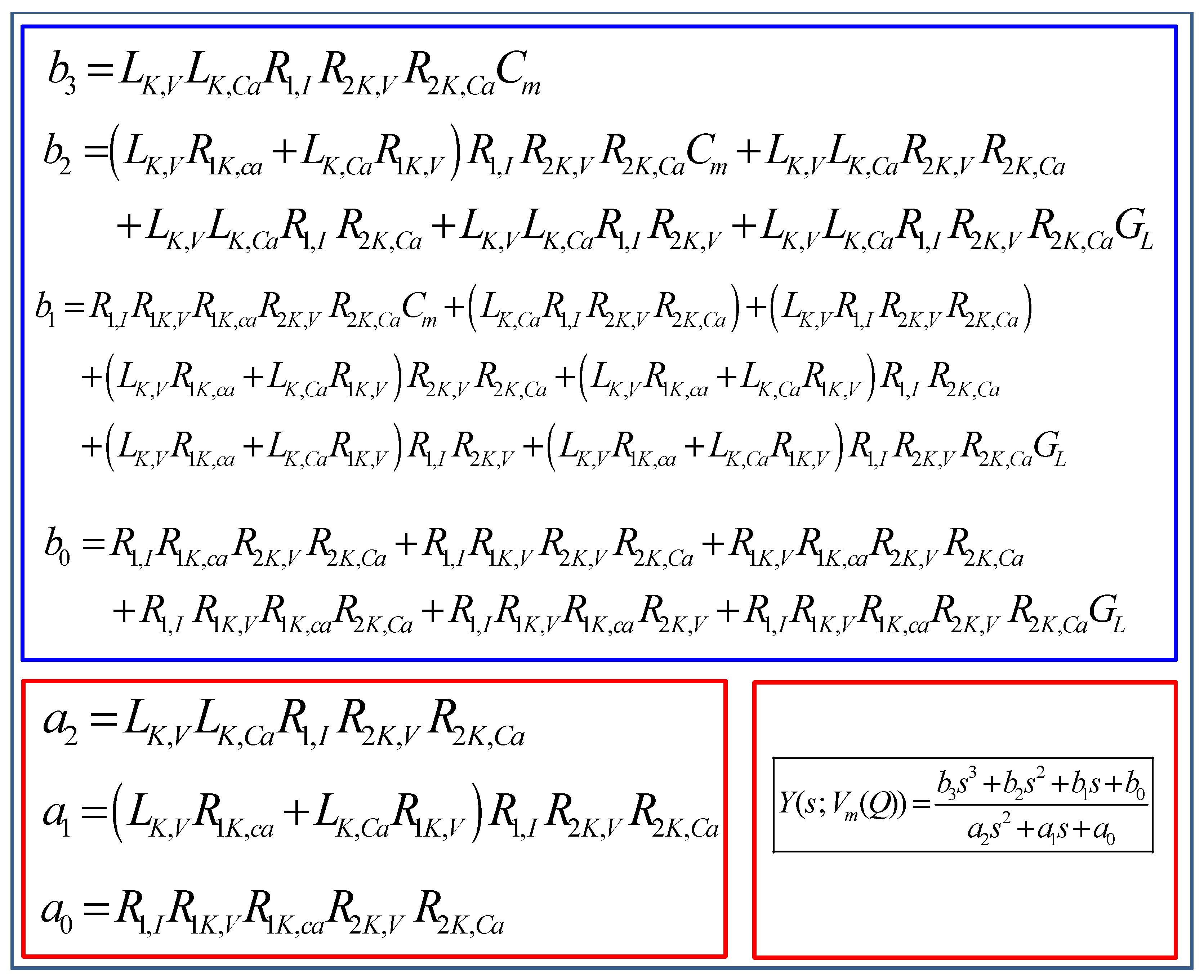
5.4. Small-Signal Circuit Model of the Memristive Chay Model
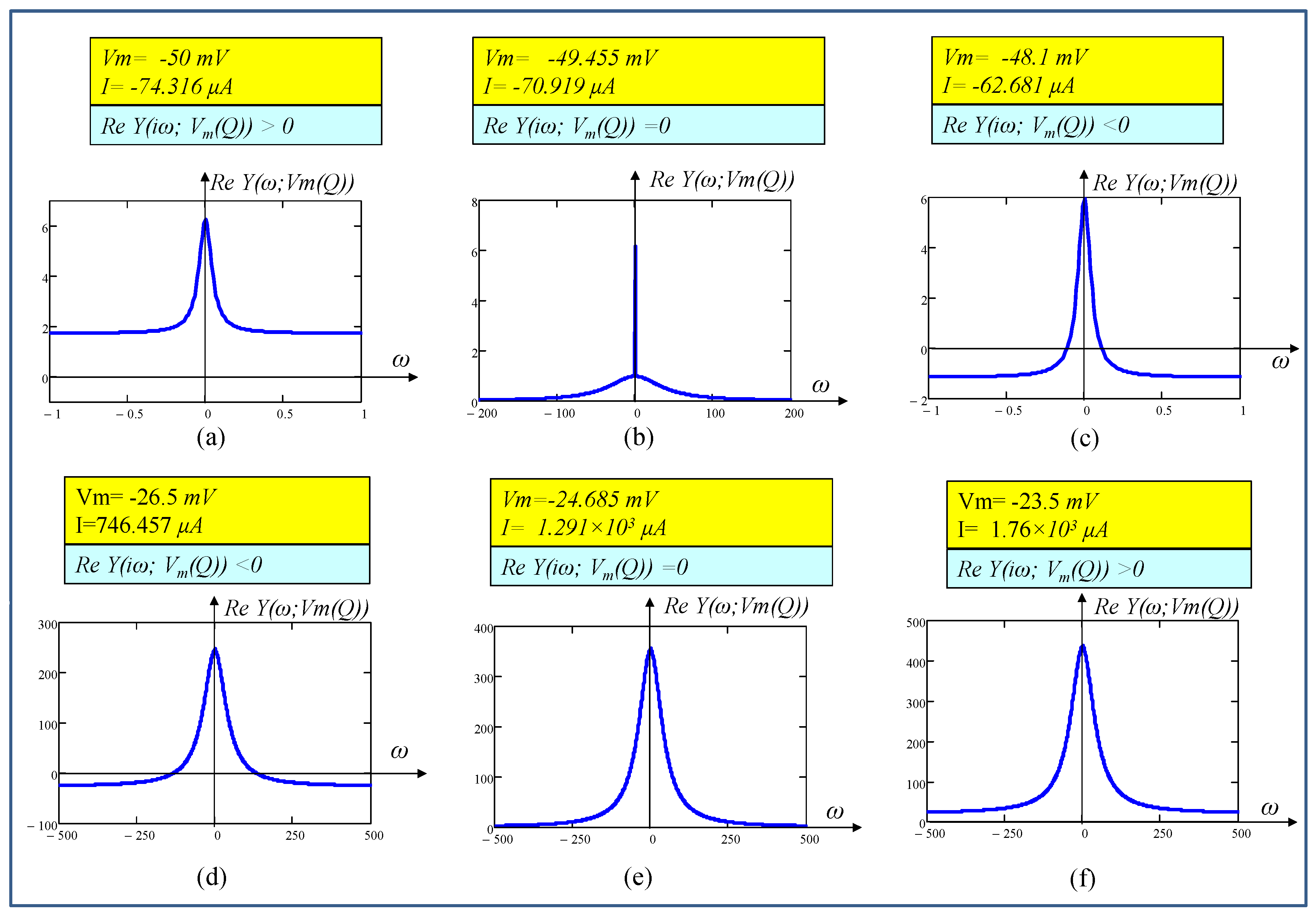
5.4.1. Frequency Response
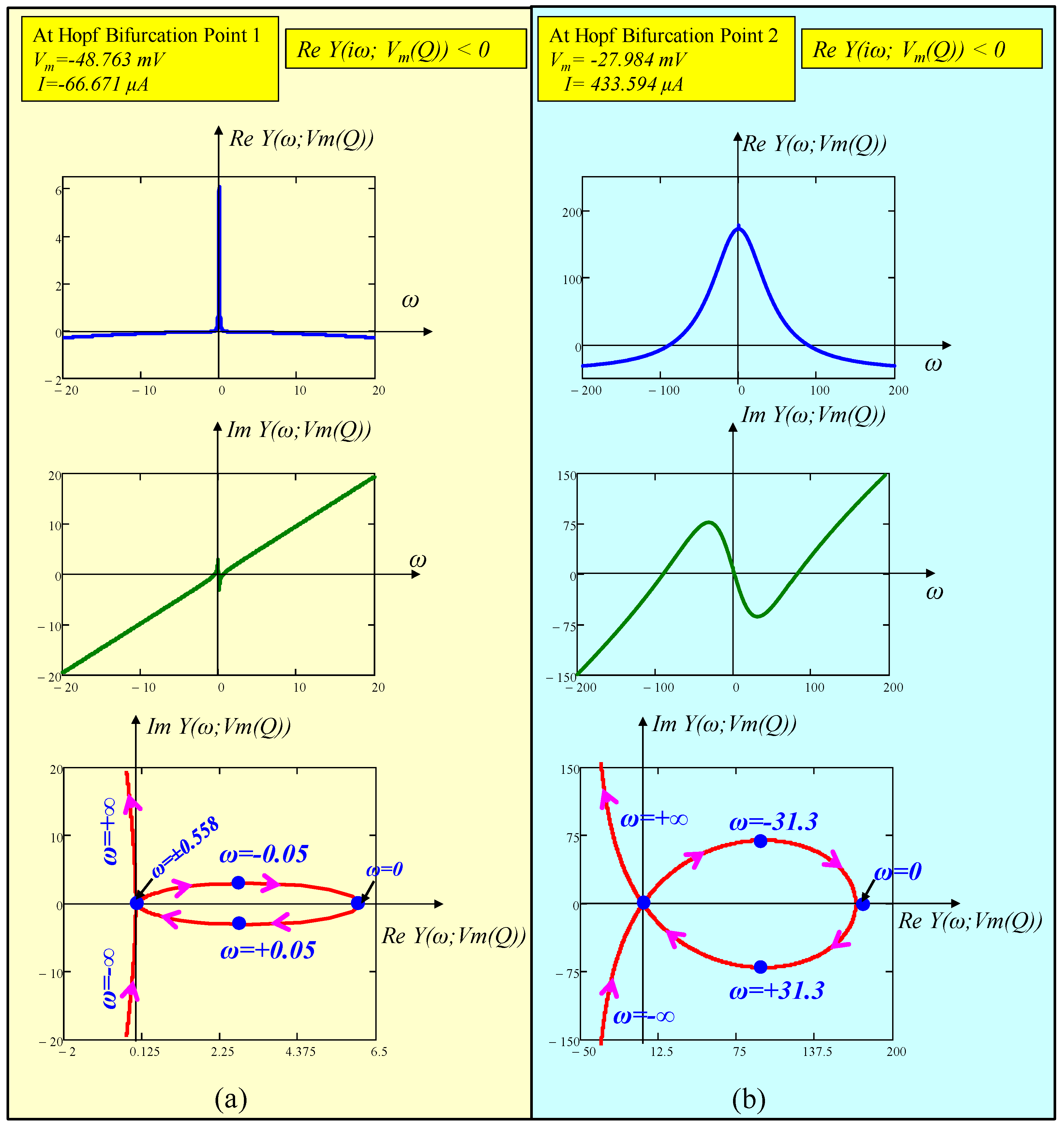
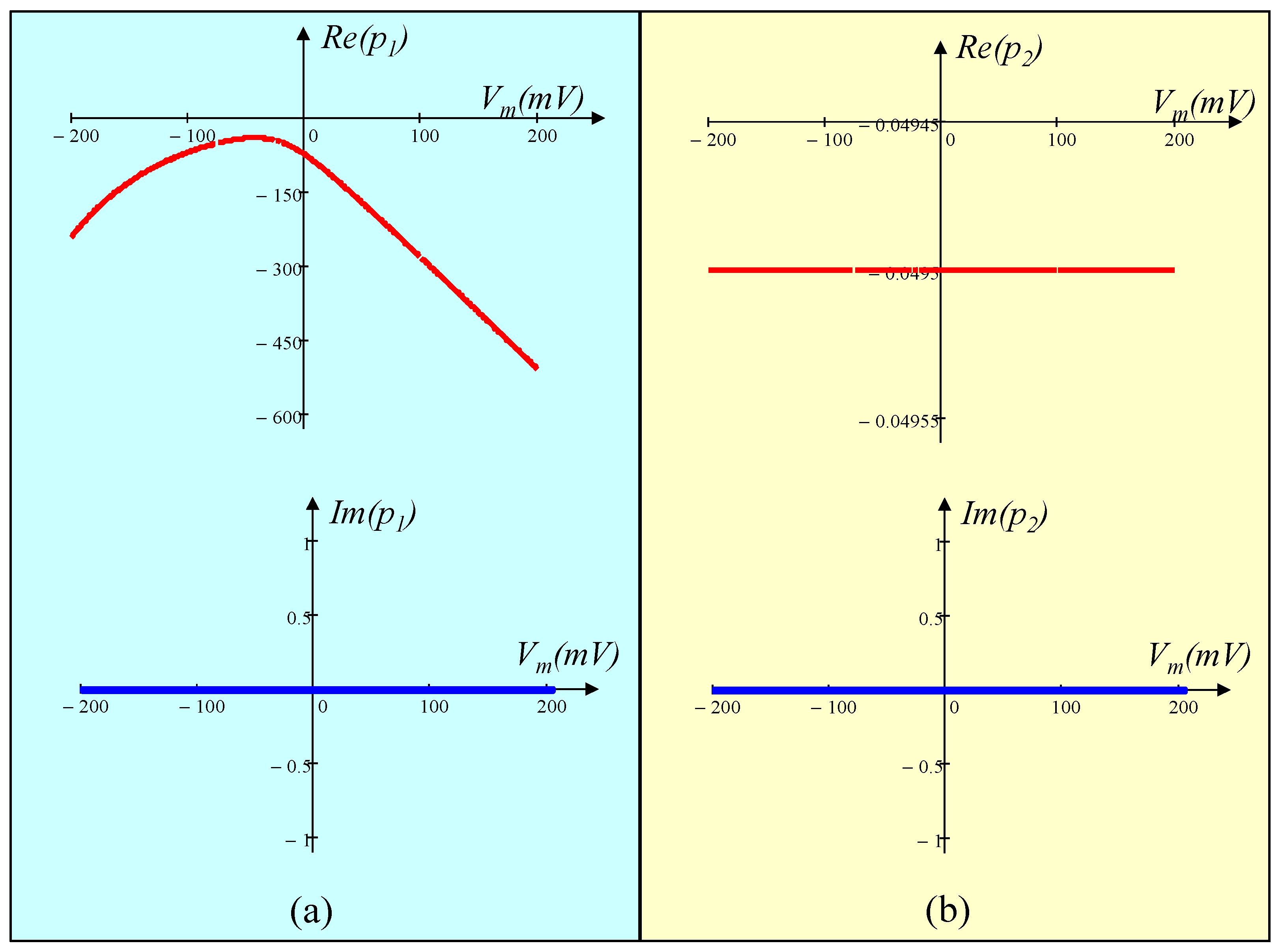
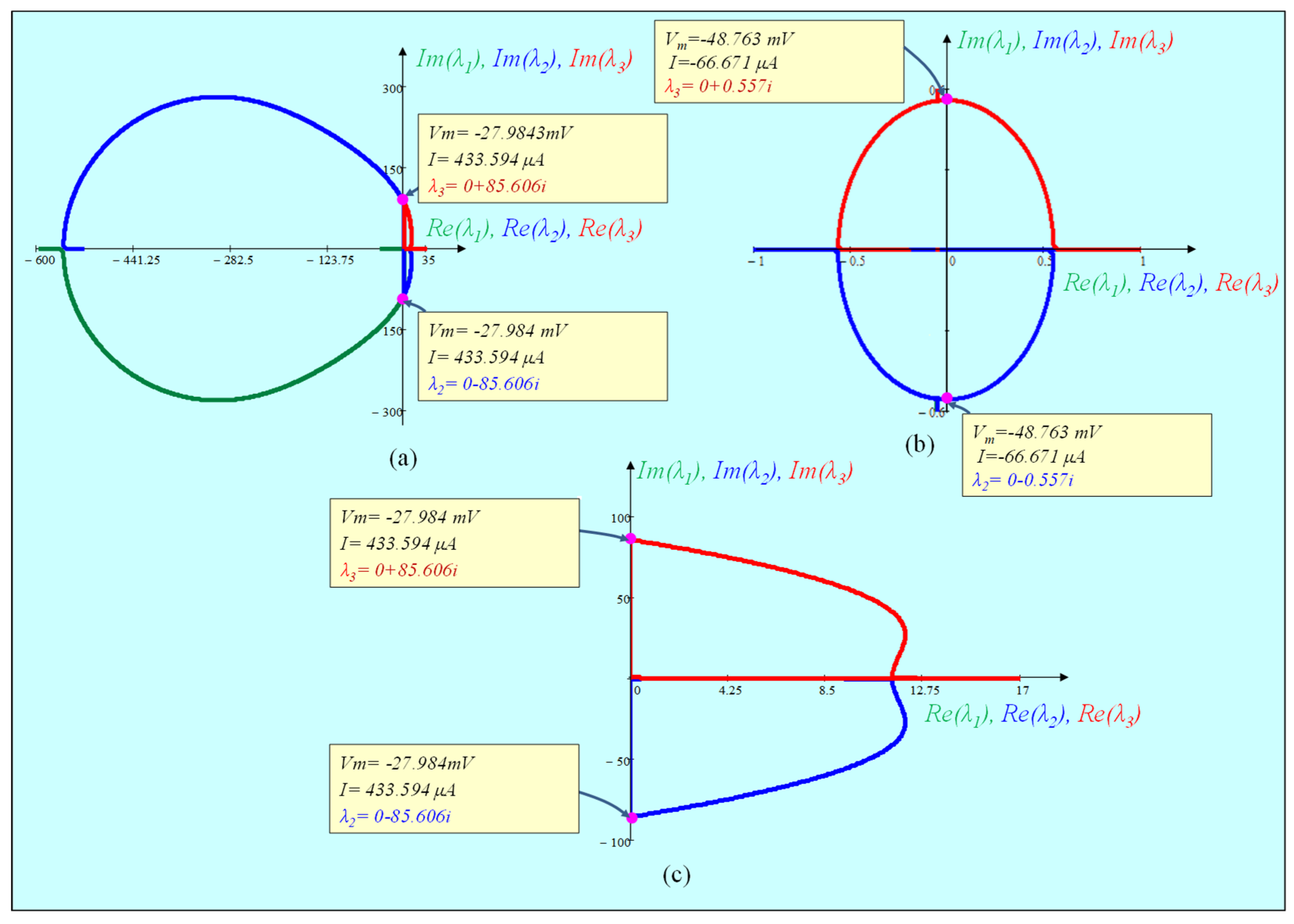
5.4.2. Pole-Zero Diagram of the Small-Signal Admittance Function Y(s; Vm(Q)) and Eigen values of the Jacobian Matrix
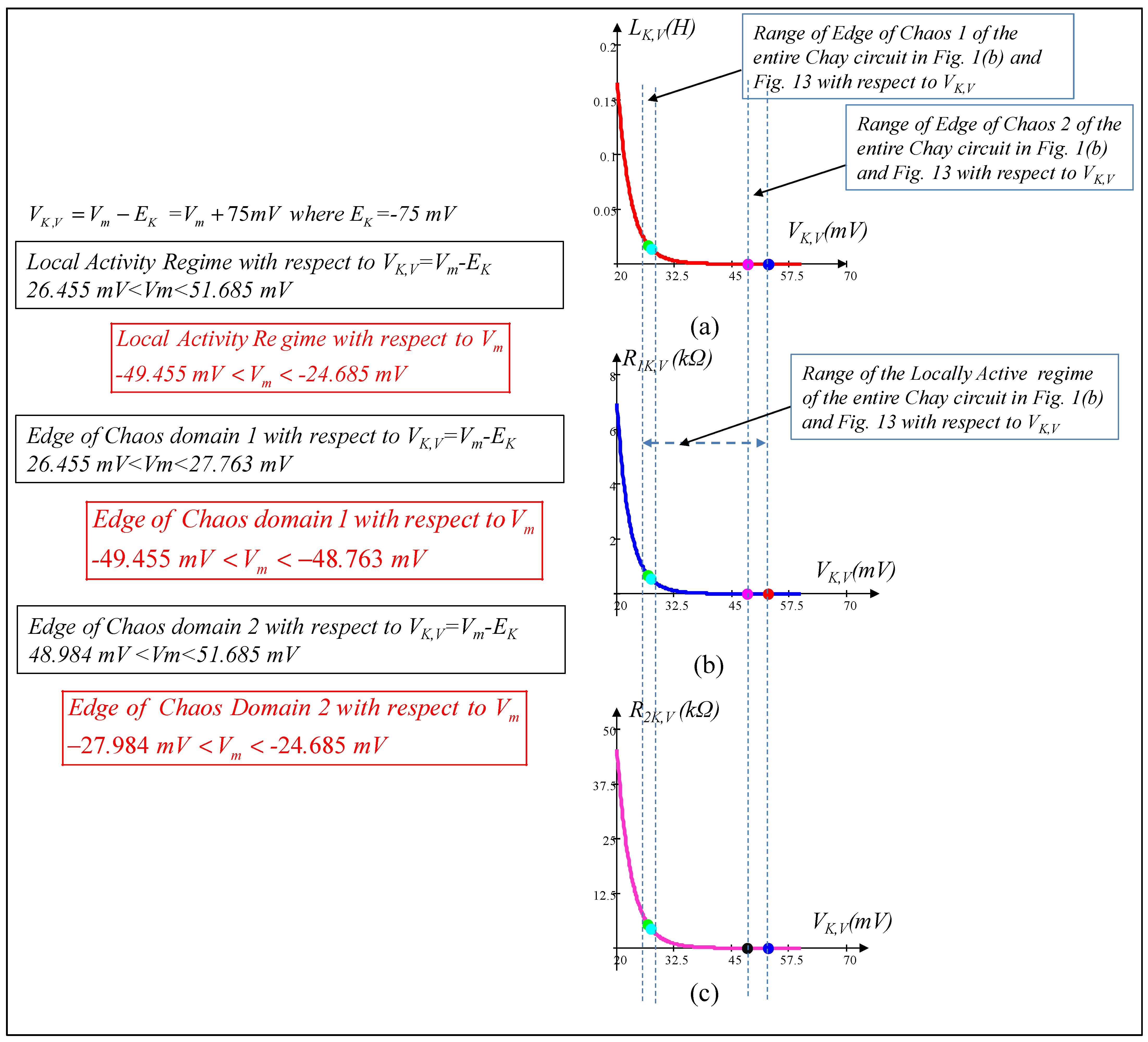
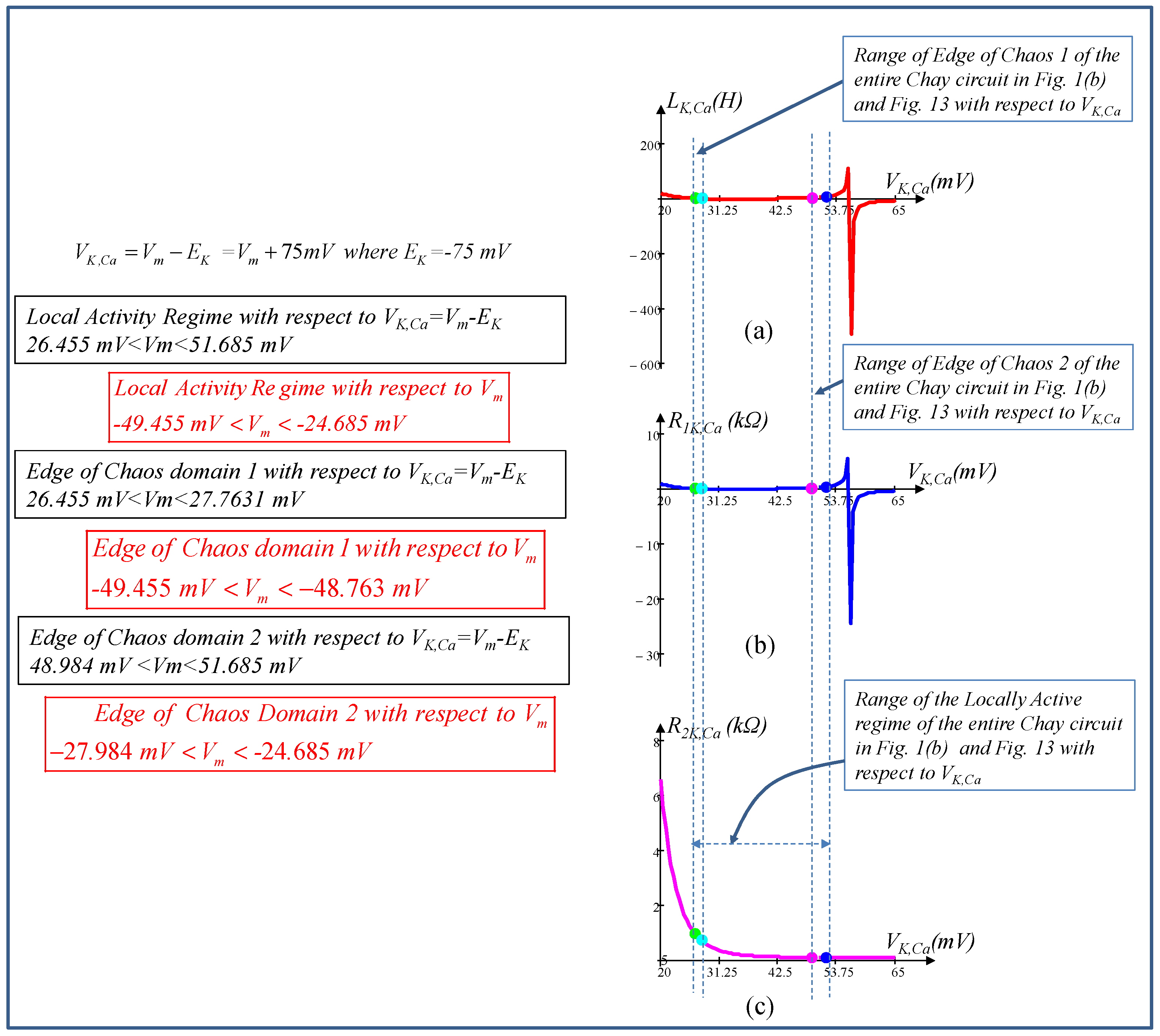
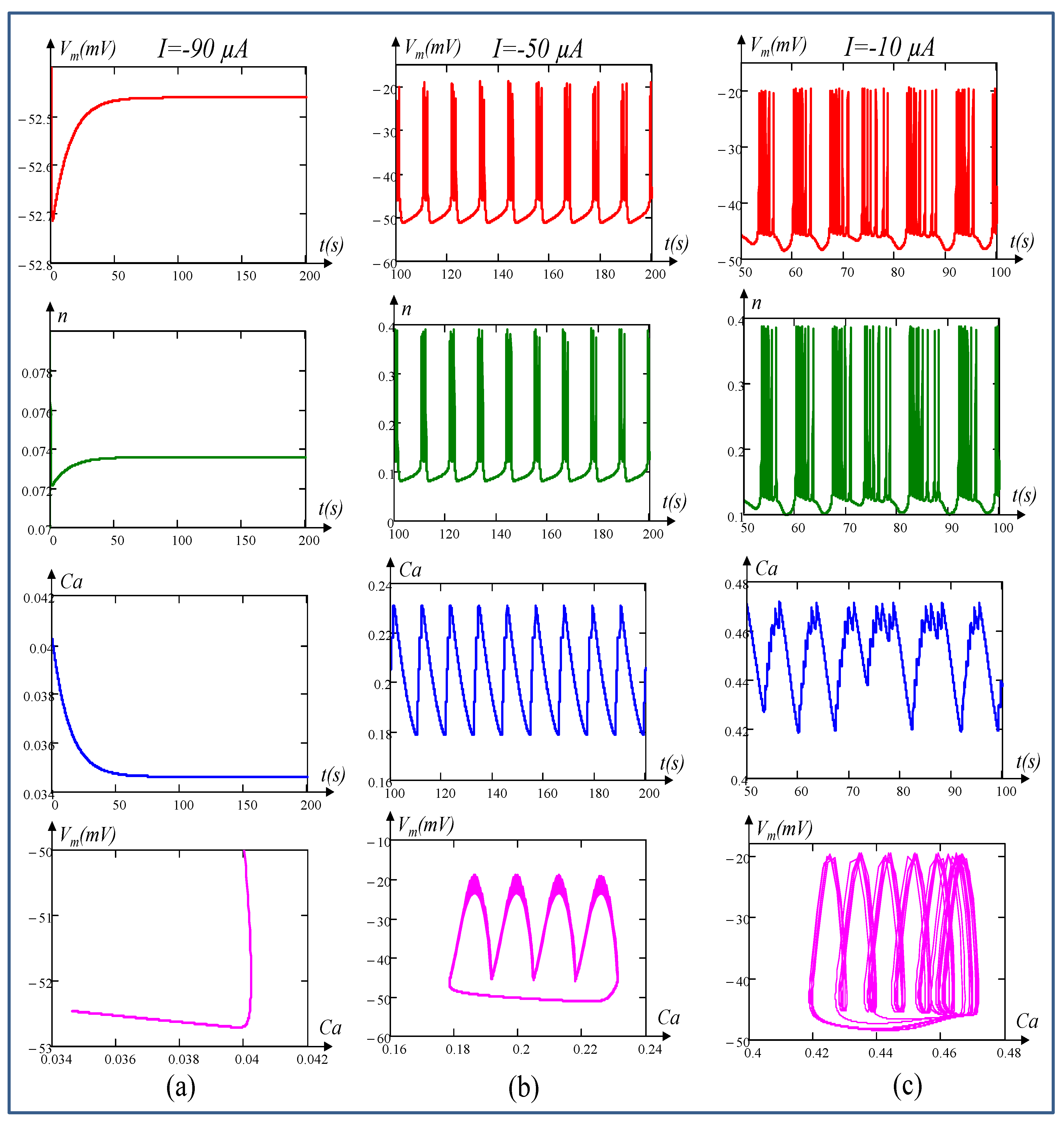
6. Local Activity, Edge of Chaos, and Hopf-Bifurcation in Memristive Chay Model
6.1. Locally Active Regime
6.2. Edge of Chaos Regime
6.3. Hopf-Bifurcation
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Abbreviations of the Model Parameters
| Cm | Membrane Capacitance |
| EK | Potential across K+ ion channel memristor |
| EI | Potential across mixed ion channel memristor |
| EL | Potential across leakage channel |
| ECa | Potential across Ca2+ ion channel memristor |
| gK,V | Voltage-sensitive K+ ion-channel conductance |
| gI | Voltage-sensitive mixed ion channel conductance |
| gL | Leakage channel conductance |
| gKCa | Calcium activated potassium conductance |
| kCa | Rate constant for the efflux of the intracellular Ca2+ ions |
| ρ | Proportionality constant |
| λn | Rate constant for K+ ion-channel opening |
| m∞ | Probability of activation of the mixed ion channel in steady state |
| αm | The rate at which the activation of the mixed ion channel closed gates transition to an open state (s−1) |
| βm | The rate at which the activation of the mixed ion channel open gates transition to the close state (s−1) |
| h∞ | Probability of inactivation of the mixed ion channel in steady state |
| αh | The rate at which the inactivation of the mixed ion channel closed gates transition to an open state (s−1) |
| βh | The rate at which the inactivation of the mixed ion channel open gates transition to the close state (s−1) |
| n | Probability of n opening of the K+ ion channel memristor |
| n∞ | Steady state value of n |
| αn | The rate at which K+ ion channel closed gates transition to an open state (s−1) |
| βn | The rate at which K+ ion channel opened gates transition to an close state (s−1) |
References
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.O.; Kang, S.M. Memristive devices and systems. Proc. IEEE 1976, 64, 209–223. [Google Scholar] [CrossRef]
- Chua, L.O.; Sbitnev, V.I.; Kim, H. Hodgkin-Huxley axon is made of memristors. Int. J. Bifurc. Chaos 2012, 22, 1230011. [Google Scholar] [CrossRef]
- Chua, L.O.; Sbitnev, V.I.; Kim, H. Neurons are poised near the edge of chaos. Int. J. Bifurc. Chaos 2012, 22, 1250098. [Google Scholar] [CrossRef]
- Chua, L. Hodgkin–Huxley equations implies Edge of chaos kernel. Jpn. J. Appl. Phys. 2022, 61, SM0805. [Google Scholar] [CrossRef]
- Chua, L. Everything you wish to know about memristor but are afraid to ask. Radioengineering 2015, 24, 319–368. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Keynes, R.D. Experiments on the injection of substances into squid giant axons by means of microsyringe. J. Physiol. 1956, 131, 592–616. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Lecar, H. Voltage oscillations in the Barnacle giant muscle fiber. J. Biophys. Soc. 1981, 35, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Sah, M.P.; Kim, H.; Eroglu, A.; Chua, L. Memristive model of the Barnacle giant muscle fibers. Int. J. Bifurc. Chaos 2016, 26, 1630001. [Google Scholar] [CrossRef]
- Rajamani, V.; Sah, M.P.; Mannan, Z.I.; Kim, H.; Chua, L. Third-order memristive Morris-Lecar model of barnacle muscle fiber. Int. J. Bifurc. Chaos 2017, 27, 1730015. [Google Scholar] [CrossRef]
- Noble, D. A modification of the Hodgkin-Huxley equations applicable to Purkinje fibre action and peacemaker potentials. J. Physiol. 1962, 160, 317–352. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J.; Lewis, R.S. A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog Rana catesbeiana. J. Physiol. 1988, 400, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Giguère, C.; Woodland, P.C. A computational model of the auditory periphery for speech and hearing research. J. Acoust. Soc. Am. 1994, 95, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, R.A.; Voyles, R.M.; Shaheen, S.E. A mini review of neuromorphic architectures and implementations. IEEE Trans. Electron. Devices 2016, 63, 3819–3829. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, T.W. Organic synapses for neuromorphic electronics: From brain inspired computing to sensorimotor nervetronics. Am. Chem. Soc. 2019, 52, 964–974. [Google Scholar] [CrossRef]
- Gentili, P.L. Photochromic and luminescent materials for the development of chemical artificial intelligence. Dye. Pigment. 2022, 205, 110547. [Google Scholar] [CrossRef]
- Peercy, B.E.; Sherman, A.S. How pancreatic beta-cells distinguish long- and short-time scale CAMP signals. Biophys. J. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Pedersen, M.G. Contributions of mathematical modeling of Beta-cells to the understanding of beta-cell oscillations and insulin secretion. Diabetes Technol. Soc. 2009, 3, 12–20. [Google Scholar] [CrossRef]
- Felix-Martinez, G.J.; Godlinez-Fernandez, J.R. Mathematical models of electrical activity of the pancreatic β-cell. Islets 2014, 6, e949195. [Google Scholar] [CrossRef]
- Kaestner, K.H.; Thompson, M.C.; Dor, Y.; Gill, R.G.; Glaser, B.; Kim, S.K.; Sander, M.; Stabler, C.; Stewart, A.F.; Powers, A.C. What is a β-cell? -Chapter I in the Human Islet Research Network (HIRN) review series. Mol. Metab. 2021, 53, 101323. [Google Scholar]
- Lenzen, S. The pancreatic beta cell: An intricate relation between anatomical structure, the signalling mechanism of glucose-induced insulin secretion, the low antioxidative defence, the high vulnerability and sensitivity to diabetic stress. ChemTexts 2021, 7, 13. [Google Scholar] [CrossRef]
- Marinelli, I.; Thompson, B.M.; Parekh, V.S.; Fletcher, P.A.; Gerardo-Giorda, L.; Sherman, A.S.; Satin, L.S.; Bertram, R. Oscillations in K(ATP) conductance drive slow calcium oscillations in pancreatic β-cells. Biophys. J. 2022, 121, 1449–1464. [Google Scholar] [CrossRef]
- Marinelli, I.; Parekh, V.; Fletcher, P.; Thompson, B.; Ren, J.; Tang, X.; Saunders, T.L.; Ha, J.; Sherman, A.; Bertram, R.; et al. Slow oscillations persist in pancreatic beta cells lacking phosphofructokinase M. Biophys. J. 2022, 121, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Mukai, E.; Fujimoto, S.; Inagaki, N. Role of Reactive Oxygen Species in Glucose Metabolism Disorder in Diabetic Pancreatic β-Cells. Biomolecules 2022, 2022, 12091228. [Google Scholar] [CrossRef] [PubMed]
- Millette, K.; Rodriguez, K.; Sheng, X.; Finley, S.D.; Georgia, S. Exogenous Lactogenic Signaling Stimulates Beta Cell Replication In Vivo and In Vitro. Biomolecules 2022, 12, 215. [Google Scholar] [CrossRef]
- Bertram, R.; Marinell, I.; Fletcher, P.A.; Satin, L.S.; Sherman, A.S. Deconstructing the integrated oscillator model for pancreatic β-cells. Math. Biosci. 2023, 365, 109085. [Google Scholar] [CrossRef]
- Plant, R.E. Bifurcation and resonance in a model for bursting nerve cells. J. Math. Biol. 1981, 11, 15–32. [Google Scholar] [CrossRef]
- Chay, T.R. Eyring rate theory in excitable membranes. Application to neural oscillations. J. Phys. Chem. 1983, 87, 2935–2940. [Google Scholar]
- Chay, T.R.; Keizer, J. Minimal model for membrane oscillations in the pancreatic β-cell. J. Biophys. Soc. 1983, 42, 181–190. [Google Scholar] [CrossRef]
- Chay, T.R. Chaos in a three-variable model of an excitable cell. Physica D 1985, 16, 233–242. [Google Scholar] [CrossRef]
- FitzHugh, R. Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1961, 1, 445–466. [Google Scholar] [CrossRef] [PubMed]
- Chua, L. Five non-volatile memristor enigmas solved. Appl. Phys. A Mater. Sci. Process. 2018, 124, 563. [Google Scholar] [CrossRef]
- Chua, L. If it’s pinched it’s a memristor. Semicond. Sci. Technol. 2014, 29, 104001. [Google Scholar] [CrossRef]
- Chua, L.O.; Desoer, C.A.; Kuh, E.S. Linear and Nonlinear Circuits; McGraw-Hill Book Co.: New York, NY, USA, 1987. [Google Scholar]
- Chua, L.O. CNN: A Paradigm for Complexity; World Scientific: Singapore, 1998. [Google Scholar]
- Chua, L.O. Local activity is the origin of complexity. Int. J. Bifurc. Chaos 2005, 15, 3435–3456. [Google Scholar] [CrossRef]
- Chua, L.O. Local activity principle: Chua’s riddle, “Turing machine, and universal computing rule 137”. In The Chua Lectures: From Memristors and Cellular Nonlinear Networks to the Edge of Chaos; World Scientific Series on Nonlinear Science; World Scientific: Singapore, 2020. [Google Scholar]
- Ascoli, A.; Demirkol, A.S.; Chua, L.O.; Tetzlaff, R. Edge of chaos theory resolves Smale paradox. IEEE Trans. Circuit Syst. I 2022, 69, 1252–1265. [Google Scholar] [CrossRef]
- Sah, M.P.; Mannan, Z.I.; Kim, H.; Chua, L. Oscillator made of only One memristor and one battery. Int. J. Bifurc. Chaos 2015, 25, 1530010. [Google Scholar] [CrossRef]
- Ascoli, A.; Demirkol, A.S.; Chua, L.O.; Tetzlaff, R. Edge of chaos is sine qua non for Turing instability. IEEE Trans. Circuit Syst. I 2022, 69, 4596–4609. [Google Scholar] [CrossRef]
- Ascoli, A.; Demirkol, A.S.; Chua, L.O.; Tetzlaff, R. Edge of chaos explains prigogine’s instability of the homogeneous. IEEE J. Emerg. Sel. Top. Circuits Syst. (JETCAS) 2022, 12, 804–820. [Google Scholar] [CrossRef]
- Ascoli, A.; Demirkol, A.S.; Tetzlaff, R.; Chua, L.O. Analysis and design of bio-inspired circuits with locally-active memristors. IEEE Trans. Circuit Syst. II 2024, 71, 1721–1726. [Google Scholar] [CrossRef]
- Ascoli, A.; Demirkol, A.S..; Tetzlaff, R.; Chua, L.O. Edge of chaos theory sheds light into the all-or-none phenomenon in neurons—Part I: On the fundamental role of the sodium ion channel. IEEE Trans. Circuit Syst. I 2024, 71, 5–19. [Google Scholar] [CrossRef]
- Ascoli, A.; Demirkol, A.S.; Tetzlaff, R.; Chua, L.O. Edge of chaos theory sheds light into the all-or-none phenomenon in neurons—Part II: On the necessary and sufficient conditions for the observation of the entire life cycle of an action potential. IEEE Trans. Circuit Syst. I, 2023; under review. [Google Scholar]





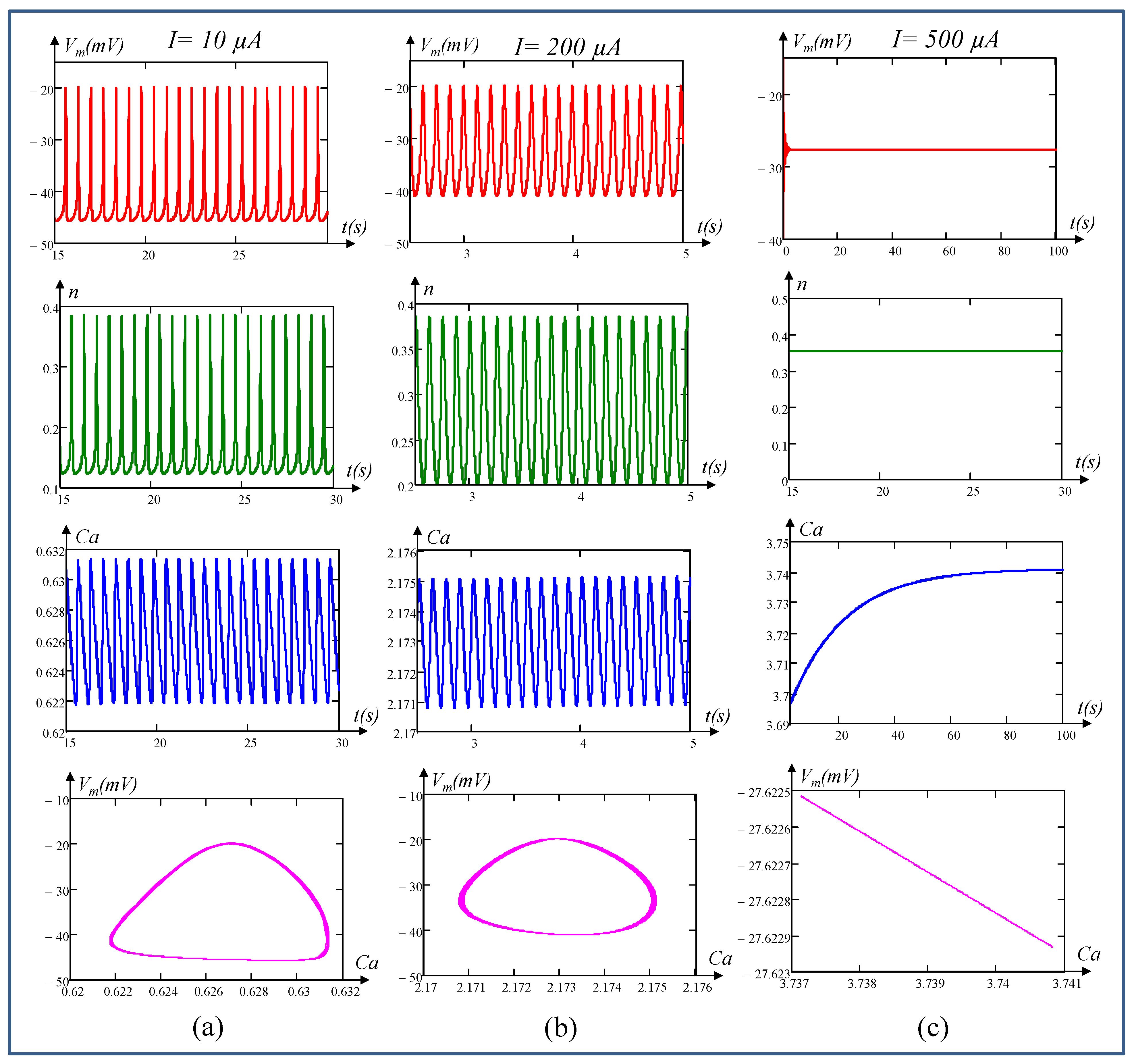
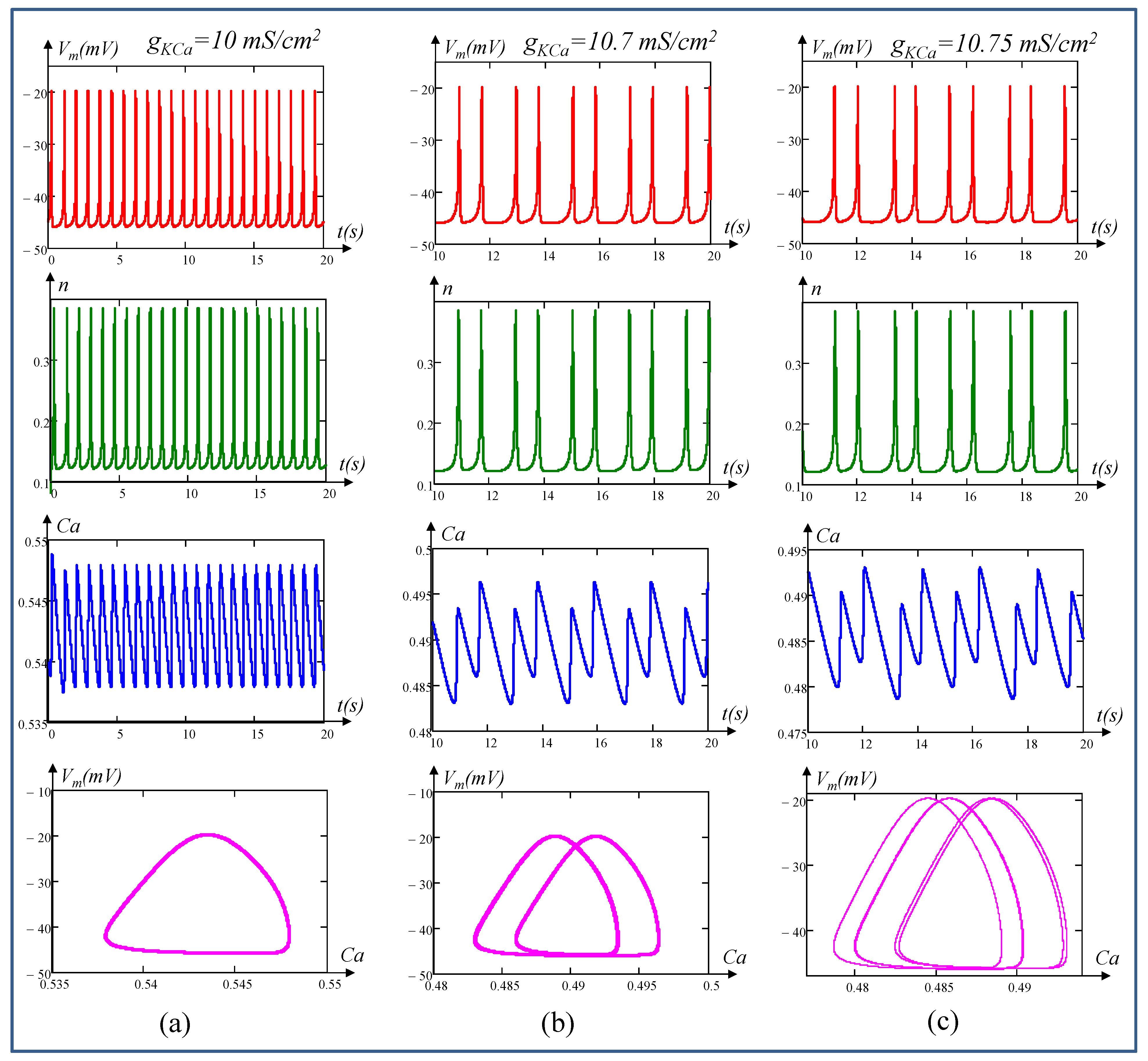
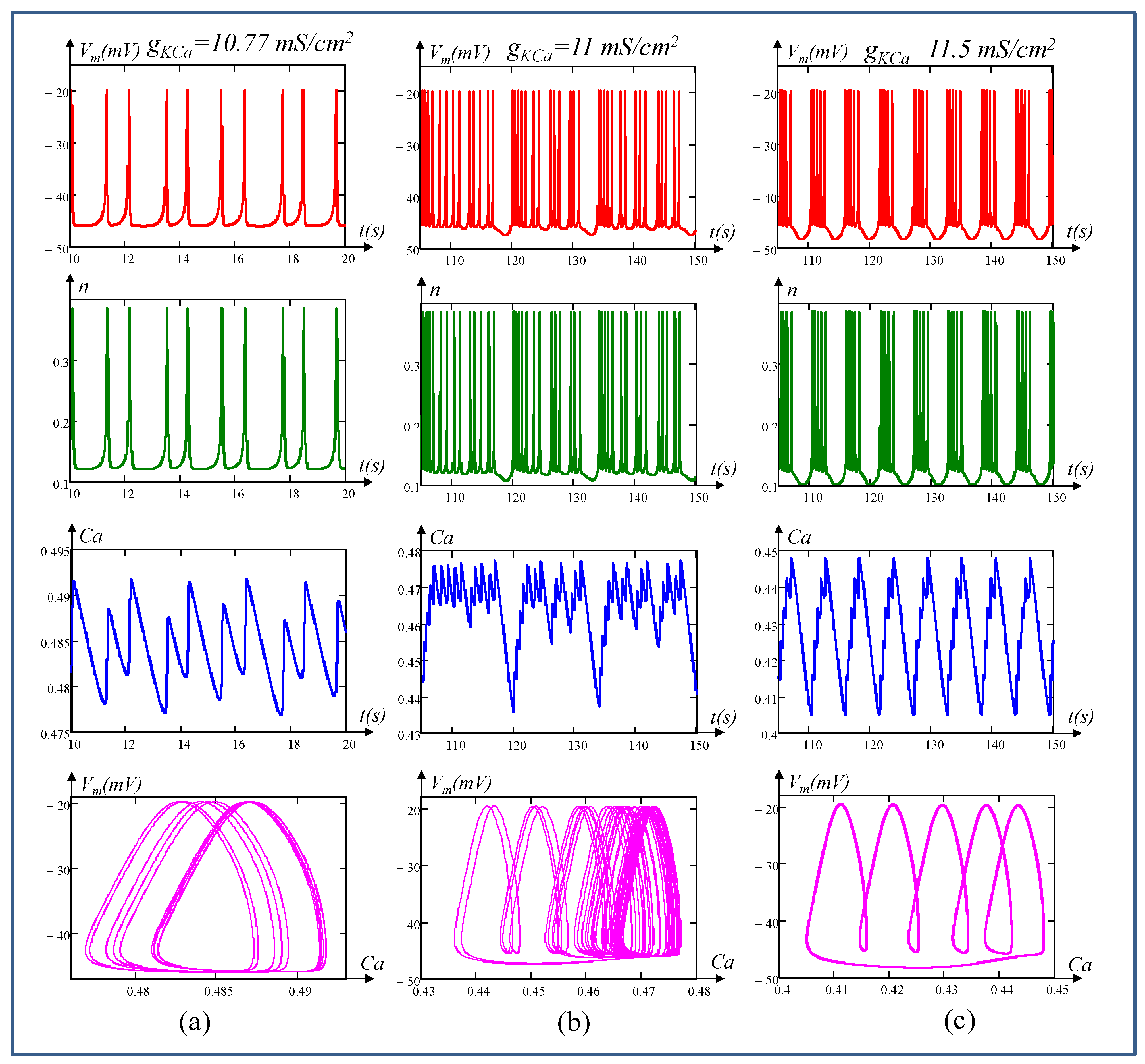
| Cm | 1 mF/cm2 | gK,V | 1700 mS/cm2 |
| EK | −75 mV | gI | 1800 mS/cm2 |
| EI | 100 mV | gL | 7 mS/cm2 |
| EL | −40 mV | gK,Ca | 10 mS/cm2 |
| ECa | 100 mV | Kca | 3.3/18 mV |
| λn | 230 | ρ | 0.27 |
| Models | Memristive Models | Strengths | Limitations |
|---|---|---|---|
| HH [1] | The potassium ion channel and sodium ion channel in the HH model are represented by generic memristors [3]. | It is a framework to understand the emergence of action potential propagation in neurons based on the experimental data of the squid giant axon. | It is difficult to generalize to all neurons. Incapable of producing complicated bursting patterns |
| FitzHugh–Nagumo [31] | It does not follow state-dependent Ohm’s law and cannot model with memristors. | Simplified model of neuronal excitation. | Not accurately represent all neuronal behaviors. Incapable of producing bursting |
| ML [8] | It was modeled that the state-independent (dependent) calcium ion channel acts as a nonlinear resistor (generic memristor) and state-dependent potassium ion channel acts as a generic memristor [9,10]. | It is initially presented a model for the barnacle muscle fiber, and later it was considered a popular and simplified representation of the neuron model. | Limited in capturing certain neuronal dynamics. Cannot produce bursting patterns. |
| Chay [30] | We are proposing a framework that the cells of excitable membranes can be modeled as the networks of memristors. | Novel model of excitable cells to capture multiple neuronal states, such as action potentials, periodic oscillations, aperiodic oscillations, spikes, and bursting patterns. | Limited validation in experimental contexts and a lack of details for some applications. |
| S.N | Vm (mV) | I (µA) | n | Ca | λ1 | λ2 | λ3 |
|---|---|---|---|---|---|---|---|
| 1. | −52.00 | −87.02 | 0.08 | 0.04 | −40.515 | −3.842 | −0.084 |
| 2. | −51.00 | −80.63 | 0.08 | 0.05 | −40.107 | −2.871 | −0.111 |
| 3. | −50.50 | −77.46 | 0.09 | 0.06 | −39.891 | −2.289 | −0.139 |
| 4. | −50.00 | −74.32 | 0.09 | 0.07 | 39.666 | −1.617 | −0.196 |
| 5. | −49.455 | −70.919 | 0.94 | 0.08 | −39.408 | −0.533 − 0.174i | −0.533 + 0.174i |
| 6. | −49.00 | −68.12 | 0.1 | 0.1 | −39.181 | −0.19 − 0.525i | −0.19 + 0.525i |
| 7. | −48.763 | −66.671 | 0.1 | 0.1 | −39.058 | 0 − 0.557i | 0 + 0.557i |
| 8. | −48.50 | −65.08 | 0.1 | 0.11 | −38.917 | 0.222 − 0.51i | 0.2215 + 0.5097i |
| 9. | −46.00 | −51.02 | 0.12 | 0.21 | −37.32 | 0.046 | 5.736 |
| 10. | −45.00 | −46.37 | 0.13 | 0.27 | −36.512 | 0.027 | 8.498 |
| 11. | −42.00 | −39.37 | 0.16 | 0.53 | −33.218 | 0.0001 | 18.604 |
| 12. | −40.00 | −42.78 | 0.18 | 0.79 | −29.899 | −0.0084 | 25.99 |
| 13. | −38.00 | −51.26 | 0.21 | 1.13 | −24.898 | −0.0112 | 31.992 |
| 14. | −32.00 | 17.59 | 0.29 | 2.57 | −0.061 | 11.669 − 38.01i | 11.669 + 38.01i |
| 15. | −30.00 | 160.68 | 0.32 | 3.12 | −0.053 | 8.049 − 61.778i | 8.049 + 61.778i |
| 16. | −28.00 | 430.84 | 0.35 | 3.65 | −0.051 | 0.08 − 85.421i | 0.08 + 85.421i |
| 17. | −27.984 | 433.594 | 0.35 | 3.65 | −0.051 | 0 − 85.606i | 0 + 85.606i |
| 18. | −27.00 | 628.91 | 0.36 | 3.89 | −5.556 − 97.197i | −5.556 + 97.197i | −0.051 |
| 19. | −25.50 | 1.02 × 103 | 0.39 | 4.22 | −15.942 − 114.607i | −15.942 + 114.607i | −0.0501 |
| 20. | −24.685 | 1.291 × 103 | 0.40 | 4.37 | −22.466 − 123.858i | −22.466 + 123.858i | −0.0499 |
| 21. | −23.00 | 1.99 × 103 | 0.43 | 4.64 | −37.643 − 142.384i | −37.643 + 142.384i | −0.0497 |
| 22. | −22.00 | 2.5 × 103 | 0.44 | 4.75 | −47.529 − 152.923i | −47.529 + 152.923i | −0.0496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, M.; Ascoli, A.; Tetzlaff, R.; Rajamani, V.; Budhathoki, R.K. Modeling Excitable Cells with Memristors. J. Low Power Electron. Appl. 2024, 14, 31. https://doi.org/10.3390/jlpea14020031
Sah M, Ascoli A, Tetzlaff R, Rajamani V, Budhathoki RK. Modeling Excitable Cells with Memristors. Journal of Low Power Electronics and Applications. 2024; 14(2):31. https://doi.org/10.3390/jlpea14020031
Chicago/Turabian StyleSah, Maheshwar, Alon Ascoli, Ronald Tetzlaff, Vetriveeran Rajamani, and Ram Kaji Budhathoki. 2024. "Modeling Excitable Cells with Memristors" Journal of Low Power Electronics and Applications 14, no. 2: 31. https://doi.org/10.3390/jlpea14020031
APA StyleSah, M., Ascoli, A., Tetzlaff, R., Rajamani, V., & Budhathoki, R. K. (2024). Modeling Excitable Cells with Memristors. Journal of Low Power Electronics and Applications, 14(2), 31. https://doi.org/10.3390/jlpea14020031







