Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. finitimus from Turkey
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Solvent Extraction
2.2. Total Flavonoid and Phenolic Contents
2.3. Liquid Chromatography–Electrospray Tandem Mass Spectrometry (LC–ESI–MS/MS) Analysis
2.4. Biological Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Composition
3.2. Antioxidant Properties
3.3. Inhibitory Effects on Amylase and Tyrosinase
3.4. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De la Parra, J.; Quave, C.L. Ethnophytotechnology: Harnessing the Power of Ethnobotany with Biotechnology. Trends Biotechnol. 2017, 35, 802–806. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, W.C.; Mahady, G.B.; Bennett, B.C.; Shiels, L.; Savo, V. Ethnobotany as a pharmacological research tool and recent developments in CNS-active natural products from ethnobotanical sources. Pharmacol. Ther. 2009, 123, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Popović, Z.; Matić, R.; Bojović, S.; Stefanović, M.; Vidaković, V. Ethnobotany and herbal medicine in modern complementary and alternative medicine: An overview of publications in the field of I&C medicine 2001–2013. J. Ethnopharmacol. 2016, 181, 182–192. [Google Scholar] [PubMed]
- Krungkrai, J.; Krungkrai, S.R. Antimalarial qinghaosu/artemisinin: The therapy worthy of a Nobel Prize. Asian Pac. J. Trop. Biomed. 2016, 6, 371–375. [Google Scholar] [CrossRef]
- Weathers, P.J.; Cambra, H.M.; Desrosiers, M.R.; Rassias, D.; Towler, M.J. Artemisinin the Nobel Molecule: From Plant to Patient. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elvesier: Amsterdam, The Netherlands, 2017; Volume 52, pp. 193–229. [Google Scholar]
- Güner, A.; Aslan, S.; Babaç, M.T.; Vural, M.; Ekim, T. Türkiye Bitkileri Listesi (Damarlı Bitkiler); Nezahat Gökyiğit Botanik Bahçesi: Istanbul, Turkey, 2012; pp. 1–1290. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the genus Astragalus: Phytochemistry and biological activity. Pharmacogn. Rev. 2016, 10, 11. [Google Scholar]
- Çeçen, Ö.; Aytac, Z.; Misirdali, H. Astragalus unalii (Fabaceae), a new species from Turkey. Turk. J. Bot. 2016, 40, 81–86. [Google Scholar] [CrossRef]
- Mükemre, M.; Behçet, L.; Çakılcıoğlu, U. Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey). J. Ethnopharmacol. 2015, 166, 361–374. [Google Scholar] [CrossRef]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Polat, R.; Cakilcioglu, U.; Satıl, F. Traditional uses of medicinal plants in Solhan (Bingöl—Turkey). J. Ethnopharmacol. 2013, 148, 951–963. [Google Scholar] [CrossRef]
- Tetik, F.; Civelek, S.; Cakilcioglu, U. Traditional uses of some medicinal plants in Malatya (Turkey). J. Ethnopharmacol. 2013, 146, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Sargin, S.A. Ethnobotanical survey of medicinal plants in Bozyazı district of Mersin, Turkey. J. Ethnopharmacol. 2015, 173, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, R.; Kirkan, B.; Sarikurkcu, C. Phenolic profile, antioxidant and enzyme inhibitory potential of methanolic extracts from different parts of Astragalus ponticus Pall. S. Afr. J. Bot. 2019, 120, 268–273. [Google Scholar] [CrossRef]
- Gülcemal, D.; Masullo, M.; Napolitano, A.; Karayıldırım, T.; Bedir, E.; Alankuş-Çalışkan, Ö.; Piacente, S. Oleanane glycosides from Astragalus tauricolus: Isolation and structural elucidation based on a preliminary liquid chromatography-electrospray ionization tandem mass spectrometry profiling. Phytochemistry 2013, 86, 184–194. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wu, K.-X.; Guo, X.-R.; Tang, Z.-H. A rapid method for sensitive profiling of bioactive triterpene and flavonoid from Astragalus mongholicus and Astragalus membranaceus by ultra-pressure liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2018, 1085, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Nalbantsoy, A.; Nesil, T.; Yılmaz-Dilsiz, Ö.; Aksu, G.; Khan, S.; Bedir, E. Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J. Ethnopharmacol. 2012, 139, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gür, C.; Onbaşılar, İ.; Atilla, P.; Genç, R.; Çakar, N.; Deliloğlu-Gürhan, İ.; Bedir, E. In vitro growth stimulatory and in vivo wound healing studies on cycloartane-type saponins of Astragalus genus. J. Ethnopharmacol. 2011, 134, 844–850. [Google Scholar] [CrossRef]
- Yesilada, E.; Bedir, E.; Çalış, İ.; Takaishi, Y.; Ohmoto, Y. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J. Ethnopharmacol. 2005, 96, 71–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.; Xia, Q.; Qi, J.; Cao, M. Pharmacological mechanism of Astragalus and Angelica in the treatment of idiopathic pulmonary fibrosis based on network pharmacology. Eur. J. Integr. Med. 2019, 32, 101003. [Google Scholar] [CrossRef]
- Adigüzel, A.; Soekmen, M.; Oezkan, H.; Ağar, G.; Guelluece, M.; Şahin, F. In vitro antimicrobial and antioxidant activities of methanol and hexane extract of Astragalus species growing in the eastern Anatolia region of Turkey. Turk. J. Biol. 2009, 33, 65–71. [Google Scholar]
- Gulluce, M.; Agar, G.; Baris, O.; Karadayi, M.; Orhan, F.; Sahin, F. Mutagenic and antimutagenic effects of hexane extract of some Astragalus species grown in the eastern Anatolia region of Turkey. Phytother. Res. 2010, 24, 1014–1018. [Google Scholar] [PubMed]
- Sokmen, M.; Gulluce, M.; Agar, G.; Sengul, M.; Sahin, F.; Baris, O. Antioxidant activities of methanol extract of some astragalus species wildly growing in Erzurum. Acta Hortic. 2009, 59. [Google Scholar] [CrossRef]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, Scotland, 1970; Volume 3. [Google Scholar]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm.: A source of multifunctional agents for the management of oxidative damage, Alzheimer’s and diabetes mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Cittan, M.; Çelik, A. Development and validation of an analytical methodology based on liquid chromatography–electrospray tandem mass spectrometry for the simultaneous determination of phenolic compounds in Olive leaf extract. J. Chromatogr. Sci. 2018, 56, 336–343. [Google Scholar] [CrossRef]
- Odabas Kose, E.; Aktaş, O.; Deniz, I.G.; Sarikürkçü, C. Chemical composition, antimicrobial and antioxidant activity of essential oil of endemic Ferula lycia Boiss. J. Med. Plants Res. 2010, 4, 1698–1703. [Google Scholar]
- Zengin, G.; Sarikurkcu, C.; Uyar, P.; Aktumsek, A.; Uysal, S.; Kocak, M.S.; Ceylan, R. Crepis foetida L. subsp rhoeadifolia (Bleb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçaǧ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Kocak, M.S.; Sarikurkcu, C.; Cengiz, M.; Kocak, S.; Uren, M.C.; Tepe, B. Salvia cadmica: Phenolic composition and biological activity. Ind. Crop. Prod. 2016, 85, 204–212. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Tepe, B.; Sarikurkcu, C.; Berk, S.; Alim, A.; Akpulat, H.A. Chemical composition, radical scavenging and antimicrobial activity of the essential oils of Thymus boveii and Thymus hyemalis. Rec. Nat. Prod. 2011, 5, 208–220. [Google Scholar]
- Zengin, G.; Sarıkürkçü, C.; Aktümsek, A.; Ceylan, R. Antioxidant potential and inhibition of key enzymes linked to Alzheimer’s diseases and diabetes mellitus by monoterpene-rich essential oil from Sideritis galatica Bornm. Endemic to Turkey. Rec. Nat. Prod. 2015, 10, 195–206. [Google Scholar]
- Sun, T.; Tanumihardjo, S. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Prosekov, A.; Zaushintsena, A.; Sukhikh, A.; Dyshlyuk, L.; Ivanova, S. Identification and quantification of phenolic compounds of Western Siberia Astragalus danicus in different regions. Heliyon 2019, 5, e02245. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, E.; Wei, Z.; Zheng, Y.; Yan, R.; Ma, X. Phytochemical analysis, cellular antioxidant and α-glucosidase inhibitory activities of various herb plant organs. Ind. Crops Prod. 2019, 141, 111771. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.-W.; Wang, H.-Q.; Yu, J.-Q.; Duan, J.-A. Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC-MS/MS. J. Pharm. Anal. 2019, 9, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, S.; Ebadi, M.-T.; Mollaei, S.; Khurizadeh, S. Evaluation of volatile and phenolic compounds, and antioxidant activity of different parts of Ferulago angulata (schlecht.) Boiss. Ind. Crops Prod. 2019, 140, 111589. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Merlin, N.; Karling, M.; Carpes, S.T.; Alencar, S.M.d.; Morales, R.G.F.; Silva, E.A.d.; Pilau, E.J. Bioguided extraction of phenolic compounds and UHPLC-ESI-Q-TOF-MS/MS characterization of extracts of Moringa oleifera leaves collected in Brazil. Food Res. Int. 2019, 125, 108647. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Li, W.; Ndolo, V.U.; Beta, T. A comparative study of the phenolic compounds and in vitro antioxidant capacity of finger millets from different growing regions in Malawi. J. Cereal Sci. 2019, 87, 143–149. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H. Antioxidant activity evaluation of dietary flavonoid hyperoside using saccharomyces cerevisiae as a model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shen, C.-Y.; Jiang, J.-G. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: Pharmacological mechanisms and prospects for drug discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ying, M.-D.; Wu, Y.-P.; Zhou, Z.-H.; Ye, Z.-M.; Li, H.; Lin, D.-S. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Pistollato, F.; Battino, M. Role of plant-based diets in the prevention and regression of metabolic syndrome and neurodegenerative diseases. Trends Food Sci. Technol. 2014, 40, 62–81. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Ceylan, R.; Guler, G.O.; Carradori, S.; Uysal, S.; Aktumsek, A. Enzyme inhibitory effect and antioxidant properties of Astragalus lagurus extracts. Curr. Enzym. Inhib. 2016, 12, 177–182. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Shkondrov, A.; Simeonova, R.; Vitcheva, V.; Krasteva, I.; Ionkova, I. In vitro/in vivo antioxidant and hepatoprotective potential of defatted extract and flavonoids isolated from Astragalus spruneri Boiss. (Fabaceae). Food Chem. Toxicol. 2018, 111, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Z.; Tan, L.; Jin, C.-G.; Lu, J.; Tian, L.; Chang, Q.-Q.; Wang, K. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crops Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, X.; Li, P.; Zhang, X.; Wu, Z.; Li, D. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Laličić-Petronijević, J.; Komes, D.; Gorjanović, S.; Belščak-Cvitanović, A.; Pezo, L.; Pastor, F.; Ostojić, S.; Popov-Raljić, J.; Sužnjević, D. Content of total phenolics, flavan-3-ols and proanthocyanidins, oxidative stability and antioxidant capacity of chocolate during storage. Food Technol. Biotechnol. 2016, 54, 13–20. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive microconstituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem.Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Rauf, A.; Jehan, N. Natural products as a potential enzyme inhibitors from medicinal plants. In Enzyme Inhibitors and Activators; InTech: Rijeka, Croatia, 2017; pp. 165–177. [Google Scholar]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015, 41, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Jung, W.-S.; Jung, H.-K.; Lee, G.-H.; Cho, J.-H.; Cho, H.-W.; Choi, I.-Y. The mixture of different parts of Nelumbo nucifera and two bioactive components inhibited tyrosinase activity and melanogenesis. J. Cosmet. Sci. 2014, 65, 377–388. [Google Scholar]
- Liao, L.; Chen, J.; Liu, L.; Xiao, A. Screening and binding analysis of flavonoids with alpha-amylase inhibitory activity from lotus leaf. J. Braz. Chem. Soc. 2018, 29, 587–593. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT-Food Sci. Technol. 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, M.-M.; Sun, Y.; Wang, L.-F.; Wang, H.; Zhang, Y.-J.; Li, H.-Y.; Zhuang, P.-W.; Yang, Z. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (−)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 23, 979–988. [Google Scholar] [CrossRef]
- Maruyama, H.; Kawakami, F.; Lwin, T.-T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.D.J.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J. Cancer Prev. 2016, 21, 41. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Q.; Yang, X.; Yan, H.B.; Lu, Q.P. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol. Med. Rep. 2016, 13, 4613–4619. [Google Scholar] [CrossRef]
- Bystrická, J.; Vollmannová, A.; Margitanová, E. Dynamics of polyphenolics formation in different plant parts and different growth phases of selected buckwheat cultivars. Acta Agric. Slov. 2010, 95, 225. [Google Scholar] [CrossRef]
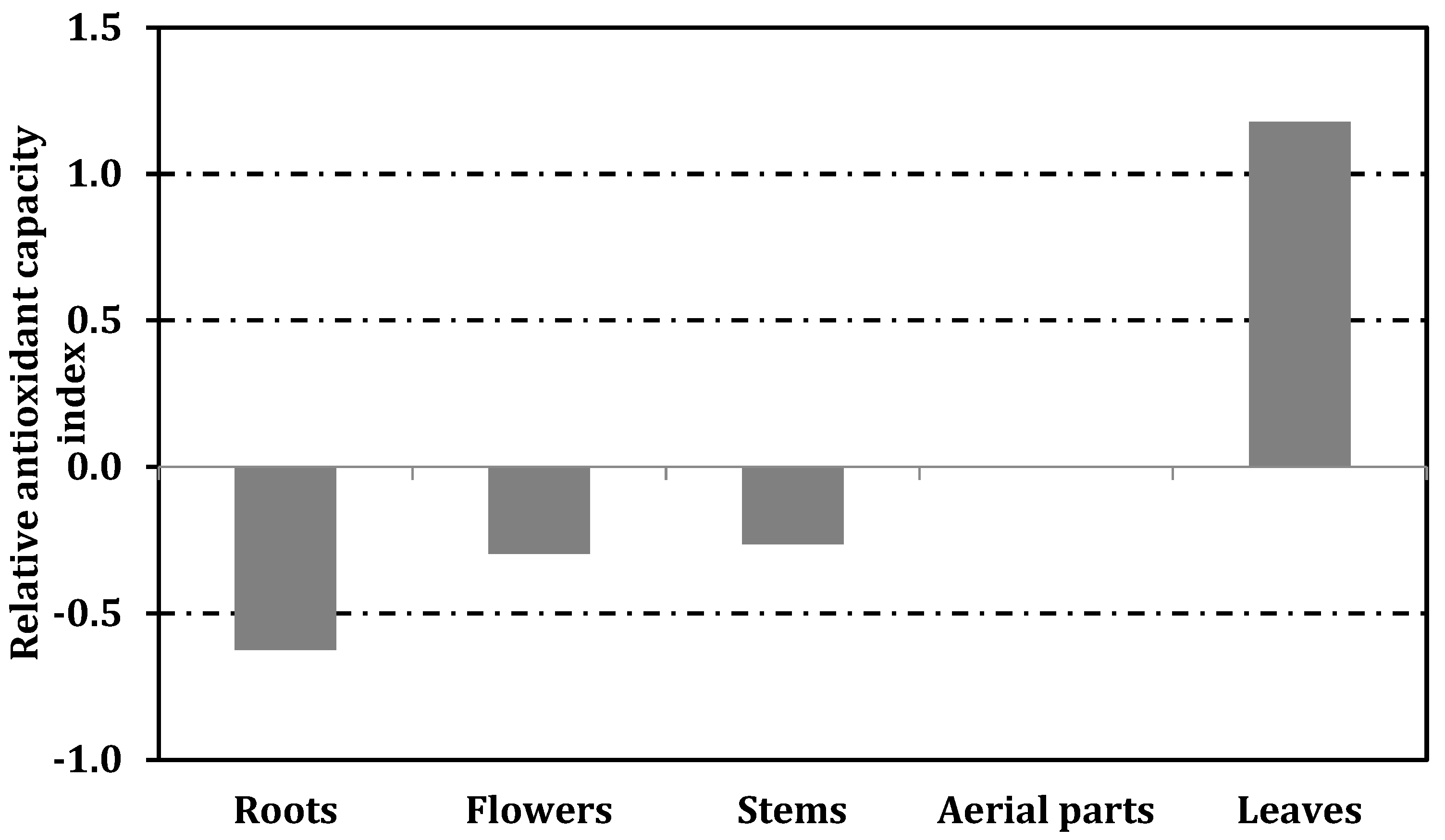
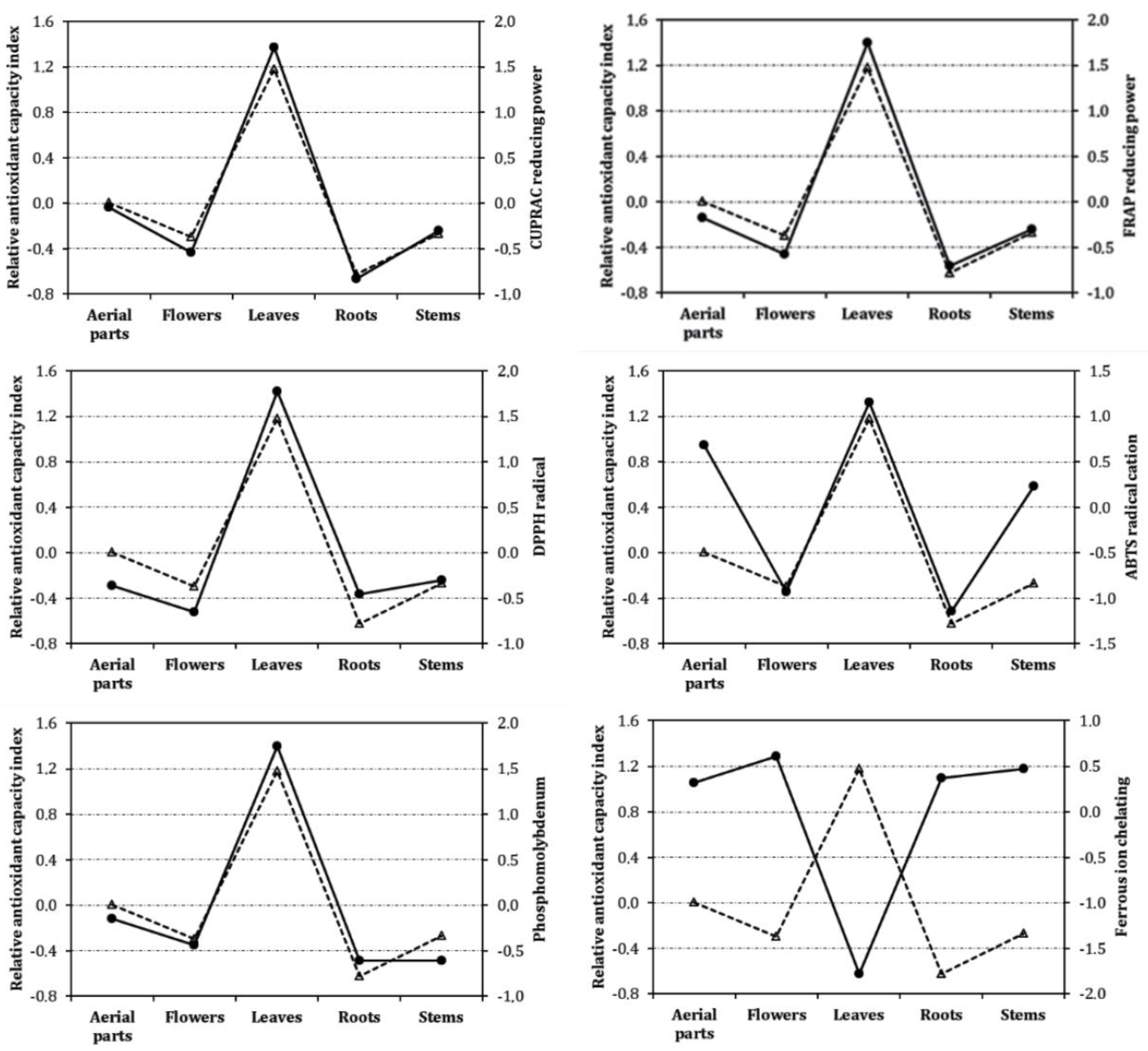
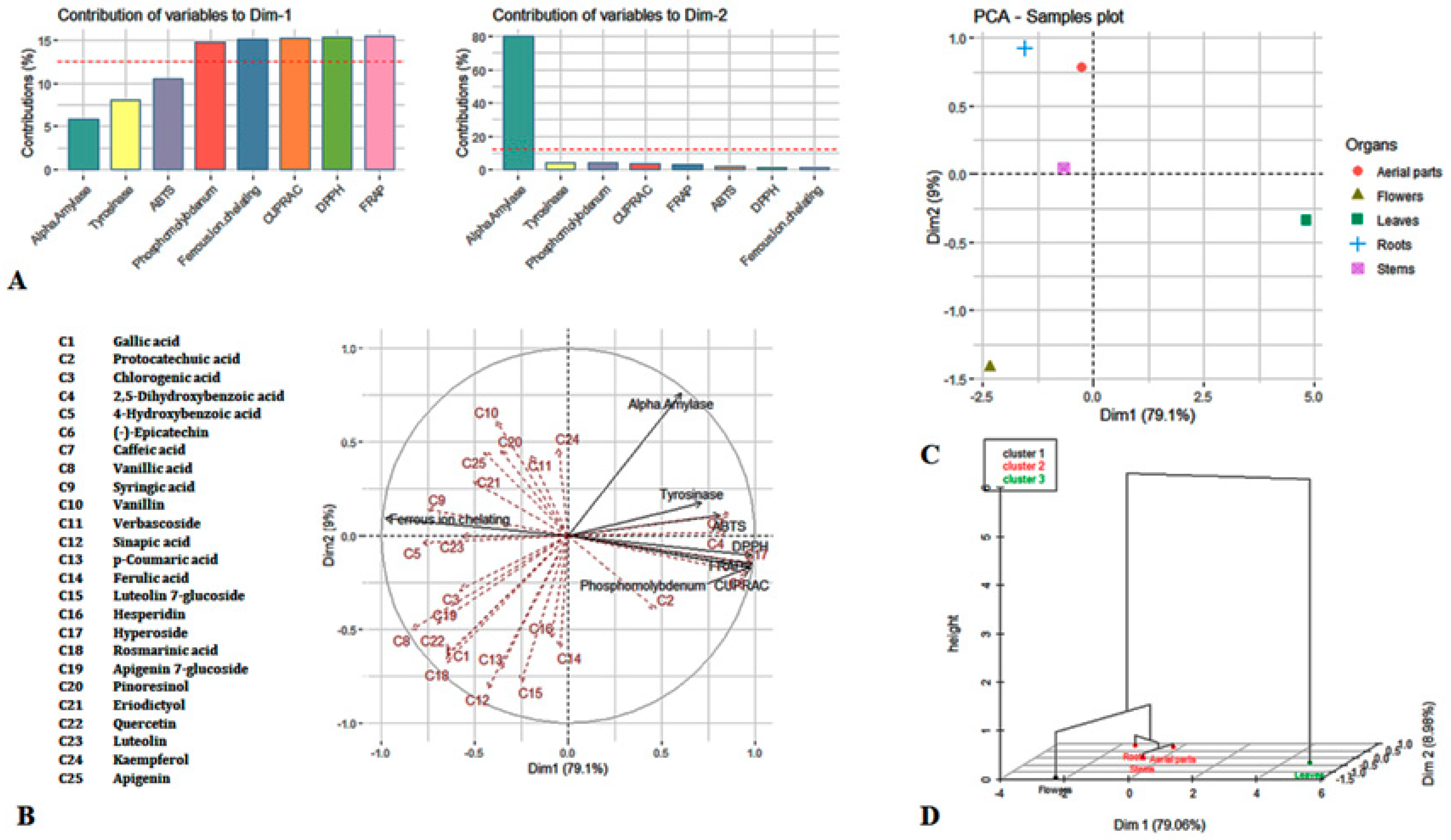
| Samples | Yield (%) | Total Flavonoids (Mg QE/g Extract) | Total Phenolics (Mg GAE/g Extract) |
|---|---|---|---|
| Aerial parts | 10.56 | 21.06 ± 0.11 c | 10.01 ± 0.17 b |
| Flowers | 6.95 | 29.90 ± 0.95 b | 6.96 ± 1.08 bc |
| Leaves | 3.46 | 39.23 ± 1.64 a | 37.68 ± 0.74 a |
| Roots | 17.83 | 6.03 ± 0.05 d | 5.60 ± 0.06 c |
| Stems | 11.78 | 7.91 ± 0.37 d | 8.29 ± 1.13 bc |
| Compounds | Aerial Parts | Flowers | Leaves | Roots | Stems |
|---|---|---|---|---|---|
| Gallic acid | 2.28 ± 0.03 d | 8.76 ± 0.18 a | 2.24 ± 0.01 d | 4.93 ± 0.12 b | 3.03 ± 0.04 c |
| Protocatechuic acid | 14.37 ± 0.08 c | 16.80 ± 0.47 b | 18.90 ± 0.16 a | 13.51 ± 0.64 c | 8.06 ± 0.05 d |
| Chlorogenic acid | 8.99 ± 0.24 a | 10.29 ± 4.02 a | 0.75 ± 0.26 b | 3.06 ± 0.69 ab | 1.59 ± 0.19 b |
| 2,5-Dihydroxybenzoic acid | 9.96 ± 1.74 c | nd | 23.14 ± 0.06 a | nd | 16.86 ± 1.09 b |
| 4-Hydroxybenzoic acid | 12.78 ± 0.16 c | 28.83 ± 0.62 b | 2.72 ± 0.07 e | 31.86 ± 0.76 a | 8.13 ± 0.28 d |
| (−)-Epicatechin | nd | nd | 5.60 ± 0.11 | nd | nd |
| Caffeic acid | 2.77 ± 0.11 b | nd | 3.94 ± 0.03 a | nd | nd |
| Vanillic acid | 23.30 ± 0.23 c | 100.53 ± 8.83 a | 4.36 ± 0.26 d | 57.78 ± 4.27 b | 51.53 ± 3.73 b |
| Syringic acid | 6.71 ± 0.03 d | 20.89 ± 0.71 b | 2.08 ± 0.06 e | 33.47 ± 1 a | 17.27 ± 1.52 c |
| Vanillin | 3.42 ± 0.11 c | nd | nd | 55.68 ± 0.68 a | 23.62 ± 0.13 b |
| Verbascoside | 43.48 ± 0.25 a | 8.68 ± 0.30 b | 1.13 ± 0.08 e | 7.29 ± 0.14 c | 2.64 ± 0.11 d |
| Sinapic acid | 7.41 ± 0.01 b | 52.66 ± 1.36 a | 3.41 ± 0.52 c | nd | nd |
| p-Coumaric acid | 76.85 ± 0.47 b | 146.78 ± 1.89 a | 33.12 ± 0.35 c | 6.81 ± 0.60 e | 22.12 ± 0.51 d |
| Ferulic acid | 52.79 ± 2.88 b | 64.20 ± 2.45 a | 37.61 ± 0.30 c | 10.53 ± 0.48 d | 17.40 ± 0.96 d |
| Luteolin 7-glucoside | 17.63 ± 0.89 ab | 28.59 ± 9.16 a | 13.45 ± 0.17 ab | 6.20 ± 0.75 b | 11.35 ± 0.16 b |
| Hesperidin | 7.69 ± 0.04 ab | 8.44 ± 1.45 a | 7.05 ± 0.03 ab | 4.99 ± 0.06 b | 9.14 ± 0.75 a |
| Hyperoside | 401.68 ± 6.72 b | 90.45 ± 1.39 d | 1828.94 ± 21 a | 2.90 ± 0.46 e | 321.43 ± 7.64 c |
| Rosmarinic acid | 2.10 ± 0.11 c | 26.95 ± 3.30 a | 1.25 ± 0.02 c | 10.46 ± 0.65 b | 5.43 ± 0.27 bc |
| Apigenin 7-glucoside | 23.68 ± 0.08 c | 63.56 ± 1.94 a | 16.85 ± 0.19 d | 44.54 ± 0.35 b | 20.24 ± 0.04 cd |
| Pinoresinol | nd | nd | nd | 6.40 ± 0.37 a | 6.80 ± 0.23 a |
| Eriodictyol | 0.44 ± 0.05 c | 2.58 ± 0.36 b | 0.30 ± 0.01 c | 6.97 ± 0.12 a | 0.73 ± 0.01 c |
| Quercetin | 6.47 ± 0.02 b | 11.11 ± 0.04 a | 3.27 ± 0.06 d | 4.58 ± 0.13 c | 4.74 ± 0.12 c |
| Luteolin | 32.06 ± 0.45 cd | 81.94 ± 0.28 b | 32.58 ± 1.07 c | 108.11 ± 1.20 a | 28.77 ± 0.73 d |
| Kaempferol | 1.58 ± 0.25 | nd | nd | nd | nd |
| Apigenin | 29.07 ± 0.50 c | 52.33 ± 1.34 b | 23 ±0.12 c | 181.90 ± 6.23 a | 42.81 ± 0.52 b |
| Assays | Aerial Parts | Flowers | Leaves | Roots | Stems | Trolox | EDTA |
|---|---|---|---|---|---|---|---|
| Effective concentration (EC50: mg/mL) | |||||||
| Phosphomolybdenum | 2.52 ± 0.23 c | 2.67 ± 0.08c | 1.81 ± 0.12 b | 2.77 ± 0.03 c | 2.77 ± 0.15 c | 1.14 ± 0.02 a | - |
| DPPH radical | 9.30 ± 0.20 c | 14.20 ± 0.12e | 2.65 ± 0.05 b | 10.54 ± 0.52 d | 8.73 ± 0.22 c | 0.26 ± 0.02 a | - |
| ABTS radical cation | 1.66 ± 0.01 b | 3.35 ± 0.01d | 1.45 ± 0.04 b | 3.88 ± 0.11 e | 1.93 ± 0.07 c | 0.25 ± 0.02 a | - |
| CUPRAC reducing power | 2.37 ± 0.06 c | 3.62 ± 0.16d | 1.06 ± 0.02 b | 5.28 ± 0.39 e | 2.90 ± 0.12 cd | 0.32 ± 0.03 a | - |
| FRAP reducing power | 1.75 ± 0.02 c | 2.46 ± 0.05d | 0.73 ± 0.01 b | 2.83 ± 0.12 e | 1.93 ± 0.08 c | 0.12 ± 0.02a | - |
| Ferrous ion chelating | 1.08 ± 0.01 b | 1.03 ± 0.01b | 1.65 ± 0.05 c | 1.07 ± 0.01 b | 1.05 ± 0.03 b | - | 0.036 ± 0.004 a |
| Antioxidant activity | |||||||
| Phosphomolybdenum (mmol TEs/g extract) | 1.85 ± 0.17 b | 1.73 ± 0.05b | 2.56 ± 0.17 a | 1.67 ± 0.02 b | 1.67 ± 0.09 b | - | - |
| DPPH radical (mg TEs/g extract) | 24.97 ± 0.61 bc | 15.42 ± 0.15d | 94.66 ± 1.97 a | 21.75 ± 1.21 c | 26.80 ± 0.76 b | - | - |
| ABTS radical cation (mg TEs/g extract) | 161.59 ± 0.86 b | 78.94 ± 0.01d | 185.65 ± 5.03 a | 67.98 ± 2.01 d | 138.54 ± 4.74 c | - | - |
| CUPRAC reducing power (mg TEs/g extract) | 126.91 ± 3.50 b | 78.76 ± 3.96d | 297.74 ± 4.90 a | 50.23 ± 4.64 e | 101.35 ± 4.66 c | - | - |
| FRAP reducing power (mg TEs/g extract) | 59.81 ± 0.67 b | 42.59 ± 0.89c | 142.69 ± 2.15 a | 37.05 ± 1.63 c | 54.37 ± 2.15 b | - | - |
| Ferrous ion chelating (mg EDTAEs/g extract) | 66.81 ± 0.36 a | 70.11 ± 0.51a | 43.41 ± 1.39 b | 67.45 ± 0.18 a | 68.59 ± 2.00 a | - | - |
| Assays and Compounds | Phosphomolybdenum | DPPH | ABTS | CUPRAC | FRAP | Ferrous Ion Chelating | Tyrosinase | α-Amylase |
|---|---|---|---|---|---|---|---|---|
| DPPH | 0.974 y | |||||||
| ABTS | 0.717 | 0.712 | ||||||
| CUPRAC | 0.980 y | 0.968 y | 0.828 | |||||
| FRAP | 0.985 y | 0.987 y | 0.789 | 0.995 y | ||||
| Ferrous ion chelating | −0.983 y | −0.995 y | −0.678 | −0.960 y | −0.980 y | |||
| Tyrosinase | 0.549 | 0.723 | 0.512 | 0.606 | 0.655 | −0.671 | ||
| α-Amylase | 0.497 | 0.525 | 0.528 | 0.477 | 0.491 | −0.552 | 0.439 | |
| Total flavonoid | 0.792 | 0.657 | 0.471 | 0.754 | 0.725 | −0.682 | 0.009 | 0.012 |
| Total phenolic | 0.992 y | 0.992 y | 0.727 | 0.985 y | 0.995 y | −0.991 y | 0.642 | 0.478 |
| Hyperoside | 0.982 y | 0.987 y | 0.794 | 0.995 y | 0.999 y | −0.979 y | 0.662 | 0.494 |
| Assays | Aerial Parts | Flowers | Leaves | Roots | Stems | Kojic Acid | Acarbose |
|---|---|---|---|---|---|---|---|
| Inhibition concentration (IC50: mg/mL) | |||||||
| Tyrosinase inhibition | 1.33 ± 0.10 cd | 1.41 ± 0.05 d | 1.02 ± 0.02 b | 1.18 ± 0.01 bc | 1.07 ± 0.03 b | 0.36 ± 0.04 a | - |
| α-Amylase inhibition | 3.40 ± 0.02 b | 4.94 ± 0.15 d | 3.36 ± 0.18 b | 3.50 ± 0.03 b | 4.12 ± 0.22 c | - | 1.24 ± 0.06 a |
| Enzyme inhibitory activities | |||||||
| Tyrosinase inhibition (mg KAEs/g extracts) | 270 ± 20 cd | 255 ± 8 d | 352 ± 7 a | 304 ± 3 bc | 336 ± 9 ab | - | - |
| α-Amylase inhibition (mg ACEs/g extracts) | 357 ± 2 a | 245 ± 8 c | 362 ± 20 a | 347 ± 3 a | 294 ± 16 b | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarikurkcu, C.; Zengin, G. Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. finitimus from Turkey. Biology 2020, 9, 231. https://doi.org/10.3390/biology9080231
Sarikurkcu C, Zengin G. Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. finitimus from Turkey. Biology. 2020; 9(8):231. https://doi.org/10.3390/biology9080231
Chicago/Turabian StyleSarikurkcu, Cengiz, and Gokhan Zengin. 2020. "Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. finitimus from Turkey" Biology 9, no. 8: 231. https://doi.org/10.3390/biology9080231
APA StyleSarikurkcu, C., & Zengin, G. (2020). Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. finitimus from Turkey. Biology, 9(8), 231. https://doi.org/10.3390/biology9080231






