Properties of Arabinogalactan Proteins (AGPs) in Apple (Malus × Domestica) Fruit at Different Stages of Ripening
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Isolation of AGPs from Fruit
2.3. Morphology Imaging of AGPs
2.4. Quantification of Monosaccharide Compositions of AGPs
2.5. Quantification of Uronic Acids in AGPs
2.6. Quantification of Protein Components in AGPs
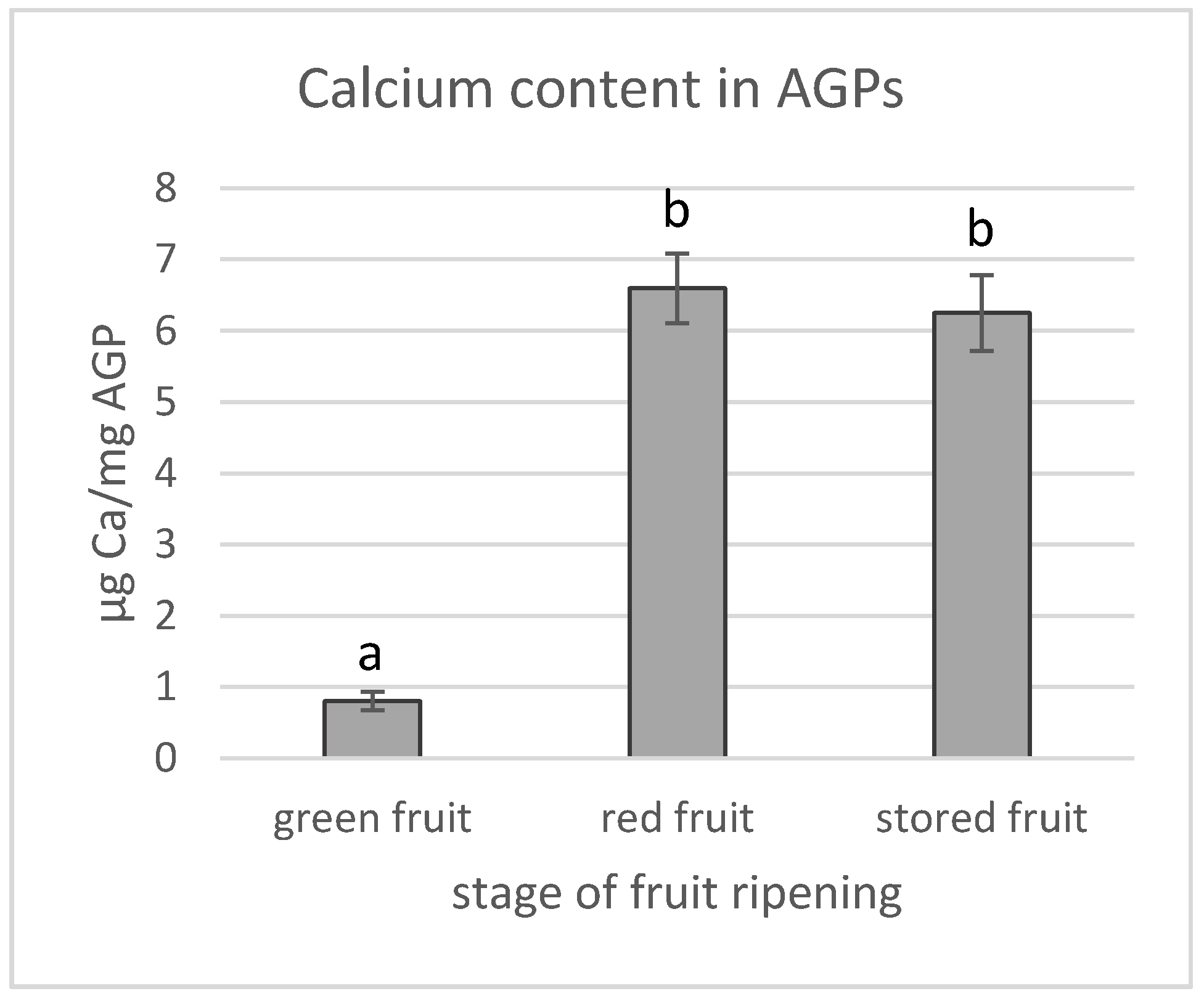
2.7. Quantification of Calcium Content in AGPs
2.8. X-ray Elemental Analysis of Calcium Ions in Apple Fruit at Tissue Level—Energy Dispersive X-ray Spectroscopy (EDS)-SEM
2.9. Analysis of Results
3. Results
3.1. Structure of AGPs
3.2. Calcium Content in AGPs and Calcium Distribution in Fruit during the Ripening Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. CMLS 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- Showalter, A.M.; Basu, D. Extensin and arabinogalactan-protein biosynthesis: Glycosyltransferases, research challenges, and biosensors. Front. Plant Sci. 2016, 7, 814. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, C.; Li, H.; Wu, W.; Liu, W.; Wang, Y.; Chen, Q.; Ma, H. Bioinformatic prediction and evolution analysis of arabinogalactan proteins in the plant kingdom. Front. Plant Sci. 2017, 8, 66. [Google Scholar] [CrossRef]
- Classen, B. Characterization of an arabinogalactan-protein from suspension culture of Echinacea purpurea. Plant Cell Tissue Organ Cult. 2007, 88, 267–275. [Google Scholar] [CrossRef]
- Volk, R.B.; Blascheck, W.; Classen, B. Characterization of an arabinogalactan protein from the pressed juice of Echinacea purpurea: Investigations into the type of linkage between the protein and polysaccharide moieties. J. Nat. Med. 2007, 61, 397–401. [Google Scholar] [CrossRef]
- Qin, X.; Yamauchi, R.; Aizawa, K.; Inakuma, T.; Kato, K. Isolation and characterization of arabinogalactan-protein from fruit of Lycium chinense Mill. J. Appl. Glycosci. 2000, 47, 155–161. [Google Scholar] [CrossRef]
- Brillouet, J.M.; Williams, P.; Will, F.; Müller, G.; Pellerin, P. Structural characterization of an apple fruits arabinogalactan-protein which aggregates following enzymic dearabinosylation. Carbohydr. Polym. 1996, 29, 271–275. [Google Scholar] [CrossRef]
- Tsumuraya, Y.; Ozeki, E.; Ooki, Y.; Yoshimi, Y.; Hashizume, K.; Kotake, T. Properties of arabinogalactan-proteins in European pear (Pyrus communis L.) fruit. Carbohydr. Res. 2019, 485, 107816. [Google Scholar] [CrossRef]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Ann. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef]
- Hijazi, M.; Roujol, D.; Nguyen-Kim, H.; Del Rocio Cisneros Castillo, L.; Saland, E.; Jamet, E.; Albenne, C. Arabinogalactan protein 31 (AGP31), a putative network-forming protein in Arabidopsis thaliana cell walls. Ann. Bot. 2014, 114, 1087–1097. [Google Scholar] [CrossRef]
- Hijazi, M.; Velasquez, S.M.; Jamet, E.; Estevez, J.M.; Albenne, C. An update on post-translational modifications of hydroxyproline-rich glycoproteins: Toward a model highlighting their contribution to plant cell wall architecture. Front. Plant Sci. 2014, 5, 395. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Šamaj, J.; Wojtaszek, P.; Volkmann, D.; Menzel, D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol. 2003, 133, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Persson, S.; Sánchez-Rodríguez, C. At the border: The plasma membrane–cell wall continuum. J. Exp. Bot. 2015, 66, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A.; Várnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Várnai, P.; Seal, C.E. Back to the future with the AGP-Ca2+ flux capacitor. Ann. Bot. 2014, 114, 1069–1085. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Amarante, C.V.T.; Labavitch, J.M.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 7, 6–13. [Google Scholar] [CrossRef]
- Lamport, D.T.A. Preparation of arabinogalactan glycoproteins from plant tissue. Bio-Protocol 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Kitazawa, K.; Tryfona, T.; Yoshimi, Y.; Hayashi, Y.; Kawauchi, S.; Antonov, L.; Tanaka, H.; Takahashi, T.; Kaneko, S.; Dupree, P.; et al. β-Galactosyl Yariv reagent binds to the β-1,3-galactan of arabinogalactan proteins. Plant Physiol. 2013, 161, 1117–1126. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Zdunek, A. Rheological and chemical properties of pectin enriched fractions from different sources extracted with citric acid. Carbohydr. Polym. 2017, 156, 443–451. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Chen, S.; Zhang, Z.; Zhao, Y.; Jin, Y.; Li, M.; Zhu, Y.; Liu, Y.; Yang, Y.; et al. A comparative study on Ca content and distribution in two Gesneriaceae species reveals distinctive mechanism to cope with high rhizospheric soluble calcium. Front. Plant Sci. 2014, 5, 647. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Baldwin, T.C.; McCann, M.; Roberts, K. A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota. Plant Physiol. 1993, 103, 115–123. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Wang, H.; Wu, H. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 1995, 82, 383–393. [Google Scholar] [CrossRef]
- Zhou, L.H.; Weizbauer, R.A.; Singamaneni, S.; Xu, F.; Genin, G.M.; Pickard, B.G. Structures formed by a cell membrane-associated arabinogalactan-protein on graphite or mica alone with Yariv phenylglycosides. Ann. Bot. 2014, 114, 1385–1397. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.; Wang, Y.; Tan, L.; Sun, L.; Petrosino, J.; Cui, M.Z.; Hao, F.; Zhang, M. Nanospherical arabinogalactan proteins are a key component of the high-strength adhesive secreted by English ivy. PNAS 2016, 113, E3193–E3202. [Google Scholar] [CrossRef] [PubMed]
- Renard, D.; Garnier, C.; Lapp, A.; Schmitt, C.; Sanchez, C. Structure of arabinogalactan-protein from Acacia gum: From porus ellipsoids to supramolecular architectures. Carbohydr. Polym. 2012, 90, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Palacio-Lόpez, K.; Tinaz, B.; Holzinger, A.; Domozych, D.Z. Arabinogalactan proteins and the extracellular matrix of Charophytes: A sticky business. Front. Plant Sci. 2019, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Perrakis, A.; Bita, C.E.; Arhondakis, S.; Krokida, A.; Mekkaoui, K.; Denic, D.; Blazakis, K.N.; Kaloudas, D.; Kalaitzis, P. Suppression of a prolyl 4 hydroxylase results in delayed abscission of overripe tomato fruits. Front. Plant Sci. 2019, 10, 348. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Dandachi, F.; Kalaitzis, P. Expression of arabinogalactan proteins during tomato fruit ripening and in response to mechanical wounding, hypoxia and anoxia. Plant Physiol. Biochem. 2012, 52, 112–118. [Google Scholar] [CrossRef]
- Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Effect of Ca2+ and cellular structure on apple firmness and acustic emission. Eur. Food Res. Technol. 2012, 235, 119–128. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Padda, M.; Wu, Q.; Park, S.; Mitcham, E.J. Dynamic Alternations in Cellular and Molecular Components during Blossom-End Rot Development in Tomatoes Expressing sCAX1, a Constitutively Active Ca2+/H+ Antiporter from Arabidopsis. Plant Physiol. 2011, 156, 844–855. [Google Scholar] [CrossRef]
- Ngouémazong, D.E.; Tengweh, F.F.; Fraeye, I.; Duvetter, T.; Cardinaels, R.; Van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of de-methylesterification on network development and nature of Ca2+ pectin gels: Towards understanding structure-function relations of pectin. Food Hydrocoll. 2012, 26, 89–98. [Google Scholar] [CrossRef]
- Braccini, I.; Perez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Leszczuk, A.; Chylińska, M.; Zięba, E.; Skrzypek, T.; Szczuka, E.; Zdunek, A. Structural network of arabinogalactan proteins (AGPs) and pectins in apple fruit during ripening and senescence processes. Plant Sci. 2018, 275, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Chylińska, M.; Zdunek, A. Distribution of arabinogalactan proteins and pectins in the cells of apple (Malus x domestica) fruit during post-harvest storage. Ann. Bot. 2019, 123, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Chylińska, M.; Zdunek, A. Enzymes and vitamin C as factors influencing the presence of arabinogalactan proteins (AGPs) in Solanum lycopersicum fruit. Plant Physiol. Biochem. 2019, 139, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Pieczywek, P.M.; Gryta, A.; Frąc, M.; Zdunek, A. Immunocytochemical studies on the distribution of arabinogalactan proteins (AGPs) as a response to fungal infection in Malus x domestica fruit. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, M.; Yang, Q.; Li, J.; Wang, X.; Zhou, Q.; Nagawa, S.; Xia, B.; Xu, T.; Huang, R.; et al. Arabinogalactan protein–rare earth element complexes activate plant endocytosis. PNAS 2019, 116, 14349–14357. [Google Scholar] [CrossRef]



| Monosaccharide Composition (mol%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | GalA | Glc | Gal | Xyl | Ara | Fuc | |
| AGPs from green fruit | 2.9 | 1.8 | 3.9 | 7.3 | 22.3 | 30.5 | 12.8 | 14.6 | 3.8 |
| AGPs from red fruit | 2.7 | 3.8 | 1.7 | 19.4 | 21.6 | 15.2 | 9.8 | 24.4 | 1.5 |
| AGPs from stored fruit | 3.7 | 5.6 | 2.0 | 20.2 | 6.3 | 16.5 | 8.6 | 34.9 | 2.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leszczuk, A.; Cybulska, J.; Skrzypek, T.; Zdunek, A. Properties of Arabinogalactan Proteins (AGPs) in Apple (Malus × Domestica) Fruit at Different Stages of Ripening. Biology 2020, 9, 225. https://doi.org/10.3390/biology9080225
Leszczuk A, Cybulska J, Skrzypek T, Zdunek A. Properties of Arabinogalactan Proteins (AGPs) in Apple (Malus × Domestica) Fruit at Different Stages of Ripening. Biology. 2020; 9(8):225. https://doi.org/10.3390/biology9080225
Chicago/Turabian StyleLeszczuk, Agata, Justyna Cybulska, Tomasz Skrzypek, and Artur Zdunek. 2020. "Properties of Arabinogalactan Proteins (AGPs) in Apple (Malus × Domestica) Fruit at Different Stages of Ripening" Biology 9, no. 8: 225. https://doi.org/10.3390/biology9080225
APA StyleLeszczuk, A., Cybulska, J., Skrzypek, T., & Zdunek, A. (2020). Properties of Arabinogalactan Proteins (AGPs) in Apple (Malus × Domestica) Fruit at Different Stages of Ripening. Biology, 9(8), 225. https://doi.org/10.3390/biology9080225






