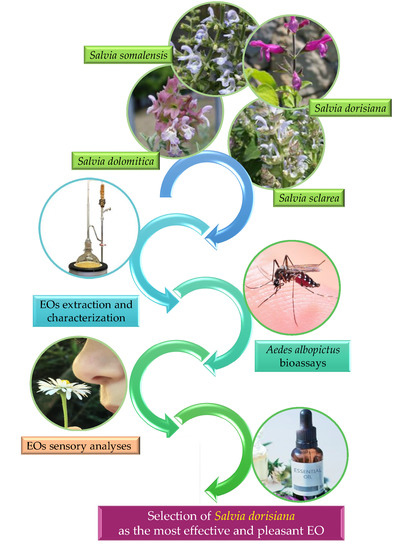
Salvia Spp. Essential Oils against the Arboviruses Vector Aedes albopictus (Diptera: Culicidae): Bioactivity, Composition, and Sensorial Profile—Stage 1
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, EOs Extraction and GC-MS Analysis
2.2. Aedes albopictus Rearing
2.3. EOs Larvicidal Activity
2.4. Essential Oils Repellent Activity
2.5. Essential Oils Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Essential Oils Chemical Composition
3.2. Essential Oils Larvicidal Activity
3.3. Essential Oils Repellent Activity
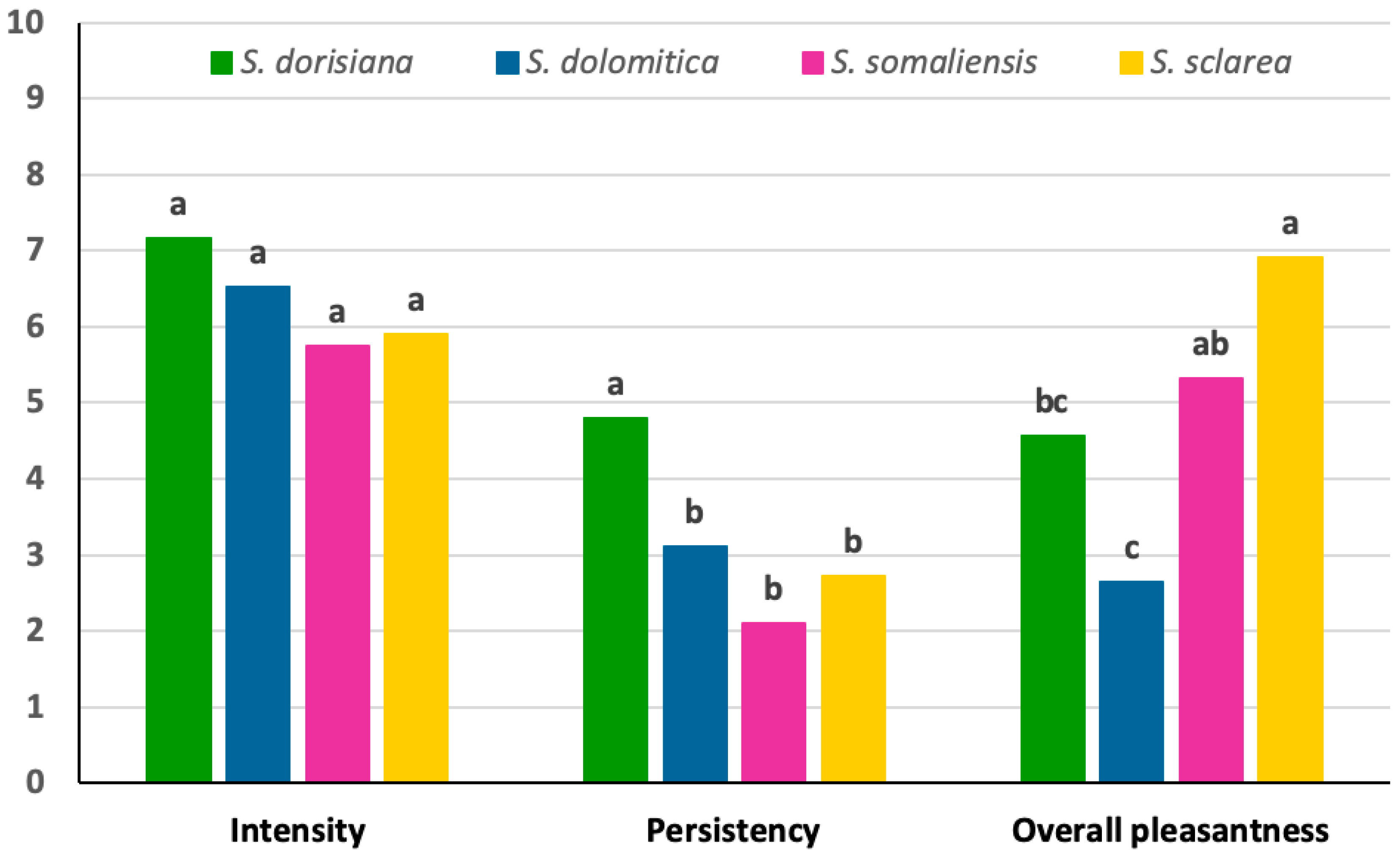
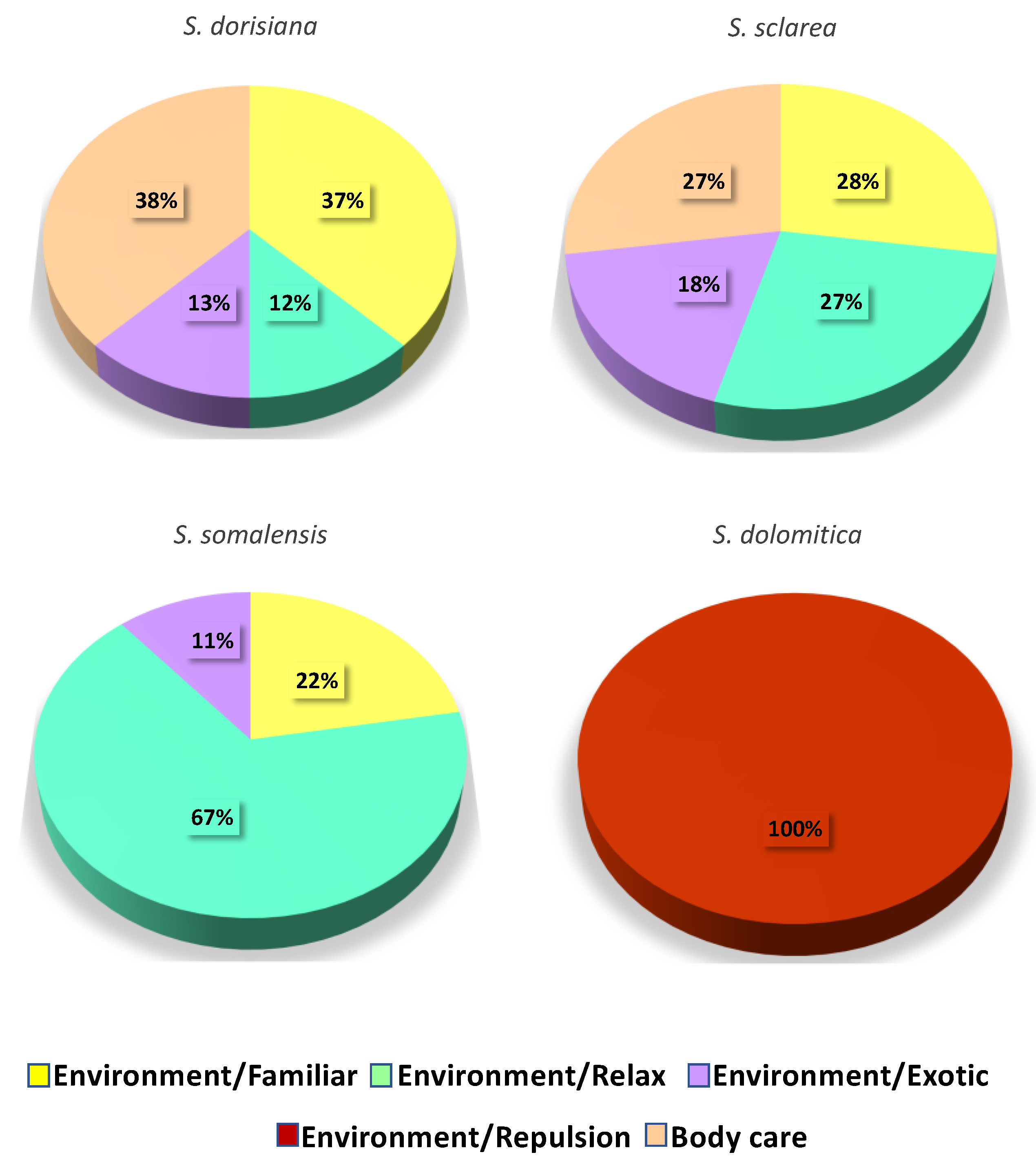
3.4. Essential Oils Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Lessler, J.; Chaisson, L.H.; Kucirka, L.M.; Bi, Q.; Grantz, K.; Salje, H.; Carcelen, A.C.; Ott, C.T.; Sheffield, J.S.; Ferguson, N.M.; et al. Assessing the global threat from Zika virus. Science 2016, 353, aaf8160-1–aaf8160-10. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Quick, J.; Claro, I.M.; Thézé, J.; de Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; da Costa, A.C.; et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017, 546, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Grubaugh, N.D.; Ladner, J.T.; Kraemer, M.U.G.; Dudas, G.; Tan, A.L.; Gangavarapu, K.; Wiley, M.R.; White, S.; Thézé, J.; Magnani, D.M.; et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature 2017, 546, 401–405. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Lymphatic Filariasis. WHO Fact Sheets; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Roy, D.N.; Goswami, R.; Pal, A. The insect repellents: A silent environmental chemical toxicant to the health. Environ. Toxicol. Pharmacol. 2017, 50, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crops Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Cioni, P.L.; Flamini, G. Repellence of essential oils from tropical and Mediterranean Lamiaceae against Sitophilus zeamais. Bull. Insectology 2010, 63, 197–202. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girardi, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- García-Díez, J.; Alheiro, J.; Pinto, A.L.; Soares, L.; Falco, V.; Fraqueza, M.J.; Patarata, L. Behaviour of food-borne pathogens on dry cured sausage manufactured with herbs and spices essential oils and their sensorial acceptability. Food Control 2016, 59, 262–270. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Golparvar, A.R.; Hadipanah, A.; Gheisari, M.M.; Naderi, D.; Rahmaniyan, S.; Khorrami, M. Chemical composition and antimicrobial activity of essential oil of Salvia officinalis L. and Salvia virgata Jacq. J. Herb. Drugs 2017, 8, 71–78. [Google Scholar] [CrossRef]
- Yilar, M.; Kadioglu, I.; Telci, I. Chemical Composition and Antifungal Activity of Salvia Officinalis (L.), S. Cryptantha (Montbret Et Aucher Ex Benth.), S. Tomentosa (Mill.) Plant Essential Oils and Extracts. Fresenius Environ. Bull. 2018, 27, 1695–1706. [Google Scholar]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Ali, Z.; Baser, K.H.C.; Khan, I.A. Chemical composition and biological activity of four salvia essential oils and individual compounds against two species of mosquitoes. J. Agric. Food Chem. 2015, 63, 447–456. [Google Scholar] [CrossRef]
- Conti, B.; Benelli, G.; Leonardi, M.; Afifi, F.U.; Cervelli, C.; Profeti, R.; Pistelli, L.; Canale, A. Repellent effect of Salvia dorisiana, S. longifolia, and S. sclarea (Lamiaceae) essential oils against the mosquito Aedes albopictus Skuse (Diptera: Culicidae). Parasitol. Res. 2012, 111, 291–299. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef]
- Mathew, J.; Thoppil, J.E. Chemical composition and mosquito larvicidal activities of Salvia essential oils. Pharm. Biol. 2011, 49, 456–463. [Google Scholar] [CrossRef][Green Version]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Biochem. Syst. Ecol. 1995, 24, 594. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography, Food/Nahrung; Academic Press: New York, NY, USA; London, UK; Sydney, Australia; Toronto, ON, Canada; San Francisco, CA, USA, 1982. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley and Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; John Wiley and Sons, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes, Aldrich Chemical Company; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- World Health Organisation. Instruction for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticide; WHO: Geneva, Switzerland, 1981. [Google Scholar]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef]
- Abbott, W.S. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- World Health Organisation. Report of the WHO Informal Consultation on the Evaluation and Testing of Insecticides CTD/WHOPES/IC/96.1; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Quartacci, M.F.; Sgherri, C.; Andrich, G.; Zinnai, A. A kinetic approach to describe the time evolution of red wine as a function of packaging conditions adopted: Influence of closure and storage position. Food Packag. Shelf Life 2017, 13, 44–48. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Xiaoguo, Y.; Andrich, G.; Zinnai, A. The influence of packaging on the sensorial evolution of white wine as a function of the operating conditions adopted during storage. Agrochimica 2016, 60, 150–160. [Google Scholar] [CrossRef]
- Martin, K.R.; Rasmussen, K.K. Comparison of Sensory Qualities of Geographically Paired Organic and Conventional Red Wines from the Southwestern US with Differing Total Polyphenol Concentrations: A Randomized Pilot Study. Food Nutr. Sci. 2011, 02, 1150–1159. [Google Scholar] [CrossRef]
- de Souza, M.A.; da Silva, L.; Macêdo, M.J.F.; Lacerda-Neto, L.J.; dos Santos, M.A.C.; Coutinho, H.D.M.; Cunha, F.A.B. Adulticide and repellent activity of essential oils against Aedes aegypti (Diptera: Culicidae)—A review. South African J. Bot. 2019, 124, 160–165. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Ruffoni, B.; D’Ascenzi, C.; Pistelli, L.; Mancianti, F. Activity of Salvia dolomitica and Salvia somalensis Essential Oils against Bacteria, Molds and Yeasts. Molecules 2018, 23, 396. [Google Scholar] [CrossRef]
- Aćimović, M.; Kiprovski, B.; Rat, M.; Sikora, V.; Popović, V.; Koren, A.; Brdar-Jokanović, M. Salvia sclarea: Chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2018, 1, 18–28. [Google Scholar]
- Kamatou, G.P.P.; Van Vuuren, S.F.; Van Heerden, F.R.; Seaman, T.; Viljoen, A.M. Antibacterial and antimycobacterial activities of South African Salvia species and isolated compounds from S. chamelaeagnea. South African J. Bot. 2007, 73, 552–557. [Google Scholar] [CrossRef]
- Bassolino, L.; Giacomelli, E.; Giovanelli, S.; Pistelli, L.; Cassetti, A.; Damonte, G.; Bisio, A.; Ruffoni, B. Tissue culture and aromatic profile in Salvia dolomitica Codd. Plant Cell. Tissue Organ Cult. 2015, 121, 83–95. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Das, N.G.; Dhiman, S.; Talukdar, P.K.; Rabha, B.; Goswami, D.; Veer, V. Synergistic mosquito-repellent activity of Curcuma longa, Pogostemon heyneanus and Zanthoxylum limonella essential oils. J. Infect. Public Health 2015, 8, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Batool, M.; Hussain, S.M.; Nasir, I.; Hafeez, F.; Debboun, M. Bioactivity of oils from medicinal plants against immature stages of dengue mosquito Aedes aegypti (Diptera: Culicidae). Int. J. Agric. Biol. 2015, 17, 843–847. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavour Chemicals. Vol. I and II; Published by author: Montclear, NJ, USA, 1969. [Google Scholar]
| N° | Compounds | Rt | Class | LRI 1 | LRI 2 | S. dolomitica | S. dorisiana | S. sclarea | S. somalensis | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Percentage (%) | |||||||||||
| 1 | Tricyclene | 5.28 | MH | 925 | 921 | - | - | 0.10 | 0.29 | ||
| 2 | α-Thujene | 5.36 | MH | 930 | 924 | - | - | 9.91 | - | ||
| 3 | α-Pinene | 5.55 | MH | 937 | 932 | 2.22 | 2.53 | 3.87 | 6.77 | ||
| 4 | Camphene | 5.94 | MH | 952 | 946 | 0.65 | 1.24 | 1.79 | 6.05 | ||
| 5 | Sabinene | 6.58 | MH | 978 | 968 | 0.21 | - | 0.10 | - | ||
| 6 | β-Pinene | 6.69 | MH | 979 | 974 | 1.23 | 0.56 | 2.86 | 0.75 | ||
| 7 | Myrcene | 7.05 | MH | 991 | 988 | 1.72 | 1.19 | 0.40 | 1.07 | ||
| 8 | α-Phellandrene | 7.49 | MH | 1005 | 1002 | 0.33 | 0.32 | - | 0.48 | ||
| 9 | δ-3-carene | 7.68 | MH | 1011 | 1008 | 3.28 | 2.16 | - | 5.19 | ||
| 10 | 1,4-Cineole | 7.81 | OM | 1016 | 1012 | - | - | 0.49 | - | ||
| 11 | α-Terpinene | 7.88 | MH | 1017 | 1014 | 0.14 | 0.27 | 0.22 | 0.45 | ||
| 12 | o-Cymene | 8.00 | MH | 1022 | 1020 | 2.02 | 0.98 | - | 0.10 | ||
| 13 | p-Cymene | 8.14 | MH | 1025 | 1022 | - | - | 1.65 | 1.76 | ||
| 14 | Limonene | 8.28 | MH | 1030 | 1024 | 3.81 | 3.70 | 1.34 | 4.13 | ||
| 15 | Eucalyptol | 8.37 | OM | 1032 | 1026 | 10.17 | 5.83 | 4.96 | - | ||
| 16 | cis-β-Ocimene | 8.55 | OM | 1038 | 1032 | 1.58 | 0.87 | - | - | ||
| 17 | trans-β-Ocimene | 8.91 | OM | 1049 | 1044 | 0.16 | 0.23 | 0.10 | - | ||
| 18 | γ-Terpinene | 9.31 | MH | 1060 | 1054 | 0.26 | 0.49 | 0.17 | 0.53 | ||
| 19 | cis-4-Thujanol | 9.56 | OM | 1070 | 1074 $ | 0.11 | - | - | - | ||
| 20 | cis-Linalool oxide (furanoid) | 9.79 | OM | 1074 | 1067 | - | - | 0.10 | - | ||
| 21 | 2-methoxyethyl-Benzene | 10.27 | NT | 1087 | 1080 | 0.15 | - | - | - | ||
| 22 | Terpinolene | 10.38 | MH | 1088 | 1086 | - | 0.29 | - | 0.84 | ||
| 23 | Fenchone | 10.40 | OM | 1096 | 1083 | 0.45 | - | 0.45 | - | ||
| 24 | Linalool | 10.78 | OM | 1099 | 1095 | - | 0.23 | 11.9 | 0.21 | ||
| 25 | iso-Amyl 2-methyl butyrate | 11.03 | NT | 1101 | 1100 $ | - | 0.26 | - | - | ||
| 26 | β-Thujone | 11.45 | OM | 1114 | 1112 | - | - | 2.40 | - | ||
| 27 | trans-Sabinol | 12.35 | OM | 1143 | 1137 | 0.39 | - | - | - | ||
| 28 | Camphor | 12.55 | OM | 1145 | 1141 | 0.27 | - | 8.10 | 12.91 | ||
| 29 | Borneol | 13.38 | OM | 1167 | 1165 | 4.41 | 3.61 | 1.08 | 3.35 | ||
| 30 | 4-Terpineol | 13.86 | OM | 1177 | 1174 | 0.61 | 0.49 | 0.20 | 0.79 | ||
| 31 | p-Cymen-8-ol | 14.16 | OM | 1183 | 1179 | - | - | - | 0.17 | ||
| 32 | α-Terpineol | 14.14 | OM | 1189 | 1186 | 0.41 | 0.48 | 2.93 | 1.87 | ||
| 33 | Myrtenol | 14.64 | OM | 1195 | 1194 | - | 0.49 | - | - | ||
| 34 | γ-Terpineol | 14.68 | OM | 1197 | 1199 | - | - | 0.18 | - | ||
| 35 | Nerol | 15.97 | OM | 1228 | 1227 | - | - | 0.22 | - | ||
| 36 | Linalyl acetate | 17.18 | OM | 1257 | 1254 | - | - | 32.03 | - | ||
| 37 | Bornyl acetate | 18.41 | OM | 1285 | 1284 | - | - | 0.84 | 18.1 | ||
| 38 | p-Mentha-1,8-dien-7-ol | 18.89 | OM | 1296 | 1297 $ | - | 1.11 | - | - | ||
| 39 | Carvacrol | 19.07 | OM | 1299 | 1298 | - | - | 0.47 | - | ||
| 40 | Myrtenyl acetate | 20.06 | OM | 1327 | 1324 | - | 4.03 | - | - | ||
| 41 | α-Cubebene | 21.06 | SH | 1351 | 1345 | 0.76 | - | - | 0.22 | ||
| 42 | Eugenol | 21.36 | OM | 1357 | 1356 | - | - | 0.23 | - | ||
| 43 | Neryl acetate | 21.71 | OM | 1364 | 1359 | - | - | 0.74 | - | ||
| 44 | Ylangene | 21.96 | SH | 1372 | 1373 | - | - | - | 0.52 | ||
| 45 | Isoledene | 22.03 | SH | 1375 | 1374 | 0.5 | - | - | - | ||
| 46 | α-Copaene | 22.15 | SH | 1376 | 1374 | 2.76 | 0.82 | 0.27 | 2.59 | ||
| 47 | Geranyl acetate | 22.51 | OM | 1382 | 1379 | - | - | 1.04 | - | ||
| 48 | β-Cubebene | 22.74 | SH | 1389 | 1387 | 0.10 | - | - | - | ||
| 49 | cis-Jasmone | 23.02 | NT | 1393 | 1392 | - | - | - | 0.37 | ||
| 50 | Methyl perillate | 23.23 | NT | 1394 | 1392 | - | 19.16 | - | - | ||
| 51 | β-Panasinsene | 23.33 | SH | 1395 | 1381 | 0.17 | - | - | - | ||
| 52 | β-Maaliene | 23.45 | SH | 1405 | 1411 $ | - | - | - | 0.57 | ||
| 53 | α-Gurjunene | 23.53 | SH | 1410 | 1409 | 1.17 | - | - | - | ||
| 54 | β-Caryophyllene | 23.92 | SH | 1419 | 1417 | 14.81 | 9.99 | 3.47 | 3.62 | ||
| 55 | β-Gurjunene | 24.27 | SH | 1432 | 1431 | 1.46 | - | - | 0.15 | ||
| 56 | 1,1,3a-Trimethyl-7-methylenedecahydro-1H-cyclopropa[a]naphthalene | 24.56 | SH | 1434 | 1435 | - | 0.18 | - | - | ||
| 57 | trans-α-Bergamotene | 24.60 | SH | 1435 | 1432 | - | - | 0.12 | - | ||
| 58 | p-Mentha-1,8-dien-7-yl acetate | 24.69 | OM | 1436 | 1436 $ | - | 21.74 | - | - | ||
| 59 | Aromadendrene | 24.84 | SH | 1440 | 1439 | 7.96 | - | - | 1.00 | ||
| 60 | α-Maaliene | 24.89 | SH | 1443 | 1442 $ | 0.96 | - | - | 0.12 | ||
| 61 | Selina-5,11-diene | 25..12 | SH | 1447 | 1447 $ | 0.95 | 0.15 | - | - | ||
| 62 | α-Humulene | 25.29 | SH | 1454 | 1452 | 1.57 | 0.76 | 2.22 | 0.27 | ||
| 63 | Cadina-3,5-diene | 25.32 | SH | 1458 | 1454 $ | - | - | - | 0.45 | ||
| 64 | Alloaromadendrene | 25.58 | SH | 1461 | 1458 | 0.87 | 0.19 | - | 0.33 | ||
| 65 | γ-Muurolene | 26.24 | SH | 1477 | 1478 | 0.72 | 0.17 | - | 1.10 | ||
| 66 | α-Amorphene | 26.32 | SH | 1482 | 1483 | - | - | - | 0.17 | ||
| 67 | Germacrene D | 26.40 | SH | 1485 | 1484 | - | - | 0.10 | - | ||
| 68 | β-Eudesmene | 26.51 | SH | 1486 | 1485 $ | 1.02 | - | - | - | ||
| 69 | Phenethyl isovalerate | 26.79 | NT | 1490 | 1491 $ | - | 0.18 | - | - | ||
| 70 | δ-Selinene | 26.83 | SH | 1493 | 1492 | 0.27 | - | - | - | ||
| 71 | epi-Bicyclosesquiphellandrene | 26.85 | SH | 1494 | 1490 $ | - | - | - | 0.42 | ||
| 72 | Viridiflorene | 27.00 | SH | 1497 | 1496 | 3.96 | 0.75 | 0.10 | - | ||
| 73 | Eremophilene | 27.07 | SH | 1498 | 1498 $ | - | - | - | 0.63 | ||
| 74 | α-Muurolene | 27.11 | SH | 1499 | 1500 | 0.53 | - | - | 0.99 | ||
| 75 | β-Bisabolene | 27.52 | SH | 1509 | 1505 | - | - | 0.10 | - | ||
| 76 | γ-Cadinene | 27.81 | SH | 1513 | 1513 | 4.36 | 1.04 | - | 2.67 | ||
| 77 | δ-Cadinene | 28.09 | SH | 1524 | 1522 | 5.86 | 2.18 | 0.11 | 5.66 | ||
| 78 | Cubenene | 28.48 | SH | 1532 | 1522 $ | 0.12 | - | - | 0.48 | ||
| 79 | α-Cadinene | 28.61 | SH | 1538 | 1537 | 0.18 | - | - | 0.17 | ||
| 80 | α-Calacorene | 28.81 | SH | 1542 | 1544 | - | 0.13 | - | 0.55 | ||
| 81 | Myrtenyl 2-methyl butyrate | 29.55 | NT | 1560 | 1559 $ | - | 0.20 | - | - | ||
| 82 | (E)-Nerolidol | 29.64 | OS | 1563 | 1561 | - | 0.27 | - | 1.49 | ||
| 83 | Spathulenol | 30.12 | OS | 1576 | 1577 | 0.50 | 0.13 | - | - | ||
| 84 | Globulol | 30.36 | OS | 1580 | 1590 | 3.36 | - | - | 0.57 | ||
| 85 | Caryophyllene oxide | 30.39 | OS | 1583 | 1582 | - | 1.62 | 0.94 | - | ||
| 86 | Viridiflorol | 30.71 | OS | 1591 | 1592 | 0.25 | - | 0.67 | 0.37 | ||
| 87 | Ledol | 30.99 | OS | 1599 | 1602 | 0.37 | - | - | - | ||
| 88 | Rosifoliol | 31.12 | OS | 1600 | 1600 | 1.55 | - | - | - | ||
| 89 | Humulene epoxide II | 31.31 | OS | 1606 | 1608 | 0.17 | 0.10 | 0.26 | - | ||
| 90 | Di-epi-1,10-cubenol | 31.54 | OS | 1614 | 1618 | 0.18 | - | - | 0.18 | ||
| 91 | Junenol | 30.77 | OS | 1617 | 1618 | - | 0.53 | - | 1.36 | ||
| 92 | (E)-Farnesene epoxide | 30.89 | OS | 1624 | 1624 $ | - | 0.35 | - | - | ||
| 93 | Epicubenol | 31.00 | OS | 1627 | 1627 | 0.63 | - | - | - | ||
| 94 | γ-Eudesmol | 31.16 | OS | 1632 | 1630 | 0.33 | - | - | - | ||
| 95 | τ-Cadinol | 32.52 | OS | 1640 | 1638 | 2.18 | 2.42 | - | 5.12 | ||
| 96 | δ-Cadinol | 32.66 | OS | 1645 | 1646 $ | 0.19 | - | - | - | ||
| 97 | octahydro-2,2,4,7a-tetramethyl-1,3a-ethano(1H)inden-4-ol | 32.78 | OS | 1648 | 1648 $ | - | 0.33 | - | - | ||
| 98 | α-Cadinol | 32.93 | OS | 1653 | 1652 | - | 0.17 | - | 0.28 | ||
| 99 | α-Eudesmol | 32.99 | OS | 1655 | 1652 | 2.48 | - | - | - | ||
| 100 | Aromadendrene oxide-(2) | 33.97 | OS | 1678 | 1678 $ | - | 0.16 | - | - | ||
| 101 | α-Bisabolol | 34.12 | OS | 1684 | 1683 | - | 0.26 | - | - | ||
| 102 | Shyobunol | 34.56 | OS | 1701 | 1686 § | 0.14 | 0.25 | - | - | ||
| 103 | Farnesyl acetone | 42.13 | AC | 1919 | 1913 | - | 0.11 | - | - | ||
| 104 | Cembrene | 43.63 | DH | 1939 | 1937 | - | 0.23 | - | - | ||
| 105 | (E)-1-(6,10-Dimethylundec-5-en-2-yl)-4-methylbenzene | 44.02 | NT | 1951 | 1950 $ | - | 0.86 | - | - | ||
| 106 | Gerany-p-cymene | 44.98 | DH | 1980 | 1980 $ | - | 0.11 | - | - | ||
| 107 | epi-13-Manool | 48.77 | OD | 2056 | 2059 | - | - | 0.10 | - | ||
| 108 | Sclareol | 51.14 | OD | 2227 | 2222 | - | - | 0.43 | - | ||
| Class of compounds | S. dolomitica | S. dorisiana | S. sclarea | S. somalensis | |||||||
| Monoterpene Hydrocarbons (MH) | 15.87 | 13.73 | 22.41 | 28.41 | |||||||
| Oxygenated Monoterpenes (OM) | 18.56 | 39.11 | 68.46 | 37.40 | |||||||
| Total monoterpenes | 34.43 | 52.84 | 90.87 | 65.81 | |||||||
| Sesquiterpene Hydrocarbons (SH) | 51.06 | 16.36 | 6.49 | 22.68 | |||||||
| Oxygenated Sesquiterpenes (OS) | 12.33 | 6.59 | 1.87 | 9.37 | |||||||
| Total sesquiterpenes | 63.39 | 22.95 | 8.36 | 32.05 | |||||||
| Diterpene Hydrocarbons (DH) | - | 0.34 | - | - | |||||||
| Oxygenated Diterpenes (OD) | - | - | 0.53 | - | |||||||
| Apocarotenoids (AC) | - | 0.11 | - | - | |||||||
| Non-terpene Derivatives (NT) | 0.15 | 20.66 | - | 0.37 | |||||||
| Total Identified | 97.97 | 96.90 | 99.76 | 98.23 | |||||||
| EO | LC50 a | LC95 b | χ2 (df) | P |
|---|---|---|---|---|
| S. dolomitica | 315.52 (293.24–338.14) | 503.04 (454.85–582.09) | 3.50 (8) | 0.899 |
| S. dorisiana | 71.08 (65.91–76.14) | 125.52 (112.70–146.54) | 4.83 (8) | 0.775 |
| S. sclarea | 559.77 (470.17–718.35) | 2159.94 (1457.00–3974.90) | 5.68 (8) | 0.683 |
| S. somalensis | 388.51 (356.59–430.74) | 686.63 (581.29–912.44) | 0.99 (8) | 0.998 |
| EO (X) | S. dolomitica | S. dorisiana | S. sclarea | S. somalensis | |
|---|---|---|---|---|---|
| EO (Y) | |||||
| S. dolomitica | - | 5.48(3.00–13.24) | 0.69 (0.45–1.01) | 0.73 (0.46–1.12) | |
| S. dorisiana | 0.18 (0.08–0.33) | - | 0.13 (0.05–0.25) | 0.13 (0.05–0.27) | |
| S. sclarea | 1.46 (1.01–2.23) | 7.98(4.07–21.81) | - | 1.07 (0.71–1.62) | |
| S. somalensis | 1.37 (0.90–2.18) | 7.49(3.77–20.47) | 0.94 (0.62–1.42) | - | |
| EO | RD50 a | RD95 b | χ2 (df) | P |
|---|---|---|---|---|
| S. dolomitica | 0.98 (0.62–1.54) | 38.68 (18.50–86.26) | 66.35 (7) | <0.001 |
| S. dorisiana | 0.56 (0.19–1.11) | 39.88 (7.84–46986.17) | 12.59 (3) | 0.006 |
| S. sclarea | 1.13 (0.74–1.74) | 12.65 (6.12–52.92) | 28.36 (4) | <0.001 |
| S. somalensis | 5.03 (3.69–7.01) | 8308.54 (3387–25371.72) | 14.91 (8) | 0.061 |
| EO (X) | S. dolomitica | S. dorisiana | S. sclarea | S. somalensis | |
|---|---|---|---|---|---|
| EO (Y) | |||||
| S. dolomitica | - | 1.78 (0.56–6.02) | 0.87 (0.29–2.63) | 0.25(0.09–0.61) | |
| S. dorisiana | 0.56 (0.17–1.80) | - | 0.49 (0.13–1.80) | 0.14(0.04–0.45) | |
| S. sclarea | 1.14 (0.38–3.50) | 2.04 (0.55–7.97) | - | 0.29(0.09–0.83) | |
| S. somalensis | 4.00 (1.63–10.68) | 7.07 (2.22–26.29) | 3.46 (1.21–10.87) | - | |
| EO. | CPT a |
|---|---|
| S. dolomitica | 21.45 ± 7.12 ab |
| S. dorisiana | 43.28 ± 3.43 a |
| S. sclarea | 13.60 ± 2.31 ab |
| S. somalensis | 4.60 ± 2.70 b |
| Species | S. dolomitica | S. dorisiana | S. sclarea | S. somalensis | |
|---|---|---|---|---|---|
| Odour Class | |||||
| Vegetative odours | Herbaceous | Herbaceous | Citronella | Herbaceous | |
| - | Mint | Fresh mint | Menthol | ||
| - | - | Citrus | Chamomille | ||
| - | - | Lime | - | ||
| Spicy | - | Sandalwood | Green spicy | Green spicy | |
| - | Licorice | Thyme | Thyme | ||
| - | - | Sage | Green tea | ||
| Other | - | Resin | - | - | |
| Off-flavors | Mould | - | - | - | |
| Wet rag | - | - | - | ||
| Old soap | - | - | - | ||
| Petrol | - | - | - | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najar, B.; Pistelli, L.; Venturi, F.; Ferroni, G.; Giovanelli, S.; Cervelli, C.; Bedini, S.; Conti, B. Salvia Spp. Essential Oils against the Arboviruses Vector Aedes albopictus (Diptera: Culicidae): Bioactivity, Composition, and Sensorial Profile—Stage 1. Biology 2020, 9, 206. https://doi.org/10.3390/biology9080206
Najar B, Pistelli L, Venturi F, Ferroni G, Giovanelli S, Cervelli C, Bedini S, Conti B. Salvia Spp. Essential Oils against the Arboviruses Vector Aedes albopictus (Diptera: Culicidae): Bioactivity, Composition, and Sensorial Profile—Stage 1. Biology. 2020; 9(8):206. https://doi.org/10.3390/biology9080206
Chicago/Turabian StyleNajar, Basma, Luisa Pistelli, Francesca Venturi, Giuseppe Ferroni, Silvia Giovanelli, Claudio Cervelli, Stefano Bedini, and Barbara Conti. 2020. "Salvia Spp. Essential Oils against the Arboviruses Vector Aedes albopictus (Diptera: Culicidae): Bioactivity, Composition, and Sensorial Profile—Stage 1" Biology 9, no. 8: 206. https://doi.org/10.3390/biology9080206
APA StyleNajar, B., Pistelli, L., Venturi, F., Ferroni, G., Giovanelli, S., Cervelli, C., Bedini, S., & Conti, B. (2020). Salvia Spp. Essential Oils against the Arboviruses Vector Aedes albopictus (Diptera: Culicidae): Bioactivity, Composition, and Sensorial Profile—Stage 1. Biology, 9(8), 206. https://doi.org/10.3390/biology9080206