Kinetics of Enzymatic Synthesis of Cyanidin-3-Glucoside Lauryl Ester and Its Physicochemical Property and Proliferative Effect on Intestinal Probiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Enzymatic Synthesis of Cy3glc-C12
2.3. Response Surface Methodology (RSM) Optimization Design
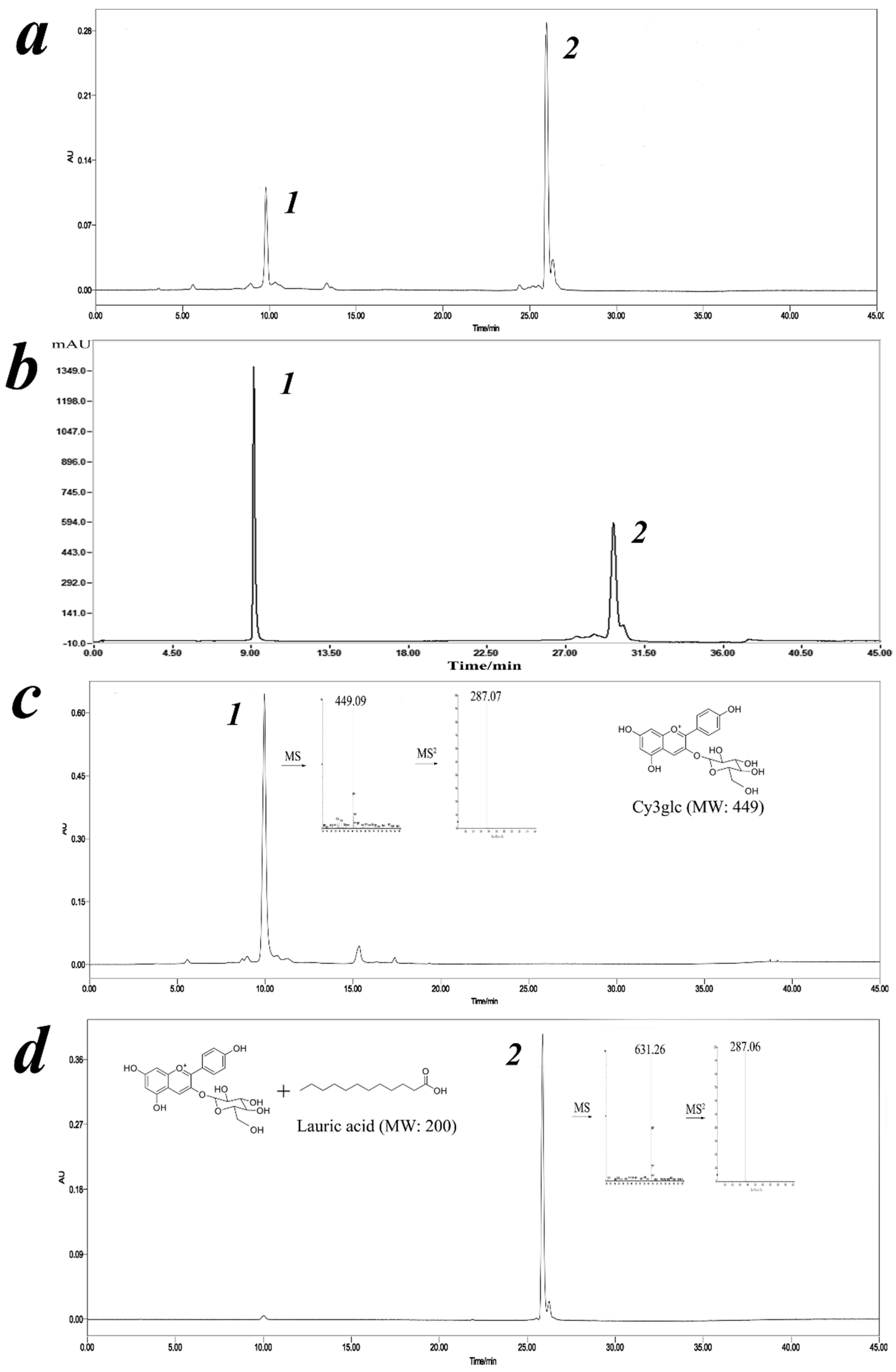
2.4. Isolation of Cy3glc-C12 by Filtrate Extraction and Semi-Preparative HPLC
2.5. Structural Identification of Cyanidin-3-Glucoside Lauryl Ester
2.5.1. Analysis by ESI-MS/MS
2.5.2. Analysis by Nuclear Magnetic Resonance (NMR)
2.6. Kinetic Model and Parameters of Enzymatic Synthesis of Cy3glc-C12
2.7. Properties of Cyanidin-3-Glucoside Lauryl Ester
2.7.1. Lipophilic Property
2.7.2. UV–VIS Absorbance Property
2.7.3. Thermostability
2.8. Effect of Cy3glc-C12 on the proliferation of Bifidobacteria and Lactobacillus
2.8.1. Sample Preparation and Cultivation of the Strains
2.8.2. Calculation of Kinetic Parameters and OD600 Values for Bifidobacteria and Lactobacillus Growth
2.8.3. Determination of Bacterial Metabolites and pH Values of Culture Media
2.9. Statistical Analysis
3. Results and Discussion
3.1. Model Fitting of Lipase-Catalyzed Synthesis of Cy3glc-C12
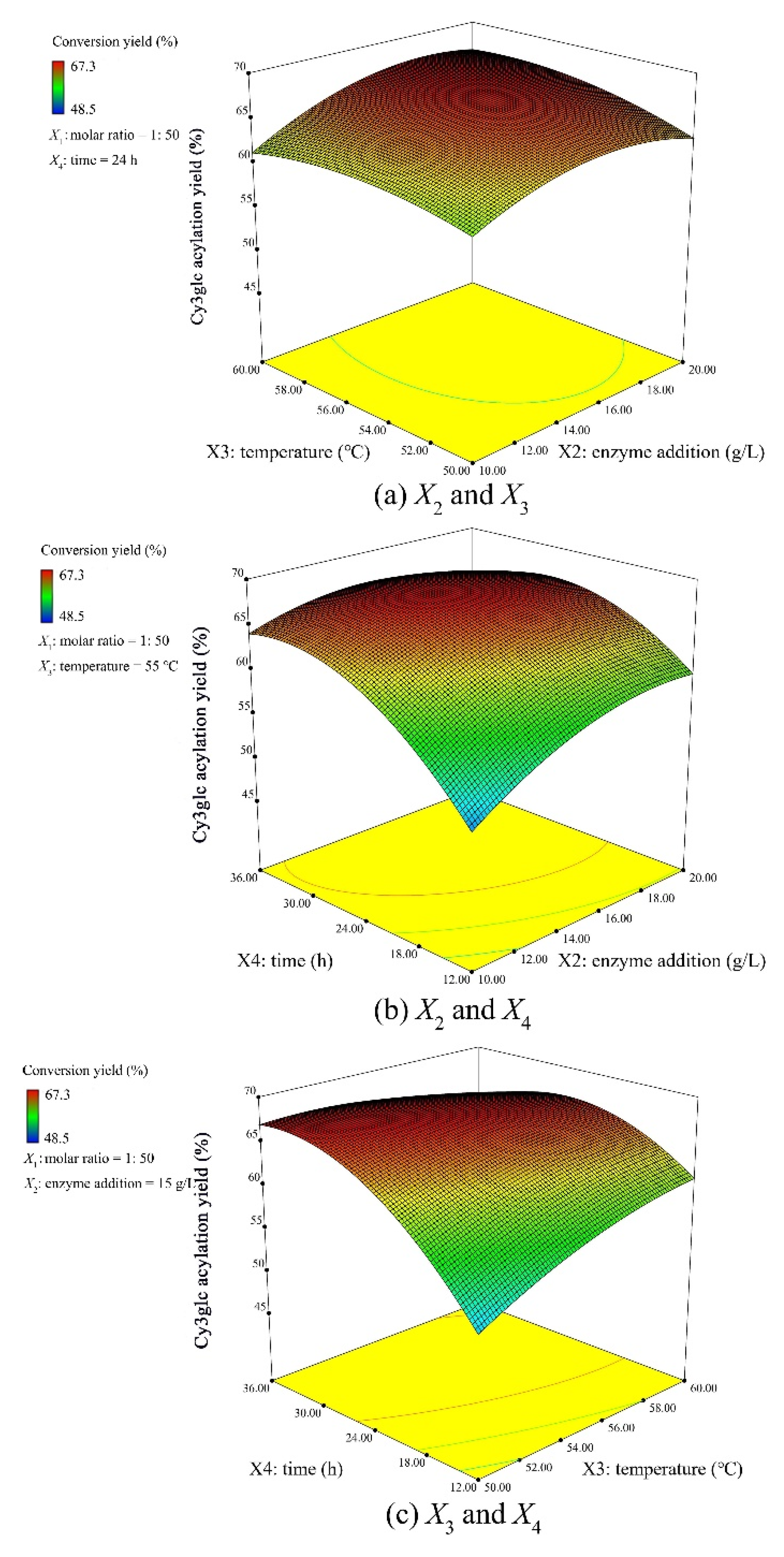
3.2. Optimization of Reaction and Model Validation
3.3. Identification of Cy3glc-C12 by ESI-MS/MS and NMR
3.4. Kinetics of Enzymatic Synthesis of Cy3glc Lauryl Ester
3.5. Properties of Cyanidin-3-Glucoside Lauryl Ester
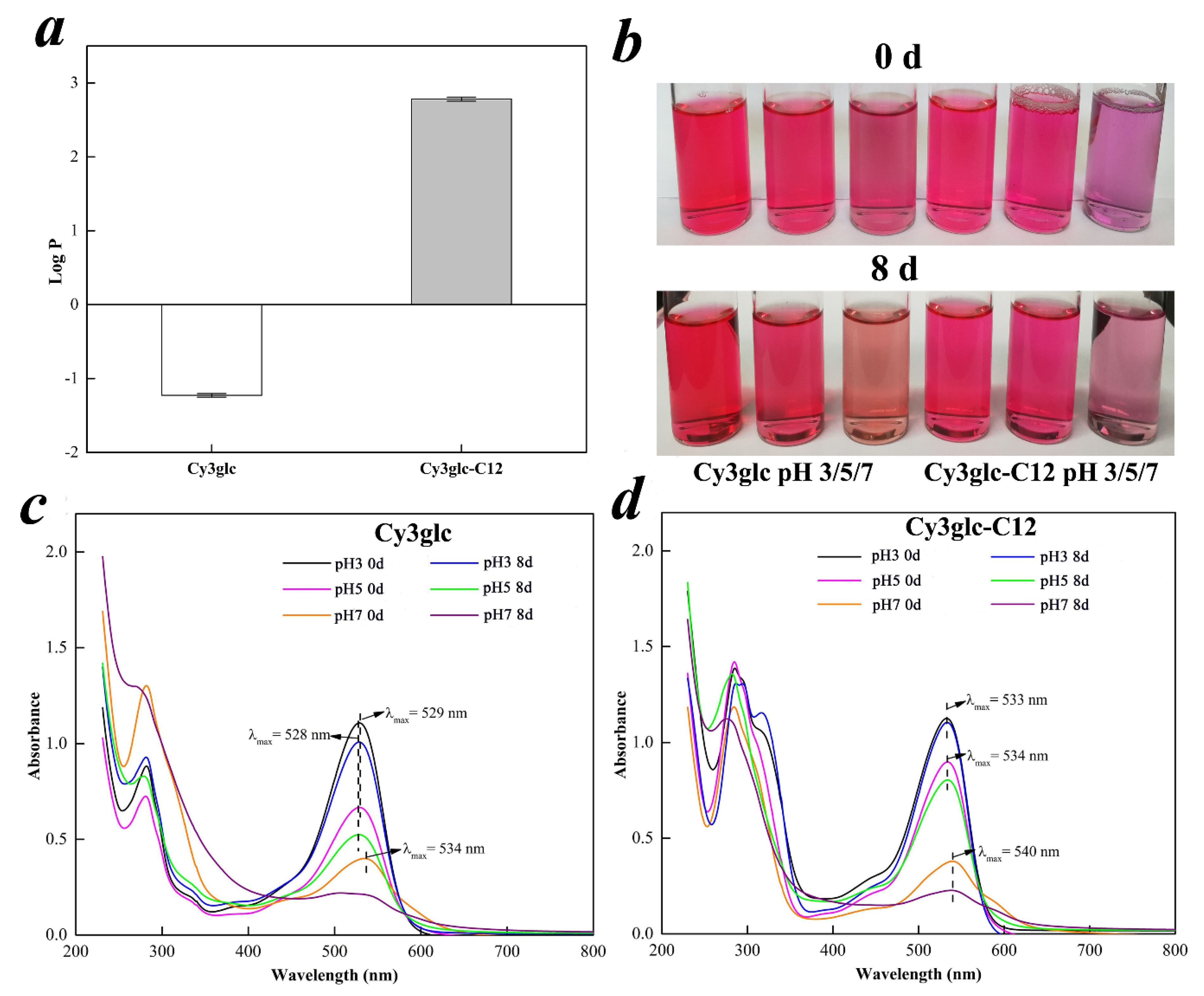
3.5.1. Lipophilic Property
3.5.2. UV-VIS Absorbance Property
3.5.3. Thermostability
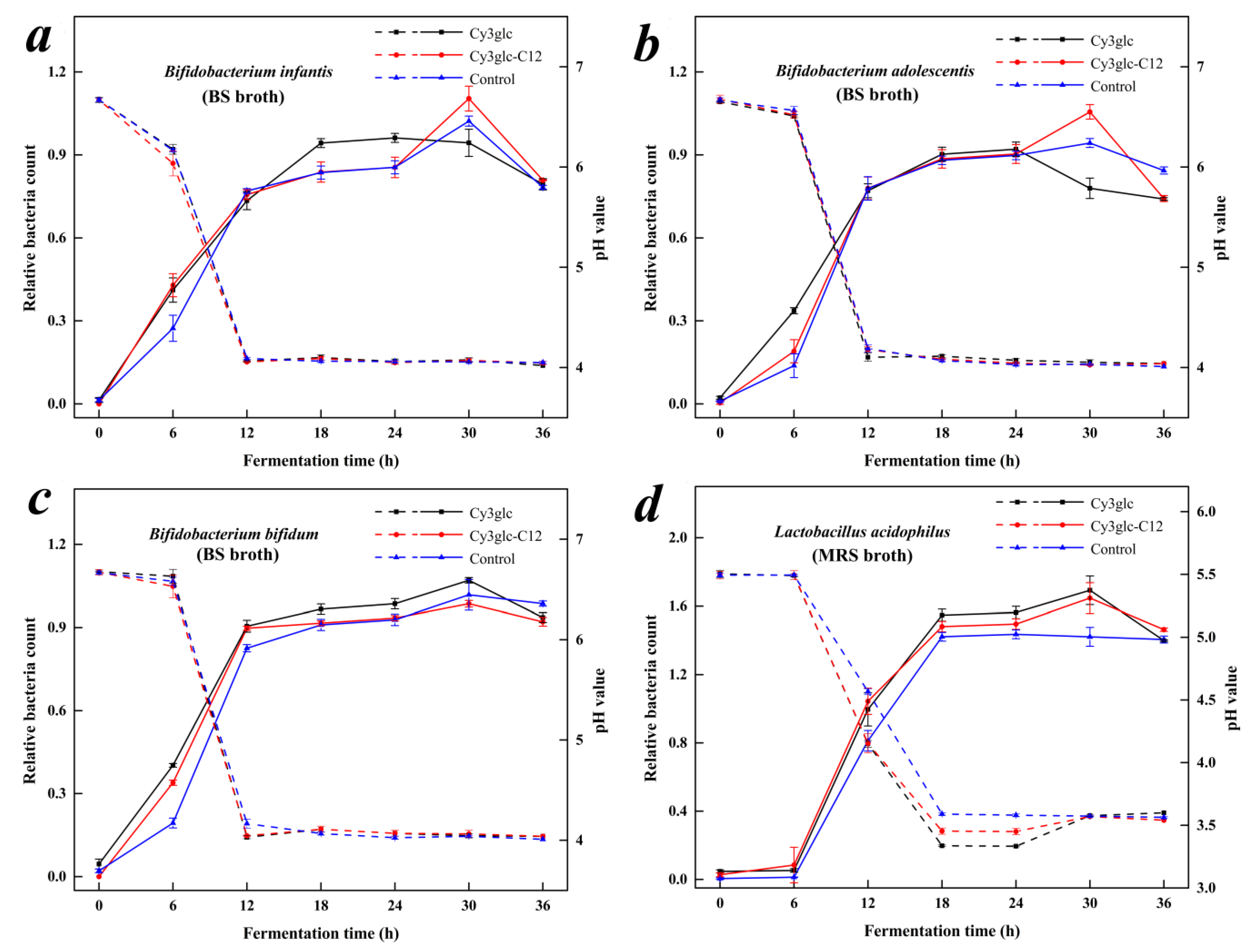
3.6. Proliferative Effect of Cy3glc-C12 on Bifidobacteria and Lactobacillus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; De Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- B¹kowska-Barczak, A. Acylated anthocyanins as stable, natural food colorants—A review. Pol. J. Food Nutr. Sci. 2005, 11, 201–247. [Google Scholar]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and quantification of phenolic acids and anthocyanins as antioxidants in bran, embryo and endosperm of white, red and black rice kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218. [Google Scholar] [CrossRef]
- He, S.; Lou, Q.; Shi, J.; Sun, H.; Zhang, M.; Li, Q. Water Extraction of Anthocyanins from Black Rice and Purification Using Membrane Separation and Resin Adsorption. J. Food Process. Preserv. 2017, 41, 1–12. [Google Scholar] [CrossRef]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef]
- Ekici, L.; Simsek, Z.; Ozturk, I.; Sağdıç, O.; Yetim, H.; Sagdic, O. Effects of Temperature, Time, and pH on the Stability of Anthocyanin Extracts: Prediction of Total Anthocyanin Content Using Nonlinear Models. Food Anal. Methods 2014, 7, 1328–1336. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; O’Donnell, B.; Tiwari, B. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ashraf, B.; Gani, A.; Gani, A. Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. Int. J. Biol. Macromol. 2018, 109, 435–442. [Google Scholar] [PubMed]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stability improvement of natural food colors: Impact of amino acid and peptide addition on anthocyanin stability in model beverages. Food Chem. 2017, 218, 277–284. [Google Scholar] [CrossRef]
- Qian, B.-J.; Liu, J.-H.; Zhao, S.-J.; Cai, J.-X.; Jing, P. The effects of gallic/ferulic/caffeic acids on colour intensification and anthocyanin stability. Food Chem. 2017, 228, 526–532. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, V.C.; Araújo, P.; Mateus, N.; De Freitas, V. Synthesis, characterisation and antioxidant features of procyanidin B4 and malvidin-3-glucoside stearic acid derivatives. Food Chem. 2015, 174, 480–486. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Chen, J.; Wang, Z.-Q.; Shen, R.-M.; Cui, N.; Sun, A. Direct Acylation of Cyanidin-3-Glucoside with Lauric Acid in Blueberry and Its Stability Analysis. Int. J. Food Prop. 2016, 19, 1–12. [Google Scholar] [CrossRef]
- Cruz, L.; Benohoud, M.; Rayner, C.M.; Mateus, N.; De Freitas, V.; Blackburn, R.S. Selective enzymatic lipophilization of anthocyanin glucosides from blackcurrant (Ribes nigrum L) skin extract and characterization of esterified anthocyanins. Food Chem. 2018, 266, 415–419. [Google Scholar] [CrossRef]
- Yan, Z.; Li, C.; Zhang, L.; Liu, Q.; Ou, S.; Zeng, X. Enzymatic Acylation of Anthocyanin Isolated from Black Rice with Methyl Aromatic Acid Ester as Donor: Stability of the Acylated Derivatives. J. Agric. Food Chem. 2016, 64, 1137–1143. [Google Scholar] [CrossRef]
- Cruz, L.; Guimarães, M.; Araújo, P.; Évora, A.; De Freitas, V.; Mateus, N. Malvidin 3-Glucoside–Fatty Acid Conjugates: From Hydrophilic toward Novel Lipophilic Derivatives. J. Agric. Food Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.M.; Fernandes, I.; Guimarães, M.; De Freitas, V.; Mateus, N. Enzymatic synthesis, structural characterization and antioxidant capacity assessment of a new lipophilic malvidin-3-glucoside-oleic acid conjugate. Food Funct. 2016, 7, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Mateus, N.; De Freitas, V.; Cruz, L. Improvement of the Color Stability of Cyanidin-3-glucoside by Fatty Acid Enzymatic Acylation. J. Agric. Food Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.K.; Yang, B.; Zheng, J. Enzymatic Acylation of Anthocyanins Isolated from Alpine Bearberry (Arctostaphylos alpina) and Lipophilic Properties, Thermo-stability and Antioxidant Capacity of the Derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qian, J.; Li, S. Enzymatic acylation of isoorientin isolated from antioxidant of bamboo leaves with palmitic acid and antiradical activity of the acylated derivatives. Eur. Food Res. Technol. 2014, 239, 661–667. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic acylation of blackcurrant (Ribes nigrum) anthocyanins and evaluation of lipophilic properties and antioxidant capacity of derivatives. Food Chem. 2019, 280, 189–196. [Google Scholar] [CrossRef]
- Krishna, S.H.; Divakar, S.; Prapulla, S.G.; Karanth, N.G. Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J. Biotechnol. 2001, 87, 193–201. [Google Scholar] [CrossRef]
- Husson, E.; Garcia-Matilla, V.; Humeau, C.; Chevalot, I.; Fournier, F.; Marc, I. Enzymatic acylation of a bifunctional molecule in 2-methyl-2-butanol: Kinetic modelling. Enzym. Microb. Technol. 2010, 46, 338–346. [Google Scholar] [CrossRef]
- Heukelem, L.; Lewitus, A.J.; Kana, T.M.; Craft, N.E. High performance liquid chromatography of phytoplankton pigments using a polymeric reversed phase C18 column. J. Phycol. 2010, 28, 867–872. [Google Scholar] [CrossRef]
- Awasthi, S.; Wilken, R.; Patel, F.; German, J.B.; Mills, D.A.; Lebrilla, C.B.; Kim, K.; Freeman, S.L.; Smilowitz, J.T.; Armstrong, A.W.; et al. Dietary supplementation with Bifidobacterium longum subsp. infantis (B. infantis) in healthy breastfed infants: Study protocol for a randomised controlled trial. Trials 2016, 17, 1–11. [Google Scholar]
- Cheng, J.-R.; Liu, X.-M.; Chen, Z.-Y.; Zhang, Y.-S.; Zhang, Y.-H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Koh, E. Stability of anthocyanins in bokbunja (Rubus occidentalis L.) under in vitro gastrointestinal digestion. Food Chem. 2018, 267, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]


| Position | Cy3glc | Cy3glc-C12 | ||
|---|---|---|---|---|
| δH (ppm)* | δC (ppm) | δH (ppm)* | δC (ppm) | |
| Cyanidin aglycone | ||||
| 4 | 7.50 (s, 1H) | 132.52 | 7.48 (s, 1H) | 134.31 |
| 6 | 6.52 (s, 1H) | 102.96 | 6.48 (s, 1H) | 103.02 |
| 8 | 6.68 (s, 1H) | 96.37 | 6.68 (s, 1H) | 96.42 |
| 2′ | 6.94 (d, J = 1.9 Hz, 1H) | 116.57 | 6.94 (d, J = 2.1 Hz, 1H) | 116.40 |
| 5′ | 6.83 (d, J = 7.4 Hz, 1H) | 117.18 | 6.85 (d, J = 7.5 Hz, 1H) | 117.27 |
| 6′ | 7.35 (dd, J = 7.4, 2.1 Hz, 1H) | 126.84 | 7.35 (dd, J = 7.4, 2.1 Hz, 1H) | 125.99 |
| 3-O-β-D-glucoside | ||||
| 1′′ | 5.07 (s, 1H) | 102.50 | 5.02 (s, 1H) | 101.16 |
| 2′′ | 3.66 (d, J = 5.1 Hz, 1H) | 73.39 | 3.61 (d, J = 4.9 Hz, 1H) | 73.47 |
| 3′′ | 3.44 (d, J = 4.9 Hz, 1H) | 76.81 | 3.78 (d, J = 5.1 Hz, 1H) | 76.90 |
| 4′′ | 3.40 (d, J = 5.1 Hz, 1H) | 71.24 | 3.55 (d, J = 5.1 Hz, 1H) | 70.82 |
| 5′′ | 3.45 (s, 1H) | 78.04 | 3.72 (s, 1H) | 75.60 |
| 6′′ | 3.82–3.67 (m, 2H) | 62.21 | 4.31 (q, J = 12.6 Hz, 2H) | 64.90 |
| 6′′-O-dodecanoate | ||||
| CH2 (2′′′) | / | / | 2.33–2.26 (m, 2H) | 34.45 |
| CH2 (3′′′) | / | / | 1.50 (s, 2H) | 25.48 |
| CH2 (C4-C11) | / | / | 1.24–1.21 (m, 16H) | 22.90–31.98 |
| CH3 (C12) | / | / | 0.88 (s, 3H) | 14.22 |
| Indicators | Cy3glc | Cy3glc-C12 | |||||
|---|---|---|---|---|---|---|---|
| 65 °C | 80 °C | 95 °C | 65 °C | 80 °C | 95 °C | ||
| pH3 | k (h−1) | 0.078 ± 0.010 | 0.210 ± 0.004 | 0.347 ± 0.004 | 0.054 ± 0.010 | 0.066 ± 0.005 | 0.074 ± 0.005 |
| t1/2 (h) | 9.04 ± 1.25 | 3.30 ± 0.06 | 2.00 ± 0.02 | 13.15 ± 2.19 | 10.48 ± 0.75 | 9.44 ± 0.61 | |
| Ea (kJ·mol−1) | 52.11 ± 5.00 a | 13.97 ± 3.23 e | |||||
| pH5 | k (h−1) | 0.150 ± 0.011 | 0.210 ± 0.011 | 0.344 ± 0.006 | 0.077 ± 0.005 | 0.096 ± 0.010 | 0.106 ± 0.011 |
| t1/2 (h) | 4.65 ± 0.33 | 3.31 ± 0.18 | 2.01 ± 0.04 | 8.97 ± 0.60 | 7.29 ± 0.80 | 6.60 ± 0.74 | |
| Ea (kJ·mol−1) | 28.70 ± 2.42 c | 10.70 ± 1.53 e | |||||
| pH7 | k (h−1) | 0.179 ± 0.010 | 0.292 ± 0.004 | 0.342 ± 0.002 | 0.088 ± 0.004 | 0.203 ± 0.004 | 0.243 ± 0.011 |
| t1/2 (h) | 3.88 ± 0.21 | 2.37 ± 0.03 | 2.03 ± 0.01 | 7.91 ± 0.33 | 3.41 ± 0.06 | 2.86 ± 0.14 | |
| Ea (kJ·mol−1) | 22.57 ± 1.79 d | 35.44 ± 1.56 b | |||||
| Parameters | X0 (OD600nm) | Xm (OD600nm) | μm (h−1) | R2 of the Model |
|---|---|---|---|---|
| Bifidobacterium infantis | ||||
| CG | 0.019 ± 0.003 | 0.847 ± 0.006 | 0.504 ± 0.023 | 0.999 |
| Cy3glc | 0.029 ± 0.012ns | 0.960 ± 0.056* | 0.319 ± 0.079* | 0.974 |
| Cy3glc-C12 | 0.015 ± 0.014ns | 0.832 ± 0.029ns | 0.501 ± 0.151ns | 0.986 |
| Bifidobacterium adolescentis | ||||
| CG | 0.008 ± 0.002 | 0.889 ± 0.006 | 0.606 ± 0.019 | 0.999 |
| Cy3glc | 0.006 ± 0.002ns | 0.915 ± 0.018ns | 0.390 ± 0.038* | 0.997 |
| Cy3glc-C12 | 0.010 ± 0.001ns | 0.901 ± 0.006ns | 0.536 ± 0.018* | 0.999 |
| Bifidobacterium bifidum | ||||
| CG | 0.008 ± 0.002 | 0.926 ± 0.010 | 0.566 ± 0.033 | 0.993 |
| Cy3glc | 0.018 ± 0.006ns | 0.979 ± 0.007* | 0.477 ± 0.025* | 0.999 |
| Cy3glc-C12 | 0.009 ± 0.010ns | 0.927 ± 0.007ns | 0.671 ± 0.044* | 0.999 |
| Lactobacillus acidophilus | ||||
| CG | 0.001 ± 0.001 | 1.433 ± 0.006 | 0.805 ± 0.072 | 0.999 |
| Cy3glc | 0.001 ± 0.001ns | 1.562 ± 0.025* | 0.655 ± 0.054* | 0.999 |
| Cy3glc-C12 | 0.002 ± 0.001ns | 1.495 ± 0.015* | 0.609 ± 0.043* | 0.999 |
| Compound (Relative Content) | Retention Time (min) | Molecular ion (m/z) | Intestinal Probiotics | |||
|---|---|---|---|---|---|---|
| B. Infantis | B. Adolescentis | B. Bifidum | L. Acidophilus | |||
| benzoic acid | 11.26 | 179 | 1.05 | 1.18 | 1.14 | 1.47 |
| Phenylacetic acid | 12.39 | 193 | 0.03 | 0.01 | 0.02 | 0.01 |
| Phenylpropanoic acid | 15.00 | 104 | 0.10 | 0.31 | 0.29 | 0.14 |
| Mandelic acid | 16.18 | 179 | 0.02 | 0.05 | 0.05 | 0.05 |
| 4-Hydroxybenzaldehyde | 16.59 | 223 | 0.07 | 0.06 | 0.05 | 0.16 |
| Phenethylamine | 17.94 | 174 | 0.02 | 0.03 | 0.13 | 0.02 |
| 4-Hydroxyphenylethanol | 18.07 | 179 | 0.06 | 0.16 | 0.18 | 0.16 |
| Phenyllactic acid | 18.26 | 193 | 9.63 | 12.04 | 14.11 | 16.67 |
| Phenylalanine | 18.96 | 218 | 4.31 | 2.20 | 3.11 | 4.50 |
| 4-Hydroxybenzoic acid | 19.01 | 267 | 0.06 | 0.05 | 0.07 | 0.16 |
| 4-Hydroxyphenylacetic acid | 19.18 | 179 | 0.09 | 0.05 | 0.05 | 0.05 |
| 4-Hydroxyphenylpropionic acid | 20.89 | 179 | 0.11 | 0.17 | 0.18 | 0.15 |
| 3-Methoxy-4-hydroxybenzoicacid | 20.93 | 297 | 0.08 | 0.03 | 0.03 | 0.12 |
| 4-Aminobenzoic acid | 21.79 | 266 | 0.02 | 0.03 | 0.03 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Sun, H.; Tu, L.; Jin, Y.; Zhang, Z.; Wang, M.; Liu, S.; Wang, Y.; He, S. Kinetics of Enzymatic Synthesis of Cyanidin-3-Glucoside Lauryl Ester and Its Physicochemical Property and Proliferative Effect on Intestinal Probiotics. Biology 2020, 9, 205. https://doi.org/10.3390/biology9080205
Yang X, Sun H, Tu L, Jin Y, Zhang Z, Wang M, Liu S, Wang Y, He S. Kinetics of Enzymatic Synthesis of Cyanidin-3-Glucoside Lauryl Ester and Its Physicochemical Property and Proliferative Effect on Intestinal Probiotics. Biology. 2020; 9(8):205. https://doi.org/10.3390/biology9080205
Chicago/Turabian StyleYang, Xi, Hanju Sun, Lijun Tu, Yuan Jin, Zuoyong Zhang, Muwen Wang, Shuyun Liu, Ying Wang, and Shudong He. 2020. "Kinetics of Enzymatic Synthesis of Cyanidin-3-Glucoside Lauryl Ester and Its Physicochemical Property and Proliferative Effect on Intestinal Probiotics" Biology 9, no. 8: 205. https://doi.org/10.3390/biology9080205
APA StyleYang, X., Sun, H., Tu, L., Jin, Y., Zhang, Z., Wang, M., Liu, S., Wang, Y., & He, S. (2020). Kinetics of Enzymatic Synthesis of Cyanidin-3-Glucoside Lauryl Ester and Its Physicochemical Property and Proliferative Effect on Intestinal Probiotics. Biology, 9(8), 205. https://doi.org/10.3390/biology9080205








