Development of a Low-Cost and High-Efficiency Culture Medium for Bacteriocin Lac-B23 Production by Lactobacillus plantarum J23
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Bacteriocin Assays and Cell Density Measurements
2.3. Influence of Medium Compositions on Bacteriocin Lac-B23 Production and Bacterial Growth
2.4. Plackett-Burman (PB) Design
2.5. Steepest Ascent Experiment
2.6. Central Composite Design (CCD)
3. Results
3.1. Effects of the Composition of MRS Broth on Bacteriocin Lac-B23 Production and Bacterial Growth
3.2. Screening of Significant Variables Using Plackett-Burman Design
3.3. Steepest Ascent Experiment
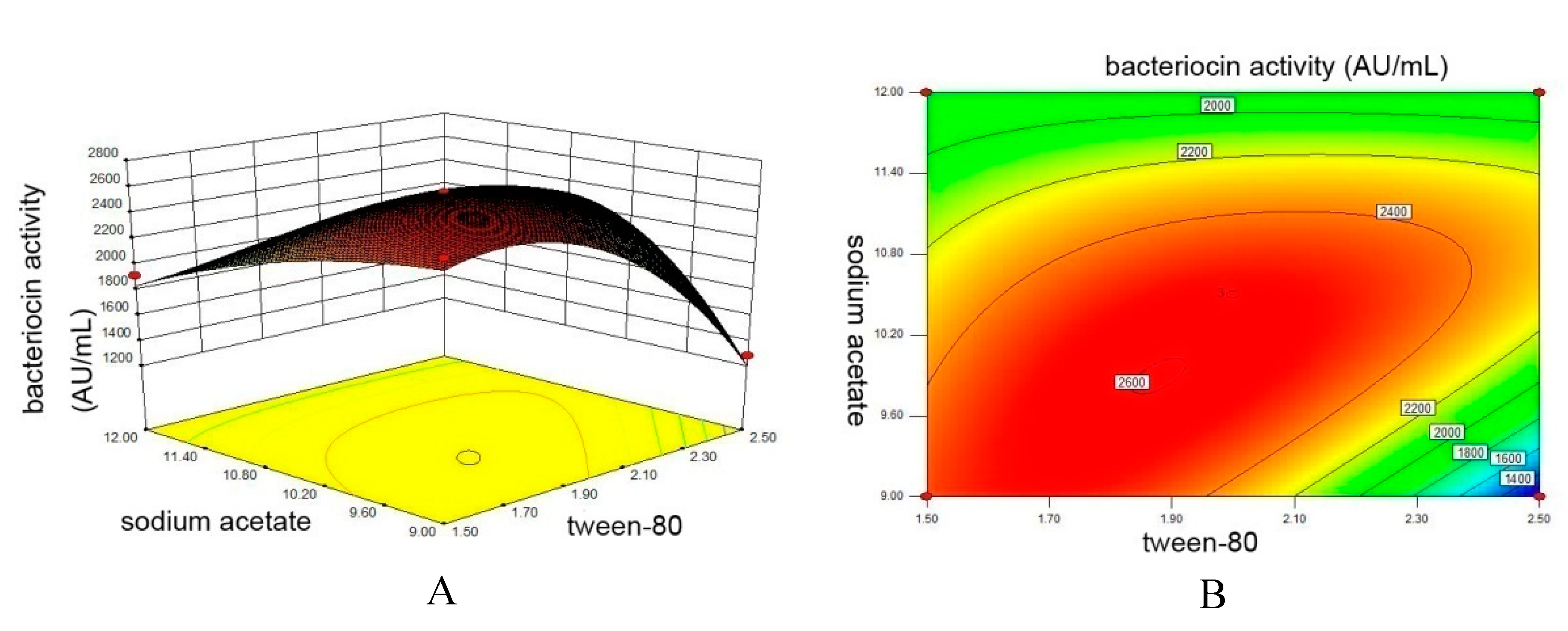
3.4. Central Composite Design Experiment
3.5. Comparison of MRS Broth and Modified MRS in Terms of Cost and Bacteriocin Lac-B23 Production Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Zhang, L.W.; Yi, H.X.; Gao, W.; Chi, C. A novel enterocin T1 with anti-Pseudomonas activity produced by Enterococcus faecium T1 from Chinese Tibet cheese. World J. Microb. Biotechnol. 2016, 32, 21. [Google Scholar] [CrossRef] [PubMed]
- Varish, A.; Mohd, S.K.; Qazi, M.S.J.; Mohammed, A.A.; Mohammad, A.A.K.; Mughees, U.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar]
- Veeresh, J.; Wu, C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar]
- Cao, S.; Du, R.P.; Zhao, K.; Xiao, H.Z.; Han, Y.; Zhou, Z.J. The mode of action of bacteriocin CHQS, a high antibacterial activity bacteriocin produced by Enterococcus faecalis TG2. Food Control 2019, 96, 470–478. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D. Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2003, 2, 82–100. [Google Scholar]
- Beshkova, D.; Frengova, G. Bacteriocins from lactic acid bacteria: Microorganisms of potential biotechnological importance for the dairy industry. Eng. Life Sci. 2012, 12, 419–432. [Google Scholar] [CrossRef]
- O’Shea, E.F.; Cotter, P.D.; Ross, R.P.; Hill, C. Strategies to improve the bacteriocin protection provided by lactic acid bacteria. Curr. Opin. Biotechnol. 2013, 24, 130–134. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Jurkiewicz, C.H.; Franco, B.D.G.M. Bacteriocin production by Lactobacillus Curvatus mbsa2 entrapped in calcium alginate during ripening of salami for control of Listeria monocytogenes. Food Control 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Zhang, J.M.; Yi, H.Y.; Gong, P.M.; Lin, K.; Chen, S.W.; Han, X.; Zhang, L.W. Adsorption of plantaricin Q7 on montmorillonite and application in feedback regulation of plantaricin Q7 synthesis by Lactobacillus plantarum Q7. Eng. Life Sci. 2018, 19, 57–65. [Google Scholar] [CrossRef]
- Bogovi-Matijai, B.; Rogelj, I. Bacteriocin complex of Lactobacillus acidophilus LF221-production studies in MRS media at different pH values and effect against Lactobacillus helveticus ATCC 15009. Process. Biochem. 1988, 33, 345–352. [Google Scholar] [CrossRef]
- Kim, M.H.; Kong, Y.J.; Baek, H.; Hyun, H.H. Optimization of culture conditions and medium composition for the production of Micrococcin go5 by Micrococcus sp. go5. J. Biotechnol. 2006, 121, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Valmorri, S.; Suzzi, G.; Corsetti, A. The role of environmental factors and medium composition on bacteriocin-like inhibitory substances (blis) production by Enterococcus mundtii strains. Food Microbiol. 2008, 25, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Rollini, M.; Manzoni, M. Development of a low cost culture medium for sakacin a production by L. sakei. Process. Biochem. 2008, 43, 1275–1280. [Google Scholar] [CrossRef]
- Gao, J.; Atiyeh, H.K.; Phillips, J.R.; Wilkins, R.M.; Huhnke, L.R. Development of low cost medium for ethanol production from syngas by Clostridium ragsdalei. Bioresour. Technol. 2013, 147, 508–515. [Google Scholar] [CrossRef]
- Zhang, J.M.; Yang, Y.Y.; Yang, H.; Bu, Y.; Yi, H.X.; Zhang, L.W.; Han, X.; Ai, L.Z. Purification and partial characterization of bacteriocin Lac-B23, a novel bacteriocin production by Lactobacillus plantarum J23, isolated from Chinese traditional fermented milk. Front. Microbiol. 2018, 9, 2165. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Dang, Y.; Shu, G. Optimization for immobilization of β-galactosidase using plackett-burman design and steepest ascent method. J. Chem. Pharm. Res. 2014, 6, 612–616. [Google Scholar]
- Song, X.; Zhang, X.; Kuang, C.; Zhu, L.; Guo, N. Optimization of fermentation parameters for the biomass and dha production of schizochytrium limacinum ouc88 using response surface methodology. Process. Biochem. 2007, 42, 1391–1397. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Chobert, J.M.; HKittikun, A. Bacteriocin-producing lactic acid bacteria isolated from mangrove forests in southern thailand as potential bio-control agents in food: Isolation, screening and optimization. Food Control 2014, 41, 202–211. [Google Scholar] [CrossRef]
- Schirru, S.; Favaro, L.; Nicoletta, P.M. Comparison of bacteriocins production from Enterococcus faecium strains in cheese whey and optimised commercial MRS medium. Ann. Microbiol. 2014, 64, 321–331. [Google Scholar] [CrossRef]
- Zhang, J.M.; Han, X.; Zhang, L.W.; Yi, H.X.; Chen, S.W.; Gong, P.M. Effects of fructose and overexpression of shock-related gene grol on plantaricin Q7 production. Probiotics Antimicrob. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Nikiforova, O.A.; Klykov, S.; Volski, A.; Dicks, L.M.T.; Chikindas, M.L. Subtilosin a production by Bacillus subtiliskatmira1933 and colony morphology are influenced by the growth medium. Ann. Microbiol. 2016, 66, 661–671. [Google Scholar] [CrossRef]
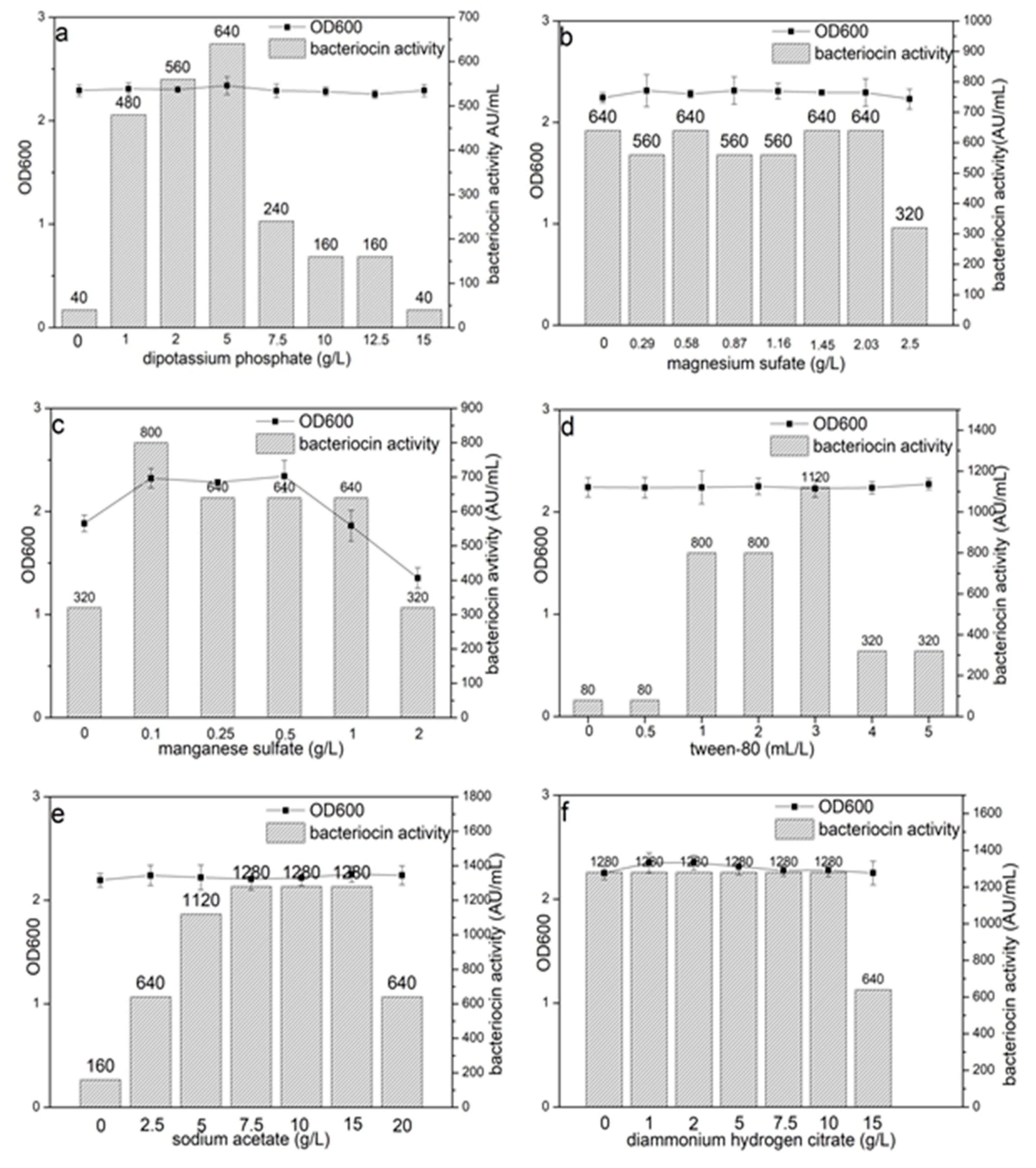
| Compositions | Concentration g/L | OD600 ± SD a | Bacteriocin Activity AU/mL a |
|---|---|---|---|
| control (without carbon source) | 0 | 0.314 ± 0.051 | 0 |
| arabinose | 20 | 0.260 ± 0.039 | 0 |
| xylose | 20 | 0.327 ± 0.022 | 0 |
| sucrose | 20 | 0.635 ± 0.047 | 20 |
| fructose | 20 | 1.994 ± 0.066 | 80 |
| galactose | 20 | 2.052 ± 0.066 | 160 |
| lactose | 20 | 2.042 ± 0.049 | 160 |
| glucose | 5 | 1.716 ± 0.076 | 80 |
| glucose | 10 | 2.023 ± 0.106 | 80 |
| glucose | 15 | 2.193 ± 0.042 | 240 |
| glucose | 20 | 2.312 ± 0.061 | 280 |
| glucose | 25 | 2.211 ± 0.069 | 280 |
| glucose | 30 | 2.260 ± 0.105 | 280 |
| glucose | 35 | 2.240 ± 0.900 | 280 |
| glucose | 40 | 2.213 ± 0.109 | 160 |
| maltose | 5 | 1.820 ± 0.073 | 160 |
| maltose | 10 | 2.128 ± 0.080 | 160 |
| maltose | 15 | 2.257 ± 0.065 | 160 |
| maltose | 20 | 2.294 ± 0.136 | 240 |
| maltose | 25 | 2.283 ± 0.061 | 240 |
| maltose | 30 | 2.302 ± 0.047 | 280 |
| maltose | 35 | 2.275 ± 0.044 | 280 |
| maltose | 40 | 2.313 ± 0.026 | 240 |
| control (without nitrogen source) | 0 | 0.214 ± 0.062 | 0 |
| beef extract powder | 7.5 | 1.918 ± 0.046 | 40 |
| peptone | 10 | 1.839 ± 0.060 | 80 |
| yeast extract | 1 | 1.460 ± 0.084 | 40 |
| yeast extract | 2.5 | 1.989 ± 0.071 | 160 |
| yeast extract | 5 | 2.223 ± 0.087 | 280 |
| yeast extract | 7.5 | 2.302 ± 0.034 | 280 |
| yeast extract | 10 | 2.318 ± 0.051 | 560 |
| yeast extract | 15 | 2.348 ± 0.093 | 280 |
| yeast extract | 20 | 2.341 ± 0.029 | 240 |
| beef extract powder + Yeast extract | 7.5 + 5 | 2.248 ± 0.050 | 240 |
| Peptone + beef extract powder | 10 + 7.5 | 2.097 ± 0.059 | 280 |
| Peptone + beef extract | 10 + 7.5 + 5 | 2.263 ± 2.263 | 280 |
| Peptone + yeast extract | 10 + 5 | 2.236 ± 0.078 | 320 |
| Peptone + yeast extract | 2.5 + 10 | 2.353 ± 0.052 | 400 |
| Peptone + yeast extract | 5 + 10 | 2.334 ± 0.078 | 240 |
| Peptone + yeast extract | 7.5 + 10 | 2.374 ± 0.053 | 240 |
| Peptone + yeast extract | 10 + 10 | 2.359 ± 0.103 | 320 |
| Peptone + yeast extract | 12.5 + 10 | 2.337 ± 0.018 | 400 |
| Peptone + yeast extract | 15 + 10 | 2.343 ± 0.056 | 480 |
| Peptone + yeast extract | 17.5 + 10 | 2.354 ± 0.055 | 480 |
| Peptone + yeast extract | 20 + 10 | 2.367 ± 0.085 | 400 |
| Variable | Real Variables/(Unit) | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| X1 | glucose/(g/L) | 15 | 25 |
| X2 | yeast extract/(g/L) | 7 | 13 |
| X3 | dipotassium phosphate/(g/L) | 3 | 6 |
| X4 | manganese sulfate/(g/L) | 0.08 | 0.16 |
| X5 | Tween 80/(mL/L) | 2 | 4 |
| X6 | sodium acetate/(g/L) | 5 | 10 |
| Variable | Real Variables/(Unit) | Level | ||||
|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | +1 | +1.414 | ||
| X5 | Tween 80/(mL/L) | 1.28 | 1.5 | 2 | 2.5 | 2.71 |
| X6 | sodium acetate/(g/L) | 8.38 | 9 | 10.5 | 12 | 12.62 |
| Run | X1 | X2 | X3 | X4 | X5 | X6 | Bacteriocin Activity (AU/mL) a |
|---|---|---|---|---|---|---|---|
| 1 | +1 | −1 | −1 | −1 | +1 | −1 | 640 |
| 2 | −1 | +1 | +1 | −1 | +1 | +1 | 960 |
| 3 | +1 | +1 | −1 | +1 | +1 | +1 | 1280 |
| 4 | +1 | −1 | +1 | +1 | +1 | −1 | 640 |
| 5 | −1 | −1 | +1 | −1 | +1 | +1 | 1280 |
| 6 | +1 | +1 | +1 | −1 | −1 | −1 | 1280 |
| 7 | +1 | −1 | +1 | +1 | −1 | +1 | 1280 |
| 8 | −1 | −1 | −1 | −1 | −1 | −1 | 960 |
| 9 | −1 | −1 | −1 | +1 | −1 | +1 | 1920 |
| 10 | −1 | +1 | +1 | +1 | −1 | −1 | 960 |
| 11 | +1 | +1 | −1 | −1 | −1 | +1 | 2560 |
| 12 | −1 | +1 | −1 | +1 | +1 | −1 | 960 |
| Factors | Coefficient Estimates | p-Values | % Contributions |
|---|---|---|---|
| Intercept | 1226.67 | 0.0465 * | |
| X1 | 53.33 | 0.2929 | 1.05 |
| X2 | 106.67 | 0.1056 | 4.21 |
| X3 | −160.00 | 0.0513 | 9.47 |
| X4 | −53.33 | 0.2929 | 1.05 |
| X5 | −266.67 | 0.0194 * | 26.32 |
| X6 | 320.00 | 0.0136 * | 37.69 |
| Experiment | Tween 80 (mL/L) | Sodium Acetate (g/L) | Bacteriocin Activity (AU/mL) a |
|---|---|---|---|
| 1 | 4 | 4.5 | 640 |
| 2 | 3.5 | 6.0 | 1280 |
| 3 | 3 | 7.5 | 1920 |
| 4 | 2.5 | 9.0 | 1920 |
| 5 | 2 | 10.5 | 2560 |
| 6 | 1.5 | 12.0 | 1920 |
| 7 | 1 | 13.5 | 640 |
| Run | X5 | X6 | Bacteriocin Activity (AU/mL) a | |
|---|---|---|---|---|
| Expected | Observed | |||
| 1 | 0 | −1.414 | 2000 | 1920 |
| 2 | +1.414 | 0 | 2000 | 1920 |
| 3 | 0 | +1.414 | 1360 | 1280 |
| 4 | −1 | −1 | 2480 | 2560 |
| 5 | −1 | +1 | 1840 | 1920 |
| 6 | +1 | −1 | 1200 | 1280 |
| 7 | 0 | 0 | 2560 | 2560 |
| 8 | −1.414 | 0 | 2000 | 1920 |
| 9 | 0 | 0 | 2560 | 2560 |
| 10 | 0 | 0 | 2560 | 2560 |
| 11 | +1 | +1 | 1840 | 1920 |
| Source | Sum of Squares | Degrees of Freedom | f-Value | Prob (p) > f |
|---|---|---|---|---|
| model | 2.257 × 106 | 7 | 18.90 | 0.0174 * |
| X5 | 0.000 | 1 | 0.00 | 1.0000 |
| X6 | 2.048 × 105 | 1 | 12.00 | 0.0405 * |
| X5 * X6 | 4.096 × 105 | 1 | 24.00 | 0.0163 * |
| X52 | 4.427 × 105 | 1 | 25.94 | 0.0146 * |
| X62 | 1.093 × 106 | 1 | 64.06 | 0.0041 ** |
| X52 * X6 | 1.024 × 105 | 1 | 6.00 | 0.0917 |
| X5 * X62 | 2.048 × 105 | 1 | 12.00 | 0.0405 * |
| R-Squared | 0.9778 | |||
| Components a | Price (RMB/g or mL) b | MRS Broth | Modified MRS (mMRS) | ||
|---|---|---|---|---|---|
| Concentration (g or mL/L) | Price of MRS Broth (RMB/L) | Concentration (g or mL/L) | Price of mMRS (RMB/L) | ||
| glucose | 59/500 | 20 | 2.36 | 20 | 2.36 |
| peptone | 139/500 | 10 | 2.78 | 0 | 0 |
| beef extract powder | 299/500 | 7.5 | 4.49 | 0 | 0 |
| yeast extract | 199/500 | 5 | 1.99 | 10 | 3.98 |
| dipotassium phosphate | 79/500 | 2 | 0.32 | 5 | 0.79 |
| Magnesium sulfate heptahydrate | 49/500 | 0.58 | 0.06 | 0 | 0 |
| manganese sulfate monohydrate | 49/500 | 0.25 | 0.02 | 0.1 | 0.01 |
| Tween 80 | 119/500 | 1 | 0.24 | 1.88 | 0.45 |
| Sodium acetate anhydrous | 49/500 | 5 | 0.49 | 9.9 | 0.97 |
| diammonium hydrogen citrate | 89/500 | 2 | 0.36 | 0 | 0 |
| Total medium cost | 13.11 RMB/L | 8.56 RMB/L | |||
| bacteriocin Lac-B23 production | 280 AU/mL | 2560 AU/mL | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Bu, Y.; Zhang, C.; Yi, H.; Liu, D.; Jiao, J. Development of a Low-Cost and High-Efficiency Culture Medium for Bacteriocin Lac-B23 Production by Lactobacillus plantarum J23. Biology 2020, 9, 171. https://doi.org/10.3390/biology9070171
Zhang J, Bu Y, Zhang C, Yi H, Liu D, Jiao J. Development of a Low-Cost and High-Efficiency Culture Medium for Bacteriocin Lac-B23 Production by Lactobacillus plantarum J23. Biology. 2020; 9(7):171. https://doi.org/10.3390/biology9070171
Chicago/Turabian StyleZhang, Jianming, Yushan Bu, Chengcheng Zhang, Huaxi Yi, Daqun Liu, and Jingkai Jiao. 2020. "Development of a Low-Cost and High-Efficiency Culture Medium for Bacteriocin Lac-B23 Production by Lactobacillus plantarum J23" Biology 9, no. 7: 171. https://doi.org/10.3390/biology9070171
APA StyleZhang, J., Bu, Y., Zhang, C., Yi, H., Liu, D., & Jiao, J. (2020). Development of a Low-Cost and High-Efficiency Culture Medium for Bacteriocin Lac-B23 Production by Lactobacillus plantarum J23. Biology, 9(7), 171. https://doi.org/10.3390/biology9070171





