Assessment of Cytocompatibility and Anti-Inflammatory (Inter)Actions of Genipin-Crosslinked Chitosan Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Material Powders
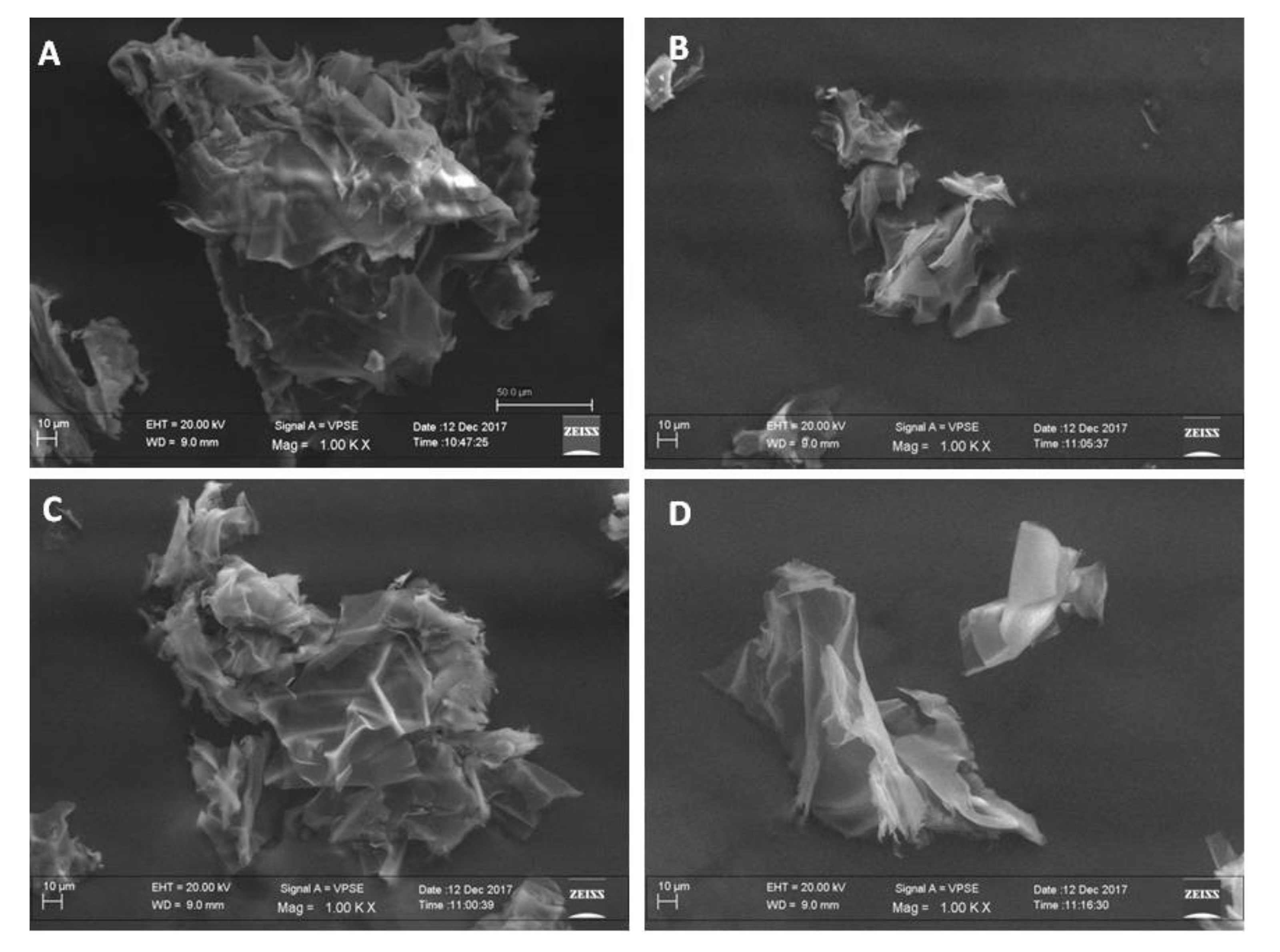
2.3. Morphological Analysis of Powders by Scanning Electron Microscopy (SEM)
2.4. Cell Culture
2.5. Evaluation of Cell Viability by MTT Assays
2.6. Hematoxylin/Eosin Staining of MG3 Cells
2.7. PMA (Phorbol 12-Myristate 13-Acetate) Treatments of THP-1 Cells
2.8. Statistical Analysis
3. Results and Discussions
3.1. Morphological Analysis of Material Powders by Using SEM
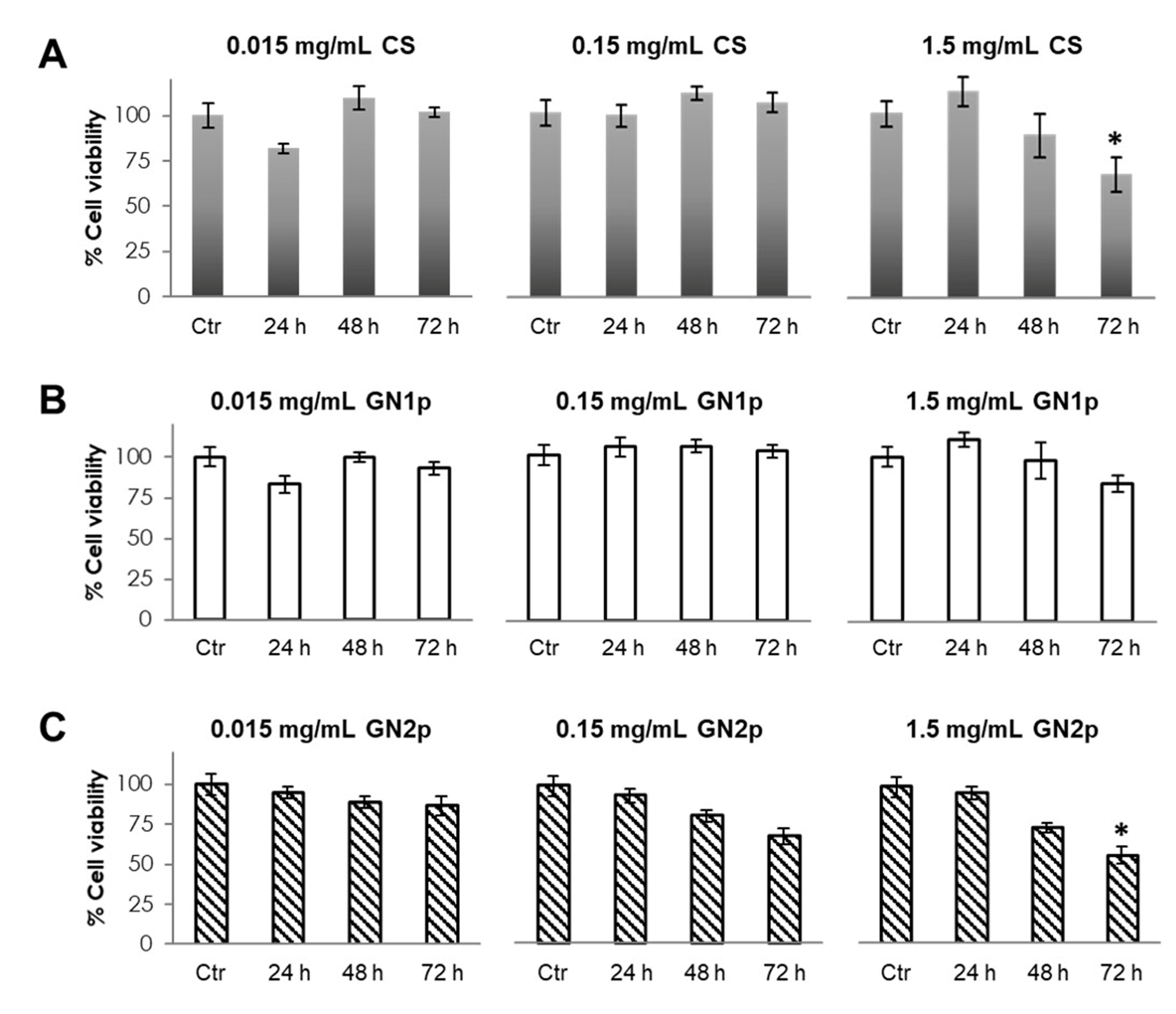
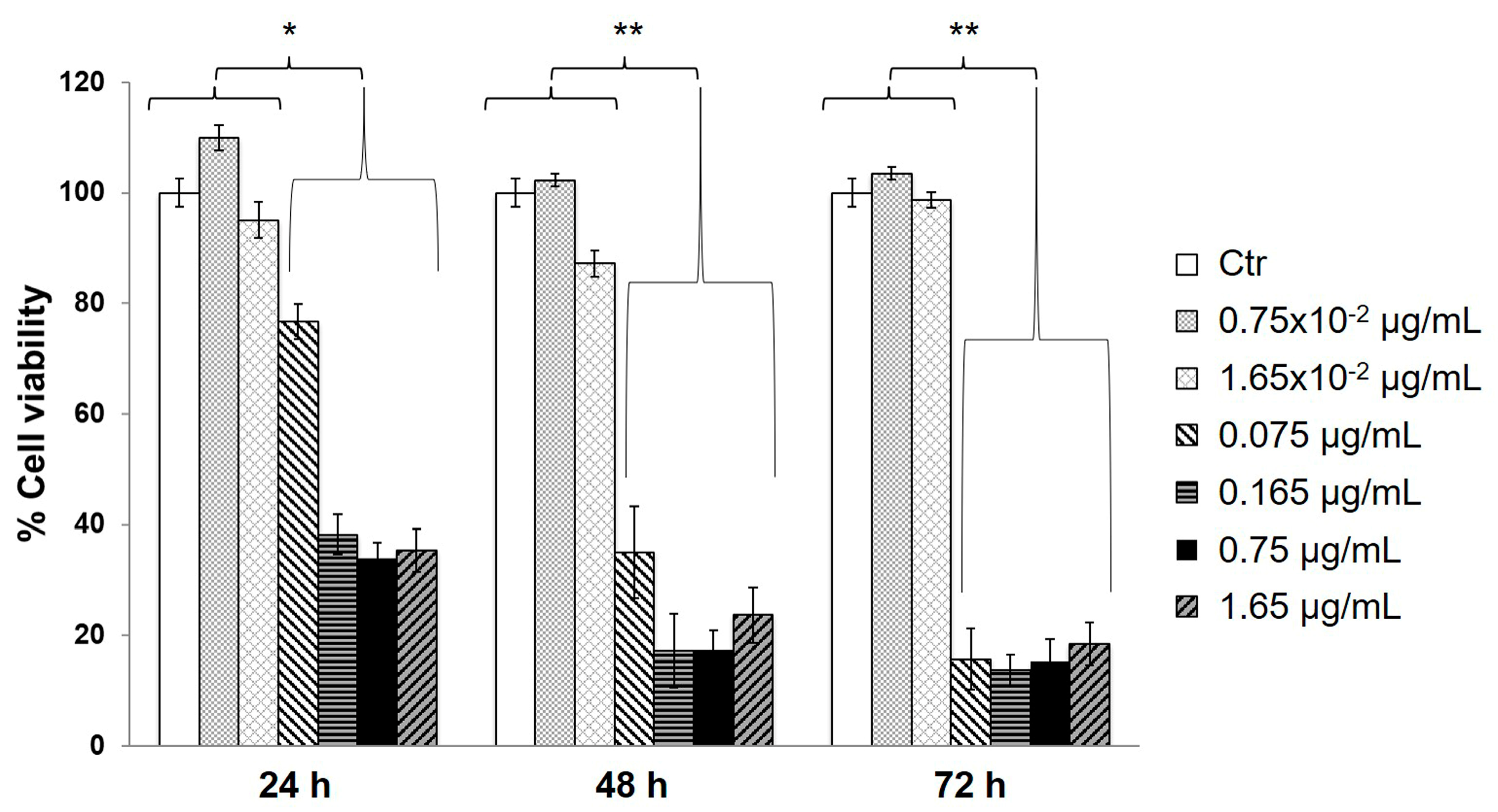
3.2. Evaluation of Cell Viability in the Presence of GNps, CS Powder and Genipin
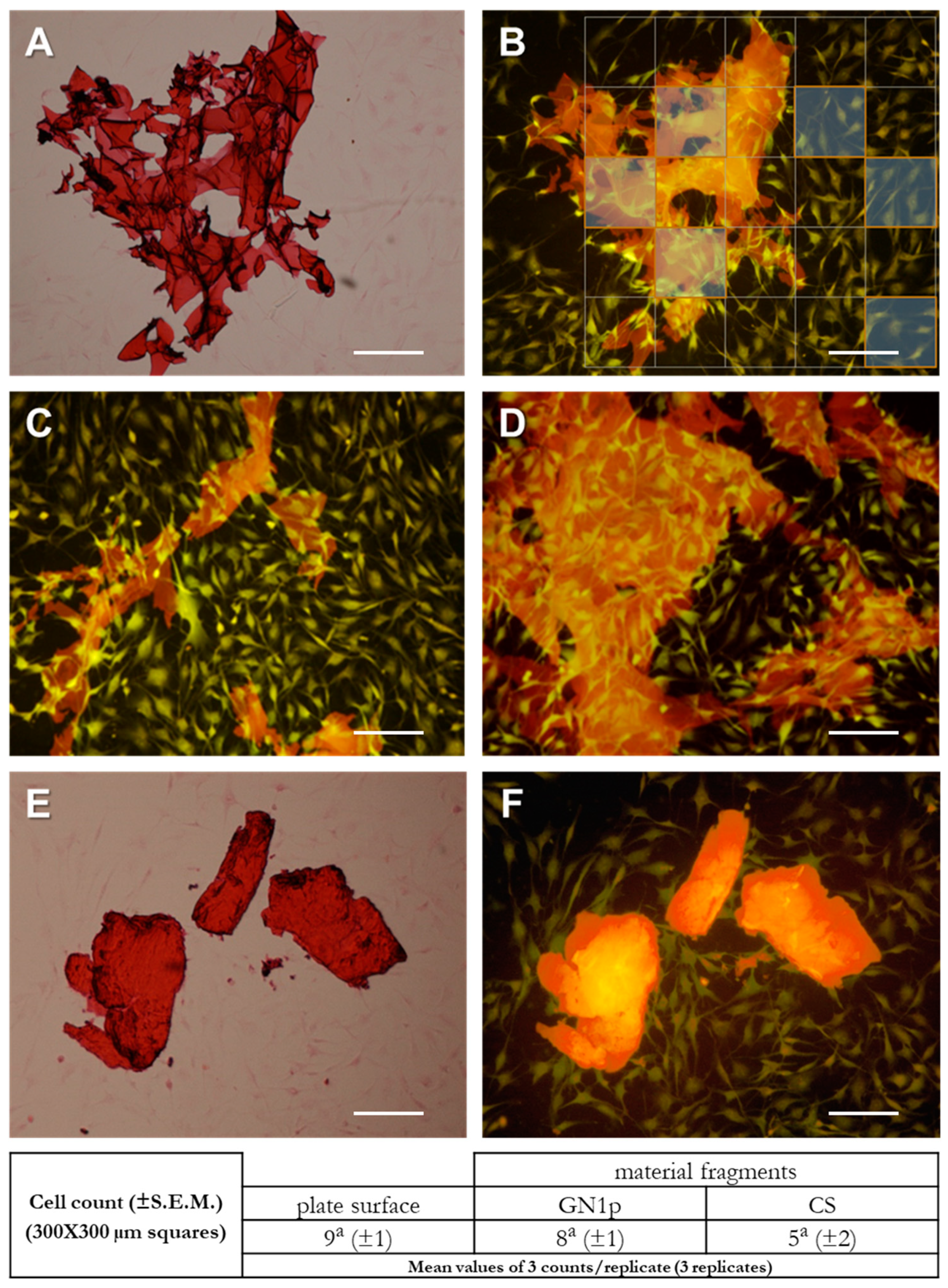
3.3. Evaluation of Cell–Material Interactions by Imaging of Osteoblast-Like Cells Cultured in the Presence of CS and GN1p Powders
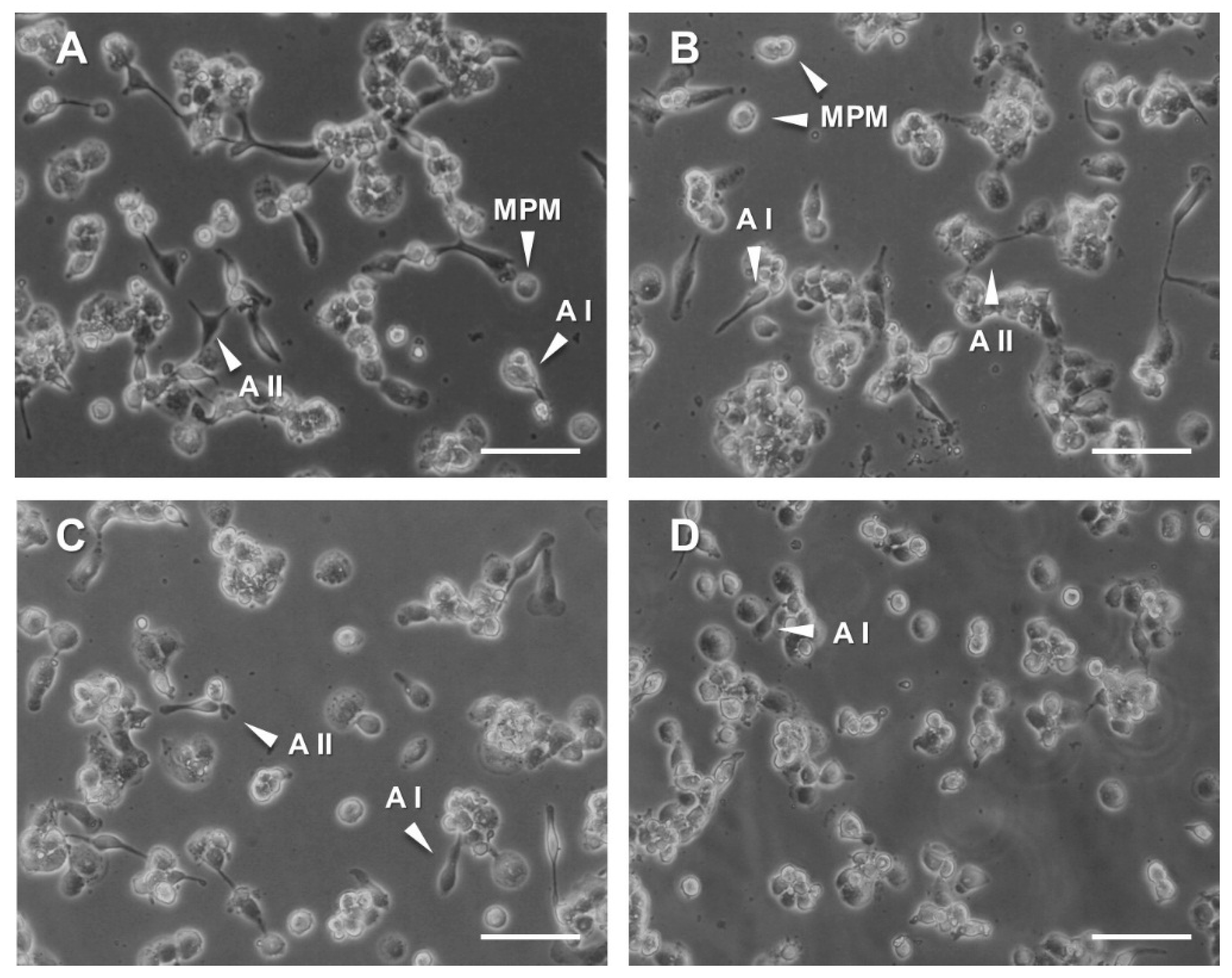
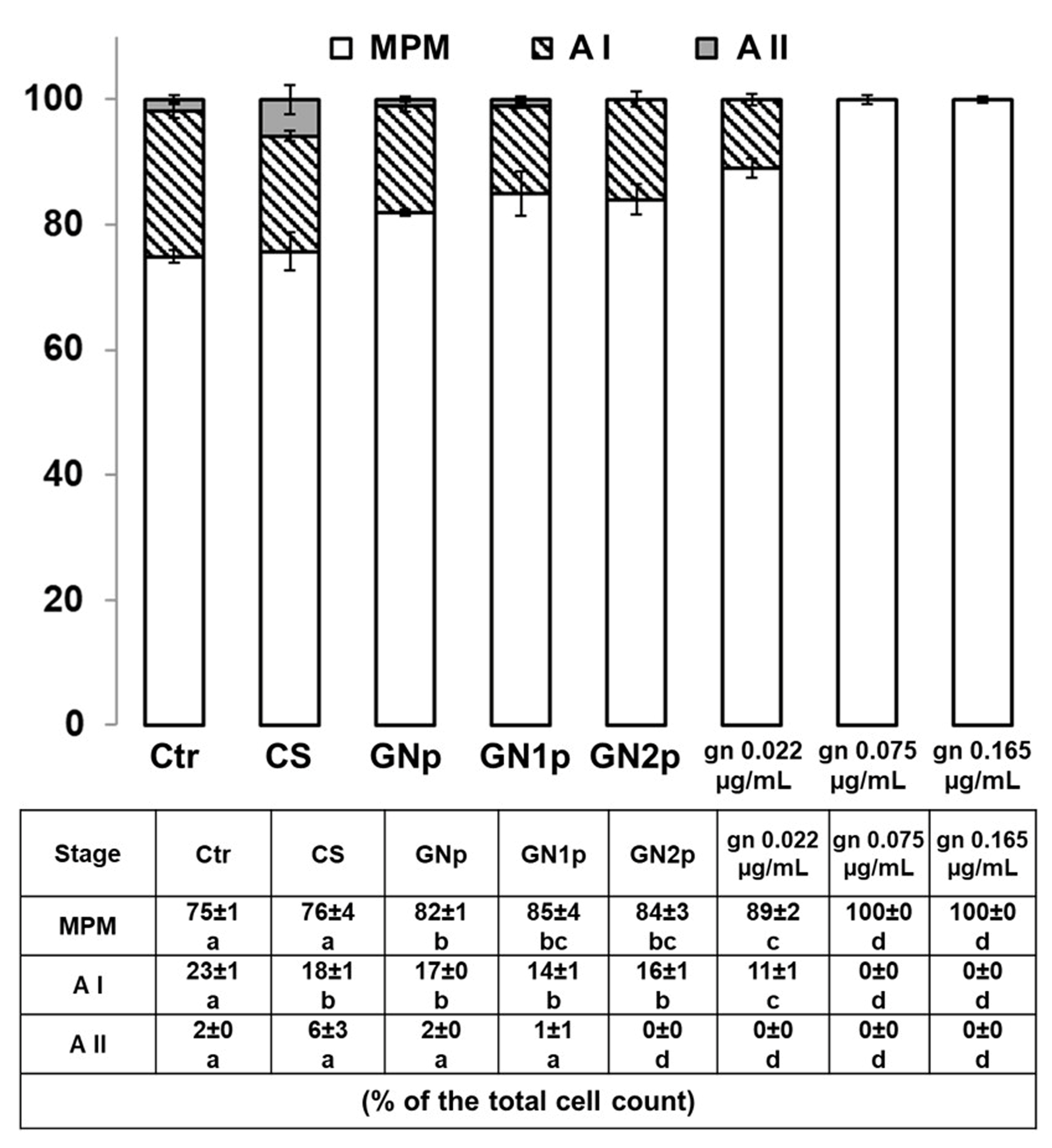
3.4. Analysis of Pro-/Anti-Inflammatory Responses in THP-1 Monocyte-Like Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramya, R.; Venkatesan, J.; Kim, S.K.; Sudha, P.N. Biomedical Applications of Chitosan: An Overview. J. Biomater. Tissue Eng. 2012, 2, 100–111. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 475–485. [Google Scholar]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Nady, N.; Kandil, S.H. Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications. Membranes 2018, 8, 2. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-Based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Seol, Y.J.; Lee, J.Y.; Park, Y.J.; Lee, Y.M.; Young-Ku Rhyu, I.C.; Lee, S.J.; Han, S.B.; Chung, C.P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-Based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Koppolu, B.P.; Smith, S.G.; Zaharoff, D.A. Effect of Chitosan Properties on Immunoreactivity. Mar. Drugs. 2016, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Future Sci. OA 2017, 4, FSO225. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015, 13, 1133–1174. [Google Scholar] [PubMed]
- Nishimura, K.; Nishimura, S.; Nishi, N.; Saiki, I.; Tokura, S.; Azuma, I. Immunological activity of chitin and its derivatives. Vaccine 1984, 2, 93–99. [Google Scholar] [CrossRef]
- Bueter, C.L.; Lee, C.K.; Wang, J.P.; Ostroff, G.R.; Specht, C.A.; Levitz, S.M. Spectrum and mechanisms of inflammasome activation by chitosan. J. Immunol. 2014, 192, 5943–5951. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Wang, C.; Lau, T.T.; Loh, W.L.; Su, K.; Wang, D.A. Cytocompatibility study of a natural biomaterial crosslinker--Genipin with therapeutic model cells. J. Biomed. Mater Res. B Appl. Biomater. 2011, 97, 58–65. [Google Scholar] [CrossRef]
- Mi, F.L.; Tan, Y.C.; Liang, H.C.; Huang, R.N.; Sung, H.W. In vitro evaluation of a chitosan membrane cross-linked with genipin. J. Biomater. Sci. Polym. Ed. 2001, 12, 835–850. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Yu, S.X.; Du, C.T.; Chen, W.; Lei, Q.Q.; Li, N.; Qi, S.; Zhang, X.J.; Hu, G.Q.; Deng, X.M.; Han, W.Y.; et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 17935. [Google Scholar] [CrossRef]
- Dimida, S.; Barca, A.; Cancelli, N.; De Benedictis, V.; Raucci, M.G.; Demitri, C. Effects of Genipin Concentration on Cross-Linked Chitosan Scaffolds for Bone Tissue Engineering: Structural Characterization and Evidence of Biocompatibility Features. Int. J. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Zhang, Z.; Winkler, T.; Mour, M.; Gunter, C.; Morlock, M.; Machens, H.G.; Schilling, A.F. Bioresorption and degradation of biomaterials. Adv. Biochem. Eng. Biotechnol. 2012, 126, 317–333. [Google Scholar] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.; Herrera, D.R.; Almeida, J.F.; Ferraz, C.C.; Gomes, B.P.; Zaia, A.A. Evaluation of cytotoxicity and up-Regulation of gelatinases in fibroblast cells by three root repair materials. Int. Endod. J. 2012, 45, 815–820. [Google Scholar] [CrossRef]
- Zhang, B.; Ni, H.; Chen, R.; Zhang, T.; Li, X.; Zhan, W.; Wang, Z.; Xu, Y. Cytotoxicity effects of three-dimensional graphene in NIH-3T3 fibroblasts. RSC Adv. 2016, 6, 45093–45102. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; No Abstract Available. Available online: http://www.ncbi.nlm.nih.gov/books/NBK500148/ (accessed on 7 July 2020).
- Castell, J.V.; Gómez-Lechón, M.J. In Vitro Methods in Pharmaceutical Research; Elsevier, Academic Press: Cambridge, MA, USA, 1996; ISBN 978-0-12-163390-5. [Google Scholar] [CrossRef]
- Fessel, G.; Cadby, J.; Wunderli, S.; van Weeren, R.; Snedeker, J.G. Dose- and time-Dependent effects of genipin crosslinking on cell viability and tissue mechanics-Toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef]
- Merten, O.W. Advances in cell culture: Anchorage dependence. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140040. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.J.; Lee, W.K.; Ko, J.S.; Kim, H.M. MG63 osteoblastic cell adhesion to the hydrophobic surface precoated with recombinant osteopontin fragments. Biomaterials 2003, 24, 1059–1066. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, J.; Wei, D.; Zhu, Y.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Establishing a cell-Affinitive interface and spreading space in a 3D hydrogel by introduction of microcarriers and an enzyme. J. Mater. Chem. B 2014, 2, 6601–6610. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, N. Mesoscopic science, where materials become life and life inspires materials. A great opportunity to push back the frontiers of life, materials, and biomaterials sciences. Biomater. Sci. 2013, 1, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Rep. 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility In Advances in Biomaterials Science and Biomedical Applications; Intech: London, UK, 2013; pp. 207–240. [Google Scholar]
- Sokol, R.J.; Hudson, G.; James, N.T.; Frost, I.J.; Wales, J. Human macrophage development: A morphometric study. J. Anat. 1987, 151, 27–35. [Google Scholar]
- Wright, P.C.; Qin, H.; Choi, M.M.; Chiu, N.H.; Jia, Z. Carbon nanodots interference with lactate dehydrogenase assay in human monocyte THP-1 cells. Springerplus 2014, 3, 615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasidhar, M.V.; Chevooru, S.K.; Eickelberg, O.; Hartung, H.P.; Neuhaus, O. Downregulation of monocytic differentiation via modulation of CD147 by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. PLoS ONE 2017, 12, e0189701. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.M.; Brachova, L.; Higgins, K.; Obermiller, L.; Sevanian, A.; Khandrika, S.; Reaven, P.D. Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J. Lipid Res. 2002, 43, 26–35. [Google Scholar]
- Gogulamudi, V.R.; Dubey, M.L.; Kaul, D.; Atluri, V.S.; Sehgal, R. Downregulation of host tryptophan-aspartate containing coat (TACO) gene restricts the entry and survival of Leishmania donovani in human macrophage model. Front. Microbiol. 2015, 6, 946. [Google Scholar] [CrossRef][Green Version]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-Stimulated THP-1 cells and monocyte-Derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimida, S.; Santin, M.; Verri, T.; Barca, A.; Demitri, C. Assessment of Cytocompatibility and Anti-Inflammatory (Inter)Actions of Genipin-Crosslinked Chitosan Powders. Biology 2020, 9, 159. https://doi.org/10.3390/biology9070159
Dimida S, Santin M, Verri T, Barca A, Demitri C. Assessment of Cytocompatibility and Anti-Inflammatory (Inter)Actions of Genipin-Crosslinked Chitosan Powders. Biology. 2020; 9(7):159. https://doi.org/10.3390/biology9070159
Chicago/Turabian StyleDimida, Simona, Matteo Santin, Tiziano Verri, Amilcare Barca, and Christian Demitri. 2020. "Assessment of Cytocompatibility and Anti-Inflammatory (Inter)Actions of Genipin-Crosslinked Chitosan Powders" Biology 9, no. 7: 159. https://doi.org/10.3390/biology9070159
APA StyleDimida, S., Santin, M., Verri, T., Barca, A., & Demitri, C. (2020). Assessment of Cytocompatibility and Anti-Inflammatory (Inter)Actions of Genipin-Crosslinked Chitosan Powders. Biology, 9(7), 159. https://doi.org/10.3390/biology9070159







