Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy
Abstract
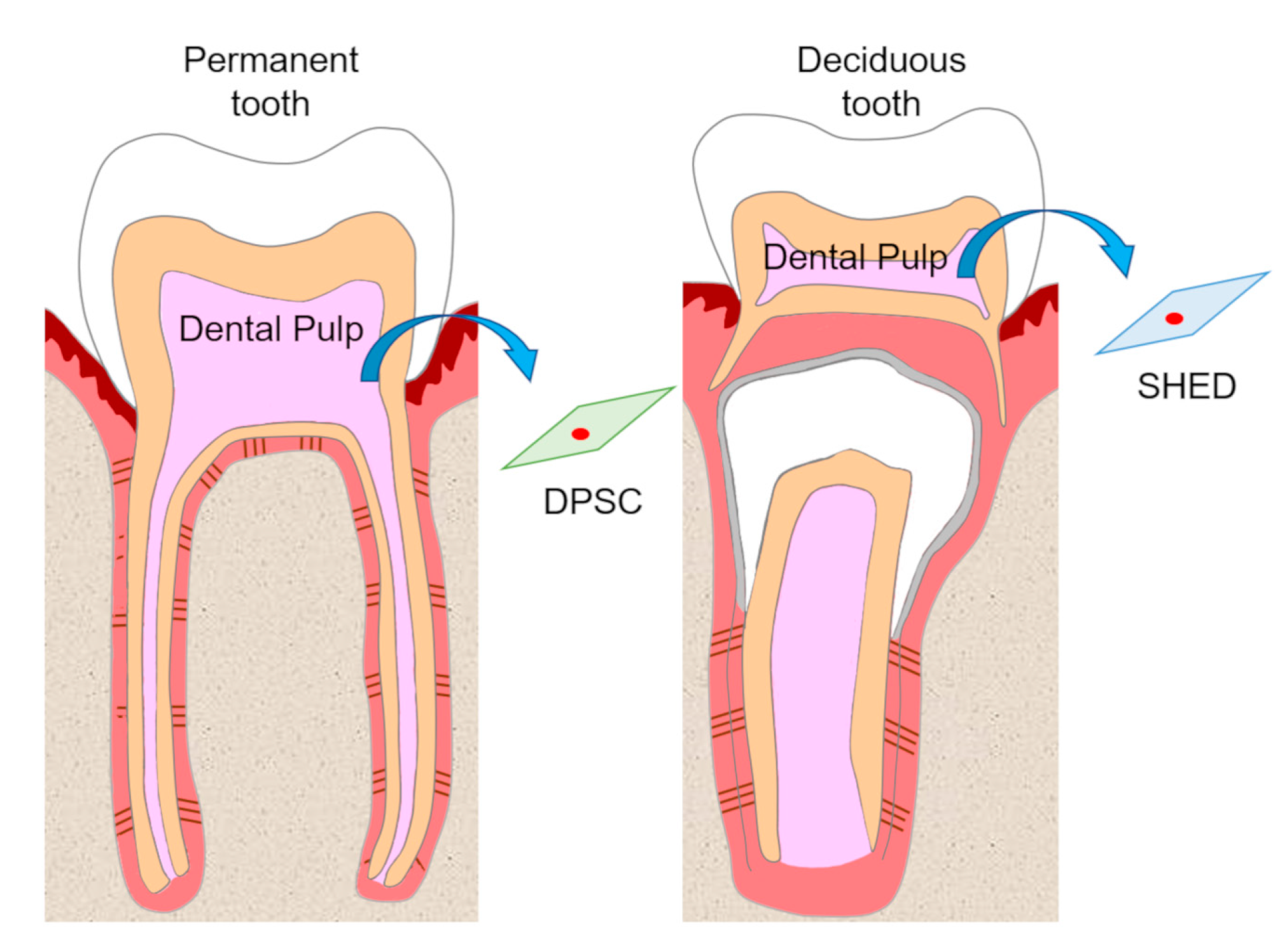
1. Introduction
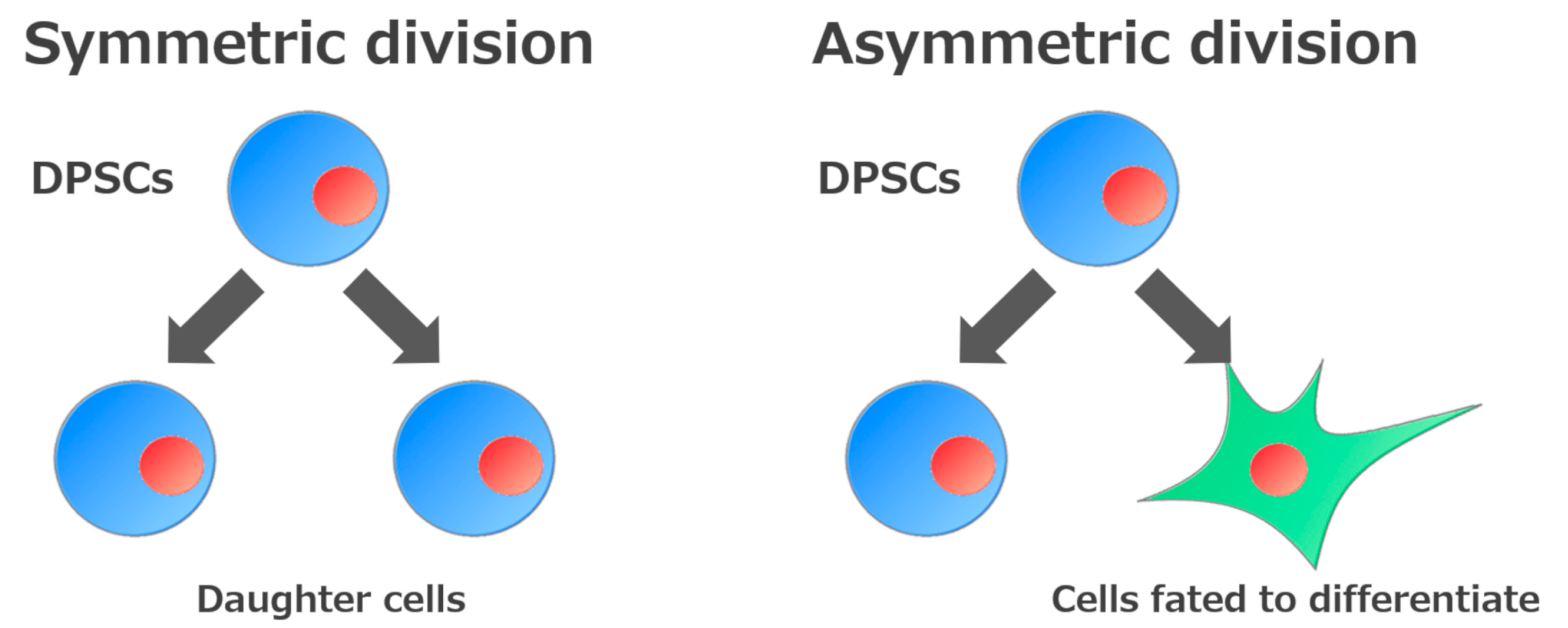
2. Self-Renewal and High Growth Capacity of DPSCs and SHEDs
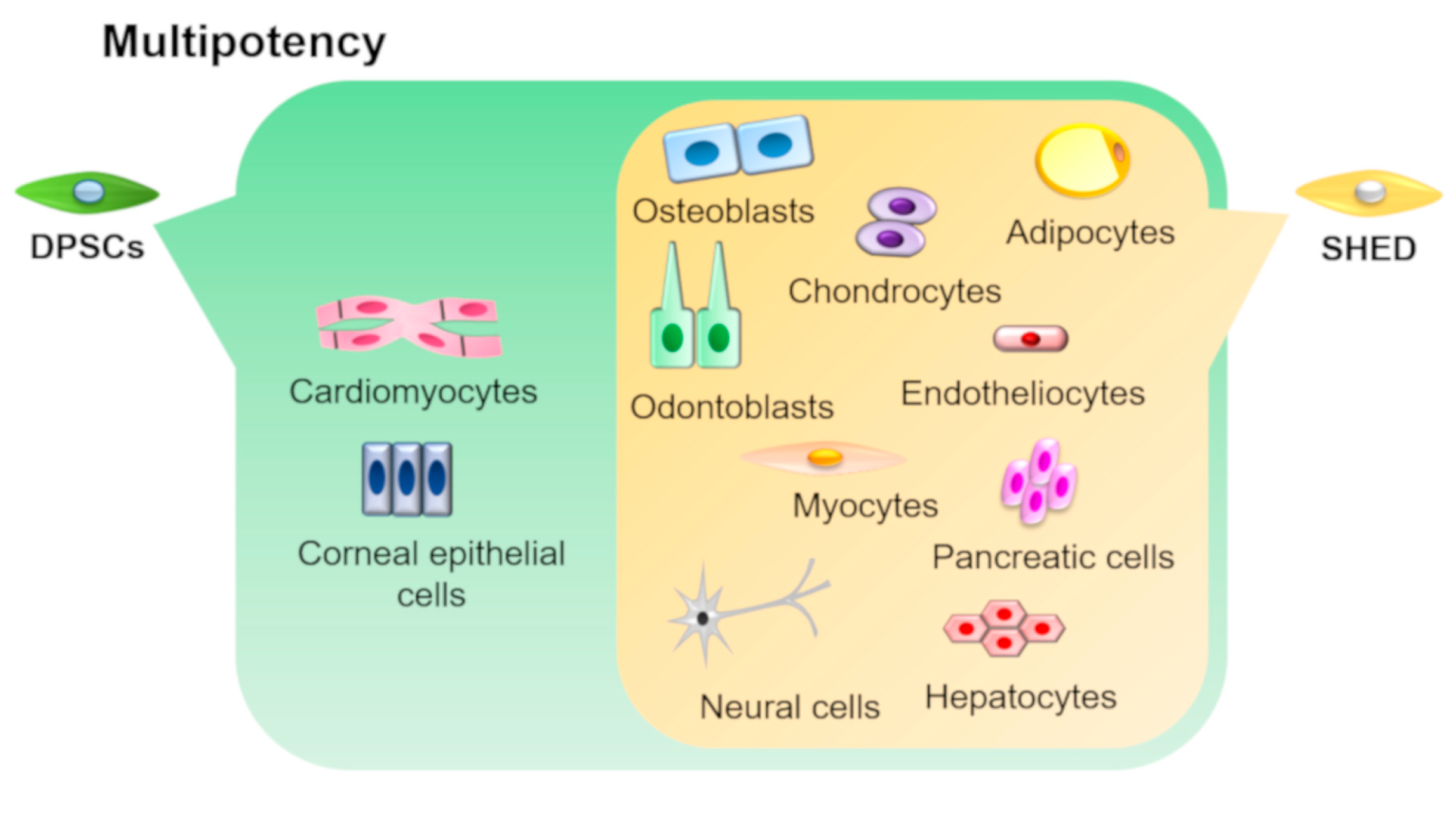
3. Multipotency of DPSCs and SHEDs
4. Cell Markers Expression in DPSCs and SHEDs
5. Immunomodulatory Effects of DPSCs and SHEDs
6. Regenerative Capacity of DPSCs and SHEDs
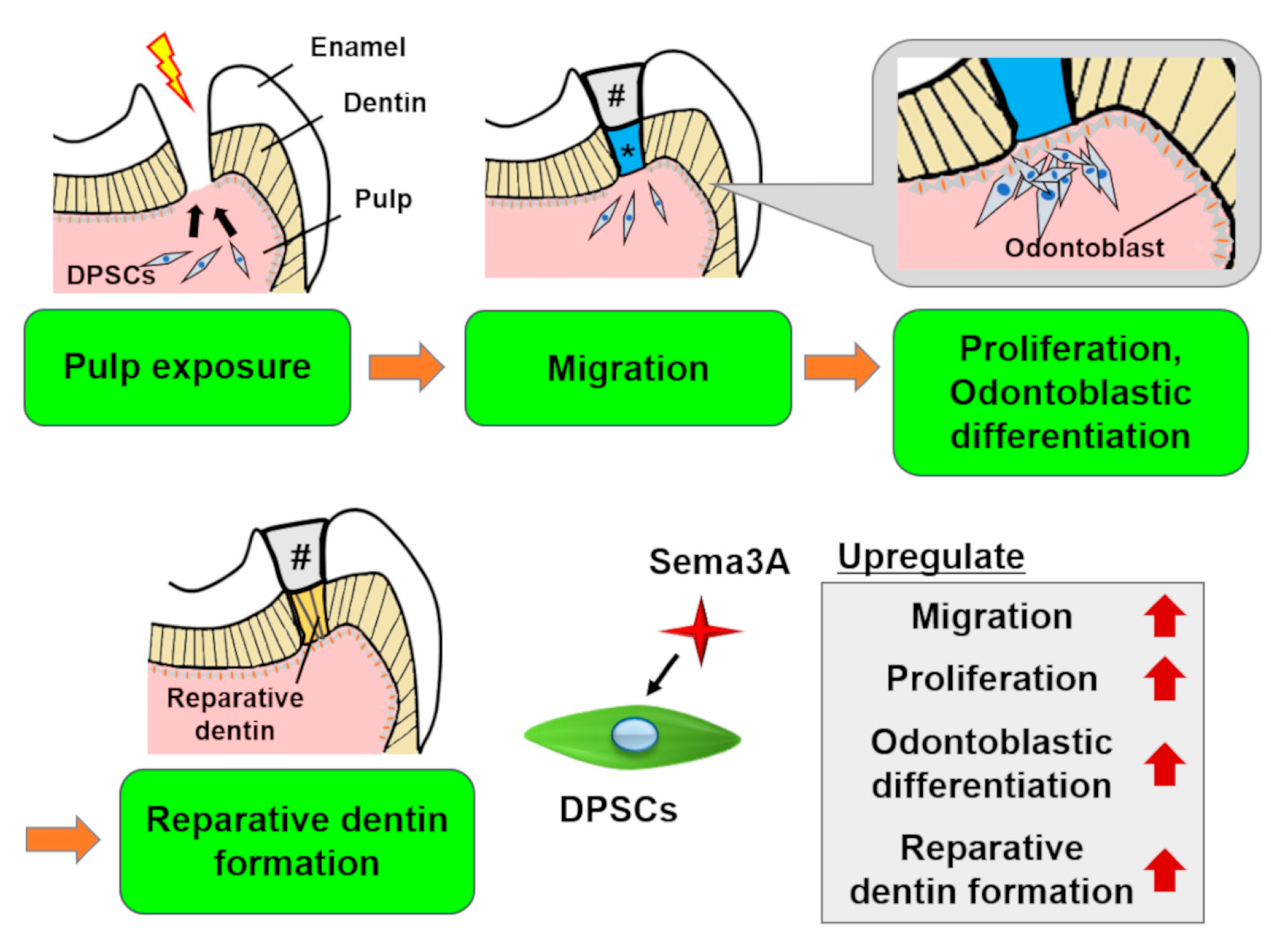
6.1. Regeneration of Dentin/Pulp Complex and Other Dental Tissues
6.2. Regeneration of Other Somatic Tissues
6.3. Cell-free Methods for Regenerative Medicine
6.3.1. Exogenous Growth Factors
6.3.2. Semaphorin 3A
6.3.3. Side Population Cells from Dental Pulp and Mobilized Dental Pulp Stem Cells
7. Cell Banking of DPSCs and SHEDs
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| bFGF | Basic fibroblastic growth factor |
| BMMSCs | Bone marrow-derived mesenchymal stem cells |
| BMP2 | Bone morphogenetic protein 2 |
| BMP7 | Bone morphogenetic protein 7 |
| Col-1 | Type 1 collagen |
| Col-2 | Type 2 collagen |
| Con A | Concanavalin A |
| DCs | Dendric cells |
| DFPCs | Dental follicle precursor cells |
| DPSCs | Dental pulp stem cells |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| EVs | Extracellular vesicles |
| FasL | Fas ligand |
| FGF | Fibroblast growth factor |
| GFAP | Glial fibrillary acidic protein |
| G-CSF | Granulocyte-colony stimulating factor |
| HGF | Hepatocyte growth factor |
| HDAC | Histone deacetylase |
| HSG | Human salivary gland |
| HA | Hydroxyapatite |
| IDO | Indoleamine 2,3-dioxygenase |
| iPSCs | Induced pluripotent stem cells |
| IGFR1 | Insulin-like growth factor 1 receptor |
| IFN-γ | Interferon gamma |
| IL-2 | Interleukin 2 |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-17A | Interleukin 17A |
| IFT80 | Intraflagellar transport 80 |
| JNK | c-Jun N-terminal kinase |
| LPL | Lipoprotein lipase |
| MSCs | Mesenchymal stem cells |
| MAP-2 | Microtubule-associated protein 2 |
| MAPK | Mitogen-activated protein kinase |
| Oct-4 | Octamer-binding transcription factor 4 |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| PDL | Periodontal ligament |
| PDLSCs | Periodontal ligament stem cells |
| PBMCs | Peripheral blood mononuclear cells |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PI3K | Phosphatidylinositol-4, 5-bisphosphate 3-kinase |
| PHA | Phytohemagglutinin |
| PLLA | Poly-L-lactic acid |
| RNA | Ribonucleic acid |
| Sema3A | Semaphorin 3A |
| SDF-1 | Stromal cell-derived factor 1 |
| SCAPs | Stem cells from apical papilla |
| SHEDs | Stem cells from human exfoliated deciduous teeth |
| Sox2 | SRY-box transcription factor 2 |
| Sox9 | SRY-box transcription factor 9 |
| Th17 | T helper 17 cells |
| TLRs | Toll-like receptors |
| TGF-β | Transforming growth factor-β |
| TCP | Tricalcium phosphate |
| TNF-α | Tumor necrosis factor alpha |
| Wnt10A | Wingless-type MMTV integration site family member 10A |
References
- Haynesworth, S.E.; Goshima, J.; Goldberg, V.M.; Caplan, A.I. Characterization of cells with osteogenic potential from human marrow. Bone 1992, 13, 81–88. [Google Scholar] [CrossRef]
- Halvorsen, Y.C.; Wilkison, W.O.; Gimble, J.M. Adipose-derived stromal cells--their utility and potential in bone formation. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24 (Suppl. 4), S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef] [PubMed]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells (Dayt. Ohio) 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Investig. 1999, 103, 697–705. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet (Lond. Engl.) 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Galler, K.; Driemel, O. Somatic stem cells for regenerative dentistry. Clin. Oral Investig. 2008, 12, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; Abdullah, A.N.; Ronald, V.S.; Musa, S.; Ab Aziz, Z.A.; Zain, R.B.; Totey, S.; Bhonde, R.R.; Abu Kasim, N.H. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J. Endod. 2010, 36, 1504–1515. [Google Scholar] [CrossRef]
- Karaoz, E.; Dogan, B.N.; Aksoy, A.; Gacar, G.; Akyuz, S.; Ayhan, S.; Genc, Z.S.; Yuruker, S.; Duruksu, G.; Demircan, P.C.; et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2010, 133, 95–112. [Google Scholar] [CrossRef]
- Sanchez-Taltavull, D. Optimal architecture of differentiation cascades with asymmetric and symmetric stem cell division. J. Theor. Biol. 2016, 407, 106–117. [Google Scholar] [CrossRef]
- Mokry, J.; Soukup, T.; Micuda, S.; Karbanova, J.; Visek, B.; Brcakova, E.; Suchanek, J.; Bouchal, J.; Vokurkova, D.; Ivancakova, R. Telomere attrition occurs during ex vivo expansion of human dental pulp stem cells. J. Biomed. Biotechnol. 2010, 2010, 673513. [Google Scholar] [CrossRef]
- Ponnaiyan, D.; Jegadeesan, V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur. J. Dent. 2014, 8, 307–313. [Google Scholar] [CrossRef]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.M.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, P.J. Telomerase and the aging process. Exp. Gerontol. 2007, 42, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Transforming Growth Factor beta-Activated Kinase 1 Regulates Mesenchymal Stem Cell Proliferation Through Stabilization of Yap1/Taz Proteins. Stem Cells (Dayt. Ohio) 2019, 37, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, Z.; Tan, Y.; Yu, N.; Wang, X.; Yao, N.; Zhao, J. Effects of Notch ligand Delta1 on the proliferation and differentiation of human dental pulp stem cells in vitro. Arch. Oral Biol. 2009, 54, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Q.; Tian, H.; Lv, P.; Zhou, C.; Gao, X. Effects of WNT10A on proliferation and differentiation of human dental pulp cells. J. Endod. 2014, 40, 1593–1599. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Y.; Li, Y.; Liu, G. Tumor Necrosis Factor Alpha Stimulates Proliferation of Dental Pulp Stem Cells via Akt/Glycogen Synthase Kinase-3beta/Cyclin D1 Signaling Pathway. J. Endod. 2015, 41, 1066–1072. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, X.; Yang, S. IFT80 is required for stem cell proliferation, differentiation, and odontoblast polarization during tooth development. Cell Death Dis. 2019, 10, 63. [Google Scholar] [CrossRef]
- Ngoc Tran, T.D.; Stovall, K.E.; Suantawee, T.; Hu, Y.; Yao, S.; Yang, L.J.; Adisakwattana, S.; Cheng, H. Transient receptor potential melastatin 4 channel is required for rat dental pulp stem cell proliferation and survival. Cell Prolif. 2017, 50, e12360. [Google Scholar] [CrossRef]
- Gao, Q.; Walmsley, A.D.; Cooper, P.R.; Scheven, B.A. Ultrasound Stimulation of Different Dental Stem Cell Populations: Role of Mitogen-activated Protein Kinase Signaling. J. Endod. 2016, 42, 425–431. [Google Scholar] [CrossRef]
- Yu, C.Y.; Boyd, N.M.; Cringle, S.J.; Alder, V.A.; Yu, D.Y. Oxygen distribution and consumption in rat lower incisor pulp. Arch. Oral Biol. 2002, 47, 529–536. [Google Scholar] [CrossRef]
- Iida, K.; Takeda-Kawaguchi, T.; Tezuka, Y.; Kunisada, T.; Shibata, T.; Tezuka, K. Hypoxia enhances colony formation and proliferation but inhibits differentiation of human dental pulp cells. Arch. Oral Biol. 2010, 55, 648–654. [Google Scholar] [CrossRef]
- Kanafi, M.M.; Ramesh, A.; Gupta, P.K.; Bhonde, R.R. Influence of hypoxia, high glucose, and low serum on the growth kinetics of mesenchymal stem cells from deciduous and permanent teeth. Cells Tissues Organs 2013, 198, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Agata, H.; Kagami, H.; Watanabe, N.; Ueda, M. Effect of ischemic culture conditions on the survival and differentiation of porcine dental pulp-derived cells. Differ. Res. Biol. Divers. 2008, 76, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, W.; Xiao, Y. The effect of hypoxia on the stemness and differentiation capacity of PDLC and DPC. Biomed Res. Int. 2014, 2014, 890675. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Coyac, B.R.; Chicatun, F.; Hoac, B.; Nelea, V.; Chaussain, C.; Nazhat, S.N.; McKee, M.D. Mineralization of dense collagen hydrogel scaffolds by human pulp cells. J. Dent. Res. 2013, 92, 648–654. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, Y.; Mohabatpour, F.; Zheng, L.; Papagerakis, S.; Chen, D.; Papagerakis, P. Dental Pulp Stem Cells: Isolation, Characterization, Expansion, and Odontoblast Differentiation for Tissue Engineering. Methods Mol. Biol. 2019, 1922, 91–101. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, F.; Zhang, X.; Wang, S.; Jin, Y.; Zhang, W.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Human Dental Pulp Stem Cells Mediated Dentin-Pulp Complex Regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef]
- Yoshida, S.; Wada, N.; Hasegawa, D.; Miyaji, H.; Mitarai, H.; Tomokiyo, A.; Hamano, S.; Maeda, H. Semaphorin 3A Induces Odontoblastic Phenotype in Dental Pulp Stem Cells. J. Dent. Res. 2016, 95, 1282–1290. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, H.; Xu, N.; Shen, Y.; Huang, Y.; Liu, C. Chondrogenic potential of stem cells from human exfoliated deciduous teeth in vitro and in vivo. Acta Odontol. Scand. 2014, 72, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; Shi, S.; Fan, M.; Jansen, J.A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006, 12, 2813–2823. [Google Scholar] [CrossRef]
- Zhang, Z.; Nor, F.; Oh, M.; Cucco, C.; Shi, S.; Nor, J.E. Wnt/beta-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells (Dayt. Ohio) 2016, 34, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Sakai, V.T.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Machado, M.A.; Shi, S.; Santos, C.F.; Nor, J.E. SHED differentiate into functional odontoblasts and endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef]
- Song, B.; Jiang, W.; Alraies, A.; Liu, Q.; Gudla, V.; Oni, J.; Wei, X.; Sloan, A.; Ni, L.; Agarwal, M. Bladder Smooth Muscle Cells Differentiation from Dental Pulp Stem Cells: Future Potential for Bladder Tissue Engineering. Stem Cells Int. 2016, 2016, 6979368. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef]
- Ishkitiev, N.; Yaegaki, K.; Calenic, B.; Nakahara, T.; Ishikawa, H.; Mitiev, V.; Haapasalo, M. Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J. Endod. 2010, 36, 469–474. [Google Scholar] [CrossRef]
- Ishkitiev, N.; Yaegaki, K.; Kozhuharova, A.; Tanaka, T.; Okada, M.; Mitev, V.; Fukuda, M.; Imai, T. Pancreatic differentiation of human dental pulp CD117(+) stem cells. Regen. Med. 2013, 8, 597–612. [Google Scholar] [CrossRef]
- Arminan, A.; Gandia, C.; Bartual, M.; Garcia-Verdugo, J.M.; Lledo, E.; Mirabet, V.; Llop, M.; Barea, J.; Montero, J.A.; Sepulveda, P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009, 18, 907–918. [Google Scholar] [CrossRef]
- Gomes, J.A.; Geraldes Monteiro, B.; Melo, G.B.; Smith, R.L.; Cavenaghi Pereira da Silva, M.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Karbanova, J.; Soukup, T.; Suchanek, J.; Pytlik, R.; Corbeil, D.; Mokry, J. Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. Cells Tissues Organs 2011, 193, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ide, R.; Saiki, C.; Kumazawa, Y.; Okamura, H. Human Dental Pulp Cells Differentiate toward Neuronal Cells and Promote Neuroregeneration in Adult Organotypic Hippocampal Slices In Vitro. Int. J. Mol. Sci. 2017, 18, 1745. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells (Dayt. Ohio) 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Yamada, Y.; Fujimoto, A.; Ito, A.; Yoshimi, R.; Ueda, M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials 2006, 27, 3766–3781. [Google Scholar] [CrossRef]
- Vishwanath, V.R.; Nadig, R.R.; Nadig, R.; Prasanna, J.S.; Karthik, J.; Pai, V.S. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: An in vitro study. J. Conserv. Dent. JCD 2013, 16, 423–428. [Google Scholar] [CrossRef]
- Akpinar, G.; Kasap, M.; Aksoy, A.; Duruksu, G.; Gacar, G.; Karaoz, E. Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014, 2014, 457059. [Google Scholar] [CrossRef]
- Patel, M.; Smith, A.J.; Sloan, A.J.; Smith, G.; Cooper, P.R. Phenotype and behaviour of dental pulp cells during expansion culture. Arch. Oral Biol. 2009, 54, 898–908. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Lee, H.J.; Kook, S.Y.; Choung, H.W.; Park, J.Y.; Chung, J.H.; Choung, Y.H.; Kim, E.S.; Yang, H.C.; Choung, P.H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007, 13, 767–773. [Google Scholar] [CrossRef]
- Lindroos, B.; Mäenpää, K.; Ylikomi, T.; Oja, H.; Suuronen, R.; Miettinen, S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem. Biophys. Res. Commun. 2008, 368, 329–335. [Google Scholar] [CrossRef]
- Kanafi, M.M.; Pal, R.; Gupta, P.K. Phenotypic and functional comparison of optimum culture conditions for upscaling of dental pulp stem cells. Cell Biol. Int. 2013, 37, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Gonmanee, T.; Thonabulsombat, C.; Vongsavan, K.; Sritanaudomchai, H. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. Arch. Oral Biol. 2018, 88, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xing, J.; Feng, G.; Sang, A.; Shen, B.; Xu, Y.; Jiang, J.; Liu, S.; Tan, W.; Gu, Z.; et al. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/beta-catenin signaling. Cell. Mol. Neurobiol. 2013, 33, 1023–1031. [Google Scholar] [CrossRef]
- Kaukua, N.; Chen, M.; Guarnieri, P.; Dahl, M.; Lim, M.L.; Yucel-Lindberg, T.; Sundström, E.; Adameyko, I.; Mao, J.J.; Fried, K. Molecular differences between stromal cell populations from deciduous and permanent human teeth. Stem Cell Res. Ther. 2015, 6, 59. [Google Scholar] [CrossRef][Green Version]
- Hara, K.; Yamada, Y.; Nakamura, S.; Umemura, E.; Ito, K.; Ueda, M. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrow-derived mesenchymal stem cells for mineralized tissue-forming cell biology. J. Endod. 2011, 37, 1647–1652. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef]
- Spath, L.; Rotilio, V.; Alessandrini, M.; Gambara, G.; De Angelis, L.; Mancini, M.; Mitsiadis, T.A.; Vivarelli, E.; Naro, F.; Filippini, A.; et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell. Mol. Med. 2010, 14, 1635–1644. [Google Scholar] [CrossRef]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Kiraly, M.; Porcsalmy, B.; Pataki, A.; Kadar, K.; Jelitai, M.; Molnar, B.; Hermann, P.; Gera, I.; Grimm, W.D.; Ganss, B.; et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009, 55, 323–332. [Google Scholar] [CrossRef]
- Ozerdem, U.; Grako, K.A.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 222, 218–227. [Google Scholar] [CrossRef]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007, 9, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Mantesso, A.; De Bari, C.; Nishiyama, A.; Sharpe, P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014, 14, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Petrini, S.; Tessa, A.; Carrozzo, R.; Verardo, M.; Pierini, R.; Rizza, T.; Bertini, E. Human melanoma/NG2 chondroitin sulfate proteoglycan is expressed in the sarcolemma of postnatal human skeletal myofibers. Abnormal expression in merosin-negative and Duchenne muscular dystrophies. Mol. Cell. Neurosci. 2003, 23, 219–231. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- An, Z.; Sabalic, M.; Bloomquist, R.F.; Fowler, T.E.; Streelman, T.; Sharpe, P.T. A quiescent cell population replenishes mesenchymal stem cells to drive accelerated growth in mouse incisors. Nat. Commun. 2018, 9, 378. [Google Scholar] [CrossRef]
- Matsui, M.; Kobayashi, T.; Tsutsui, T.W. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum. Cell 2018, 31, 127–138. [Google Scholar] [CrossRef]
- Suchánek, J.; Visek, B.; Soukup, T.; El-Din Mohamed, S.K.; Ivancakova, R.; Mokry, J.; Aboul-Ezz, E.; Omran, A. Stem cells from human exfoliated deciduous teeth-isolation, long term cultivation and phenotypical analysis. Acta Med. (Hradec Kral.) 2010, 53, 93–99. [Google Scholar]
- Li, Z.; Jiang, C.M.; An, S.; Cheng, Q.; Huang, Y.F.; Wang, Y.T.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef]
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Özdemir, A.T.; Özgul Özdemir, R.B.; Kirmaz, C.; Sariboyaci, A.E.; Ünal Halbutoğllari, Z.S.; Özel, C.; Karaöz, E. The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell. Immunol. 2016, 310, 108–115. [Google Scholar] [CrossRef]
- Demircan, P.C.; Sariboyaci, A.E.; Unal, Z.S.; Gacar, G.; Subasi, C.; Karaoz, E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: Comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 2011, 13, 1205–1220. [Google Scholar] [CrossRef]
- Kim, J.M.; Rasmussen, J.P.; Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kwack, K.H.; Lee, J.M.; Park, S.H.; Lee, H.W. Human Dental Pulp Stem Cells Suppress Alloantigen-induced Immunity by Stimulating T Cells to Release Transforming Growth Factor Beta. J. Endod. 2017, 43, 100–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Jin, Y.; Shi, S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J. Dent. Res. 2012, 91, 948–954. [Google Scholar] [CrossRef]
- Caseiro, A.R.; Pereira, T.; Ivanova, G.; Luis, A.L.; Mauricio, A.C. Neuromuscular Regeneration: Perspective on the Application of Mesenchymal Stem Cells and Their Secretion Products. Stem Cells Int. 2016, 2016, 9756973. [Google Scholar] [CrossRef]
- Tomic, S.; Djokic, J.; Vasilijic, S.; Vucevic, D.; Todorovic, V.; Supic, G.; Colic, M. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev. 2011, 20, 695–708. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Bronneke, H.S.; et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Kobayashi, Y.; Kamiya, H.; Nakamura, J.; Ozawa, S.; Tanaka, Y.; Takebe, J.; et al. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J. Diabetes Investig. 2016, 7, 485–496. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Wang, X.; Key, B.; Lee, B.H.; Li, H.; Ye, Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018, 2018, 1731289. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.Y.; Ni, S.Y.; Ma, K.; Ma, Y.S.; Wang, Z.S.; Zhao, X.L. Stem cells from human exfoliated deciduous teeth correct the immune imbalance of allergic rhinitis via Treg cells in vivo and in vitro. Stem Cell Res. Ther. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Silva Fde, S.; Ramos, R.N.; de Almeida, D.C.; Bassi, E.J.; Gonzales, R.P.; Miyagi, S.P.; Maranduba, C.P.; Sant’Anna, O.A.; Marques, M.M.; Barbuto, J.A.; et al. Mesenchymal stem cells derived from human exfoliated deciduous teeth (SHEDs) induce immune modulatory profile in monocyte-derived dendritic cells. PLoS ONE 2014, 9, e98050. [Google Scholar] [CrossRef]
- Yamaza, T.; Kentaro, A.; Chen, C.; Liu, Y.; Shi, Y.; Gronthos, S.; Wang, S.; Shi, S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res. Ther. 2010, 1, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, S.; Liu, D.; Chen, C.; Xu, X.; Chen, X.; Shi, S. Transplantation of SHED prevents bone loss in the early phase of ovariectomy-induced osteoporosis. J. Dent. Res. 2014, 93, 1124–1132. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Du, Z.H.; Ding, C.; Zhang, Q.; Zhang, Y.; Ge, X.Y.; Li, S.L.; Yu, G.Y. Stem cells from exfoliated deciduous teeth alleviate hyposalivation caused by Sjogren syndrome. Oral Dis. 2019, 25, 1530–1544. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef] [PubMed]
- Panitvisai, P.; Messer, H.H. Cuspal deflection in molars in relation to endodontic and restorative procedures. J. Endod. 1995, 21, 57–61. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Robbins, J.W. Post placement and restoration of endodontically treated teeth: A literature review. J. Endod. 2004, 30, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.S.; Choung, H.W.; Shon, W.J.; Seo, B.M.; Lee, E.H.; Cho, J.Y.; Park, J.C. Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials 2011, 32, 9696–9706. [Google Scholar] [CrossRef]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, P.G.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef]
- Sun, H.H.; Chen, B.; Zhu, Q.L.; Kong, H.; Li, Q.H.; Gao, L.N.; Xiao, M.; Chen, F.M.; Yu, Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 2014, 35, 9459–9472. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Lee, H.J.; Choi, Y.A.; Kim, K.M.; Baek, S.H.; Park, H.S.; Kim, J.Y.; Ahn, J.M.; Cho, J.Y.; Cho, D.W.; et al. Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng. Part A 2011, 17, 181–191. [Google Scholar] [CrossRef]
- Takeda, T.; Tezuka, Y.; Horiuchi, M.; Hosono, K.; Iida, K.; Hatakeyama, D.; Miyaki, S.; Kunisada, T.; Shibata, T.; Tezuka, K. Characterization of dental pulp stem cells of human tooth germs. J. Dent. Res. 2008, 87, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nor, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nor, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef]
- Janebodin, K.; Reyes, M. Neural crest-derived dental pulp stem cells function as ectomesenchyme to support salivary gland tissue formation. Dentistry S 2012, 13, 5. [Google Scholar]
- Otaki, S.; Ueshima, S.; Shiraishi, K.; Sugiyama, K.; Hamada, S.; Yorimoto, M.; Matsuo, O. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol. Int. 2007, 31, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Cricenti, L.; Mariani, G.M.; Romano, F. Autologous dental pulp stem cells in periodontal regeneration: A case report. Int. J. Periodontics Restor. Dent. 2014, 34 (Suppl. 3), s27–s33. [Google Scholar] [CrossRef]
- Seo, B.M.; Sonoyama, W.; Yamaza, T.; Coppe, C.; Kikuiri, T.; Akiyama, K.; Lee, J.S.; Shi, S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- De Mendonca Costa, A.; Bueno, D.F.; Martins, M.T.; Kerkis, I.; Kerkis, A.; Fanganiello, R.D.; Cerruti, H.; Alonso, N.; Passos-Bueno, M.R. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J. Craniofacial Surg. 2008, 19, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Jin, L.; Ma, P.; Fan, Z.; Wang, S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J. Periodontol. 2014, 85, 845–851. [Google Scholar] [CrossRef]
- Syed-Picard, F.N.; Du, Y.; Lathrop, K.L.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L. Dental pulp stem cells: A new cellular resource for corneal stromal regeneration. Stem Cells Transl. Med. 2015, 4, 276–285. [Google Scholar] [CrossRef]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Martinez-Sarra, E.; Montori, S.; Gil-Recio, C.; Nunez-Toldra, R.; Costamagna, D.; Rotini, A.; Atari, M.; Luttun, A.; Sampaolesi, M. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res. Ther. 2017, 8, 175. [Google Scholar] [CrossRef]
- Gandia, C.; Arminan, A.; Garcia-Verdugo, J.M.; Lledo, E.; Ruiz, A.; Minana, M.D.; Sanchez-Torrijos, J.; Paya, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells (Dayt. Ohio) 2008, 26, 638–645. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Cho, Y.A.; Lee, Y.M.; Lee, S.Y.; Bae, W.J.; Kim, E.C. PIN1 Suppresses the Hepatic Differentiation of Pulp Stem Cells via Wnt3a. J. Dent. Res. 2016, 95, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, J.H.; Bae, J.; Bu, Y.; Kim, E.C. Human Dental Pulp Stem Cells Are More Effective Than Human Bone Marrow-Derived Mesenchymal Stem Cells in Cerebral Ischemic Injury. Cell Transplant. 2017, 26, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Datta, I.; Bhadri, N.; Shahani, P.; Majumdar, D.; Sowmithra, S.; Razdan, R.; Bhonde, R. Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy 2017, 19, 1208–1224. [Google Scholar] [CrossRef]
- Kong, F.; Shi, X.; Xiao, F.; Yang, Y.; Zhang, X.; Wang, L.S.; Wu, C.T.; Wang, H. Transplantation of Hepatocyte Growth Factor-Modified Dental Pulp Stem Cells Prevents Bone Loss in the Early Phase of Ovariectomy-Induced Osteoporosis. Hum. Gene Ther. 2018, 29, 271–282. [Google Scholar] [CrossRef]
- Yamagata, M.; Yamamoto, A.; Kako, E.; Kaneko, N.; Matsubara, K.; Sakai, K.; Sawamoto, K.; Ueda, M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke 2013, 44, 551–554. [Google Scholar] [CrossRef]
- Sonoda, S.; Tomoda, E.; Tanaka, Y.; Yamaza, T. Properties and possibilities of human dental pulp-derived stem cells. Arch. Stem Cell Res. 2015, 2, 1012. [Google Scholar]
- Yamaza, T.; Alatas, F.S.; Yuniartha, R.; Yamaza, H.; Fujiyoshi, J.K.; Yanagi, Y.; Yoshimaru, K.; Hayashida, M.; Matsuura, T.; Aijima, R.; et al. In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res. Ther. 2015, 6, 171. [Google Scholar] [CrossRef]
- Taguchi, T.; Yanagi, Y.; Yoshimaru, K.; Zhang, X.-Y.; Matsuura, T.; Nakayama, K.; Kobayashi, E.; Yamaza, H.; Nonaka, K.; Ohga, S. Regenerative medicine using stem cells from human exfoliated deciduous teeth (SHED): A promising new treatment in pediatric surgery. Surg. Today 2019, 49, 316–322. [Google Scholar] [CrossRef]
- Aboutaleb, N.; Faezi, M.; Nasseri Maleki, S.; Nazarinia, D.; Razavi Tousi, S.M.T.; Hashemirad, N. Conditioned medium obtained from mesenchymal stem cells attenuates focal cerebral ischemia reperfusion injury through activation of ERK1/ERK2-BDNF signaling pathway. J. Chem. Neuroanat. 2019, 97, 87–98. [Google Scholar] [CrossRef]
- Kay, A.G.; Long, G.; Tyler, G.; Stefan, A.; Broadfoot, S.J.; Piccinini, A.M.; Middleton, J.; Kehoe, O. Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Sci. Rep. 2017, 7, 18019. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zuzzio, K.; Walker, C.L. Systemic Dental Pulp Stem Cell Secretome Therapy in a Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci. 2019, 9, 165. [Google Scholar] [CrossRef]
- Makino, E.; Nakamura, N.; Miyabe, M.; Ito, M.; Kanada, S.; Hata, M.; Saiki, T.; Sango, K.; Kamiya, H.; Nakamura, J.; et al. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J. Diabetes Investig. 2019, 10, 1199–1208. [Google Scholar] [CrossRef]
- Asadi-Golshan, R.; Razban, V.; Mirzaei, E.; Rahmanian, A.; Khajeh, S.; Mostafavi-Pour, Z.; Dehghani, F. Sensory and Motor Behavior Evidences Supporting the Usefulness of Conditioned Medium from Dental Pulp-Derived Stem Cells in Spinal Cord Injury in Rats. Asian Spine J. 2018, 12, 785–793. [Google Scholar] [CrossRef]
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part A 2013, 19, 24–29. [Google Scholar] [CrossRef]
- Hu, X.; Zhong, Y.; Kong, Y.; Chen, Y.; Feng, J.; Zheng, J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFbeta1/smads signaling pathway via transfer of microRNAs. Stem Cell Res. Ther. 2019, 10, 170. [Google Scholar] [CrossRef]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.Y.; Ren, J.L.; Xu, F.; Chen, F.M.; Li, A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res. Ther. 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Hayashi, Y.; Murakami, M.; Alvarez, F.J.; Horibe, H.; Iohara, K.; Nakata, K.; Nakamura, H.; Nakashima, M. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral Dis. 2015, 21, 113–122. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Wake, H.; Ito, M.; Nabekura, J.; Wakita, H.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem Cells (Dayt. Ohio) 2008, 26, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Zhang, Y.F.; Wan, C.Y.; Sun, Z.Y.; Nie, S.; Jian, S.J.; Zhang, L.; Song, G.T.; Chen, Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials 2015, 44, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Murray, P.E.; Namerow, K.N. Dental pulp stem cell migration. J. Endod. 2010, 36, 1963–1966. [Google Scholar] [CrossRef]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bardet, C.; Mouraret, S.; Liu, B.; Singh, G.; Sadoine, J.; Dhamdhere, G.; Smith, A.; Tran, X.V.; Joy, A.; et al. Wnt Acts as a Prosurvival Signal to Enhance Dentin Regeneration. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 1150–1159. [Google Scholar] [CrossRef]
- Luo, L.; Albashari, A.A.; Wang, X.; Jin, L.; Zhang, Y.; Zheng, L.; Xia, J.; Xu, H.; Zhao, Y.; Xiao, J.; et al. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int. 2018, 2018, 2398521. [Google Scholar] [CrossRef]
- Aksel, H.; Huang, G.T. Combined Effects of Vascular Endothelial Growth Factor and Bone Morphogenetic Protein 2 on Odonto/Osteogenic Differentiation of Human Dental Pulp Stem Cells In Vitro. J. Endod. 2017, 43, 930–935. [Google Scholar] [CrossRef]
- La Noce, M.; Mele, L.; Laino, L.; Iolascon, G.; Pieretti, G.; Papaccio, G.; Desiderio, V.; Tirino, V.; Paino, F. Cytoplasmic Interactions between the Glucocorticoid Receptor and HDAC2 Regulate Osteocalcin Expression in VPA-Treated MSCs. Cells 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef]
- Paino, F.; La Noce, M.; Tirino, V.; Naddeo, P.; Desiderio, V.; Pirozzi, G.; De Rosa, A.; Laino, L.; Altucci, L.; Papaccio, G. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: Evidence for HDAC2 involvement. Stem Cells (Dayt. Ohio) 2014, 32, 279–289. [Google Scholar] [CrossRef]
- Jin, H.; Park, J.Y.; Choi, H.; Choung, P.H. HDAC inhibitor trichostatin A promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng. Part A 2013, 19, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Smith, A.J.; Fleming, G.J.; Partridge, N.C.; Shimizu, E.; Moran, G.P.; Cooper, P.R. The Histone-Deacetylase-Inhibitor Suberoylanilide Hydroxamic Acid Promotes Dental Pulp Repair Mechanisms Through Modulation of Matrix Metalloproteinase-13 Activity. J. Cell. Physiol. 2016, 231, 798–816. [Google Scholar] [CrossRef]
- Wright, D.E.; White, F.A.; Gerfen, R.W.; Silos-Santiago, I.; Snider, W.D. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J. Comp. Neurol. 1995, 361, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Taylor, G.I.; Minichiello, J.; Farlie, P.; Cichowitz, A.; Watson, N.; Klagsbrun, M.; Mamluk, R.; Newgreen, D.F. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev. Biol. 2003, 255, 77–98. [Google Scholar] [CrossRef]
- Eichmann, A.; Le Noble, F.; Autiero, M.; Carmeliet, P. Guidance of vascular and neural network formation. Curr. Opin. Neurobiol. 2005, 15, 108–115. [Google Scholar] [CrossRef]
- Gomez, C.; Burt-Pichat, B.; Mallein-Gerin, F.; Merle, B.; Delmas, P.D.; Skerry, T.M.; Vico, L.; Malaval, L.; Chenu, C. Expression of Semaphorin-3A and its receptors in endochondral ossification: Potential role in skeletal development and innervation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 234, 393–403. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74. [Google Scholar] [CrossRef]
- Tomokiyo, A.; Maeda, H.; Fujii, S.; Wada, N.; Shima, K.; Akamine, A. Development of a multipotent clonal human periodontal ligament cell line. Differ. Res. Biol. Divers. 2008, 76, 337–347. [Google Scholar] [CrossRef]
- Wada, N.; Maeda, H.; Hasegawa, D.; Gronthos, S.; Bartold, P.M.; Menicanin, D.; Fujii, S.; Yoshida, S.; Tomokiyo, A.; Monnouchi, S.; et al. Semaphorin 3A induces mesenchymal-stem-like properties in human periodontal ligament cells. Stem Cells Dev. 2014, 23, 2225–2236. [Google Scholar] [CrossRef]
- Yamada, D.; Takahashi, K.; Kawahara, K.; Maeda, T. Autocrine Semaphorin3A signaling is essential for the maintenance of stem-like cells in lung cancer. Biochem. Biophys. Res. Commun. 2016, 480, 375–379. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells (Dayt. Ohio) 2006, 24, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Iohara, K.; Wakita, H.; Hattori, H.; Ueda, M.; Matsushita, K.; Nakashima, M. Dental pulp-derived CD31⁻/CD146⁻ side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part A 2011, 17, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of CD31(-)/CD146(-) side population cells from a canine tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, R.; Hayashi, Y.; Iohara, K.; Sugiyama, M.; Murakami, M.; Yamamoto, T.; Fukuta, O.; Nakashima, M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 2013, 34, 1888–1897. [Google Scholar] [CrossRef]
- Murakami, M.; Horibe, H.; Iohara, K.; Hayashi, Y.; Osako, Y.; Takei, Y.; Nakata, K.; Motoyama, N.; Kurita, K.; Nakashima, M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 2013, 34, 9036–9047. [Google Scholar] [CrossRef]
- Nakayama, H.; Iohara, K.; Hayashi, Y.; Okuwa, Y.; Kurita, K.; Nakashima, M. Enhanced regeneration potential of mobilized dental pulp stem cells from immature teeth. Oral Dis. 2017, 23, 620–628. [Google Scholar] [CrossRef]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef]
- Gioventu, S.; Andriolo, G.; Bonino, F.; Frasca, S.; Lazzari, L.; Montelatici, E.; Santoro, F.; Rebulla, P. A novel method for banking dental pulp stem cells. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 2012, 47, 199–206. [Google Scholar] [CrossRef]
- Ma, L.; Makino, Y.; Yamaza, H.; Akiyama, K.; Hoshino, Y.; Song, G.; Kukita, T.; Nonaka, K.; Shi, S.; Yamaza, T. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS ONE 2012, 7, e51777. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yan, X.; Qin, H.; Qu, C.; Tuan, R.S.; Shi, S.; Huang, G.T. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010, 19, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Dubey, N.; Shim, W.; Ramachandra, C.J.A.; Min, K.S.; Cao, T.; Rosa, V. Functional Odontoblastic-Like Cells Derived from Human iPSCs. J. Dent. Res. 2018, 97, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Pisal, R.V.; Suchanek, J.; Siller, R.; Soukup, T.; Hrebikova, H.; Bezrouk, A.; Kunke, D.; Micuda, S.; Filip, S.; Sullivan, G.; et al. Directed reprogramming of comprehensively characterized dental pulp stem cells extracted from natal tooth. Sci. Rep. 2018, 8, 6168. [Google Scholar] [CrossRef] [PubMed]
- Hamano, S.; Tomokiyo, A.; Hasegawa, D.; Yoshida, S.; Sugii, H.; Mitarai, H.; Fujino, S.; Wada, N.; Maeda, H. Extracellular Matrix from Periodontal Ligament Cells Could Induce the Differentiation of Induced Pluripotent Stem Cells to Periodontal Ligament Stem Cell-Like Cells. Stem Cells Dev. 2018, 27, 100–111. [Google Scholar] [CrossRef] [PubMed]
| Marker Type | Expression Markers | DPSCs | SHEDs | Reference Numbers |
|---|---|---|---|---|
| MSC-related | CD13, CD44, CD73, CD90, CD146, CD166, STRO-1 | + | + | [9,10,16,17,54,55,56,57,58,59,60,61,62] |
| Osteogenic | BMP2, OCN, OPN, Osteonectin, Col-1 | + | + | [9,17,58,65] |
| Adipogenic | PPARγ, LPL | + | + | [10,14,17,58,66] |
| Chondrogenic | Col-2, Sox9 | + | + | [17,58] |
| Myogenic | α-SMA, Myogen, Myosin | + | + | [9,17,58,67] |
| Neurogenic | Nestin, GFAP, β-III tubulin, MAP-2 | + | + | [14,16,17,56,58,63,68,69] |
| Pluripotent | Oct-4, Nanog, Sox2 | + | ++ | [16,17,56,64] |
| IGF1R | + | + | ||
| Monocytic/hematopoietic | CD14, CD19, CD34, CD45, HLA-DR | - | - | [9,16,17,55,56,57,63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy. Biology 2020, 9, 160. https://doi.org/10.3390/biology9070160
Yoshida S, Tomokiyo A, Hasegawa D, Hamano S, Sugii H, Maeda H. Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy. Biology. 2020; 9(7):160. https://doi.org/10.3390/biology9070160
Chicago/Turabian StyleYoshida, Shinichiro, Atsushi Tomokiyo, Daigaku Hasegawa, Sayuri Hamano, Hideki Sugii, and Hidefumi Maeda. 2020. "Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy" Biology 9, no. 7: 160. https://doi.org/10.3390/biology9070160
APA StyleYoshida, S., Tomokiyo, A., Hasegawa, D., Hamano, S., Sugii, H., & Maeda, H. (2020). Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy. Biology, 9(7), 160. https://doi.org/10.3390/biology9070160





