Evidence of Modular Responsiveness of Osteoblast-Like Cells Exposed to Hydroxyapatite-Containing Magnetic Nanostructures
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. DM and DM/n-HA Nanostructures Synthesis
2.3. Cell Culture and Treatments for Analysis of Cell-Nanocomposites Interaction
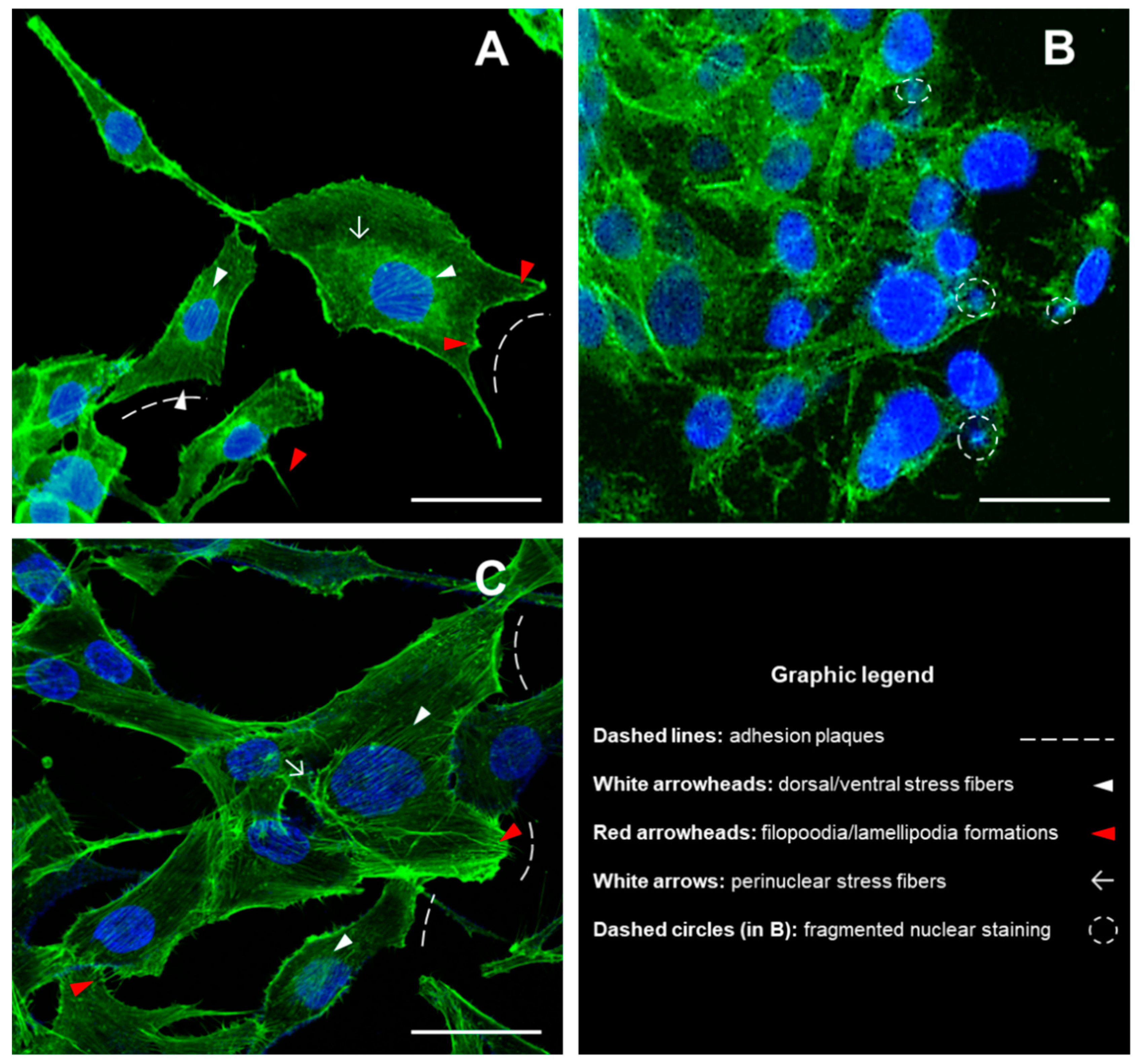
2.4. Fluorescence Imaging of Cell Cytoskeleton and Nuclei by Phalloidin-FITC/DAPI Double Staining
2.5. RNAIsolation and Reverse Transcription
2.6. Real-Time PCR (qPCR)
3. Results
3.1. Detection of Cytoskeletal Morphology of MG-63 Cells Exposed to DM and DM/n-HA
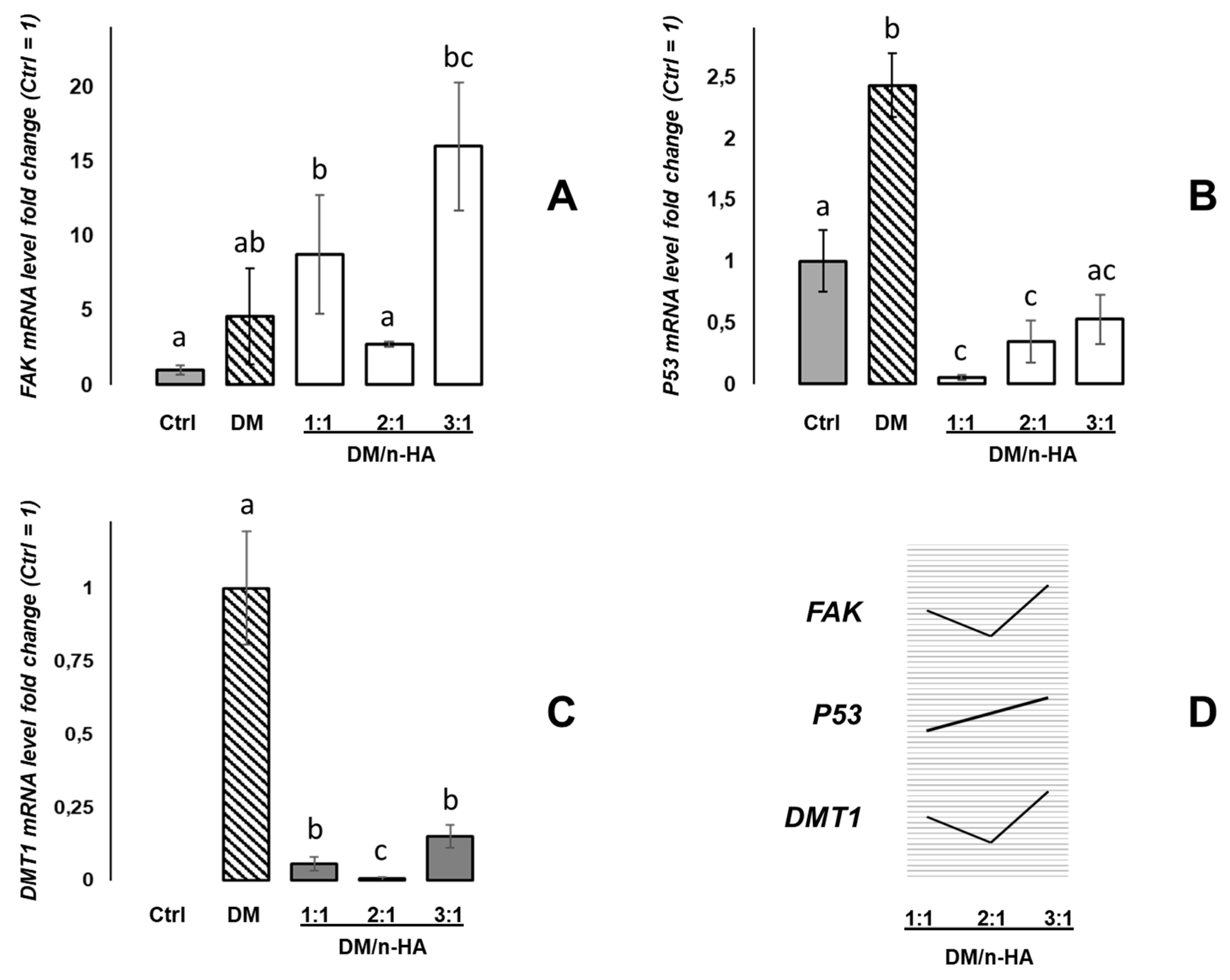
3.2. Effects of DM and DM/n-HA on mRNA Expression of FAK, p53, and DMT1 Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Monzel, C.; Vicario, C.; Piehler, J.; Coppey, M.; Dahan, M. Magnetic control of cellular processes using biofunctional nanoparticles. Chem. Sci. 2017, 8, 7330–7338. [Google Scholar] [CrossRef]
- Gil, S.; Mano, J.F. Magnetic composite biomaterials for tissue engineering. Biomater. Sci. 2014, 2, 812–818. [Google Scholar] [CrossRef]
- Balmert, S.C.; Little, S.R. Biomimetic Delivery with Micro- and Nanoparticles. Adv. Mater. 2012, 24, 3757–3778. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Hyeon, T. Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications. Small 2012, 9, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Mattix, B.; Olsen, T.R.; Moore, T.L.; Casco, M.; Simionescu, D.; Visconti, R.P.; Alexis, F. Accelerated Iron Oxide Nanoparticle Degradation Mediated by Polyester Encapsulation within Cellular Spheroids. Adv. Funct. Mater. 2013, 24, 800–807. [Google Scholar] [CrossRef]
- Abraham, R.; Walton, J.; Russell, L.; Wolman, R.; Wardley-Smith, B.; Green, J.R.; Mitchell, A.; Reeve, J. Dietary determinants of post-menopausal bone loss at the lumbar spine: A possible beneficial effect of iron. Osteoporos. Int. 2006, 17, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.M.; Houtkooper, L.B.; Farrell, V.A.; Parkhill, C.; Weber, J.L.; Flint-Wagner, H.; Weiss, L.; Going, S.B.; Lohman, T. Dietary iron is associated with bone mineral density in healthy postmenopausal women. J. Nutr. 2003, 133, 3598–3602. [Google Scholar] [CrossRef]
- Amirfazli, A. Magnetic nanoparticles hit the target. Nat. Nanotechnol. 2007, 2, 467–468. [Google Scholar] [CrossRef]
- Kim, K.; Fisher, J.P. Nanoparticle technology in bone tissue engineering. J. Drug Target. 2007, 15, 241–252. [Google Scholar] [CrossRef]
- Buyukhatipoglu, K.; Chang, R.; Sun, W.; Clyne, A.M. Bioprinted Nanoparticles for Tissue Engineering Applications. Tissue Eng. Part C Methods 2010, 16, 631–642. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Hajalilou, A.; Ahsanul, K. Synthesis, Characterization, and Cytotoxicity of Iron Oxide Nanoparticles. Adv. Mater. Sci. Eng. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Kim, H.H.; Seo, H.; Lee, K.D.; Oh, J. Magnetic hydroxyapatite: A promising multifunctional platform for nanomedicine application. Int. J. Nanomed. 2017, 12, 8389–8410. [Google Scholar] [CrossRef]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomater. 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Webster, T.J. Enhanced functions of osteoblasts on nanophase ceramics. Biomater. 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Pareta, R.A.; Taylor, E.; Webster, T.J. Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnol. 2008, 19, 265101. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011, 7, 1298–1306. [Google Scholar] [CrossRef]
- Scialla, S.; Palazzo, B.; Barca, A.; Carbone, L.; Fiore, A.; Monteduro, A.G.; Maruccio, G.; Sannino, A.; Gervaso, F. Simplified preparation and characterization of magnetic hydroxyapatite-based nanocomposites. Mater. Sci. Eng. C 2017, 76, 1166–1174. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Walsh, D.; Hall, S.R.; Moir, A.; Wimbush, S.C.; Palazzo, B. Carbonated Water Mediated Preparation of Poly(N-isopropylacrylamide) Thermoresponsive Gels and Liquids. Biomacromolecules 2007, 8, 3800–3805. [Google Scholar] [CrossRef]
- Bique, A.-M.; Kaivosoja, E.; Mikkonen, M.; Paulasto-Kröckel, M. Choice of osteoblast model critical for studying the effects of electromagnetic stimulation on osteogenesis in vitro. Electromagn. Biol. Med. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. Part A 2010, 96, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef]
- Wang, B.; Du, T.; Wang, Y.; Yang, C.; Zhang, S.; Cao, X. Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 Cells. Biochem. Biophys. Res. Commun. 2011, 410, 671–676. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, L.-W.; Liao, E.-Y.; Wang, Q.-J.; Wang, B.-B.; Lei, J.-X. The role of integrin-β/FAK in cyclic mechanical stimulation in MG-63 cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7451–7459. [Google Scholar] [PubMed]
- Fernandes, C.J.; Bezerra, F.; Ferreira, M.R.; Andrade, A.F.; Pinto, T.S.; Zambuzzi, W.F. Nano hydroxyapatite-blasted titanium surface creates a biointerface able to govern Src-dependent osteoblast metabolism as prerequisite to ECM remodeling. Colloids Surfaces B Biointerfaces 2018, 163, 321–328. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Catledge, S.A.; Xu, Y.; Hennessy, K.M.; Thomas, V.; Jablonsky, M.J.; Chowdhury, S.; Stanishevsky, A.V.; Vohra, Y.K.; et al. Mesenchymal Stem Cell Responses to Bone-Mimetic Electrospun Matrices Composed of Polycaprolactone, Collagen I and Nanoparticulate Hydroxyapatite. PLoS ONE 2011, 6, e16813. [Google Scholar] [CrossRef] [PubMed]
- Soussi, T. The TP53 Gene Network in a Postgenomic Era. Hum. Mutat. 2014, 35, 641–642. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 1–13. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.-Z. Cellular Iron Metabolism and Regulation. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; Volume 1173, pp. 21–32. [Google Scholar]
- Piperno, A.; Pelucchi, S.; Mariani, R. Inherited iron overload disorders. Transl. Gastroenterol. Hepatol. 2020, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, H.; Feng, L.; Zhu, Q.; Hancharou, A.; Liu, B.; Yan, C.; Xu, Y.; Guo, R. Preparation and characterization of 3D porous conductive scaffolds with magnetic resonance enhancement in tissue engineering. Biomed. Mater. 2019, 14, 045013. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef] [PubMed]
- Sajesh, K.M.; Ashokan, A.; Gowd, G.S.; Sivanarayanan, T.B.; Unni, A.; Nair, S.; Koyakutty, M. Magnetic 3D scaffold: A theranostic tool for tissue regeneration and non-invasive imaging in vivo. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Vlad, M.D.; Del Valle, L.J.; Poeată, I.; Barracó, M.; Lopez, J.; Torres, R.; Fernández, E.; Valle, L.J. Injectable iron-modified apatitic bone cement intended for kyphoplasty: Cytocompatibility study. J. Mater. Sci. Mater. Electron. 2008, 19, 3575–3583. [Google Scholar] [CrossRef]
- Khatua, C.; Sengupta, S.; Kundu, B.; Bhattacharya, D.; Balla, V.K. Enhanced strength, in vitro bone cell differentiation and mineralization of injectable bone cement reinforced with multiferroic particles. Mater. Des. 2019, 167. [Google Scholar] [CrossRef]
- Zhang, K.; Li, G.; Pei, Z.; Zhao, S.; Jing, A.; Liang, G. Injectable graphite-modified Fe3O4/calcium phosphate bone cement with enhanced heating ability for hyperthermia. Mater. Technol. 2019, 1–9. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic Hydroxyapatite Bone Substitutes to Enhance Tissue Regeneration: Evaluation In Vitro Using Osteoblast-Like Cells and In Vivo in a Bone Defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef]
- D’Amora, U.; Russo, T.; Gloria, A.; Rivieccio, V.; D’Antò, V.; Negri, G.; Ambrosio, L.; De Santis, R. 3D additive-manufactured nanocomposite magnetic scaffolds: Effect of the application mode of a time-dependent magnetic field on hMSCs behavior. Bioact. Mater. 2017, 2, 138–145. [Google Scholar] [CrossRef]
- Russo, A.; Bianchi, M.; Sartori, M.; Boi, M.; Giavaresi, G.; Salter, D.M.; Jelic, M.; Maltarello, M.C.; Ortolani, A.; Sprio, S.; et al. Bone regeneration in a rabbit critical femoral defect by means of magnetic hydroxyapatite macroporous scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 546–554. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Torres, P.M.; Barros, M.J.; Presa, R.; Ribeiro, N.; Abrantes, J.; Belo, J.H.; Amaral, J.; Amaral, V.S.; Bañobre-López, M.; et al. Effective production of multifunctional magnetic-sensitive biomaterial by an extrusion-based additive manufacturing technique. Biomed. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
| Human Gene | Ref Seq mRNA acc. n. | 5′-3′ Sense | 5′-3′ Antisense | PCR Product Size (bp) |
|---|---|---|---|---|
| SLC11A2/DMT1 | NM_001174125.1 | CACTGTGAACTAAAATCAT | CTCCTCCTCAGGAATGGAGA | 235 |
| P53 | NM_000546.5 | CCTCCTCAGCATCTTATCCG | GCACAAACACGCACCTCAAA | 250 |
| FAK | NM_005607.4 | ATTAAATGGATGGCTCCAGA | CTCCCACATACACACACCAA | 89 |
| 28S | M27830.1 | GACGAGAGGGCGTGCATTC | TAAAATCCCGCGGACGCAAA | 138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scialla, S.; Palazzo, B.; Sannino, A.; Verri, T.; Gervaso, F.; Barca, A. Evidence of Modular Responsiveness of Osteoblast-Like Cells Exposed to Hydroxyapatite-Containing Magnetic Nanostructures. Biology 2020, 9, 357. https://doi.org/10.3390/biology9110357
Scialla S, Palazzo B, Sannino A, Verri T, Gervaso F, Barca A. Evidence of Modular Responsiveness of Osteoblast-Like Cells Exposed to Hydroxyapatite-Containing Magnetic Nanostructures. Biology. 2020; 9(11):357. https://doi.org/10.3390/biology9110357
Chicago/Turabian StyleScialla, Stefania, Barbara Palazzo, Alessandro Sannino, Tiziano Verri, Francesca Gervaso, and Amilcare Barca. 2020. "Evidence of Modular Responsiveness of Osteoblast-Like Cells Exposed to Hydroxyapatite-Containing Magnetic Nanostructures" Biology 9, no. 11: 357. https://doi.org/10.3390/biology9110357
APA StyleScialla, S., Palazzo, B., Sannino, A., Verri, T., Gervaso, F., & Barca, A. (2020). Evidence of Modular Responsiveness of Osteoblast-Like Cells Exposed to Hydroxyapatite-Containing Magnetic Nanostructures. Biology, 9(11), 357. https://doi.org/10.3390/biology9110357







