Effectivity of Two Cell Proliferation Markers in Brain of a Songbird Zebra Finch
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. BrdU/EdU Injections and Tissue Processing
2.3. Immunohistochemistry
2.4. Analysis and Statistics
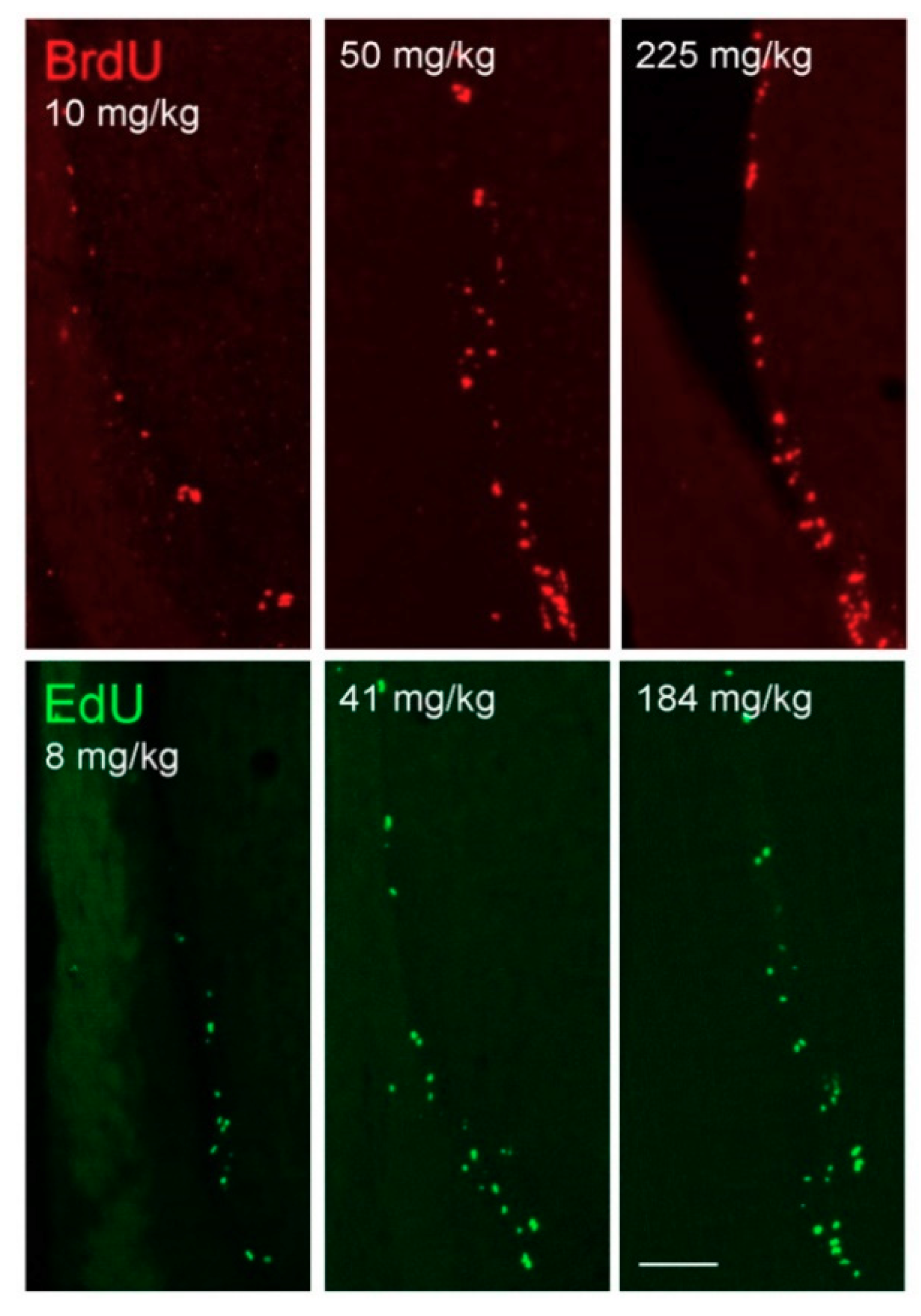
3. Results
3.1. Saturation Analyses
3.2. Comparison of Doses
3.3. Comparison of BrdU and EdU Labeling in the Same Animal
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Nottebohm, F. Migration of young neurons in adult avian brain. Nature 1988, 335, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Ligasova, A.; Koberna, K. DNA Replication: From Radioisotopes to Click Chemistry. Molecules 2018, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Nowakowski, R.S. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988, 457, 44–52. [Google Scholar] [CrossRef]
- Chehrehasa, F.; Meedeniya, A.C.; Dwyer, P.; Abrahamsen, G.; Mackay-Sim, A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J. Neurosci. Methods 2009, 177, 122–130. [Google Scholar] [CrossRef]
- Llorens-Martin, M.; Trejo, J.L. Multiple birthdating analyses in adult neurogenesis: A line-up of the usual suspects. Front. Neurosci. 2011, 5, 76. [Google Scholar] [CrossRef]
- Cavanagh, B.L.; Walker, T.; Norazit, A.; Meedeniya, A.C. Thymidine analogues for tracking DNA synthesis. Molecules 2011, 16, 7980–7993. [Google Scholar] [CrossRef]
- Anda, S.; Boye, E.; Grallert, B. Cell-cycle analyses using thymidine analogues in fission yeast. PLoS ONE 2014, 9, e88629. [Google Scholar] [CrossRef]
- Shevchouk, O.T.; Ball, G.F.; Cornil, C.A.; Balthazart, J. Studies of HVC Plasticity in Adult Canaries Reveal Social Effects and Sex Differences as Well as Limitations of Multiple Markers Available to Assess Adult Neurogenesis. PLoS ONE 2017, 12, e0170938. [Google Scholar] [CrossRef]
- Leuner, B.; Glasper, E.R.; Gould, E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J. Comp. Neurol. 2009, 517, 123–133. [Google Scholar] [CrossRef]
- Liboska, R.; Ligasova, A.; Strunin, D.; Rosenberg, I.; Koberna, K. Most anti-BrdU antibodies react with 2′-deoxy-5-ethynyluridine—The method for the effective suppression of this cross-reactivity. PLoS ONE 2012, 7, e51679. [Google Scholar] [CrossRef]
- Polomova, J.; Lukacova, K.; Bilcik, B.; Kubikova, L. Is neurogenesis in two songbird species related to their song sequence variability? Proc. Biol. Sci. 2019, 286, 20182872. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.A.; Thatra, N.M.; Hou, D.; Hu, R.A.; Brenowitz, E.A. Seasonal changes in neuronal turnover in a forebrain nucleus in adult songbirds. J. Comp. Neurol. 2019, 527, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Eadie, B.D.; Redila, V.A.; Christie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005, 486, 39–47. [Google Scholar] [CrossRef]
- Cameron, H.A.; McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Burns, K.A.; Kuan, C.Y. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur. J. Neurosci. 2005, 21, 803–807. [Google Scholar] [CrossRef]
- Zeng, C.; Pan, F.; Jones, L.A.; Lim, M.M.; Griffin, E.A.; Sheline, Y.I.; Mintun, M.A.; Holtzman, D.M.; Mach, R.H. Evaluation of 5-ethynyl-2’-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010, 1319, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Breunig, J.J.; Arellano, J.I.; Macklis, J.D.; Rakic, P. Everything that glitters isn’t gold: A critical review of postnatal neural precursor analyses. Cell Stem Cell 2007, 1, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef]
- Balthazart, J.; Ball, G.F. Endogenous versus exogenous markers of adult neurogenesis in canaries and other birds: Advantages and disadvantages. J. Comp. Neurol. 2014, 522, 4100–4120. [Google Scholar] [CrossRef]
- Mandyam, C.D.; Harburg, G.C.; Eisch, A.J. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 2007, 146, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Huntoon, S. Specificity and sodium dependence of the active nucleoside transport system in choroid plexus. J. Neurochem. 1984, 42, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.A.; Segal, M.B. Saturation kinetics, specificity and NBMPR sensitivity of thymidine entry into the central nervous system. Brain Res. 1997, 760, 59–67. [Google Scholar] [CrossRef]
- Bradbury, M. Why a blood-brain barrier? Trends Neurosci. 1979, 2, 36–38. [Google Scholar] [CrossRef]
- Hammond, J.R.; Clanachan, A.S. Species differences in the binding of [3H] nitrobenzylthioinosine to the nucleoside transport system in mammalian central nervous system membranes: Evidence for interconvertible conformations of the binding site/transporter complex. J. Neurochem. 1985, 45, 527–535. [Google Scholar] [CrossRef]
- Hayes, N.L.; Nowakowski, R.S. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res. Dev. Brain Res. 2002, 134, 77–85. [Google Scholar] [CrossRef]
- Cattan, A.; Ayali, A.; Barnea, A. The cell birth marker BrdU does not affect recruitment of subsequent cell divisions in the adult avian brain. Biomed. Res. Int. 2015, 2015, 126078. [Google Scholar] [CrossRef]
- Staroscik, R.N.; Jenkins, W.H.; Mendelsohn, M.L. Availability of Tritiated Thymidine after Intravenous Administration. Nature 1964, 202, 456–458. [Google Scholar] [CrossRef]
- Nowakowski, R.S.; Rakic, P. Clearance rate of exogenous 3H-thymidine from the plasma of pregnant rhesus monkeys. Cell Tissue Kinet. 1974, 7, 189–194. [Google Scholar] [CrossRef]
- Packard, D.S., Jr.; Menzies, R.A.; Skalko, R.G. Incorportaiton of thymidine and its analogue, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation 1973, 1, 397–404. [Google Scholar] [CrossRef]
- Barker, J.M.; Charlier, T.D.; Ball, G.F.; Balthazart, J. A new method for in vitro detection of bromodeoxyuridine in serum: A proof of concept in a songbird species, the canary. PLoS ONE 2013, 8, e63692. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Theelen, M.; Nottebohm, F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron 1990, 5, 101–109. [Google Scholar] [CrossRef]
- Vellema, M.; van der Linden, A.; Gahr, M. Area-specific migration and recruitment of new neurons in the adult songbird brain. J. Comp. Neurol. 2010, 518, 1442–1459. [Google Scholar] [CrossRef]
- Post, J.; Huang, C.Y.; Hoffman, J. The replication time and pattern of the liver cell in the growing rat. J. Cell Biol. 1963, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Melendez, J.; Liu, M.; Sampson, L.; Akunuru, S.; Han, X.; Vallance, J.; Witte, D.; Shroyer, N.; Zheng, Y. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology 2013, 145, 808–819. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubikova, L.; Polomova, J.; Mikulaskova, V.; Lukacova, K. Effectivity of Two Cell Proliferation Markers in Brain of a Songbird Zebra Finch. Biology 2020, 9, 356. https://doi.org/10.3390/biology9110356
Kubikova L, Polomova J, Mikulaskova V, Lukacova K. Effectivity of Two Cell Proliferation Markers in Brain of a Songbird Zebra Finch. Biology. 2020; 9(11):356. https://doi.org/10.3390/biology9110356
Chicago/Turabian StyleKubikova, Lubica, Justina Polomova, Viktoria Mikulaskova, and Kristina Lukacova. 2020. "Effectivity of Two Cell Proliferation Markers in Brain of a Songbird Zebra Finch" Biology 9, no. 11: 356. https://doi.org/10.3390/biology9110356
APA StyleKubikova, L., Polomova, J., Mikulaskova, V., & Lukacova, K. (2020). Effectivity of Two Cell Proliferation Markers in Brain of a Songbird Zebra Finch. Biology, 9(11), 356. https://doi.org/10.3390/biology9110356




