Phenotypic Responses, Reproduction Mode and Epigenetic Patterns under Temperature Treatments in the Alpine Plant Species Ranunculus kuepferi (Ranunculaceae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Morphological Growth Variables/Seed Set/Reproductive Fitness
2.3. Methylation-Sensitive Amplified Fragment-Length Polymorphisms (MS-AFLPs)
2.4. Flow Cytometric Seed Screening (FCSS)
2.5. Statistical Analyses
3. Results
3.1. Morphological Growth Data
3.2. Seed Set (Reproductive Fitness) and Reproduction Mode
3.3. Comparison of Morphological Growth Data with MS-AFLP Data
4. Discussion
4.1. Phenotypic Plasticity and Morphological Traits
4.2. Epigenetic Patterns and Morphological Traits
4.3. Reproduction Mode under Temperature Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- West-Eberhard, M.J. Phenotypic plasticity. In Encyclopedia of Ecology; Jorgensen, E., Fath, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 2701–2707. [Google Scholar]
- Körner, C.H. Alpine Plant Life, 2nd ed.; Springel: Berlin, Germany, 2003. [Google Scholar]
- Nagy, L.; Grabherr, G. The Biology of Alpine Habitats; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Brandshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Schlichting, C.D. The evolution of phenotypic plasticity in plants. Annu. Rev. Ecol. Syst. 1986, 17, 667–693. [Google Scholar] [CrossRef]
- Price, T.D.; Qvarnström, A.; Irwin, D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. Ser. B 2003, 270, 1433–1440. [Google Scholar] [CrossRef]
- Whitman, D.W.; Agrawal, A.A. What is phenotypic plasticity and why is it important? In Phenotypic Plasticity of Insects; Ananthakrishnan, T.N., Ed.; Science Publishers: Enfield, NH, USA, 2009; pp. 1–63. [Google Scholar]
- Munns, R. Plant adaptations to salt and water stress: Differences and commonalities. Adv. Bot. Res. 2011, 57, 1–32. [Google Scholar]
- Laland, K.N.; Uller, T.; Feldman, M.W.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablinka, E.; Odling-Smee, J. The extended evolutionary synthesis:its structure, assumptions and predictions. Proc. R. Soc. B 2015, 282, 20151019. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Alonso, C.; Becker, C.; Bossdorf, O.; Bucher, E.; Colome-Tatche, M.; Durka, W.; Engelhardt, J.; Gaspar, B.; Gogol-Döring, A.; et al. Ecological plant epigenetics: Evidence from model and non-model species, and the way forward. Ecol. Lett. 2017, 20, 1576–1590. [Google Scholar] [CrossRef]
- Kopp, M.; Matuszewski, S. Rapid evolution of quantitative traits: Theoretical perspectives. Evol. Appl. 2013, 7, 169–191. [Google Scholar] [CrossRef]
- Chevin, L.M.; Hoffmann, A.A. Evolution of phenotypic plasticity in extreme environments. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160138. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Merilä, J.; Hendry, P. Climate change, adaptation and phenotypic plasticity: The problem and the evidence. Evol. Appl. 2013, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Via, S.; Lande, R. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 1985, 39, 505–522. [Google Scholar] [CrossRef]
- Sterns, S. The evolutionary significance of phenotypic plasticity. Bioscience 1989, 39, 436–445. [Google Scholar] [CrossRef]
- Pigliucci, M. Phenotypic Plasticity: Beyond Nature and Nurture; John Hopkins Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2013, 7, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M. Adaptation to climate change through genetic accommodation and assimilation of plastic phenotypes. Philos. Trans. R. Soc. B 2019, 374, 20180176. [Google Scholar] [CrossRef] [PubMed]
- Angers, B.; Castonguay, E.; Massicotte, R. Environmentally induced phenotypes and DNA methylation: How to deal with unpredictable conditions until the next generation and after. Mol. Ecol. 2010, 19, 1283–1295. [Google Scholar] [CrossRef]
- Kooke, R.; Johannes, F.; Wardenaar, R.; Becker, F.; Etcheverry, M.; Colot, V.; Vreugdenhil, D.; Keurentjes, J.B. Epigenetic Basis of Morphological Variation and Phenotypic Plasticity in Arabidopsis thaliana. Plant Cell 2015, 27, 337–348. [Google Scholar] [CrossRef]
- Donelson, J.M.; Salinas, S.; Munday, P.L.; Shama, L.N.S. Transgenerational plasticity and climate change experiments: Where do we go from here? Glob. Chang. Biol. 2017, 24, 13–24. [Google Scholar] [CrossRef]
- Yeh, P.J.; Price, T.D. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 2004, 164, 531–542. [Google Scholar] [CrossRef]
- Sol, D.; Duncan, R.P.; Blackburn, T.M.; Cassey, P.; Lefebvre, L. Big brains, enhanced cognition and response of birds to novel environments. Proc. Natl. Acad. Sci. USA 2005, 102, 5460–6465. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer: Sunderland, MA, USA, 1998. [Google Scholar]
- Lande, R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 2009, 22, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Chevin, L.M.; Lande, R. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 2010, 64, 1143–1150. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Metcalf, J.C.; Rose, K.; Rees, M. Evolutionary demography of monocarpic perennials. Trends Ecol. Evol. 2003, 18, 471–480. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploidy formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Mirouze, M.; Paskzowski, J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef]
- Lacaze, X.; Hayes, P.M.; Korol, A. Genetics of phenotypic plasticity: QTL analysis in barley Hordeum vulgare. Heredity 2009, 102, 163–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Fischer, M.; Colot, V.; Bossdorf, O. Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol. 2013, 197, 314–322. [Google Scholar] [CrossRef]
- Nogler, G.A. Gametophytic apomixis. In Embryology of Angiosperms, 1st ed.; Johri, B.M., Ed.; Springer: Berlin, Germany, 1984; pp. 475–518. [Google Scholar]
- Ozias-Akins, P.; van Dijk, P.J. Mendelian genetics of apomixis in plants. Annu. Rev. Genet. 2007, 41, 509–537. [Google Scholar] [CrossRef]
- Hojsgaard, D.; Greilhuber, J.; Pellino, M.; Paun, O.; Sharbel, T.F.; Hörandl, E. Emergence of apospory and bypass of meiosis via apomixis after sexual hybridization and polyploidisation. New Phytol. 2014, 204, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A. Controlling Apomixis: Shared features and distinct characteristics of gene regulation. Genes 2020, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Klatt, S.; Schinkel, C.C.-F.; Kirchheimer, B.; Dullinger, S.; Hörandl, E. Effects of cold treatments on fitness and mode of reproduction in the diploid and polyploid alpine plant Ranunculus kuepferi (Ranunculaceae). Ann. Bot. 2018, 121, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Koltunow, A.M.; Grossniklaus, U. Apomixis: A developmental perspective. Annu. Rev. Plant Biol. 2003, 54, 547–574. [Google Scholar] [CrossRef]
- Grimanelli, D. Epigenetic regulation of reproductive development and the emergence of apomixis in angiosperms. Curr. Opin. Plant Biol. 2012, 15, 57–62. [Google Scholar] [CrossRef]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubesova, M.; Pysck, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef]
- Carman, J.G. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory and polyembryony. Biol. J. Linn. Soc. 1997, 61, 51–94. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Schinkel, C.C.-F.; Kirchheimer, B.; Dellinger, A.S.; Klatt, S.; Winkler, M.; Dullinger, S.; Hörandl, E. Correlations of polyploidy and apomixis with elevation and associated environmental gradients in an alpine plant. AoB Plants 2016, 8, plw064. [Google Scholar] [CrossRef]
- Levin, D.A. Minority cytotype exclusion in local plant populations. Taxon 1975, 24, 35–43. [Google Scholar] [CrossRef]
- Levin, D.A. The cytoplasmatic factor in plant speciation. Syst. Bot. 2003, 28, 5–11. [Google Scholar]
- Vandel, A. La parthenogenese geographique: Contribution a l’etude biologique et cytologique de la parthenogenese naturelle. Bull Biol. France Belg. 1928, 62, 164–281. [Google Scholar]
- Bierzychudek, P. Patterns in plant parthenogenesis. Experientia 1985, 41, 1255–1264. [Google Scholar] [CrossRef]
- Hörandl, E. The complex causality of geographical parthenogenesis. New Phytol. 2006, 171, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Hörandl, E.; Hadacek, F. The oxidative damage initiation hypothesis for meiosis. Plant Reprod. 2013, 26, 351–367. [Google Scholar] [CrossRef]
- Klatt, S.; Hadacek, F.; Hodac, L.; Brinkmann, G.; Eilerts, M.; Hojsgaard, D.; Hörandl, E. Photoperiod extension enhances sexual megaspore formation and triggers metabolic reprogramming in facultative apomictic Ranunculus auricomus. Front. Plant Sci. 2016, 7, 728. [Google Scholar] [CrossRef]
- Ulum, F.B.; Castro, C.C.; Hörandl, E. Ploidy-dependent effects of light stress on the mode of reproduction in the Ranunculus auricomus complex (Ranunculaceae). Front. Plant Sci. 2020, 11, 104. [Google Scholar] [CrossRef]
- Verhoeven, K.J.F.; Jansen, J.J.; van Dijk, P.J.; Biere, A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol. 2010, 185, 1108–1118. [Google Scholar] [CrossRef]
- Verhoeven, K.J.F.; van Dijk, P.J.; Biere, A. Changes in genomic methylation patterns during the formation of triploid asexual dandelion lineages. Mol. Ecol. 2010, 19, 315–324. [Google Scholar] [CrossRef]
- Verhoeven, K.J.F.; Preite, V. Epigenetic variation in asexually reproducing organisms. Evolution 2013, 68, 644–655. [Google Scholar] [CrossRef]
- Preite, V.; Snoek, L.; Oplaat, C.; Biere, A.; Putten, W.; Verhoeven, K.J.F. The epigenetic footprint of poleward range-expanding plants in apomictic dantelions. Mol. Ecol. 2015, 24, 4406–4418. [Google Scholar] [CrossRef] [PubMed]
- Cosendai, A.C.; Hörandl, E. Cytotype stability, facultative apomixis and geographical parthenogenesis in Ranunculus kuepferi (Ranunculaceae). Ann. Bot. 2010, 105, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Cosendai, A.C.; Rodewald, J.; Hörandl, E. Origin and distribution of autopolyploids via apomixis in the alpine species Ranunculus kuepferi (Ranunculaceae). Taxon 2011, 60, 355–364. [Google Scholar] [CrossRef]
- Burnier, J.; Buerki, S.; Arrigo, N.; Küpfer, P.; Alvarez, N. Genetic structure and evolution of Alpine polyploid complexes: Ranunculus kuepferi (Ranunculaceae) as a case study. Mol. Ecol. 2009, 18, 3730–3744. [Google Scholar] [CrossRef] [PubMed]
- Kirchheimer, B.; Schinkel, C.C.-F.; Dellinger, A.S.; Klatt, S.; Moser, D.; Winkler, M.; Lenoir, J.; Caccianiga, M.; Guisan, A.; Nieto-lugilde, D.; et al. A matter of scale: Apparent niche differentiation of diploid and tetraploid plants may depend on extent and grain of analysis. J. Biogeogr. 2016, 43, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Cosendai, A.C.; Wagner, J.; Ladinig, U.; Rosche, C.; Hörandl, E. Geographical parthenogenesis and population genetic structure in the alpine species Ranunculus kuepferi (Ranunculaceae). Heredity 2013, 110, 560–569. [Google Scholar] [CrossRef]
- Küpfer, P. Recherches sur les liens de parente entre la flore orophile des Alpes et celle des Pyrenees. Boissiera 1974, 23, 1–322. [Google Scholar]
- Kirchheimer, B.; Wessely, J.; Gattringer, A.; Hülber, K.; Moser, D.; Schinkel, C.C.-F.; Appelhans, M.; Klatt, S.; Caccianiga, M.; Dellinger, A.; et al. Reconstructing geographical parthenogenesis: Effects of niche differentiation and reproductive mode on Holocene range expansion of an alpine plant. Ecol. Lett. 2018, 21, 392–401. [Google Scholar] [CrossRef]
- Schinkel, C.C.-F.; Kirchheimer, B.; Dullinger, S.; Geelen, D.; De Storme, N.; Hörandl, E. Pathways to polyploidy: Indications of a female triploid bridge in the alpine species Ranunculus kuepferi (Ranunculaceae). Plant Syst. Evol. 2017, 303, 1093–1108. [Google Scholar] [CrossRef]
- Schinkel, C.C.-F.; Syngelaki, E.; Kirchheimer, B.; Dullinger, S.; Klatt, S.; Hörandl, E. Epigenetic patterns and geographical parthenogenesis in the alpine plant species Ranunculus kuepferi (Ranunculaceae). Int. J. Mol. Sci. 2020, 21, 3318. [Google Scholar] [CrossRef]
- Syngelaki, E.; Schinkel, C.C.-F.; Klatt, S.; Hörandl, E. Effects of temperature treatments on cytosine-methylation profiles of diploid and autotetraploid plants of the alpine species Ranunculus kuepferi (Ranunculaceae). Front. Plant Sci. 2020, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Bossdorf, O.; Arcuri, D.; Richards, C.L.; Pigliucci, M. Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol. Ecol. 2010, 24, 541–553. [Google Scholar] [CrossRef]
- Ladinig, U.; Hacker, J.; Neuner, G.; Wagner, J. How endangered is sexual reproduction of high-mountain plants by summer frosts? Frost resistance, frequency of frost events and risk assessments. Oecologia 2013, 171, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Huber, W. Natürliche Bastardierungen Zwischen Weissblühenden Ranunculus-Arten in den Alpen [Natural Hybridizations between White-Flowered Species of Ranunculus in the Alps]. Ph.D. Thesis, Federal Institute of Technology, Stiftung Rübel, Zürich, Switzerland, 1988. [Google Scholar]
- Paun, O.; Schönswetter, P. Amplified Fragment Length Polymorphism (AFLP)—An invaluable fingerprinting technique for genomic, transcriptomic and epigenetic studies. Methods Mol. Biol. 2012, 862, 75–87. [Google Scholar] [PubMed]
- Arrigo, N.; Tuszynski, J.W.; Ehrich, D.; Gerdes, T.; Alvarez, N. Evaluating the impact of scoring parameters on the structure of intra-specific genetic variation using RawGeno, an R package for automating AFLP scoring. BMC Bioinform. 2009, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Eckstein, R.L.; Durka, W. Scoring and analysis of methylation-sensitive amplification polymorphisms for epigenetic population studies. Mol. Ecol. Resour. 2013, 13, 642–653. [Google Scholar] [CrossRef]
- Matzk, F.; Meister, A.; Schubert, I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000, 21, 97–108. [Google Scholar] [CrossRef]
- Otto, F.J. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. Methods Cell Biol. 1990, 33, 105–110. [Google Scholar]
- Dolezel, J.; Bartos, J. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 2005, 95, 99–110. [Google Scholar] [CrossRef]
- Dolezel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 17 September 2020).
- RStudio Team. RStudio: Integrated Development for R.; RStudio, PBC: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 17 September 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; Version 2.2-1. 2019. Available online: https://CRAN.R-project.org/package=vegan/ (accessed on 17 September 2020).
- Bolker, B.; Brooks, M.; Clark, C.; Geange, S.; Poulsen, J.; Stevens, H.; White, J.-S. Generalized Linear Mixed Models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bateson, P. Why are individuals so different from each other? Heredity 2015, 115, 285–292. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fisher, M. Adaptive evolution of plastic foraging responses in a clonal plant. Ecology 2001, 82, 3309–3319. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fisher, M. Effects of four generations of density-dependent selection on life history traits and their plasticity in a clonally propagated plant. J. Evol. Biol. 2003, 16, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Bossdorf, O.; Pigliucci, M. Plasticity to wind is modular and genetically variable in Arabidopsis thaliana. Evol. Ecol. 2009, 23, 669–685. [Google Scholar] [CrossRef]
- Miryeganeh, M.; Saze, H. Epigenetic inheritance in plant evolution. Popul. Ecol. 2019, 62, 17–27. [Google Scholar] [CrossRef]
- Wyles, J.S.; Kunkel, J.G.; Wilson, A.C. Birds, behavior and anatomical evolution. Proc. Natl. Acad. Sci. USA 1983, 80, 4394–4397. [Google Scholar] [CrossRef] [PubMed]
- Pfenning, D.W.; McGee, M. Resource polyphenism increases species richness: A test of the hypothesis. Philos. Trans. R. Soc. Ser. B 2010, 365, 577–591. [Google Scholar] [CrossRef]
- Vrijenhoek, R.C.; Parker, E.D. Geographical parthenogenesis: General purpose genotypes and frozen niche variation BT. In Lost Sex: The Evolutionary Biology of Parthenogenesis; Schön, I., Martens, K., Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 99–131. [Google Scholar]
- Noble, D.; Jablonka, E.; Joyner, M.; Muller, G.; Omholt, S.W. Evolution evolves: Physiology returns to centre stage. J. Physiol. 2014, 592, 2237–2244. [Google Scholar] [CrossRef]
- Johannes, F.; Porcher, E.; Teixeira, F.K.; Saliba-Colombani, V.; Simon, M.; Agier, N.; Bulski, A.; Albuisson, J.; Heredia, F.; Audigier, P.; et al. Assessing the Impact of Transgenerational Epigenetic Variation on Complex Traits. PLoS Genet. 2009, 5, e1000530. [Google Scholar] [CrossRef]
- Cortijo, S.; Wandenaar, R.; Colome-Tatche, M.; Gilly, A.; Etcheverry, M.; Labadie, K.; Caillieux, E.; Hospital, F.; Aury, J.-M.; Wincker, P.; et al. Mapping the epigenetic basis of complex traits. Science 2014, 343, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Paskowski, J.; Grossniklaus, U. Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Curr. Opin. Plant Biol. 2011, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Milkos, I. Epigenetic inheritance, genetic assimilation and speciation. J. Theor. Biol. 1999, 200, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Crews, D.; Gore, A.C.; Hsu, T.S.; Dangleben, N.L.; Spinetta, M.; Schallert, T.; Anway, M.D.; Skinner, M.K. Transgenerational epigenetic imprints and mate preference. Proc. Natl. Acad. Sci. USA 2007, 104, 5942–5946. [Google Scholar] [CrossRef]
- Castonguay, E.; Angers, B. The key role of epigenetics in the persistence of a sexual lineage. Genet. Res. Int. 2012, 2012, 534289. [Google Scholar]
- Herrera, C.M.; Bazaga, P. Epigenetic correlates of plant phenotypic plasticity: DNA methylation differs between prickly and nonprickly leaves in heterophyllous Ilex aquifolium (Aquifoliaceae) trees. Bot. J. Linn. Soc. 2013, 171, 441–452. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Segal, D.L.; Hoyle, G.L.; Schrey, A.W.; Verhoeven, K.J.F.; Richards, C.L. Adaptive plasticity and epigenetic variation in response to warming in an alpine plant. Ecol. Evol. 2015, 5, 634–647. [Google Scholar] [CrossRef]
- Rubenstein, D.R.; Skolnik, H.; Berrio, A.; Champagne, F.A.; Phelps, S.; Solomon, J. Sex-specific fitness effects of unpredictable early life conditions are associated with DNA methylation in the avian glucocorticoid receptor. Mol. Ecol. 2016, 25, 1714–1728. [Google Scholar] [CrossRef]
- Verhoeven, K.J.F.; von Holdt, B.M.; Sork, V.L. Epigenetics in ecology and evolution: What we know and what we need to know. Mol. Ecol. 2016, 25, 1631–1638. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E. Adaptive transgenerational plasticity in plants: Case studies, mechanisms and implications for natural populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef]
- Jablonka, E.; Lamb, M.J. Epigenetic Inheritance and Evolution; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Richards, E.J. Inherited epigenetic variation-revisiting soft inheritance. Nat. Rev. 2006, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Maugarny-Calès, A.; Laufs, P. Getting leaves into shape: A molecular, cellular, environmental and evolutionary view. Development 2018, 145, dev161646. [Google Scholar] [CrossRef] [PubMed]
- Tholen, D.; Boom, C.; Zhu, X.-G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photsynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Aspinwall, M.J.; Loik, M.E.; De Dios, V.R.; Tjoelker, M.G.; Payton, P.R.; Tissue, D.T. Utilizing intraspecific variation in phenotypic plasticity to bolster agricultural and forest productivity under climate change. Plant Cell Environ. 2015, 38, 1752–1764. [Google Scholar] [CrossRef]
- Steward, N.; Ito, M.; Yamaguchi, Y.; Koizumi, N.; Sano, H. DNA methylation in maize nucleosomes and demethylation by environmental stress. J. Biol. Chem. 2002, 277, 37741–37746. [Google Scholar] [CrossRef]
- Shan, X.; Wang, X.; Yang, G.; Wu, Y.; Su, S.; Li, S.; Liu, H.; Yuan, Y. Analysis of the DNA Methylation of Maize (Zea mays L.) in response to cold stress Based on methylation-sensitive amplified polymorphisms. J. Plant Biol. 2013, 56, 32–38. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J. Dynamics and function of DNA methylation in plants. Nat. Rev. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Palmer, A.R. Waltzing with Asymmetry. BioScience 1996, 46, 518–532. [Google Scholar] [CrossRef]
- Moller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability and Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Dongen, S.V. Fluctuating asymmetry and development instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Angers, B.; Perez, M.; Menicucci, T.; Leung, C. Sources of epigenetic variation and their applications in natural populations. Evol. Appl. 2020, 13, 1262–1278. [Google Scholar] [CrossRef]
- Ladinig, U.; Wagner, J. Timing of sexual reproduction and reproductive success in the high-mountain plant Saxifraga bryoides L. Plant Biol. 2007, 9, 683–693. [Google Scholar] [CrossRef]
- Kumar, P.; Singhal, V.K. Male meiosis, morphometric analysis and distribution pattern of 2x and 4x cytotypes of Ranunculus hirtellus Royle (Ranunculaceae) from the cold regions of northwest Himalayas (India). Comp. Cytogenet. 2011, 5, 143–161. [Google Scholar] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Dewitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]

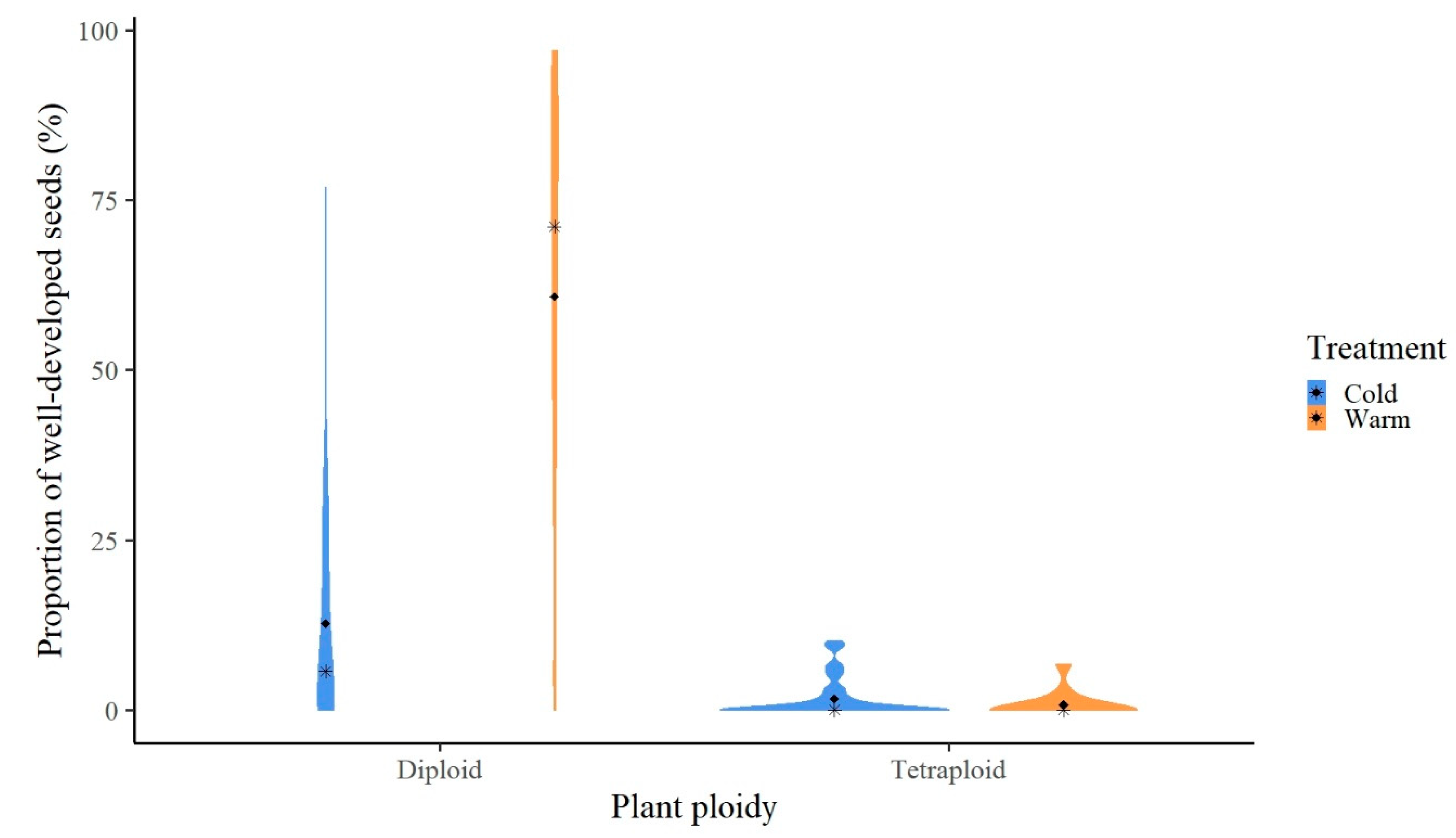
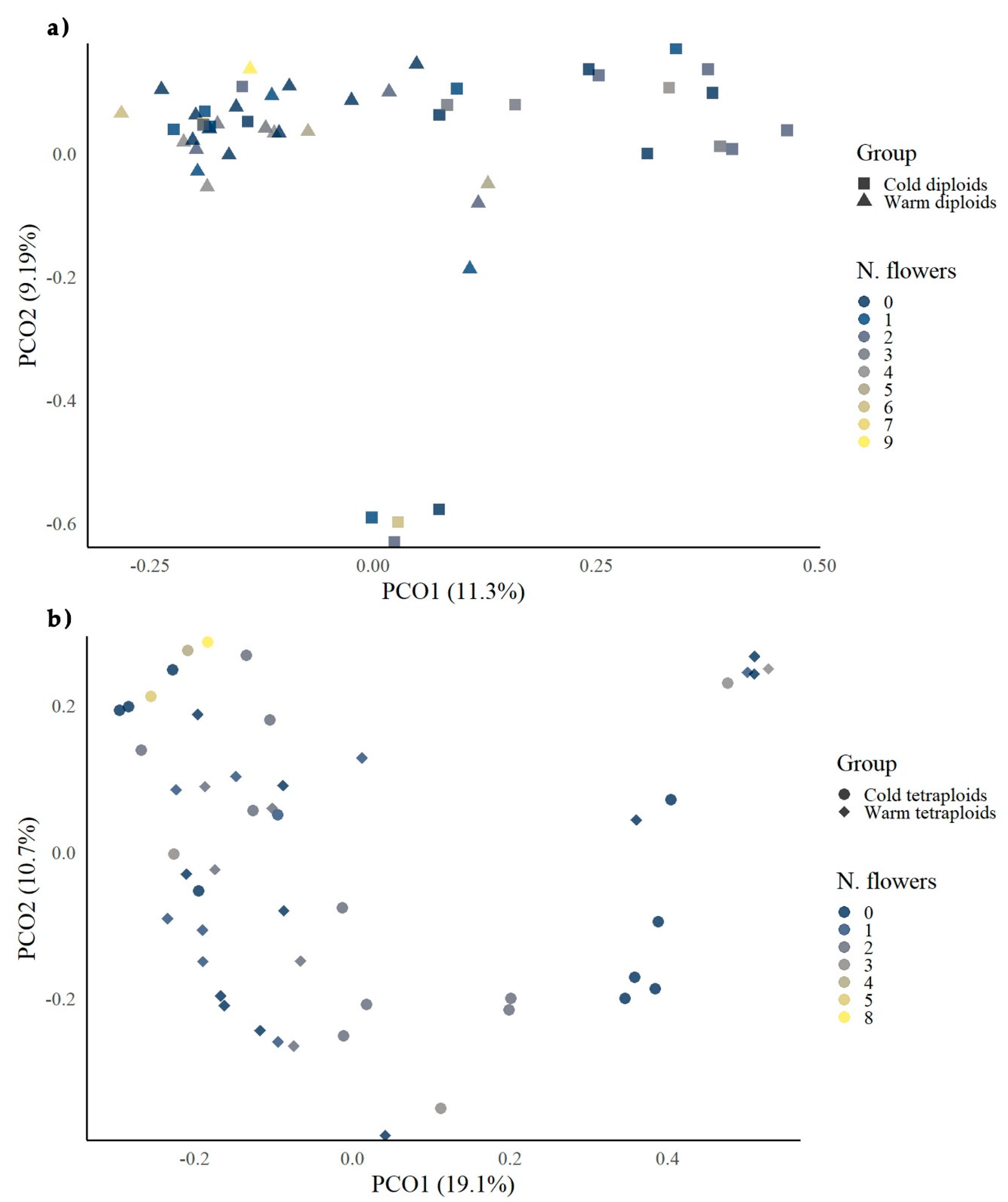
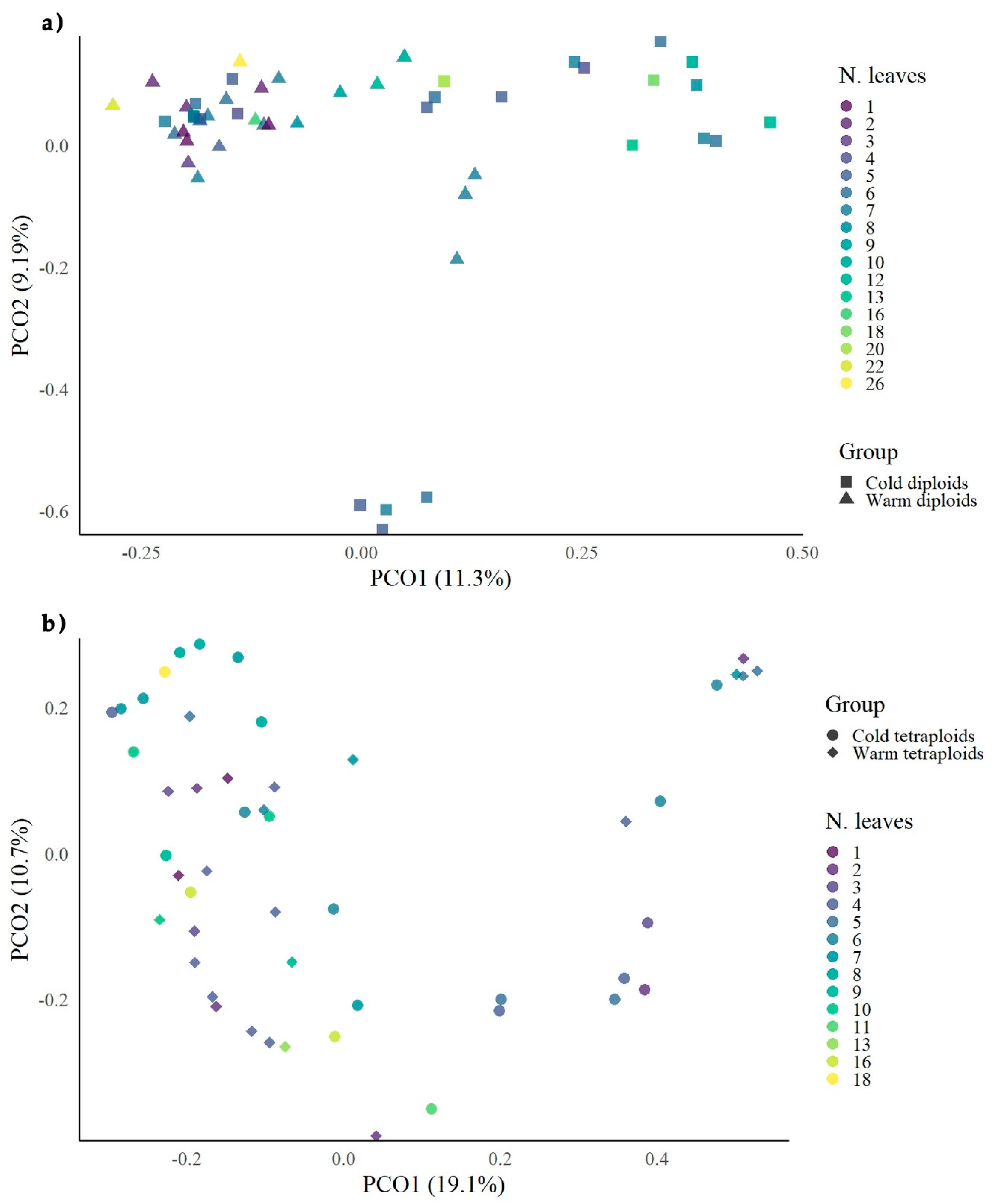
| Plant Ploidy | Cold Treatment | Warm Treatment | ||
|---|---|---|---|---|
| Diploid | Tetraploid | Diploid | Tetraploid | |
| No. plants | 164 | 189 | ||
| 74 | 90 | 92 | 97 | |
| Light regime (μmol·m−2·s−1, PAR) | ca. 700 * | |||
| Photoperiod | 16 h; 10 h of full light and 3 + 3 of twilight | |||
| Temperature during the light/dark period (°C) | +7 °C day/+ 2 °C night; frost treatment; −1 °C cold shocks for three nights per week | +15 °C day/+ 10 °C night | ||
| Nonmethylated Epiloci | Internally Methylated Epiloci | Externally Methylated Epiloci | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EST | SE | t | p | EST | SE | t | p | EST | SE | t | p | |
| (a) Linear Models (LMs) | ||||||||||||
| Stem height | ||||||||||||
| Epiloci’s arcsin | 0.3109 | 0.9664 | 0.322 | 0.749 | 0.317 | 0.7598 | 0.417 | 0.678 | −0.5181 | 0.7724 | −0.671 | 0.505 |
| Group WD | 21.0011 | 2.4582 | 8.543 | <0.0001 | 20.9473 | 2.2096 | 9.48 | <0.0001 | 22.7812 | 2.2576 | 10.091 | <0.0001 |
| Group WT | 14.9239 | 2.4573 | 6.073 | <0.0001 | 15.14 | 1.8074 | 8.376 | <0.0001 | 16.9496 | 2.5926 | 6.538 | <0.0001 |
| Group CD | 11.3751 | 2.6498 | 4.293 | <0.0001 | 11.3147 | 2.3252 | 4.866 | <0.0001 | 13.3097 | 2.209 | 6.025 | <0.0001 |
| Group CT | 18.653 | 2.3273 | 8.015 | <0.0001 | 18.836 | 1.7315 | 10.879 | <0.0001 | 20.5472 | 2.4101 | 8.525 | <0.0001 |
| Leaf length | ||||||||||||
| Epiloci’s arcsin | 0.7476 | 0.3653 | 2.047 | 0.0435 | −0.0095 | 0.3222 | −0.029 | 0.977 | −0.2165 | 0.3482 | −0.622 | 0.535 |
| Group WD | 8.1467 | 0.9546 | 8.534 | <0.0001 | 9.7367 | 0.9122 | 10.674 | <0.0001 | 10.1492 | 0.9062 | 11.199 | <0.0001 |
| Group WT | 8.9927 | 0.9476 | 9.49 | <0.0001 | 10.5581 | 0.7544 | 13.996 | <0.0001 | 11.1192 | 1.0916 | 10.186 | <0.0001 |
| Group CD | 7.5579 | 0.9898 | 7.636 | <0.0001 | 9.2389 | 0.9716 | 9.509 | <0.0001 | 9.7112 | 0.9849 | 9.86 | <0.0001 |
| Group CT | 9.4605 | 0.8641 | 10.948 | <0.0001 | 10.8027 | 0.6849 | 15.772 | <0.0001 | 11.371 | 1.0967 | 10.369 | <0.0001 |
| (b) Generalized Linear Models (GLMs) | ||||||||||||
| No. of flowers | ||||||||||||
| Epiloci’s arcsin | 0.0019 | 0.1519 | 0.012 | 0.9901 | −0.0077 | 0.1327 | −0.058 | 0.9536 | 0.0973 | 0.1514 | 0.643 | 0.5202 |
| Group WD | 2.0764 | 0.4299 | 4.83 | <0.0001 | 2.0971 | 0.4089 | 5.128 | <0.0001 | 1.8705 | 0.4126 | 4.533 | <0.0001 |
| Group WT | 0.836 | 0.3644 | 2.294 | <0.05 | 0.8495 | 0.2705 | 3.14 | <0.01 | 0.5777 | 0.4362 | 1.324 | 0.1854 |
| Group CD | 1.8357 | 0.4325 | 4.245 | <0.0001 | 1.8601 | 0.4206 | 4.423 | <0.0001 | 1.6387 | 0.4363 | 3.756 | <0.001 |
| Group CT | 1.7965 | 0.381 | 4.715 | <0.0001 | 1.8091 | 0.3075 | 5.884 | <0.0001 | 1.5369 | 0.4789 | 3.21 | <0.01 |
| No. of leaves | ||||||||||||
| Epiloci’s arcsin | −0.5111 | 0.3085 | −1.657 | 0.0975 | −0.3444 | 0.287 | −1.2 | 0.23 | 0.0663 | 0.3181 | 0.208 | 0.835 |
| Group WD | 8.4683 | 0.8596 | 9.852 | <0.0001 | 8.1792 | 0.8334 | 9.814 | <0.0001 | 7.2984 | 0.8359 | 8.731 | <0.0001 |
| Group WT | 5.7235 | 0.7948 | 7.201 | <0.0001 | 5.1557 | 0.6189 | 8.331 | <0.0001 | 4.4241 | 0.9462 | 4.676 | <0.0001 |
| Group CD | 8.8534 | 0.8903 | 9.945 | <0.0001 | 8.4953 | 0.896 | 9.482 | <0.0001 | 7.5825 | 0.9142 | 8.294 | <0.0001 |
| Group CT | 8.6126 | 0.7854 | 10.966 | <0.0001 | 8.1359 | 0.6513 | 12.493 | <0.0001 | 7.5378 | 1.0138 | 7.436 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syngelaki, E.; Daubert, M.; Klatt, S.; Hörandl, E. Phenotypic Responses, Reproduction Mode and Epigenetic Patterns under Temperature Treatments in the Alpine Plant Species Ranunculus kuepferi (Ranunculaceae). Biology 2020, 9, 315. https://doi.org/10.3390/biology9100315
Syngelaki E, Daubert M, Klatt S, Hörandl E. Phenotypic Responses, Reproduction Mode and Epigenetic Patterns under Temperature Treatments in the Alpine Plant Species Ranunculus kuepferi (Ranunculaceae). Biology. 2020; 9(10):315. https://doi.org/10.3390/biology9100315
Chicago/Turabian StyleSyngelaki, Eleni, Mareike Daubert, Simone Klatt, and Elvira Hörandl. 2020. "Phenotypic Responses, Reproduction Mode and Epigenetic Patterns under Temperature Treatments in the Alpine Plant Species Ranunculus kuepferi (Ranunculaceae)" Biology 9, no. 10: 315. https://doi.org/10.3390/biology9100315
APA StyleSyngelaki, E., Daubert, M., Klatt, S., & Hörandl, E. (2020). Phenotypic Responses, Reproduction Mode and Epigenetic Patterns under Temperature Treatments in the Alpine Plant Species Ranunculus kuepferi (Ranunculaceae). Biology, 9(10), 315. https://doi.org/10.3390/biology9100315





