Abstract
Recent advances in vitamin D research indicate that this vitamin, a secosteroid hormone, has beneficial effects on several body systems other than the musculoskeletal system. Both 25 dihydroxy vitamin D [25(OH)2D] and its active hormonal form, 1,25-dihydroxyvitamin D [1,25(OH)2D] are essential for human physiological functions, including damping down inflammation and the excessive intracellular oxidative stresses. Vitamin D is one of the key controllers of systemic inflammation, oxidative stress and mitochondrial respiratory function, and thus, the aging process in humans. In turn, molecular and cellular actions form 1,25(OH)2D slow down oxidative stress, cell and tissue damage, and the aging process. On the other hand, hypovitaminosis D impairs mitochondrial functions, and enhances oxidative stress and systemic inflammation. The interaction of 1,25(OH)2D with its intracellular receptors modulates vitamin D–dependent gene transcription and activation of vitamin D-responsive elements, which triggers multiple second messenger systems. Thus, it is not surprising that hypovitaminosis D increases the incidence and severity of several age-related common diseases, such as metabolic disorders that are linked to oxidative stress. These include obesity, insulin resistance, type 2 diabetes, hypertension, pregnancy complications, memory disorders, osteoporosis, autoimmune diseases, certain cancers, and systemic inflammatory diseases. Vitamin D adequacy leads to less oxidative stress and improves mitochondrial and endocrine functions, reducing the risks of disorders, such as autoimmunity, infections, metabolic derangements, and impairment of DNA repair; all of this aids a healthy, graceful aging process. Vitamin D is also a potent anti-oxidant that facilitates balanced mitochondrial activities, preventing oxidative stress-related protein oxidation, lipid peroxidation, and DNA damage. New understandings of vitamin D-related advances in metabolomics, transcriptomics, epigenetics, in relation to its ability to control oxidative stress in conjunction with micronutrients, vitamins, and antioxidants, following normalization of serum 25(OH)D and tissue 1,25(OH)2D concentrations, likely to promise cost-effective better clinical outcomes in humans.
1. Introduction
Vitamin D is a micronutrient that is metabolized into a multifunctional secosteroid hormone that is essential for human health. Globally, its deficiency is a major public health problem affecting all ages and ethnic groups; it has surpassed iron deficiency as the most common nutritional deficiency in the world. The increasing prevalence of vitamin D deficiency and its associated complications are prominent in countries furthest from the equator. However, incidence is also high among those who live within 1,000 km of the equator (e.g., in Sri Lanka, India, and Far Eastern, Middle Eastern, Central American, and Persian Gulf countries) because of a combination of climatic conditions, ethnic and cultural habits, and having darker skin color [1,2,3,4].
Most of the vitamin D the human body requires can be generated by an individual’s exposure to summer-like sunlight, with dietary sources playing a supporting role when sunlight exposure is limited or ineffective for vitamin D production. Despite the above, more than 50% of the population in the group of countries mentioned has vitamin D deficiency [5,6]. If effective public health guidelines are implemented, vitamin D deficiency can be easily and cost-effectively treated and prevented, saving millions of dollars and lives. Although excessive sun exposure does not cause hypervitaminosis D, it can cause other harm because of dermal cell DNA damage [7,8,9]. Thus, guidelines for safe sun exposure are needed for each country.
During the past decade, many advances in the understanding of the physiology and biology of vitamin D and its receptor ecology have emerged [10]. Vitamin D metabolism and functions are modulated by many factors. Accumulating evidence supports biological associations of vitamin D with disease risk reduction and improved physical and mental functions. The field is rapidly advancing, including the knowledge of the physiology of vitamin D-vitamin D receptor (VDR) interactions and the biology and metabolism of vitamin D and their effects on vitamin D axis and gene polymorphisms [11]. Together these data have facilitated our understanding of new pathways to intervene to prevent and treat human diseases.
However, what is lacking is the adequately powered, conducted for sufficient duration, well-designed randomized controlled clinical studies (RCTs) conducted in an unbiased manner with the nutrient vitamin D as the key intervention and having predefined hard endpoints/primary outcomes [12]. Moreover, such studies must recruit persons with vitamin D deficiency [i.e., serum 25(OH)D concentrations less than 20 ng/mL (50 nmol/L)] and achieve a pre-determined target serum 25(OH)D concentration through daily oral administration and/or safe exposure to ultraviolet rays; not merely by relying on oral administered doses. The goal of this review is to explore the effects from vitamin D-modulated gene interactions and the effects of hypovitaminosis-induced, mitochondria-based oxidative stress on aging.
1.1. Extrarenal Generation of 1,25(OH)2D
The active form of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D], is generated not only in renal tubular cells (endocrine functions as a hormone) but also in extrarenal target tissue cells, providing autocrine and paracrine functions. However, because it remains within the target tissue cells, the intra-cellular concentrations achieved are unclear. In addition, the catabolic activity of 24-hydroxylase in target tissues plays an important part in regulating both 25-hydroxy vitamin D [25(OH)D; calcidiol], and 1,25(OH)2D (calcitriol) concentrations and their availability.
The amounts of 1,25(OH)2D generated in renal tubules and target cells can vary from person to person and day to day and are hard to quantify. Although the calcitriol in the circulation is modulated by parathyroid hormone (PTH) and serum ionized calcium concentrations [13], the intracellular content is regulated largely through serum 25(OH)D availability and calcidiol and calcitriol catabolism through hydroxylation at the molecular positions C-24 and C-23 by a specific 24-hydroxylase (CYP24A1) [14].
1.2. Excess Sun Exposure Does Not Cause Hypervitaminosis D
After exposure to ultraviolet B (UVB) rays, dermal cells actively synthesize vitamin D. As a feedback mechanism, excess precursors produced are catabolized within the dermal cells by the same UVB rays. In addition, skin also contains the inactivating enzyme 24-hydroxylase, which also prevents over-production of vitamin D by 24-hydroxylation of vitamin D [15]. This process of homeostasis is regulated by UVB, PTH, and serum ionized calcium concentrations [16]. When an individual is overexposed to ultraviolet rays, the mentioned built-in protective mechanism prevents excessive retention of vitamin D in the skin. Therefore, sun exposure does not raise serum 25(OH)D to pathological levels, cause hypervitaminosis D or its complications, such as hypercalcemia.
In addition, vitamin D synthesized in the skin from UVB exposure in excess of need is catabolized in part through 20-hydroxylation by the cholesterol side chain cleavage enzyme CYP11A1 [17]. Thus, there are multiple intrinsic mechanisms present to prevent excess vitamin D from reaching the circulation. The efficiency of vitamin D synthesis in the skin is affected by many factors, including the density of melanin pigment, condition of the skin and age, and the use of sunscreen and UV-blocking makeup, creams, and ointments and clothing. In addition, older age or scarred skin, as well as time of day or year and the duration of sun exposure affect vitamin D synthesis in the skin [18,19,20,21].
Although solar radiation is the source of vitamin D generation in the skin, excessive exposure, particularly in those who are genetically vulnerable, may cause skin cancers [22,23,24]. On the other hand, optimal vitamin D status protects against several types of internal cancers, melanoma, and several other diseases. Therefore, one needs to balance sun exposure in favor of benefits while avoiding potential harmful effects [25,26,27].
2. Vitamin D and Gene Regulation
1,25(OH)2D regulates many genes within the human genome [28]. Tissue vitamin D concentrations and its receptor gene polymorphisms, not only influence mechanism of actions and modulate second messenger systems, but also modulate the ability of the ligand-bound VDR to bind to vitamin D response elements (VDREs) on promoter regions in genes and initiate second messenger systems [29]. The National Human Genome Research Institute (NHGRI) launched a public research consortium, ENCODE [Encyclopedia of DNA Elements; http://www.genome.gov/10005107] to answer pertinent questions [30].
ENCODE has demonstrated the genome-wide actions of 1,25(OH)2D3 on the formation rates of proteins, such as the insulator protein CTCF (transcriptional repressor CTCF; 11-zinc finger protein, CCCTC-binding factor, etc.) and the VDR [31,32]. These findings suggest the presence of numerous functional VDRE regions across the human genome [29,33]. In addition, it has been suggested that the expression of these genes could be used as biomarkers for different actions of vitamin D in varied tissues and cells and assessment of vulnerabilities [34].
Hormone, 1,25(OH)2D modulates cell proliferation through direct and indirect pathways. For example, vitamin D inhibits the pathways related to transcription factor NF-κB [35]. People with chronic non-communicable diseases, such as cardiovascular disease, type 2 diabetes, autoimmune diseases, arthritis, and osteoporosis are reported to have chronically elevated NF-κB [36]. NF-κB enhances the oxidative stress and cellular responses to inflammation and injury; including following head injury [37]. Whereas, 1,25(OH)2D (calcitriol) suppresses NF-κB and thereby reduces chronic diffuse somatic inflammation [38,39]. In addition, calcitriol also reduce cell proliferation and enhance cell differentiation—key anti-cancer effects of vitamin D [40].
2.1. Epigenetic Mechanisms Influence Cancer Genesis
Epigenetic mechanisms influence cancer genesis, growth, dissemination, and aging phenomena [41,42,43,44]. For example, the epigenetic modifications of VDR–1,25(OH)2D effects can be mediated through complex processes involving CYP27A1 and CYP27B1 and via the vitamin D-catabolizing enzyme CYP24 [14,44]. These actions can be favorably influenced by modifications of VDREs across the genome modulated by both histone acetylases and deacetylases [28,29,45].
Epigenetic regulation of vitamin D metabolism influences several physiological mechanisms and modulate outcomes of some human diseases. Example of diseases include adenocarcinoma of the lung [44], specific gene mutations in Asians with advanced non-small cell lung cancer [41], and genetic alterations in the effectiveness of systemic therapy for lung cancer induced by cigarette smoking [42]. In severely obese children, low 25(OH)D concentrations are associated with increased markers of oxidative and nitrosative stress, inflammation, and endothelial over-activation [46].
CYP27B1-mediated target tissue production of 1,25(OH)D is critically important for the paracrine and autocrine functions of calcitriol to obtain the full biological potential of vitamin D. Taken together, the benefits of having adequate serum 25(OH)D concentrations and maintaining vitamin D repletion in the long run and considering the overall health benefits of vitamin D, there is an urgent need to create national policies to combat hypovitaminosis D. The savings derived from reducing the risks and severity of infectious and parasitic diseases alone would pay-off the cost of this public health approach. This can be achieved through targeting to raise the population serum 25(OH)D concentration, leading to a tangible positive impact on humans and on the economy.
2.2. Epigenetics and Molecular Genetics of Vitamin D
Molecular and genetic studies confirm that vitamin D also modulates risks of several other human diseases, including autoimmune disorders such as multiple sclerosis [47]. Although the predominant cause of cancer is modulation of the underlying metabolic abnormalities through genes, such as p53 and c-myc modifying the metastatic risks, the responsiveness to therapy is in part determined by epigenetic modifications of genes.
Tumor-related key metabolic abnormalities include imbalance between glucose fermentation and oxidative phosphorylation (under aerobic and anaerobic conditions—the Warburg effect); dysregulation of metabolic enzymes, such as pyruvate kinase, fumarate hydratase, and succinate dehydrogenase; isocitrate dehydrogenase mutations; and alterations of gene expression levels linked to tumorigenesis that are influenced by the vitamin D status [48].
Examples related to activity of the vitamin D axis include epigenetic changes that affect the expression of the CYP24A1 gene and VDR polymorphisms. Although epigenetic enhancement can occur through methylation and repression by histone-modifications of DNA, vitamin D markedly influences the regulation of cell replication [42,44]. This substantiates targeting of CYP24A1 to optimize the antiproliferative effects of 1,25(OH)2D in a target-specific manner [49].
In addition, gene activation following the interaction of 1,25(OH)2D with VDR is important for mitochondrial integrity and respiration, and many other physiological activities. Moreover, the vitamin D signaling pathway plays a central role in protecting cells from elevated mitochondrial respiration and associated damage and overproduction of reactive oxygen species (ROS), which can lead to cellular and DNA damage [50].
3. Vitamin D–Oxidative Stress
1,25(OH)2D is involved in many intracellular genomic activities and biochemical and enzymatic reactions, whereas 25(OH)D concentrations are important in overcoming inflammation, the destruction of invading microbes and parasites, the minimization of oxidative stress following the day-to-day exposure to toxic agents, and controlling the aging process [51,52].
For example, the presence of a physiologic 25(OH)D concentration enhances the expression of the nuclear factor, erythroid-2(Nf-E2)-related factor 2(Nrf2) [53,54,55] and enhances Klotho, a phosphate regulating hormone and also an antiaging protein [56,57]. It also facilitates protein stabilization [11]. Klotho also regulates cellular signaling systems, including the formation of antioxidants [58]. Consequently, in mice, functional abnormalities of the Klotho gene or removal of it through gene knock-out procedures induce premature aging syndrome [59]. In animal studies, inefficient FGF23 and/or Klotho expression have shown to cause premature aging. Figure 1 is a schematic representation of various key factors and their interactions that influence aging and death.
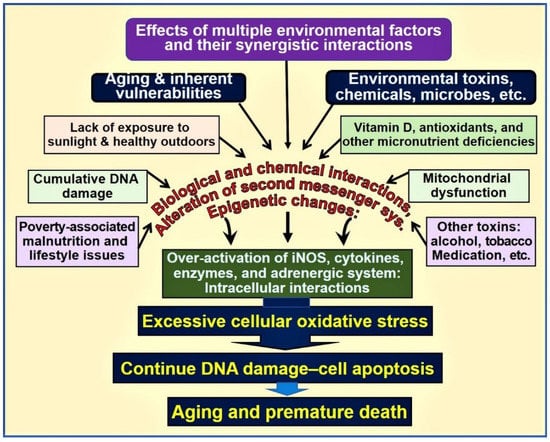
Figure 1.
Environmental, microbial, biological and chemical interactions that modify the DNA and mitochondrial functions and epigenetics, which modifies the aging process. Vitamin D deficiency is one of the factors that enhances this oxidative-stress cycle and accelerating premature cell death [abbreviations used: DNA = deoxyribonucleic acid; iNOS = inducible nitric oxide enzyme].
Influences of Vitamin D on Oxidative Stress
When vitamin D status is adequate, many of the intracellular oxidative stress-related activities are downregulated. Having suboptimal concentrations of serum 25(OH)D fails to subdue oxidative stress conditions, augment intracellular oxidative damage and the rate of apoptosis. The intracellular Nrf2 level is inversely correlated with the accumulation of mitochondrial ROS [51,60] and the consequent escalation of oxidative stress. Thus, Nrf2 plays a key role in protecting cells against oxidative stress; this is modulated by vitamin D [61,62].
In addition, vitamin D supports cellular oxidation and reduction (redox) control by maintaining normal mitochondrial functions [63,64,65]. Loss in the redox control of the cell cycle may lead to aberrant cell proliferation, cell death, the development of neurodegenerative diseases, and accelerated aging [65,66,67,68]. Peroxisome proliferator-activated receptor-coactivator 1α (PGC-1α) is bound to mitochondrial deacetylase (SIRT3). PGC-1α directly couples to the oxidative stress cycle [69] and interacts with Nrf2. This complex regulates the expression of SIRT3; this process is influenced by vitamin D metabolites [70]. In addition, the activation of the mitochondrial Nrf2/PGC-1α-SIRT3 path is dependent on intracellular calcitriol concentrations.
Calcitriol has overarching beneficial effects in upregulating the expression of certain antioxidants and anti-inflammatory cytokines [71], thereby protecting the tissues from toxins, micronutrient deficiency-related abnormalities, and parasitic and intracellular microbe-induced harm [72]. It regulates ROS levels through its anti-inflammatory effects and mitochondrial-based expression of antioxidants through cell-signaling pathways [67,73].
4. Role of Vitamin D in Neutralization of Toxins and Aging-Related Compounds
Following 1,25(OH)2D—VDR interaction, the transcription factor Nrf2 translocates from the cytoplasm to the nucleus. Nrf2 activates the expression of several genes that have antioxidant activity [52,54,67]. When Nrf2 activity is insufficient, risks from oxidative stress-related tissue damage increases [61,74]. The resultant excessive ROS formation by dysregulated mitochondria leads to a pathologic oxidative stress cycle, a key cause of toxin-induced and age-related cell death [68,75,76].
Meanwhile, the Nrf2 activity in part is controlled by the cytosolic protein Keap1 [77], another transcription factor and a negative regulator of Nrf2 [55,61]. Keap1 also controls the subcellular distribution of Nrf2 that correlates with its antioxidant activity [53,54]. When confronted with intracellular oxidative and/or electrophilic stresses, as a protective mechanism, the Nrf2-antioxidant response path is activated. This response enhances gene transcription and translation of protein products that are necessary to eliminate and/or neutralize toxins, ROS, and cumulating aging-related products through conjugation [78,79].
4.1. The Concept and the Process of Aging
Aging generally refers to the biological process of growing older, also known as cellular senescence, and is a complex process. Advancing age is, especially after adulthood, is associated with a gradual decline of physiological functions and capacities [80]. Aging has also been quantified from mortality curves using mathematical modeling; for example, by using Gompertz equation m(t) = AeGt, for which, m(t) = the mortality rate as a function of time or age (t); A = extrapolated constant to birth or maturity; G = the exponential (Gompertz) mortality rate coefficient] [79].
Moreover, efficiency and the functions of the body decline after sexual maturity, suggesting a connection between the aging process after fulfilling the procreation needs. Most age-related functions are irreversible, in part due to accumulation of oxidative stress-related toxic products, methylation of DNA, and mitochondrial damage, leading to reduced viability of cells and consequent accelerated cell death [81]. There is also a parallel decline in the immune system functions (i.e., immune-senescence) and an increase in inflammation, demonstrable with increased circulating pro-inflammatory cytokines [82,83]. These are likely to contribute to several age-related disorders, such as Alzheimer’s disease, cardiovascular and pulmonary diseases, and susceptibility to autoimmunity and infections [82,83].
Many bodily functions slow with aging, including response and reaction time; access to and the capacity of memory; pulmonary, gastrointestinal, and cardiovascular capacities; and even the ability to generate vitamin D in the skin. While age is perhaps the strongest risk factors for death, age-related disorders are the number one cause of death among the adults. This scenario is aggravated in the presence of vitamin D deficiency.
Chronic hypovitaminosis D is associated with cardiovascular and metabolic dysfunctions and premature deaths [84], even among children [85]. Overall data suggest that vitamin D deficiency could be considered an important comorbidity or a risk factor for premature death [84,85,86,87]. In fact, inverse relationships have been reported with vitamin D adequacy, with reduced all-cause mortality [88,89,90], and cancer [90,91,92,93].
4.2. Effects of Vitamin D on Apoptosis and Aging
The generalized inflammatory process is known to cause cellular damage and increase apoptosis [94], as in the case of interstitial tubular cell damage in chronic kidney disease, and thus is a part of the aging process [52,55,95]. In addition, hypovitaminosis D and dysfunctional mitochondrial activity increase inflammation [73,96,97]. Thus, the anti-inflammatory effects from having adequate, physiological vitamin D concentrations are important [95,98]. Hypovitaminosis D increases the expression of inflammatory cytokines [71,99] such as tumor necrosis factor-α (TNF-α), increasing the expression of the InsP3Rs and resulting in increased intracellular Ca2+ and accelerating cellular damage, apoptosis, and aging [66,75].
Many of the genes in the Klotho–Nrf2 regulatory system have multiple functions that are regulated by calcitriol [57,62,65]. These include, increasing intracellular antioxidant concentration, maintaining the redox homeostasis and, normal intracellular-reduced environment by removing excess ROS, and thereby down-regulating the oxidative stress [100]. In addition, the vitamin D-dependent expression of γ-glutamyl transpeptidase, glutamate cysteine ligase, and glutathione reductase contribute to the synthesis of the key redox agent glutathione (an essential antioxidant of low–molecular-weight thiol) [99,101].
Vitamin D also upregulates the expression of glutathione peroxidase that converts the ROS molecule H2O2 to water [101]. Vitamin D also effect the formation of glutathione through activation of the enzyme glucose-6-phosphate dehydrogenase [101]—which downregulates nitrogen oxide (NOx), a potent precursor for generating ROS that converts O2− to H2O2 and upregulating superoxide dismutase (SOD). These vitamin D-related actions collectively reduce the burden of intracellular ROS.
Telomeres are repetitive DNA sequences that caps end of linear chromosomes protecting DNA molecules [102]. Aging is associated with shortening of telomeres, including in stem cells. The amount of telomerase present is gradually become too short to maintain its protective effects on DNA during cell division, and thus cell apoptosis. While vitamin D deficiency increases inflammation and the intracellular oxidative stress, the latter enhances the rate of telomere shortening during cell proliferation, resulting in genomic instability [36].
4.3. Hypovitaminosis D Leads to Deranged Mitochondrial Respiration
Activated vitamin D is an essential component for maintaining physiological respiratory chain activity in mitochondria, facilitating the generation of energy [103,104]. In addition, 25(OH)D regulates the expression of the uncoupling protein that is attached to the inner membrane of mitochondria that regulates thermogenesis [105,106,107]. Chronic vitamin D deficiency reduces the capacity of mitochondrial respiration through modulating nuclear mRNA [108,109,110]. The latter also downregulates the expression of complex I of the electron transport chain and thus reduces the formation of adenosine triphosphate (ATP) [67,75], another mechanism that increases cancer risks. Consequently, a low level of electron transport chain increases the formation of ROS and oxidative stress, a common phenomenon following acute and chronic exposure to toxins and many chronic diseases and seen in aging [66,111,112].
The accumulation of intracellular toxins and/or age-related products disrupts signaling pathways, including the G protein–coupled systems, caspases, mitochondria, and the death receptor-linked mechanisms, triggering cell apoptosis and causing premature cell death [113,114]. The process is aggravated by stimulating G proteins, leading to activation of downstream pathways, including protein kinase A and C (PKA and PKC), phosphatidylinositol 3 kinase (PI3-kinase), Ca2+ and MAP kinase-dependent systems, tyrosine phosphorylation [75,114], and work additively, aiding cancer genesis [93] and accelerating the aging process.
4.4. Calcitriol Protects Mitochondrial Functions
Toxins, chronic metabolic abnormalities, and the aging process are known to cause mitochondrial dysfunction [66,106,107,108,115]. Abnormal mitochondria produce suboptimal amounts of ATP while generating excess ROS, creating a vicious cycle of enhanced and persisting the effects from excessive oxidative stress [106,107,116]. These events cause DNA damage (and impair DNA repair systems), premature cell death, and accelerated aging [62,66]. Data are accumulating that suggest that mitochondrial dysfunction is likely fueled by sustained intracellular inflammation, as in the case with vitamin D deficiency [79,95,97,117].
Based on animal studies, researchers have reported that mitochondrial decay, a part of the aging process, can be slowed by micronutrient supplementation (e.g., by lipoic acid, acetyl carnitine, vitamin K, and vitamin D) and by boosting coenzyme levels through high doses of vitamin B, such as pantothenic acid [118]. The NAD+-dependent protein deacetylases sirtuins function as antiaging proteins that also neutralize excess ROS [11]. For example, sirtuin 1 (SIRT) is essential to maintaining normal mitochondrial functions; meanwhile, calcitriol and SIRT1 work synergistically to regenerate mitochondria [119,120]. Their actions facilitate the removal and neutralization of toxins and thereby reduce the rate and the effects of aging [121,122].
Dysfunctional mitochondria also have reduced intracellular Ca2+ buffering capacity, resulting in increased (and fluctuating) intracellular Ca2+ levels, which are cytotoxic and contribute to sustenance of several chronic diseases [108,115]. Sub physiological concentrations of calcitriol, at least in part, enhance and maintain oxidative stress, autophagy, inflammation, mitochondrial dysfunction, epigenetic changes, DNA damage, intracellular Ca2+, and generation and signaling of ROS. Therefore, sustained, adequate serum 25(OH)D concentrations should allow target tissues to keep many of these harmful processes under control [68,71,73]. Multiple benefits of controlling excessive oxidative stress are illustrated in Figure 2.
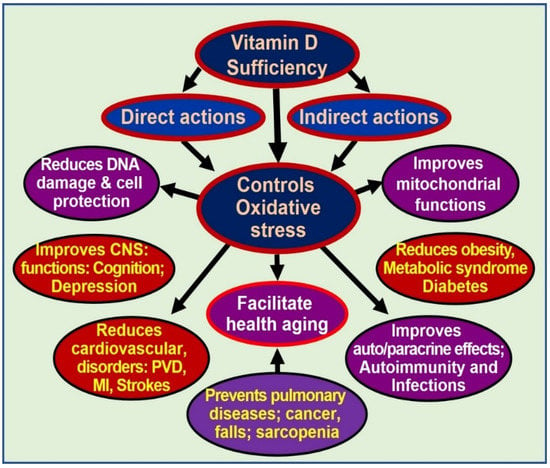
Figure 2.
Oxidative stress is harmful to cells. Controlling oxidative stresses through vitamin D adequacy leads to cellular and organ protection and reduces the effects of aging [abbreviations used: CNS = central nervous system; DNA = deoxyribonucleic acid; MI = myocardial infarction; PVD = peripheral vascular diseases].
5. Discussion
The proper functioning of the vitamin D endocrine, paracrine, and autocrine systems is essential for many human physiological functions. Vitamin D deficiency, as determined by serum 25(OH)D concentrations of less than 30 ng/mL, is associated with increased risks of illnesses and disorders and increased all-cause mortality even among apparently healthy individuals, including those with normal serum 1,25(OH)2D. Some of the key functions of vitamin D include subduing oxidative stress and chronic inflammation and maintaining mitochondrial respiratory functions. Through its targeted mitochondrial activity and subduing of ROS through multiple mechanisms, vitamin D has key beneficial effects on controlling oxidative stress, inflammation, and energy metabolism.
Normal serum concentrations of both 25(OH)D and 1,25(OH)2D are essential for optimal cellular function and protect from the excessive oxidative stress-related DNA damage. However, increased risk for illnesses and reduced longevity can occur despite the presence of physiologic concentrations of calcitriol because this is not the only mechanism protecting cells from oxidative stress. Physiologic serum 25(OH)D and 1,25(OH)2D levels in target tissues allow exertion of the homeostatic modulatory effects on enzymatic reactions, mitochondrial activities, and functioning of optimal second messenger systems. These are essential parts of the actions of vitamin D mediated through the mentioned mechanisms.
Vitamin D metabolism and functions are modulated by many factors, including physical activities and lifestyles, certain medications, environmental pollutants, and epigenetics, all of which also modify the balance between energy intake and expenditure through mitochondrial metabolic control [123]. For reductions in the incidence of diseases, longer-term maintenance of a steady state of the serum 25(OH)D concentration is necessary [124]. The minimal level is considered to be 30 ng/mL (50 nmol/L).
After correction of vitamin D deficiency through loading doses of oral vitamin D (or safe sun exposure), adequate maintenance doses of vitamin D3 are needed. This can be achieved in approximately 90% of the adult population with vitamin D supplementation between 1000 to 4000 IU/day, 10,000 IU twice a week, or 50,000 IU twice a month [10,125]. On a population basis, such doses would allow approximately 97% of people to maintain their serum 25(OH)D concentrations above 30 ng/mL [19,126]. Others, such as persons with obesity, those with gastrointestinal disorders, and during pregnancy and lactation, are likely to require doses of 6,000 IU/day [127,128].
Funding
This research received no funding.
Acknowledgments
The author greatly appreciates the helpful comments of Eugene L. Heyden.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxy vitamin D |
| Nrf2 | Nuclear factor erythroid 2 (Nf-E2)-related factor 2 |
| PTH | Parathyroid hormone |
| ROS | Reactive oxygen species |
| UVB | Ultraviolet B |
| VDR | Vitamin D receptor |
| VDRE | Vitamin D response element |
References
- Van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Eggemoen, A.R.; Knutsen, K.V.; Dalen, I.; Jenum, A.K. Vitamin D status in recently arrived immigrants from Africa and Asia: A cross-sectional study from Norway of children, adolescents and adults. BMJ Open 2013, 3, e003293. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef]
- Haq, A.; Wimalawansa, S.J.; Carlberg, C. Highlights from the 5th International Conference on Vitamin D Deficiency, Nutrition and Human Health, Abu Dhabi, United Arab Emirates, March 24-25, 2016. J. Steroid Biochem. Mol. Biol. 2018, 175, 1–3. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Felton, S.J.; Cooke, M.S.; Kift, R.; Berry, J.L.; Webb, A.R.; Lam, P.M.; de Gruijl, F.R.; Vail, A.; Rhodes, L.E. Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low-level summer sunlight exposures. Br. J. Dermatol. 2016, 175, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Thomson, C.; Tongkao-on, W.; Song, E.J.; Carter, S.E.; Dixon, K.M.; Mason, R.S. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. Adv. Exp. Med. Biol. 2014, 810, 303–328. [Google Scholar] [PubMed]
- Petersen, B.; Wulf, H.C.; Triguero-Mas, M.; Philipsen, P.A.; Thieden, E.; Olsen, P.; Heydenreich, J.; Dadvand, P.; Basagana, X.; Liljendahl, T.S.; et al. Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage. J. Investig. Dermatol. 2014, 134, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D. What clinicians would like to know. Sri Lanka Journal of Diabetes, Endocrinology and Metabolism 2012, 1, 73–88. [Google Scholar] [CrossRef]
- Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.R.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes skn-1, ire-1, and xbp-1. Cell Rep. 2016, 17, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; DeCosta, B.R.; Holick, M.F. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882–887. [Google Scholar] [CrossRef]
- Armbrecht, H.J.; Hodam, T.L.; Boltz, M.A.; Partridge, N.C.; Brown, A.J.; Kumar, V.B. Induction of the vitamin D 24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 is regulated by parathyroid hormone in UMR106 osteoblastic cells. Endocrinology 1998, 139, 3375–3381. [Google Scholar] [CrossRef]
- Miller, W.L. Genetic disorders of Vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2017, 165, 101–108. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr. Osteoporos. Rep. 2012, 10, 4–15. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F. Vitamin D supplements and reasonable solar UVB should be recommended to prevent escalating incidence of chronic diseases. Br. Med. J. 2015, 350, h321. [Google Scholar]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Ortel, B.; Venturini, M. Non-melanoma skin cancer, sun exposure and sun protection. Giornale Italiano di Dermatologia e Venereologia 2015, 150, 369–378. [Google Scholar]
- Cascinelli, N.; Krutmann, J.; MacKie, R.; Pierotti, M.; Prota, G.; Rosso, S.; Young, A. European School of Oncology advisory report. Sun exposure, UVA lamps and risk of skin cancer. Eur. J. Cancer 1994, 30A, 548–560. [Google Scholar] [PubMed]
- Moan, J.; Porojnicu, A.C.; Dahlback, A.; Setlow, R.B. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc. Natl. Acad. Sci. USA 2008, 105, 668–673. [Google Scholar] [CrossRef]
- Omura, Y. Clinical Significance of Human Papillomavirus Type 16 for Breast Cancer & Adenocarcinomas of Various Internal Organs and Alzheimer’s Brain with Increased beta-amyloid (1-42); Combined Use of Optimal Doses of Vitamin D3 and Taurine 3 times/day Has Significant Beneficial Effects of Anti-Cancer, Anti-Ischemic Heart, and Memory & Other Brain Problems By Significant Urinary Excretion of Viruses, Bacteria, and Toxic Metals & Substances. Acupunct. Electro-Ther. Res. 2016, 41, 127–134. [Google Scholar]
- Gilad, L.A.; Bresler, T.; Gnainsky, J.; Smirnoff, P.; Schwartz, B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J. Endocrinol. 2005, 185, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Robsahm, T.E.; Tretli, S.; Dahlback, A.; Moan, J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 2004, 15, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M. The physiology of vitamin D receptor activation. Contrib. Nephrol. 2009, 163, 206–212. [Google Scholar] [PubMed]
- Farnham, P.J. Thematic minireview series on results from the ENCODE Project: Integrative global analyses of regulatory regions in the human genome. J. Biol. Chem. 2012, 287, 30885–30887. [Google Scholar] [CrossRef]
- Washington, S.D.; Edenfield, S.I.; Lieux, C.; Watson, Z.L.; Taasan, S.M.; Dhummakupt, A.; Bloom, D.C.; Neumann, D.M. Depletion of the insulator protein CTCF results in HSV-1 reactivation in vivo. J. Virol. 2018. [Google Scholar] [CrossRef]
- MacPherson, M.J.; Sadowski, P.D. The CTCF insulator protein forms an unusual DNA structure. BMC Mol. Biol. 2010, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; LaPorta, E.; Simmons, K.M.; Beaudin, S.; Welsh, J. The impact of vitamin D in breast cancer: Genomics, pathways, metabolism. Front. Physiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Robinson, A.R.; Wang, J.; Gregg, S.Q.; Clauson, C.L.; Reay, D.P.; Nasto, L.A.; St Croix, C.M.; Usas, A.; Vo, N.; et al. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Investig. 2012, 122, 2601–2612. [Google Scholar] [CrossRef]
- Pusceddu, I.; Farrell, C.J.; Di Pierro, A.M.; Jani, E.; Herrmann, W.; Herrmann, M. The role of telomeres and vitamin D in cellular aging and age-related diseases. Clin. Chem. Lab. Med. 2015, 53, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hua, F.; Wang, J.; Sayeed, I.; Wang, X.; Chen, Z.; Yousuf, S.; Atif, F.; Stein, D.G. Progesterone and vitamin D: Improvement after traumatic brain injury in middle-aged rats. Horm. Behav. 2013, 64, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef] [PubMed]
- Myszka, M.; Klinger, M. The immunomodulatory role of Vitamin D. Postepy Hig. Med. Dosw. 2014, 68, 865–878. [Google Scholar] [CrossRef]
- Watanabe, R.; Inoue, D. Current Topics on Vitamin D. Anti-cancer effects of vitamin D. Clin. Calcium 2015, 25, 373–380. [Google Scholar]
- Shi, Y.; Au, J.S.; Thongprasert, S.; Srinivasan, S.; Tsai, C.M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef]
- O’Malley, M.; King, A.N.; Conte, M.; Ellingrod, V.L.; Ramnath, N. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J. Thorac. Oncol. 2014, 9, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Iswariya, G.T.; Paital, B.; Padma, P.R.; Nirmaladevi, R. MicroRNAs: Epigenetic players in cancer and aging. Front. Biosci. (Schol Ed) 2019, 11, 29–55. [Google Scholar]
- Ramnath, N.; Nadal, E.; Jeon, C.K.; Sandoval, J.; Colacino, J.; Rozek, L.S.; Christensen, P.J.; Esteller, M.; Beer, D.G.; Kim, S.H. Epigenetic regulation of vitamin D metabolism in human lung adenocarcinoma. J. Thorac. Oncol. 2014, 9, 473–482. [Google Scholar] [CrossRef]
- Karlic, H.; Varga, F. Impact of vitamin D metabolism on clinical epigenetics. Clin. Epigenet. 2011, 2, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Codoner-Franch, P.; Tavarez-Alonso, S.; Simo-Jorda, R.; Laporta-Martin, P.; Carratala-Calvo, A.; Alonso-Iglesias, E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J. Pediatr. 2012, 161, 848–854. [Google Scholar] [CrossRef]
- Zeitelhofer, M.; Adzemovic, M.Z.; Gomez-Cabrero, D.; Bergman, P.; Hochmeister, S.; N’Diaye, M.; Paulson, A.; Ruhrmann, S.; Almgren, M.; Tegner, J.N.; et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2017, 114, E1678–E1687. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, S. Metabolic changes in cancer: Beyond the Warburg effect. Acta Biochim. Biophys. Sin. 2013, 45, 18–26. [Google Scholar] [CrossRef]
- Garcia-Quiroz, J.; Garcia-Becerra, R.; Barrera, D.; Santos, N.; Avila, E.; Ordaz-Rosado, D.; Rivas-Suarez, M.; Halhali, A.; Rodriguez, P.; Gamboa-Dominguez, A.; et al. Astemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: A novel approach for breast cancer therapy. PLoS ONE 2012, 7, e45063. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Holmes, S.; Abbassi, B.; Su, C.; Singh, M.; Cunningham, R.L. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology 2013, 154, 4281–4292. [Google Scholar] [CrossRef]
- Petersen, K.S.; Smith, C. Ageing-Associated Oxidative Stress and Inflammation Are Alleviated by Products from Grapes. Oxid. Med. Cell. Longev. 2016, 2016, 6236309. [Google Scholar] [CrossRef]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.N.; Mele, J.; Hayes, J.D.; Buffenstein, R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr. Comp. Biol. 2010, 50, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.E.; Jurutka, P.W.; Hsieh, J.C.; Haussler, C.A.; Lowmiller, C.L.; Kaneko, I.; Haussler, M.R.; Kerr Whitfield, G. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem. Biophys. Res. Commun. 2011, 414, 557–562. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D: A custodian of cell signalling stability in health and disease. Biochem. Soc. Trans. 2015, 43, 349–358. [Google Scholar] [CrossRef]
- Razzaque, M.S. FGF23, klotho and vitamin D interactions: What have we learned from in vivo mouse genetics studies? Adv. Exp. Med. Biol. 2012, 728, 84–91. [Google Scholar]
- Kuro-o, M. Klotho and aging. Biochim. Biophys. Acta 2009, 1790, 1049–1058. [Google Scholar] [CrossRef]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef]
- Wang, L.; Lewis, T.; Zhang, Y.L.; Khodier, C.; Magesh, S.; Chen, L.; Inoyama, D.; Chen, Y.; Zhen, J.; Hu, L.; et al. The identification and characterization of non-reactive inhibitor of Keap1-Nrf2 interaction through HTS using a fluorescence polarization assay. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Berridge, M.J. Vitamin D deficiency: Infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am. J. Physiol. Cell Physiol. 2018, 314, C135–C151. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1alpha,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef]
- Bouillon, R.; Verstuyf, A. Vitamin D, mitochondria, and muscle. J. Clin. Endocrinol. Metab. 2013, 98, 961–963. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D cell signalling in health and disease. Biochem. Biophys. Res. Commun. 2015, 460, 53–71. [Google Scholar] [CrossRef]
- Ureshino, R.P.; Rocha, K.K.; Lopes, G.S.; Bincoletto, C.; Smaili, S.S. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid. Redox Signal. 2014, 21, 123–137. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Song, C.; Fu, B.; Zhang, J.; Zhao, J.; Yuan, M.; Peng, W.; Zhang, Y.; Wu, H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2017, 7, 672. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- George, N.; Kumar, T.P.; Antony, S.; Jayanarayanan, S.; Paulose, C.S. Effect of vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic rats. Br. J. Nutr. 2012, 108, 1410–1418. [Google Scholar] [CrossRef]
- Shelton, R.C.; Claiborne, J.; Sidoryk-Wegrzynowicz, M.; Reddy, R.; Aschner, M.; Lewis, D.A.; Mirnics, K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry 2011, 16, 751–762. [Google Scholar] [CrossRef]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.H.; Verkhratsky, A. Calcium and ATP control multiple vital functions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150418. [Google Scholar] [CrossRef]
- Petrosillo, G.; Di Venosa, N.; Moro, N.; Colantuono, G.; Paradies, V.; Tiravanti, E.; Federici, A.; Ruggiero, F.M.; Paradies, G. In vivo hyperoxic preconditioning protects against rat-heart ischemia/reperfusion injury by inhibiting mitochondrial permeability transition pore opening and cytochrome c release. Free Radic. Biol. Med. 2011, 50, 477–483. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Finch, C.E.; Pike, M.C. Maximum life span predictions from the Gompertz mortality model. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, B183–B194. [Google Scholar] [CrossRef]
- Macedo, J.C.; Vaz, S.; Logarinho, E. Mitotic Dysfunction Associated with Aging Hallmarks. Adv. Exp. Med. Biol. 2017, 1002, 153–188. [Google Scholar]
- Jallali, N.; Ridha, H.; Thrasivoulou, C.; Underwood, C.; Butler, P.E.; Cowen, T. Vulnerability to ROS-induced cell death in ageing articular cartilage: The role of antioxidant enzyme activity. Osteoarthr. Cartil. 2005, 13, 614–622. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Pilz, S.; Marz, W.; Wellnitz, B.; Seelhorst, U.; Fahrleitner-Pammer, A.; Dimai, H.P.; Boehm, B.O.; Dobnig, H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J. Clin. Endocrinol. Metab. 2008, 93, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.C.; Offiah, A.; Sprigg, A.; Al-Adnani, M. Vitamin D deficiency and sudden unexpected death in infancy and childhood: A cohort study. Pediatr. Dev. Pathol. 2013, 16, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Vitamin D Deficiency May Explain Comorbidity as an Independent Risk Factor for Death Associated with Cancer in Taiwan. Asia Pac. J. Public Health 2015, 27, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.A.; Albuquerque, M.; Arida, R.M.; Terra, V.C.; Machado, H.R.; Cavalheiro, E.A. Benefits of sunlight: Vitamin D deficiency might increase the risk of sudden unexpected death in epilepsy. Med. Hypotheses 2010, 74, 158–161. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Brenner, H.; Jansen, L.; Saum, K.U.; Holleczek, B.; Schottker, B. Vitamin D Supplementation Trials Aimed at Reducing Mortality Have Much Higher Power When Focusing on People with Low Serum 25-Hydroxyvitamin D Concentrations. J. Nutr. 2017, 147, 1325–1333. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Krstic, G.; Wetterslev, J.; Gluud, C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Marz, W.; Drechsler, C.; Ritz, E.; Zittermann, A.; Cavalier, E.; Pieber, T.R.; Lappe, J.M.; Grant, W.B.; et al. Vitamin D, cardiovascular disease and mortality. Clin. Endocrinol. 2011, 75, 575–584. [Google Scholar] [CrossRef]
- Van der Reest, J.; Gottlieb, E. Anti-cancer effects of vitamin C revisited. Cell Res. 2016, 26, 269–270. [Google Scholar] [CrossRef]
- Da Luz Dias, R.; Basso, B.; Donadio, M.V.F.; Pujol, F.V.; Bartrons, R.; Haute, G.V.; Gassen, R.B.; Bregolin, H.D.; Krause, G.; Viau, C.; et al. Leucine reduces the proliferation of MC3T3-E1 cells through DNA damage and cell senescence. Toxicol. In Vitro 2018, 48, 1–10. [Google Scholar] [CrossRef]
- Cevenini, E.; Caruso, C.; Candore, G.; Capri, M.; Nuzzo, D.; Duro, G.; Rizzo, C.; Colonna-Romano, G.; Lio, D.; Di Carlo, D.; et al. Age-related inflammation: The contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr. Pharm. Des. 2010, 16, 609–618. [Google Scholar] [CrossRef]
- Talmor, Y.; Bernheim, J.; Klein, O.; Green, J.; Rashid, G. Calcitriol blunts pro-atherosclerotic parameters through NFkappaB and p38 in vitro. Eur. J. Clin. Investig. 2008, 38, 548–554. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Beilfuss, J.; Berg, V.; Sneve, M.; Jorde, R.; Kamycheva, E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine 2012, 60, 870–874. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Soares, M.J.; Rowlands, J.; Newsholme, P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016, 10, 243–250. [Google Scholar] [CrossRef]
- Liu, Y.; Hyde, A.S.; Simpson, M.A.; Barycki, J.J. Emerging regulatory paradigms in glutathione metabolism. Adv. Cancer Res. 2014, 122, 69–101. [Google Scholar]
- Weipoltshammer, K.; Schofer, C.; Almeder, M.; Philimonenko, V.V.; Frei, K.; Wachtler, F.; Hozak, P. Intranuclear anchoring of repetitive DNA sequences: Centromeres, telomeres, and ribosomal DNA. J. Cell Biol. 1999, 147, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, M.; Viano, M.; Casarin, S.; Castagnoli, C.; Pescarmona, G.; Silvagno, F. Mitochondrial and lipogenic effects of vitamin D on differentiating and proliferating human keratinocytes. Exp. Dermatol. 2015, 24, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Wyckelsma, V.L.; Levinger, I.; McKenna, M.J.; Formosa, L.E.; Ryan, M.T.; Petersen, A.C.; Anderson, M.J.; Murphy, R.M. Preservation of skeletal muscle mitochondrial content in older adults: Relationship between mitochondria, fibre type and high-intensity exercise training. J. Physiol. 2017, 595, 3345–3359. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, A.A.; Abbas, M.; Kassem, M.; Gleizes, C.; Kreutter, G.; Schini-Kerth, V.; Mitrea, I.L.; Toti, F.; Kessler, L. Differential influence of tacrolimus and sirolimus on mitochondrial-dependent signaling for apoptosis in pancreatic cells. Mol. Cell. Biochem. 2016, 418, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Matera, M.; Casanova, G.; Ruggiero, F.M.; Paradies, G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem. Int. 2008, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Matera, M.; Moro, N.; Ruggiero, F.M.; Paradies, G. Mitochondrial complex I dysfunction in rat heart with aging: Critical role of reactive oxygen species and cardiolipin. Free Radic. Biol. Med. 2009, 46, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.; Kim, A.; Yoshihara, T.; Tobita, S.; Takeuchi, T.; Higuchi, M. Mitochondrial respiratory function induces endogenous hypoxia. PLoS ONE 2014, 9, e88911. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Rezin, G.T.; Carvalho, A.F.; Streck, E.L.; Berk, M.; Quevedo, J. Mitochondrial dysfunction in bipolar disorder: Evidence, pathophysiology and translational implications. Neurosci. Biobehav. Rev. 2016, 68, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.S.; Xia, L.; Mills, G.B.; Lowell, C.A.; Touw, I.P.; Corey, S.J. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 2006, 107, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Berg, A.H.; Iyengar, P.; Lam, T.K.; Giacca, A.; Combs, T.P.; Rajala, M.W.; Du, X.; Rollman, B.; Li, W.; et al. The hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. J. Biol. Chem. 2005, 280, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, A.A.; Gusev, G.P. Molecular mechanisms of cytotoxicity and apoptosis induced by inorganic fluoride. ISRN Cell Biol. 2012, 2012, 403835. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Gusev, G.P. Fluoride induces oxidative stress and ATP depletion in the rat erythrocytes in vitro. Environ. Toxicol. Pharmacol. 2012, 34, 334–337. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Liu, Z.; Cadenas, E. Mitochondrial function in ageing: Coordination with signalling and transcriptional pathways. J. Physiol. 2016, 594, 2025–2042. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015, 13, 68. [Google Scholar] [CrossRef]
- Ames, B.N. Optimal micronutrients delay mitochondrial decay and age-associated diseases. Mech. Ageing Dev. 2010, 131, 473–479. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, E.A.; Sanita, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. Ten years of NAD-dependent SIR2 family deacetylases: Implications for metabolic diseases. Trends Pharmacol. Sci. 2010, 31, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low vitamin D status: Definition, prevalence, consequences, and correction. Rheum. Dis. Clin. N. Am. 2012, 38, 45–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know; Karunaratne & Sons: Homagama, Sri Lanka, 2012; ISBN 978-955-9098-94-2. [Google Scholar]
- Heyden, E.L.; Wimalawansa, S.J. Vitamin D: Effects on human reproduction, pregnancy, and fetal well-being. J. Steroid Biochem. Mol. Biol. 2018, 180, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Wagner, C.L.; Hollis, B.W. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N. Engl. J. Med. 2018, 379, 1880–1881. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).