The Expression of Adipose Tissue-Derived Cardiotrophin-1 in Humans with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.1.1. Study 1
2.1.2. Study 2
2.2. Study Procedures (Baseline and Post-Overfeeding Intervention)
2.2.1. Body Composition
2.2.2. Oral Glucose Tolerance Test
2.2.3. Hyperinsulinemic Euglycemic Clamp
2.2.4. Adipose Tissue Biopsies
2.2.5. RNA Isolation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Febbraio, M.A. gp130 receptor ligands as potential therapeutic targets for obesity. J. Clin. Investig. 2007, 117, 841–849. [Google Scholar] [CrossRef]
- Pennica, D.; King, K.L.; Shaw, K.J.; Luis, E.; Rullamas, J.; Luoh, S.M.; Darbonne, W.C.; Knutzon, D.S.; Yen, R.; Chien, K.R.; et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc. Natl. Acad. Sci. USA 1995, 92, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Pennica, D.; Wood, W.I.; Chien, K.R. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development 1996, 122, 419–428. [Google Scholar]
- Talwar, S.; Squire, I.B.; Downie, P.F.; O’Brien, R.J.; Davies, J.E.; Ng, L.L. Elevated circulating cardiotrophin-1 in heart failure: Relationship with parameters of left ventricular systolic dysfunction. Clin. Sci. (Lond.) 2000, 99, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Lopez, B.; Martin-Raymondi, D.; Lozano, E.; Varo, N.; Barba, J.; Serrano, M.; Diez, J. Usefulness of plasma cardiotrophin-1 in assessment of left ventricular hypertrophy regression in hypertensive patients. J. Hypertens. 2005, 23, 2297–2304. [Google Scholar] [CrossRef]
- Lopez, N.; Diez, J.; Fortuno, M.A. Differential hypertrophic effects of cardiotrophin-1 on adult cardiomyocytes from normotensive and spontaneously hypertensive rats. J. Mol. Cell Cardiol. 2006, 41, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Ermetici, F.; Morricone, L.; Delnevo, A.; Coman, C.; Ambrosi, B.; Corsi, M.M. Association of increased plasma cardiotrophin-1 with left ventricular mass indexes in normotensive morbid obesity. Hypertension 2008, 51, 8–10. [Google Scholar] [CrossRef]
- Natal, C.; Fortuno, M.A.; Restituto, P.; Bazan, A.; Colina, I.; Diez, J.; Varo, N. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2008, 294, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Lu, F.H.; Ou, H.Y.; Wu, H.T.; Wu, J.S.; Yang, Y.C.; Chang, C.J. Increased cardiotrophin-1 in subjects with impaired glucose tolerance and newly diagnosed diabetes. Int. J. Cardiol. 2013, 169, 33–34. [Google Scholar] [CrossRef]
- Vespasiani-Gentilucci, U.; De Vincentis, A.; Argemi, J.; Galati, G.; Anso, E.; Patti, G.; Picardi, A. Cardiotrophin-1 is not associated with carotid or coronary disease and is inversely associated with obesity in patients undergoing coronary angiography. Arch. Med. Sci. 2013, 9, 635–639. [Google Scholar] [CrossRef]
- Hung, H.C.; Lu, F.H.; Wu, H.T.; Ou, H.Y.; Yang, Y.C.; Wu, J.S.; Chang, C.J. Cardiotrophin-1 is inversely associated with obesity in non-diabetic individuals. Sci. Rep. 2015, 5, 17438. [Google Scholar] [CrossRef]
- Moreno-Aliaga, M.J.; Perez-Echarri, N.; Marcos-Gomez, B.; Larequi, E.; Gil-Bea, F.J.; Viollet, B.; Gimenez, I.; Martinez, J.A.; Prieto, J.; Bustos, M. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell. Metab. 2011, 14, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Castano, D.; Larequi, E.; Belza, I.; Astudillo, A.M.; Martinez-Anso, E.; Balsinde, J.; Argemi, J.; Aragon, T.; Moreno-Aliaga, M.J.; Muntane, J.; et al. Cardiotrophin-1 eliminates hepatic steatosis in obese mice by mechanisms involving AMPK activation. J. Hepatol. 2014, 60, 1017–1025. [Google Scholar] [CrossRef]
- Lopez-Yoldi, M.; Moreno-Aliaga, M.J.; Bustos, M. Cardiotrophin-1: A multifaceted cytokine. Cytokine Growth Factor Rev. 2015, 26, 523–532. [Google Scholar] [CrossRef]
- White, U.A.; Fitch, M.D.; Beyl, R.A.; Hellerstein, M.K.; Ravussin, E. Association of In Vivo Adipose Tissue Cellular Kinetics With Markers of Metabolic Health in Humans. J. Clin. Endocrinol. Metab. 2017, 102, 2171–2178. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Tchoukalova, Y.; Tam, C.S.; Covington, J.D.; Xie, W.; Schwarz, J.M.; Bajpeyi, S.; Ravussin, E. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: Testing the adipose tissue expandability hypothesis. Diabetes Care 2014, 37, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, 214–223. [Google Scholar] [CrossRef]
- Lillioja, S.; Bogardus, C. Obesity and insulin resistance: Lessons learned from the Pima Indians. Diabetes Metab. Rev. 1988, 4, 517–540. [Google Scholar] [CrossRef]
- Tam, C.S.; Covington, J.D.; Bajpeyi, S.; Tchoukalova, Y.; Burk, D.; Johannsen, D.L.; Zingaretti, C.M.; Cinti, S.; Ravussin, E. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J. Clin. Endocrinol. Metab. 2014, 99, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aliaga, M.J.; Romero-Lozano, M.A.; Castano, D.; Prieto, J.; Bustos, M. Role of cardiotrophin-1 in obesity and insulin resistance. Adipocyte 2012, 1, 112–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Limongelli, G.; Calabro, P.; Maddaloni, V.; Russo, A.; Masarone, D.; D’Aponte, A.; Roselli, T.; Bonauro, R.; D’Alessandro, R.; D’Andrea, A.; et al. Cardiotrophin-1 and TNF-alpha circulating levels at rest and during cardiopulmonary exercise test in athletes and healthy individuals. Cytokine 2010, 50, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Vague, J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, atherosclerosis, gout and uric calculous disease. Am. J. Clin. Nutr. 1956, 4, 20–34. [Google Scholar] [CrossRef]
- Krotkiewski, M.; Bjorntorp, P.; Sjostrom, L.; Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Investig. 1983, 72, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. (Lond.) 2010, 34, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Yoldi, M.; Marcos-Gomez, B.; Romero-Lozano, M.A.; Sainz, N.; Prieto, J.; Martinez, J.A.; Bustos, M.; Moreno-Aliaga, M.J. Cardiotrophin-1 Regulates Adipokine Production in 3T3-L1 Adipocytes and Adipose Tissue From Obese Mice. J. Cell Physiol. 2017, 232, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.M.; Belza, I.; Holtmann, B.; Pennica, D.; Prieto, J.; Bustos, M. Cardiotrophin-1 is an essential factor in the natural defense of the liver against apoptosis. Hepatology 2007, 45, 639–648. [Google Scholar] [CrossRef]
- Iniguez, M.; Berasain, C.; Martinez-Anso, E.; Bustos, M.; Fortes, P.; Pennica, D.; Avila, M.A.; Prieto, J. Cardiotrophin-1 defends the liver against ischemia-reperfusion injury and mediates the protective effect of ischemic preconditioning. J. Exp. Med. 2006, 203, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Perretta-Tejedor, N.; Munoz-Felix, J.M.; Duwel, A.; Quiros-Luis, Y.; Fernandez-Martin, J.L.; Morales, A.I.; Lopez-Hernandez, F.J.; Lopez-Novoa, J.M.; Martinez-Salgado, C. Cardiotrophin-1 opposes renal fibrosis in mice: Potential prevention of chronic kidney disease. Acta. Physiol. (Oxf.) 2018, e13247. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; Gonzalez, A.; Lasarte, J.J.; Sarobe, P.; Borras, F.; Diaz, A.; Barba, J.; Tomas, L.; Lozano, E.; Serrano, M.; et al. Is plasma cardiotrophin-1 a marker of hypertensive heart disease? J. Hypertens 2005, 23, 625–632. [Google Scholar] [CrossRef]
- Tsutamoto, T.; Asai, S.; Tanaka, T.; Sakai, H.; Nishiyama, K.; Fujii, M.; Yamamoto, T.; Ohnishi, M.; Wada, A.; Saito, Y.; et al. Plasma level of cardiotrophin-1 as a prognostic predictor in patients with chronic heart failure. Eur. J. Heart Fail. 2007, 9, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
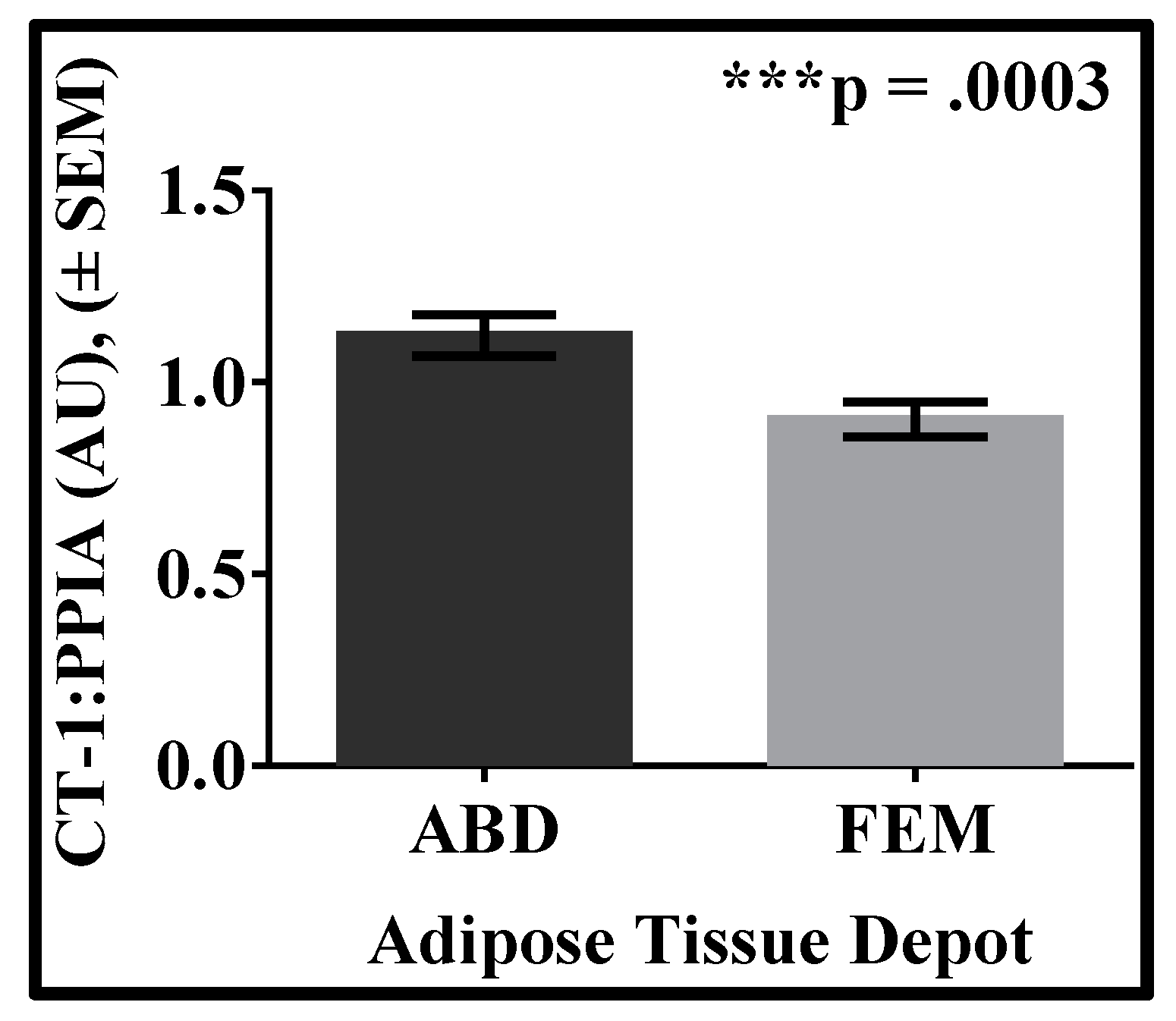
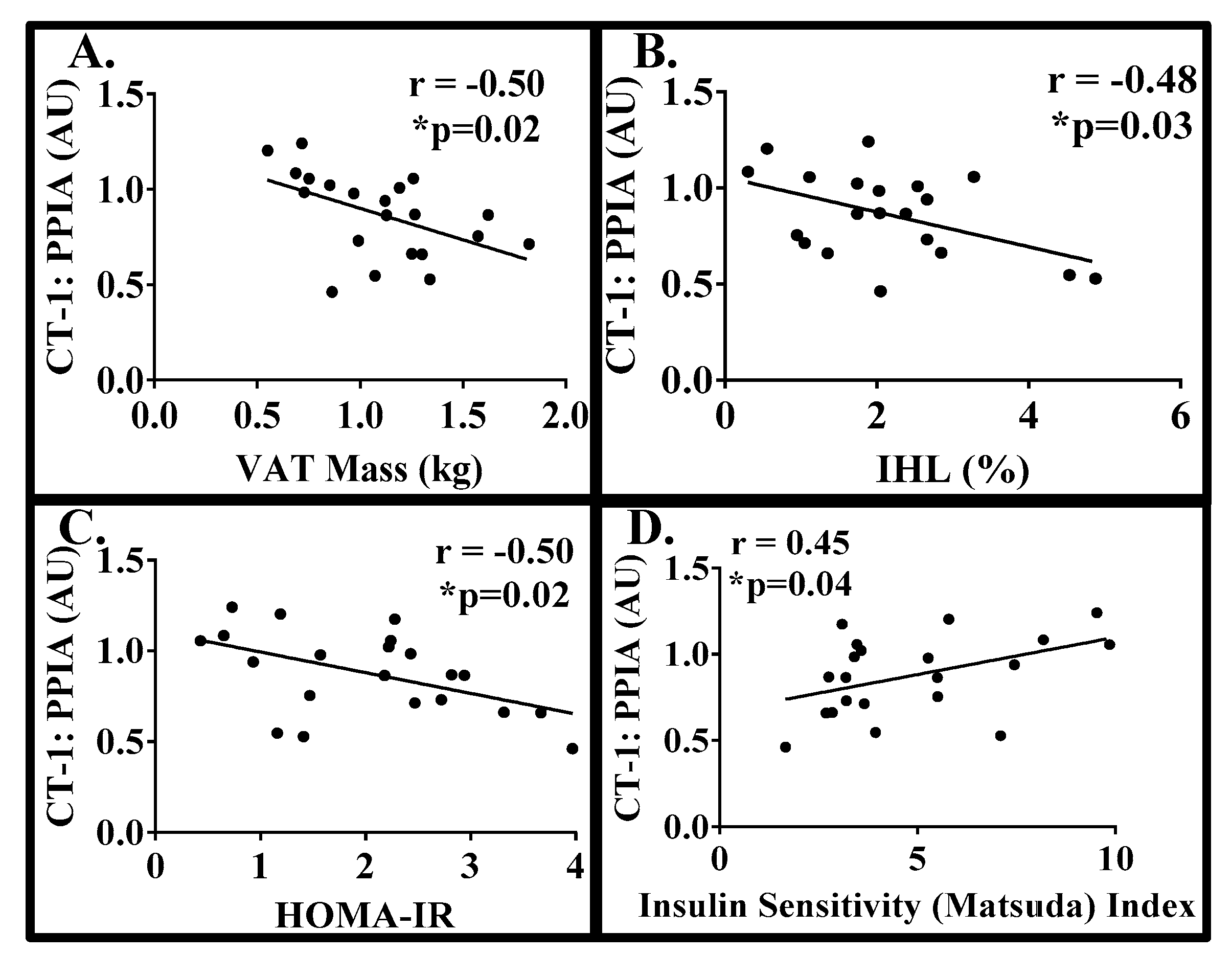
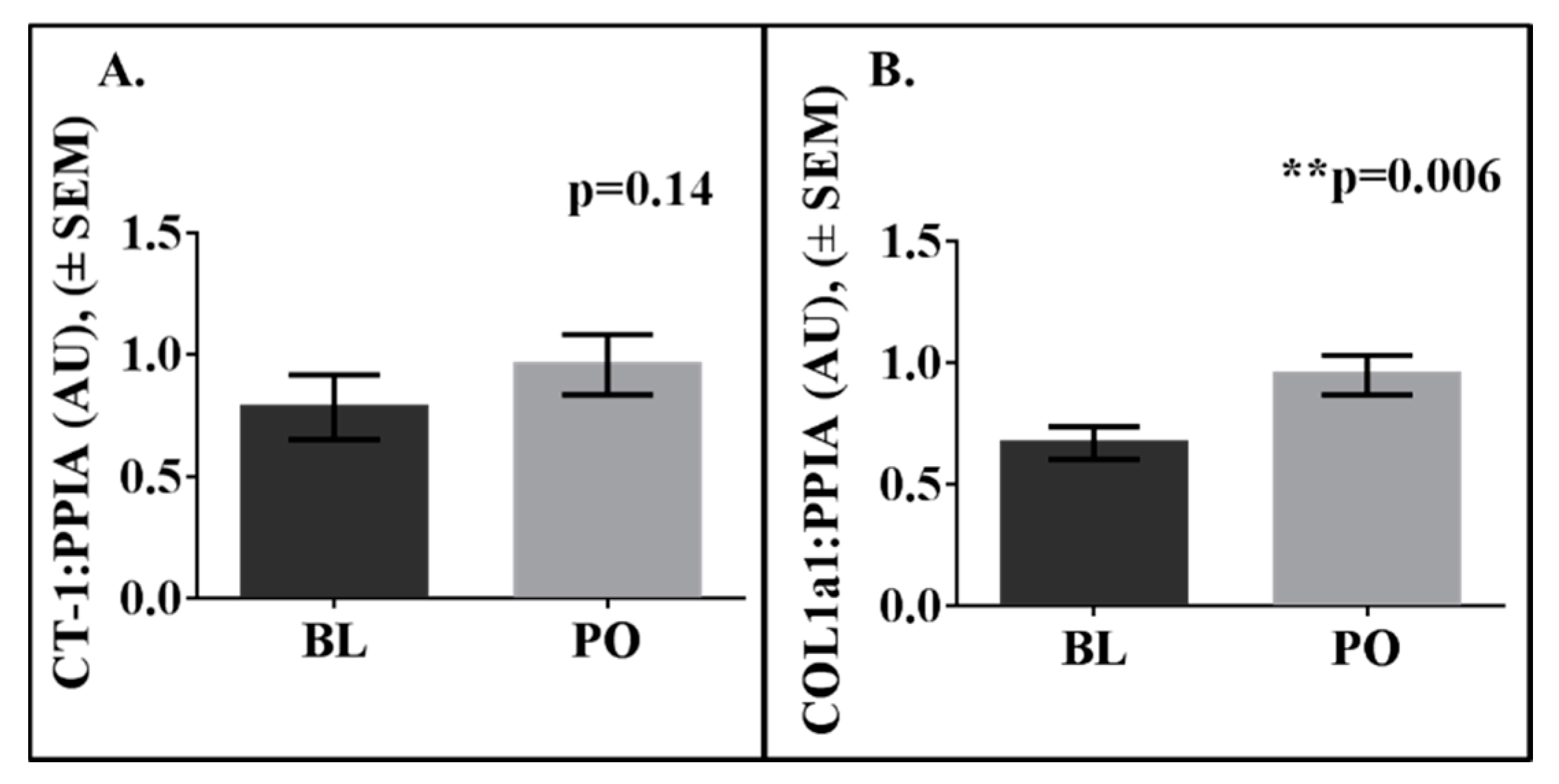
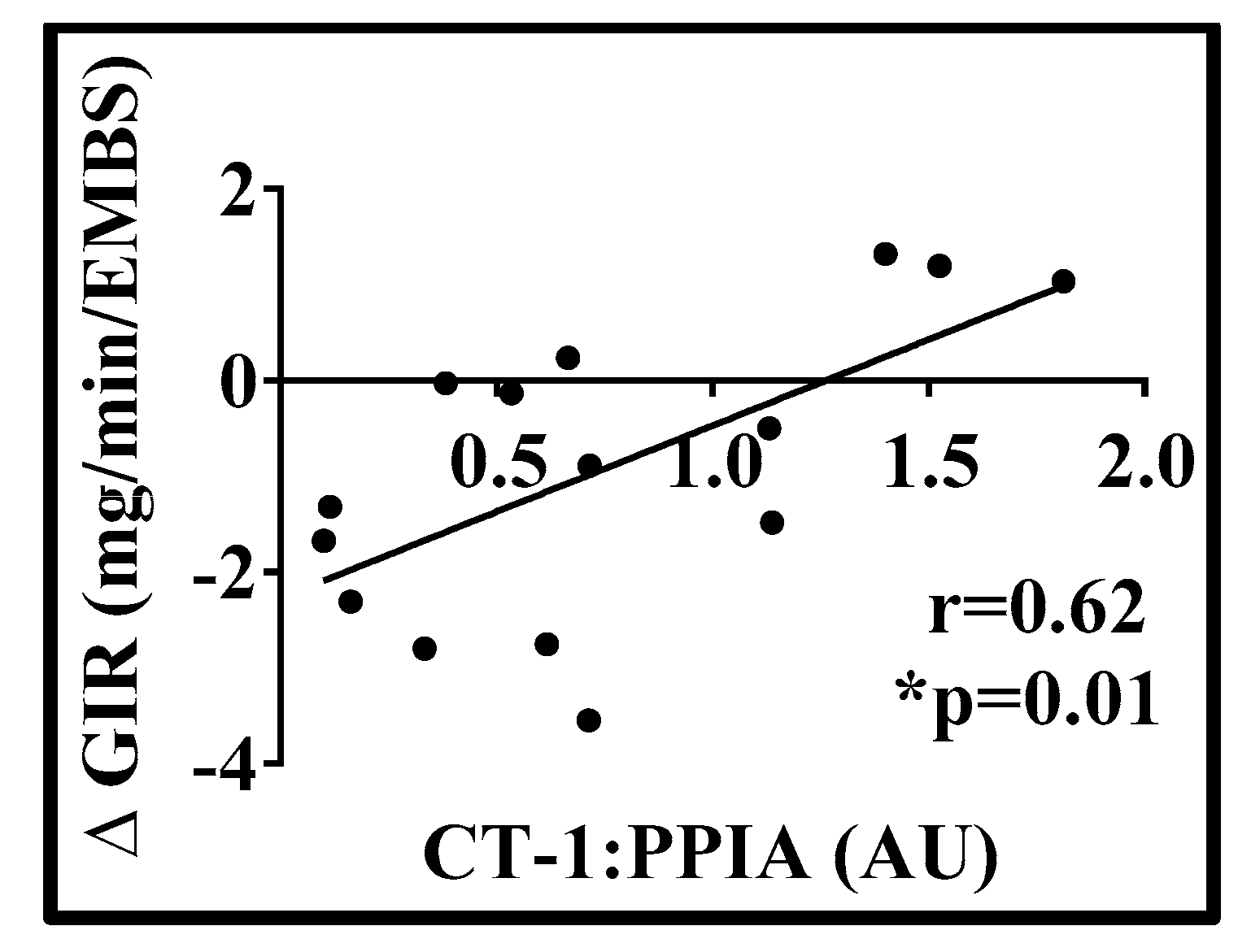
| Variable | Mean | SD |
|---|---|---|
| Age | 30 | 6 |
| Body weight (kg) | 87.16 | 8.17 |
| BMI (kg/m2) | 32.40 | 2.48 |
| Body Fat (%) | 45.19 | 4.29 |
| scABD AT (kg) | 7.84 | 1.60 |
| VAT (kg) | 1.09 | 0.33 |
| IHL (%) | 2.13 | 1.18 |
| Matsuda Index | 4.66 | 2.44 |
| HOMA-IR | 2.04 | 1.00 |
| Mean BP (mmHg) | 76.19 | 6.74 |
| TRIG (mg/dL) | 82.45 | 41.44 |
| Total CHOL (mg/dL) | 180.13 | 41.79 |
| LDL (mg/dL) | 109.9 | 32.71 |
| HDL (mg/dL) | 53.74 | 13.82 |
| Total CHOL:HDL | 3.47 | 0.93 |
| VARIABLE | BASELINE | POST-OVERFEEDING | Change | p value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Body Weight (kg) | 79.71 ± 7.33 | 87.44 ± 7.83 | 7.73 ± 1.92 | <0.0001 |
| BMI (kg/m2) | 24.72 ± 2.09 | 27.37 ± 2.24 | 2.65 ± 0.91 | <0.0001 |
| Body Fat (%) | 21.20 ± 4.28 | 24.55 ± 4.57 | 3.35 ± 0.96 | <0.0001 |
| scABD AT (kg) | 4.38 ± 1.34 | 5.88 ± 1.67 | 1.50 ± 0.50 | <0.0001 |
| VAT (kg) | 0.54 ± 0.34 | 0.93 ± 0.45 | 0.39 ± 0.16 | <0.0001 |
| IHL (%) | 0.69 ± 0.41 | 1.27 ± 1.11 | 0.58 ± 0.88 | 0.02 |
| Glucose Infusion (mg/min∙(FFM+17.7)) | ||||
| 10 (mU/min·m2 insulin) | 2.62 ± 0.87 | 2.08 ± 0.67 | −0.54 ± 0.53 | 0.001 |
| 50 (mU/min·m2 insulin) | 11.61 ± 2.83 | 10.70 ± 2.82 | −0.90 ± 1.53 | 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephens, J.; Ravussin, E.; White, U. The Expression of Adipose Tissue-Derived Cardiotrophin-1 in Humans with Obesity. Biology 2019, 8, 24. https://doi.org/10.3390/biology8020024
Stephens J, Ravussin E, White U. The Expression of Adipose Tissue-Derived Cardiotrophin-1 in Humans with Obesity. Biology. 2019; 8(2):24. https://doi.org/10.3390/biology8020024
Chicago/Turabian StyleStephens, Jacqueline, Eric Ravussin, and Ursula White. 2019. "The Expression of Adipose Tissue-Derived Cardiotrophin-1 in Humans with Obesity" Biology 8, no. 2: 24. https://doi.org/10.3390/biology8020024
APA StyleStephens, J., Ravussin, E., & White, U. (2019). The Expression of Adipose Tissue-Derived Cardiotrophin-1 in Humans with Obesity. Biology, 8(2), 24. https://doi.org/10.3390/biology8020024






