The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance
Abstract
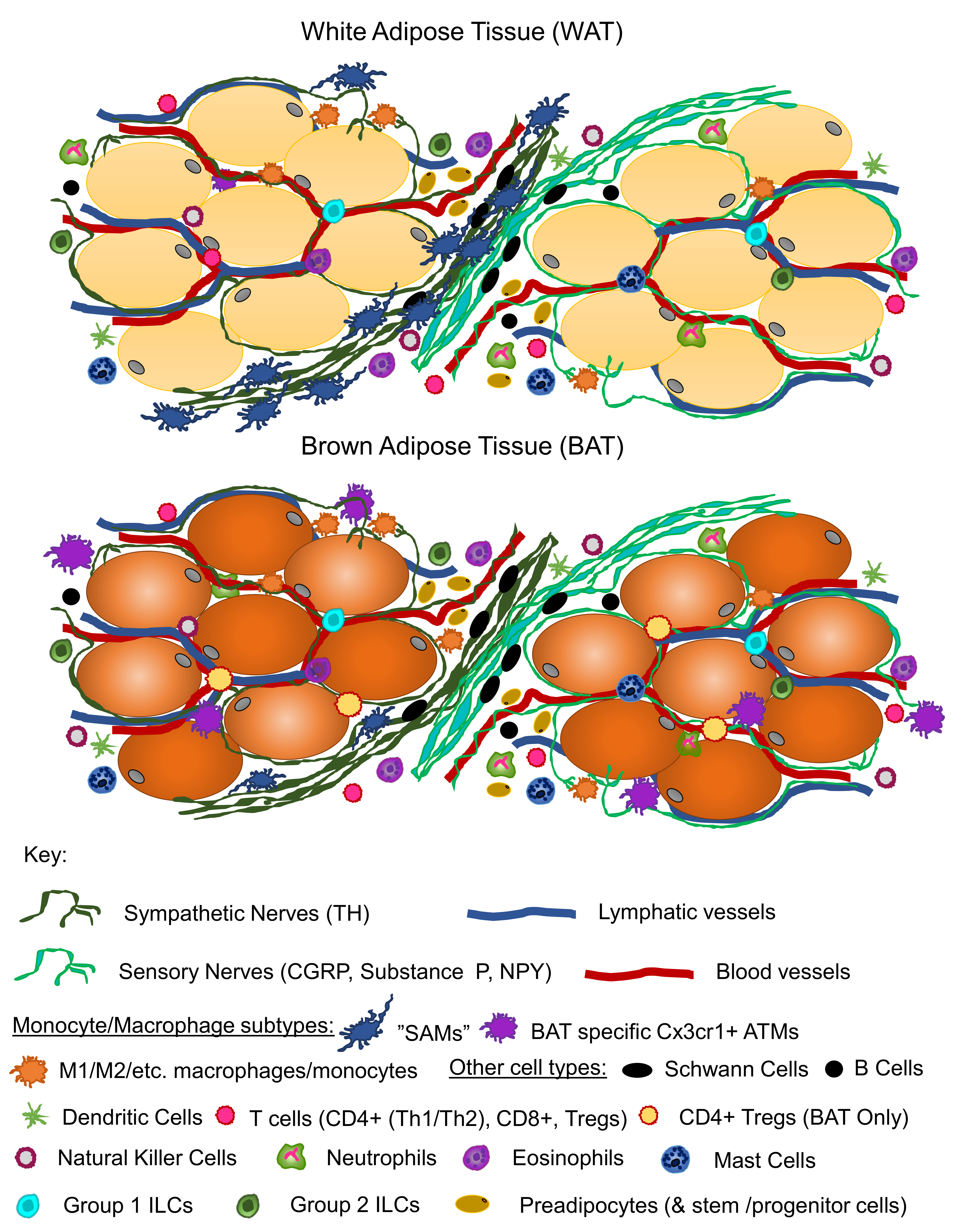
:1. Introduction
2. Innervation of Adipose Tissues
2.1. Innervation of Brown Adipose Tissue (BAT)
2.2. Innervation of White Adipose Tissue (WAT)
3. Lessons from Adipose Denervation Studies
3.1. Denervation of BAT
3.2. Denervation of WAT
3.3. Potential for Nerve-Independent Thermogenesis in Brown Adipocytes
4. Advancements in Imaging/Analysis Techniques for Visualizing Adipose Innervation
4.1. What Has Been Learned from Tissue Sectioning
4.2. Whole-Tissue Processing and Imaging
4.3. Discoveries Using Whole-Adipose Tissue Imaging
5. Peripheral Nerve Regulation in the Pancreas, Liver, and Gut
5.1. Pancreas
5.2. Liver
5.3. Gut
5.4. Perspective
6. Adipose Neuroimmune Interactions
7. Perspective
8. Open Questions in the Field
- Which nerve subtypes (including sensory vs. sympathetic and myelinated vs. unmyelinated) are present in BAT vs. WAT (and distinct depots thereof) and are these nerve types “plastic” (i.e., undergo remodeling in response to physiological or pathophysiological stimuli).
- Are similar mechanisms of neural regulation and brain cross-talk conserved between different metabolic tissues and organs?
- What is the role of tissue-resident immune cells in mediating nerve health, function, and plasticity?
- What is the functional significance of the heterogeneity of nerve distribution across an adipose depot and which cell types have synaptic connections?
Funding
Conflicts of Interest
References
- Dogiel, A.S. Die sensiblen Nervenendigungen im Herzen und in den Blutgefässen der Säugethiere. Arch. Mikrosk. Anat. 1898, 52, 44–70. [Google Scholar] [CrossRef]
- Sidman, R.L.; Fawcett, D.W. The effect of peripheral nerve section on some metabolic responses of brown adipose tissue in mice. Anat. Rec. 1954, 118, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Wirsen, C. Adrenergic innervation of adipose tissue examined by fluorescence microscopy. Nature 1964, 202, 913. [Google Scholar] [CrossRef] [PubMed]
- Wirsen, C. Distribution of adrenergic nerve fibers in brown and white adipose tissue. In Handbook of Physiology; American Physiological Society: Washington, DC, USA, 1965; pp. 197–199. [Google Scholar]
- Bargmann, W.; Hehn, G.V.; Lindner, E. Über die Zellen des braunen Fettgewebes und ihre Innervation. Z. Zellforch. Mikrosk. Anat. 1968, 85, 601–613. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Alson, L.D. Role of adrenaline and noradrenaline in chemical regulation of heat production. Am. J. Physiol. 1957, 190, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Depocas, F. The calorigenic response of cold-acclimated white rats to infused noradrenaline. Can. J. Biochem. Physiol. 1960, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Heim, T.; Hull, D. The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J. Physiol. 1966, 187, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Young, J.B.; Saville, E.; Rothwell, N.J.; Stock, M.J.; Landsberg, L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J. Clin. Invest. 1982, 69, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Frisbee, J.C. Obesity and vascular dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Pirzgalska, R.M.; Pereira, M.M.; Kubasova, N.; Barateiro, A.; Seixas, E.; Lu, Y.H.; Kozlova, A.; Voss, H.; Martins, G.G.; et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 2015, 163, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Blaszkiewicz, M.; Willows, J.W.; Dubois, A.L.; Waible, S.; Johnson, C.P.; DiBello, K.; Lyons, L.L.; Breeding, W.P.; Tilbury, K.B.; Michael, M.; et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. bioRxiv 2018, 480095. [Google Scholar] [CrossRef]
- Chi, J.; Wu, Z.; Choi, C.H.J.; Nguyen, L.; Tegegne, S.; Ackerman, S.E.; Crane, A.; Marchildon, F.; Tessier-Lavigne, M.; Cohen, P. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 2018, 27, 226–236.e223. [Google Scholar] [CrossRef] [PubMed]
- Fishman, R.B.; Dark, J. Sensory innervation of white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987, 253, R942–R944. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Schwartz, G.J.; Bartness, T.J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R501–R511. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.T.; Schwartz, G.J.; Nguyen, N.L.T.; Mendez, J.M.; Ryu, V.; Bartness, T.J. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1338–E1347. [Google Scholar] [CrossRef] [PubMed]
- Garretson, J.T.; Szymanski, L.A.; Schwartz, G.J.; Xue, B.; Ryu, V.; Bartness, T.J. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol. Metab. 2016, 5, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Youngstrom, T.G.; Bartness, T.J. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1995, 268, R744–R751. [Google Scholar] [CrossRef] [PubMed]
- Bamshad, M.; Aoki, V.T.; Adkison, M.G.; Warren, W.S.; Bartness, T.J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 1998, 275, R291–R299. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Demas, G. Special issue dedicated to Dr. Timothy J. Bartness. Physiol. Behav. 2018, 190, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S. Denervation as a tool for testing sympathetic control of white adipose tissue. Physiol. Behav. 2018, 190, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.H.; Zarebidaki, E.; Ehlen, J.C.; Bartness, T.J. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol. 2014, 537, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H.; Tranzer, J. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-hydroxydopamine. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968, 261, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Demas, G.E.; Bartness, T.J. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1499–R1505. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Song, C.K.; Giordano, A.; Cinti, S.; Bartness, T.J. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1028–R1037. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.A.; Kettelhut, I.C.; Roselino, J.S.; Migliorini, R.H. Effect of acute cold exposure on norpinephrine turnover rates in rat white adipose tissue. J. Autonom. Nerv. Syst. 1996, 60, 206–208. [Google Scholar] [CrossRef]
- Migliorini, R.H.; Garofalo, M.A.; Kettelhut, I.C. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am. J Physiol 1997, 272, R656–R661. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.O.; Depocas, F.; Behrens, G.Z. Unilaterality of the sympathetic innervation of each pad of rat interscapular brown adipose tissue. Can. J. Physiol. Pharm. 1982, 60, 107–113. [Google Scholar] [CrossRef]
- Morrison, S.F.; Ramamurthy, S.; Young, J.B. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J. Neurosci. 2000, 20, 9264–9271. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Miller, D.S. Energy balance following sympathetic denervation of brown adipose tissue. Can. J. Physiol. Pharmacol. 1984, 62, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.L.; Madden, C.J.; Blaszkiewicz, M.; McDougall, L.; Tupone, D.; Lynes, M.D.; Mishina, Y.; Yu, P.; Morrison, S.F.; Tseng, Y.-H. Reestablishment of energy balance in a male mouse model with POMC neuron deletion of BMPR1A. Endocrinology 2017, 158, 4233–4245. [Google Scholar] [CrossRef] [PubMed]
- Klingenspor, M.; Meywirth, A.; Stohr, S.; Heldmaier, G. Effect of unilateral surgical denervation of brown adipose tissue on uncoupling protein mRNA level and cytochrom-c-oxidase activity in the Djungarian hamster. J. Comp. Physiol. B. 1994, 163, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.W.; Boucher, J.; Cheong, J.K.; Vernochet, C.; Koh, H.-J.; Mallol, C.; Townsend, K.; Langin, D.; Kawamori, D.; Hu, J. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat. Med. 2013, 19, 217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.L.T.; Barr, C.L.; Ryu, V.; Cao, Q.; Xue, B.; Bartness, T.J. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 312, R132–R145. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.H.; Bartness, T.J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1049–R1058. [Google Scholar] [CrossRef] [PubMed]
- Cousin, B.; Casteilla, L.; Lafontan, M.; Ambid, L.; Langin, D.; Berthault, M.; Penicaud, L. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology 1993, 133, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.R.; Festuccia, W.T.; Song, C.K.; Shi, H.; Migliorini, R.H.; Bartness, T.J. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1167–R1175. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.T.; Bartness, T.J. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1630–R1637. [Google Scholar] [CrossRef] [PubMed]
- Rooks, C.R.; Penn, D.M.; Kelso, E.; Bowers, R.R.; Bartness, T.J.; Harris, R.B. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R92–R102. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity 2012, 20, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Oeckl, J.; Westermeier, J.; Li, Y.; Klingenspor, M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wu, J.; Cohen, P.; Kazak, L.; Khandekar, M.J.; Jedrychowski, M.P.; Zeng, X.; Gygi, S.P.; Spiegelman, B.M. Fat cells directly sense temperature to activate thermogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 12480–12485. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Between brown and white: novel aspects of adipocyte differentiation. Ann. Med. 2011, 43, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.; Kay Song, C. Innervation of brown adipose tissue and its role in thermogenesis. Can. J. Diabetes 2005, 29, 420–428. [Google Scholar]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. (Lond). 2010, 34, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.O.; Depocas, F.; Zuker, M. Heterogeneity of the sympathetic innervation of rat interscapular brown adipose tissue via intercostal nerves. Can. J. Physiol. Pharmacol. 1982, 60, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Diculescu, I.; Stoica, M. Fluorescence histochemical investigation on the adrenergic innervation of the white adipose tissue in the rat. J. Neurovisc. Relat. 1970, 32, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Frontini, A.; Murano, I.; Tonello, C.; Marino, M.A.; Carruba, M.O.; Nisoli, E.; Cinti, S. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J. Histochem. Cytochem. 2005, 53, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Morroni, M.; Santone, G.; Marchesi, G.F.; Cinti, S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: An immunohistochemical and ultrastructural investigation. J. Neurocytol. 1996, 25, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Murano, I.; Barbatelli, G.; Giordano, A.; Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 2009, 214, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Chang, J.S.; Jo, Y.H. Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Sci. Rep. 2018, 8, 6672. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.A.; Brito, M.N.; Bartness, T.J. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1445–R1452. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid. Res. 2012, 53, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Koza, R.A.; Yamashita, H.; Walsh, K.; Kozak, L.P. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 1998, 102, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Jung, Y.S.; Kwon, H.J.; Seong, J.K.; Granneman, J.G.; Lee, Y.H. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol. Sex Differ. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Shrestha, Y.B.; Vaughan, C.H.; Schwartz, G.J.; Song, C.K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 2010, 318, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S. Role of Substance P Neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.K.; Eiden, L.E.; Weihe, E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience 1998, 84, 361–376. [Google Scholar] [CrossRef]
- Giordano, A.; Frontini, A.; Castellucci, M.; Cinti, S. Presence and distribution of cholinergic nerves in rat mediastinal brown adipose tissue. J. Histochem. Cytochem. 2004, 52, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kreier, F.; Fliers, E.; Voshol, P.J.; Van Eden, C.G.; Havekes, L.M.; Kalsbeek, A.; Van Heijningen, C.L.; Sluiter, A.A.; Mettenleiter, T.C.; Romijn, J.A.; et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat–functional implications. J. Clin. Invest. 2002, 110, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Song, C.K.; Bowers, R.R.; Ehlen, J.C.; Frontini, A.; Cinti, S.; Bartness, T.J. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1243–R1255. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Fox, E.A.; Neuhuber, W.L. Vagaries of adipose tissue innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1240–R1242. [Google Scholar] [CrossRef] [PubMed]
- Kreier, F.; Buijs, R.M. Evidence for parasympathetic innervation of white adipose tissue, clearing up some vagaries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R548–R549; author reply R550–R552, discussion R553–R554. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R.; Fox, E.A.; Neuhuber, W.L. Rebuttal: Controversial white adipose tissue innervation by the vagus nerve: Seeing is believing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R553–R554. [Google Scholar] [CrossRef]
- Giordano, A.; Kay Song, C.; Bowers, R.R.; Christopher Ehlen, J.; Frontini, A.; Cinti, S.; Bartness, T.J. Reply to Kreier and Buijs: no sympathy for the claim of parasympathetic innervation of white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R550–R552. [Google Scholar] [CrossRef]
- Richardson, D.S.; Lichtman, J.W. Clarifying tissue clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Spalteholz, W. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen, nebst Anhang: Über Knochenfärbung; S. Hirzel: Leipzig, Germany, 1914. [Google Scholar]
- Azaripour, A.; Lagerweij, T.; Scharfbillig, C.; Jadczak, A.E.; Willershausen, B.; Van Noorden, C.J. A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog. Histochem. Cytochem. 2016, 51, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Dodt, H.U.; Leischner, U.; Schierloh, A.; Jahrling, N.; Mauch, C.P.; Deininger, K.; Deussing, J.M.; Eder, M.; Zieglgansberger, W.; Becker, K. Ultramicroscopy: Three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 2007, 4, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Renier, N.; Wu, Z.; Simon, D.J.; Yang, J.; Ariel, P.; Tessier-Lavigne, M. iDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 2014, 159, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Choe, M.; Kim, S.Y. Clearing and labeling techniques for large-scale biological tissues. Mol. Cells 2016, 39, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ding, X.; Cao, Y.; Wang, H.; Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 2017, 26, 686–692.e683. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yeung, J.L.; Lee, J.H.; An, J.; Steadman, P.E.; Kim, J.R.; Sung, H.K. Visualization of 3D white adipose tissue structure using whole-mount staining. J. Vis. Exp. 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, H.; Wang, Q.; Han, X.; Zeng, W. Three-dimensional volume fluorescence-imaging of vascular plasticity in adipose tissues. Mol. Metab. 2018, 14, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, H.; Zeng, W. Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein Cell 2018, 9, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Crane, A.; Wu, Z.; Cohen, P. Adipo-clear: A tissue clearing method for three-dimensional imaging of adipose tissue. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Cai, R.; Pan, C.; Ghasemigharagoz, A.; Todorov, M.; Foerstera, B.; Zhao, S.; Bhatia, H.; Mrowka, L.; Theodorou, D.; Rempfler, M.; et al. Panoptic vDISCO Imaging Reveals Neuronal Connectivity, Remote Trauma Effects and Meningeal Vessels in Intact Transparent Mice. 2018. Available online: https://www.biorxiv.org/content/10.1101/374785v1 (accessed on 29 November 2018).
- Belle, M.; Godefroy, D.; Couly, G.; Malone, S.A.; Collier, F.; Giacobini, P.; Chedotal, A. Tridimensional visualization and analysis of early human development. Cell 2017, 169, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Mayrand, D.; Fradette, J. High definition confocal imaging modalities for the characterization of tissue-engineered substitutes. Methods Mol. Biol. 2018, 1773, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Santibanez, G.; Cho, K.W.; Lumeng, C.N. Imaging white adipose tissue with confocal microscopy. Methods Enzymol. 2014, 537, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Christie, B.R. Isolectin-IB 4 as a vascular stain for the study of adult neurogenesis. J. Neurosci. Methods 2006, 150, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Djouhri, L.; McMullan, S.; Berry, C.; Waxman, S.G.; Okuse, K.; Lawson, S.N. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J. Neurosci. 2006, 26, 7281–7292. [Google Scholar] [CrossRef] [PubMed]
- Tannenberg, R.K.; Dodd, P.R. CELL DAMAGE/EXCITOTOXICITY|Excitotoxicity and Neurodegenerative Disease. In Encyclopedia of Basic Epilepsy Research; Schwartzkroin, P.A., Ed.; Academic Press: Oxford, UK, 2009; pp. 114–119. [Google Scholar] [CrossRef]
- Verstraelen, P.; Van Dyck, M.; Verschuuren, M.; Kashikar, N.D.; Nuydens, R.; Timmermans, J.P.; De Vos, W.H. Image-based profiling of synaptic connectivity in primary neuronal cell culture. Front. Neurosci. 2018, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Miller, R.E. Direct enhancement of insulin secretion by vagal stimulation of the isolated pancreas. Am. J. Physiol. 1973, 225, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Langerhans, P. Über die Nerven der menschlichen Haut. Virchows Arch. 1868, 44, 325–337. [Google Scholar] [CrossRef]
- Langerhans, P.; Morrison, H. Contributions to the microscopic anatomy of the pancreas. Bull. Ins. History Med. 1937, 5, 259–297. [Google Scholar]
- Rodriguez-Diaz, R.; Abdulreda, M.H.; Formoso, A.L.; Gans, I.; Ricordi, C.; Berggren, P.-O.; Caicedo, A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Caicedo, A. Neural control of the endocrine pancreas. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Dolenšek, J.; Rupnik, M.S.; Stožer, A.J.I. Structural similarities and differences between the human and the mouse pancreas. Islets 2015, 7, e1024405. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B. Autonomic regulation of islet hormone secretion -implications for health and disease. Diabetologia 2000, 43, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P.; Henquin, J.-C. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [PubMed]
- Molina, J.; Rodriguez-Diaz, R.; Fachado, A.; Jacques-Silva, M.C.; Berggren, P.-O.; Caicedo, A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014, DB_131371. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Hua, T.-E.; Fu, Y.-Y.; Pasricha, P.; Tang, S.-C. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 2012, 55, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Satin, L.S.; Kinard, T.A. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas. Endocrine 1998, 8, 213–223. [Google Scholar] [CrossRef]
- Robbins, M.S.; Grouse, L.H.; Sorenson, R.L.; Elde, R.P. Effect of muscimol on glucose-stimulated somatostatin and insulin release from the isolated, perfused rat pancreas. Diabetes 1981, 30, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.C., III; Hare, T.A. GABA in peripheral tissues: Presence and actions in endocrine pancreatic function. Brain Res. Bull. 1980, 5, 341–346. [Google Scholar] [CrossRef]
- Ikegami, R.; Shimizu, I.; Sato, T.; Yoshida, Y.; Hayashi, Y.; Suda, M.; Katsuumi, G.; Li, J.; Wakasugi, T.; Minokoshi, Y.; et al. Gamma-aminobutyric acid signaling in brown adipose tissue promotes systemic metabolic derangement in obesity. Cell Rep. 2018, 24, 2827–2837.e2825. [Google Scholar] [CrossRef] [PubMed]
- Mundinger, T.O.; Mei, Q.; Foulis, A.K.; Fligner, C.L.; Hull, R.L.; Taborsky, G.J. Human type 1 diabetes is characterized by an early, marked, sustained and islet-selective loss of sympathetic nerves. Diabetes 2016, db160284. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Gadeberg, P.; Brock, B.; Jakobsen, J. Muscular atrophy in diabetic neuropathy: A stereological magnetic resonance imaging study. Diabetologia 1997, 40, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.S.; Jakobsen, J.; Andersen, H. Muscle weakness: A progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 2006, 55, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Hasan, M.; Alshuaib, W. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J. App. Physiol. 2000, 89, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Furuno, T.; McKay, D.M.; Wolvers, D.; Teshima, R.; Nakanishi, M.; Bienenstock, J. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J. Immunol. 1999, 163, 2410–2415. [Google Scholar] [PubMed]
- Hoogerwerf, W.A.; Gondesen, K.; Xiao, S.-Y.; Winston, J.H.; Willis, W.D.; Pasricha, P.J. The role of mast cells in the pathogenesis of pain in chronic pancreatitis. BMC Gastroenterol. 2005, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and its receptors in the regulation of inflammatory response. IJMS 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Schorn, S.; Schremmer-Danninger, E.; Wang, K.; Kehl, T.; Giese, N.A.; Algül, H.; Friess, H.; Ceyhan, G.O. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS ONE 2013, 8, e60529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mehta, K.; Li, C.; Xu, G.-Y.; Liu, L.; Colak, T.; Shenoy, M.; Pasricha, P.J. Systemic administration of anti-NGF increases A-type potassium currents and decreases pancreatic nociceptor excitability in a rat model of chronic pancreatitis. Am. J. Physiol.-Gastr. L. 2011, 302, G176–G181. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Wang, K.; Tieftrunk, E.; Giese, N.A.; Xing, B.; Friess, H.; Kehl, T.; Ceyhan, G.O. Neuronal plasticity in chronic pancreatitis is mediated via the neurturin/GFRα2 axis. Am. J. Physiol.-Gastr. L. 2012, 303, G1017–G1028. [Google Scholar] [CrossRef] [PubMed]
- Su, H.C.; Bishop, A.E.; Power, R.F.; Hamada, Y.; Polak, J.M. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J. Neurosci. 1987, 7, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, W.H. On the structure, distribution and function of the nerves which innervate the visceral and vascular systems. J. Physiol. 1886, 7, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, F. On a large-fibred sensory supply of the thoracic and abdominal viscera. J. Physiol. 1892, 13, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kreier, F.; Kap, Y.S.; Mettenleiter, T.C.; van Heijningen, C.; van der Vliet, J.; Kalsbeek, A.; Sauerwein, H.P.; Fliers, E.; Romijn, J.A.; Buijs, R.M. Tracing from fat tissue, liver, and pancreas: A neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 2006, 147, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Imoto, M.; Koyama, Y.; Miyazawa, Y.; Hayakawa, T. Demonstration of noradrenaline-immunoreactive nerve fibres in the liver. J. Int. Med. Res. 1996, 24, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kandilis, A.N.; Papadopoulou, I.P.; Koskinas, J.; Sotiropoulos, G.; Tiniakos, D.G. Liver innervation and hepatic function: New insights. J. Surg. Res. 2015, 194, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Uyama, N.; Geerts, A.; Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. Part A Discov. Mol. Cell Evol. Biol. 2004, 280, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [PubMed]
- Seseke, F.G.; Gardemann, A.; Jungermann, K. Signal propagation via gap junctions, a key step in the regulation of liver metabolism by the sympathetic hepatic nerves. FEBS Lett. 1992, 301, 265–270. [Google Scholar] [CrossRef]
- Hertzberg, E.; Gilula, N. Isolation and characterization of gap junctions from rat liver. J. Biol. Chem. 1979, 254, 2138–2147. [Google Scholar] [PubMed]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obesity 2002, 26, 1459. [Google Scholar] [CrossRef] [PubMed]
- Naples, G.G.; Mortimer, J.T.; Scheiner, A.; Sweeney, J.D. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE T. Bio.-Med. Eng. 1988, 35, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Foldes, E.L.; Ackermann, D.M.; Bhadra, N.; Kilgore, K.L.; Bhadra, N. Design, fabrication and evaluation of a conforming circumpolar peripheral nerve cuff electrode for acute experimental use. J. Neurosci. Meth. 2011, 196, 31–37. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 2016, 594, 5791–5815. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; He, Z.; Li, K.; Wang, X.; Dong, J.J.H.R. Evaluation of the role of sympathetic denervation on hepatic function. Hepatol. Res. 2006, 36, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Moreno-Cuevas, J.E.; González-Garza, M.T.; Maldonado-Bernal, C.; Cruz-Vega, D.E. Liver fibrosis and mechanisms of the protective action of medicinal plants targeting inflammation and the immune response. Int. J. Inflam. 2015, 2015, PMC4411506. [Google Scholar] [CrossRef] [PubMed]
- Gard, A.L.; White, F.P.; Dutton, G.R. Extra-neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of rat liver. J. Neuroimmunol. 1985, 8, 359–375. [Google Scholar] [CrossRef]
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the developing nervous system. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliver. Rev. 2015, 82, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.; Carlson, N.; Powley, T. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991, 260, R200–R207. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Chen, J.; Behles, R.R.; Hyun, J.; Kopin, A.S.; Moran, T.H. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R55–R63. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Paulino, G.; Raybould, H.E. CCK1 receptor is essential for normal meal patterning in mice fed high fat diet. Physiol. Behav. 2007, 92, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Paulino, G.; Barbier de la Serre, C.; Knotts, T.A.; Oort, P.J.; Newman, J.W.; Adams, S.H.; Raybould, H.E. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E898–E903. [Google Scholar] [CrossRef] [PubMed]
- Diepenbroek, C.; Quinn, D.; Stephens, R.; Zollinger, B.; Anderson, S.; Pan, A.; de Lartigue, G. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am. J. Physiol.-Gastr. L. 2017, 313, G342–G352. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, G.; Ronveaux, C.C.; Raybould, H.E. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol. Metab. 2014, 3, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Lourenssen, S.; Stanzel, R.; Blennerhassett, M. Selective loss of NGF-sensitive neurons following experimental colitis. Exp. Neurol. 2005, 191, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Reinshagen, M.; Rohm, H.; Steinkamp, M.; Lieb, K.; Geerling, I.; Von Herbay, A.; Flamig, G.; Eysselein, V.E.; Adler, G. Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology 2000, 119, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.M.; Brierley, S.M.; Isaacs, N.; Hughes, P.A.; Castro, J.; Blackshaw, L.A. Sprouting of colonic afferent central terminals and increased spinal mitogen-activated protein kinase expression in a mouse model of chronic visceral hypersensitivity. J. Comp. Neurol. 2012, 520, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.; Stebbing, M.J.N.; Motility. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol. Motil. 2018, 30, e13234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Ruiz, H.H.; Jhun, K.; Finan, B.; Oberlin, D.J.; van der Heide, V.; Kalinovich, A.V.; Petrovic, N.; Wolf, Y.; Clemmensen, C. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017, 23, 623. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.S.; Parichy, D.M. A macrophage relay for long-distance signaling during postembryonic tissue remodeling. Science 2017, 355, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Balter, N.J.; Schwartz, S.L. Accumulation of norepinephrine by macrophages and relationships to known uptake processes. J. Pharmacol. Exp. Ther. 1977, 201, 636–643. [Google Scholar] [PubMed]
- Pirzgalska, R.M.; Seixas, E.; Seidman, J.S.; Link, V.M.; Sánchez, N.M.; Mahú, I.; Mendes, R.; Gres, V.; Kubasova, N.; Morris, I. Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 2017, 23, 1309. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.D.; Sander, J.; Spadaro, O.; Lee, A.; Nguyen, K.Y.; Wing, A.; Goldberg, E.L.; Youm, Y.H.; Brown, C.W.; Elsworth, J.; et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 2017, 550, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Boura-Halfon, S.; Cortese, N.; Haimon, Z.; Shalom, H.S.; Kuperman, Y.; Kalchenko, V.; Brandis, A.; David, E.; Segal-Hayoun, Y. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 2017, 18, 665. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.C.; Du, F.; Felice, C.A.; Shan, X.; Nigam, A.; Mandel, G.; Robinson, J.K.; Ballas, N. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J. Neurosci. 2012, 32, 10021–10034. [Google Scholar] [CrossRef] [PubMed]
- Suto, F.; Ito, K.; Uemura, M.; Shimizu, M.; Shinkawa, Y.; Sanbo, M.; Shinoda, T.; Tsuboi, M.; Takashima, S.; Yagi, T. Plexin-A4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J. Neurosci. 2005, 25, 3628–3637. [Google Scholar] [CrossRef] [PubMed]
- Perez-Branguli, F.; Zagar, Y.; Shanley, D.K.; Graef, I.A.; Chédotal, A.; Mitchell, K.J. Reverse signaling by Semaphorin-6A regulates cellular aggregation and neuronal morphology. PLoS ONE 2016, 11, e0158686. [Google Scholar] [CrossRef] [PubMed]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Bracci-Laudiero, L.; Bonini, S.; Bonini, S.; Starace, G.; D'elios, M.M.; De Carli, M.; Aloe, L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J. Allergy Clin. Immun. 1997, 100, 408–414. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Gallmeier, E.; Behrens, L.; Leal, V.V.; Misgeld, T.; Klinkert, W.E.; Kolbeck, R.; Hoppe, E.; Oropeza-Wekerle, R.-L.; Bartke, I. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J. Exp. Med. 1999, 189, 865–870. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, R.; Ricquier, D.; Cinti, S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: An immunohistochemical study. J. Neurocytol. 1998, 27, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Liu, Y.; Shrestha, Y.B.; Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 2014, 35, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J.; Lundberg, J.M.; Hokfelt, T.; Terenius, L.; Goldstein, M. ‘Neuropeptide tyrosine’ (NPY) is co-stored with noradrenaline in vascular but not in parenchymal sympathetic nerves of brown adipose tissue. Exp. Cell Res. 1986, 164, 546–550. [Google Scholar] [CrossRef]
- Chen, L.; Jin, Y.; Yang, X.; Liu, Z.; Wang, Y.; Wang, G.; Qi, Z.; Shen, Z. Fat tissue, a potential Schwann cell reservoir: Isolation and identification of adipose-derived Schwann cells. Am. J. Transl. Res. 2017, 9, 2579. [Google Scholar] [PubMed]
- Ferrante, A.W., Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Chaldakov, G.N.; Fiore, M.; Ghenev, P.I.; Beltowski, J.; Ranćić, G.; Tunçel, N.; Aloe, L. Triactome: Neuro–immune–adipose interactions. Implication in vascular biology. Front. Immunol. 2014, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gleich, G.J.; Butterfield, J.H.; Kita, H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood 2002, 99, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Noga, O.; Englmann, C.; Hanf, G.; Grutzkau, A.; Seybold, J.; Kunkel, G. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin. Exp. Allergy 2003, 33, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Medrikova, D.; Sijmonsma, T.P.; Sowodniok, K.; Richards, D.M.; Delacher, M.; Sticht, C.; Gretz, N.; Schafmeier, T.; Feuerer, M.; Herzig, S. Brown adipose tissue harbors a distinct sub-population of regulatory T cells. PLoS ONE 2015, 10, e0118534. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaszkiewicz, M.; Willows, J.W.; Johnson, C.P.; Townsend, K.L. The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance. Biology 2019, 8, 10. https://doi.org/10.3390/biology8010010
Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL. The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance. Biology. 2019; 8(1):10. https://doi.org/10.3390/biology8010010
Chicago/Turabian StyleBlaszkiewicz, Magdalena, Jake W. Willows, Cory P. Johnson, and Kristy L. Townsend. 2019. "The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance" Biology 8, no. 1: 10. https://doi.org/10.3390/biology8010010
APA StyleBlaszkiewicz, M., Willows, J. W., Johnson, C. P., & Townsend, K. L. (2019). The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance. Biology, 8(1), 10. https://doi.org/10.3390/biology8010010



