Investigation of the Neurotoxic Effects and Mechanisms of Michler’s Ketone as Investigated by Network Toxicology and Transcriptomics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Husbandry
2.3. Construction of Neurotoxicity Targets for MK
2.4. Construction of the Protein–Protein Interaction (PPI) Network and KEGG Pathway Analysis Were Performed
2.5. MK Exposure and General Developmental Toxicity Assessment
2.6. Locomotor Behavior Testing in Zebrafish Larvae
2.7. Investigation of Neurodevelopmental Toxicity in Zebrafish Larvae
2.8. Transcriptome Analysis and Quantitative Real-Time PCR
2.9. Data Analytics
3. Results
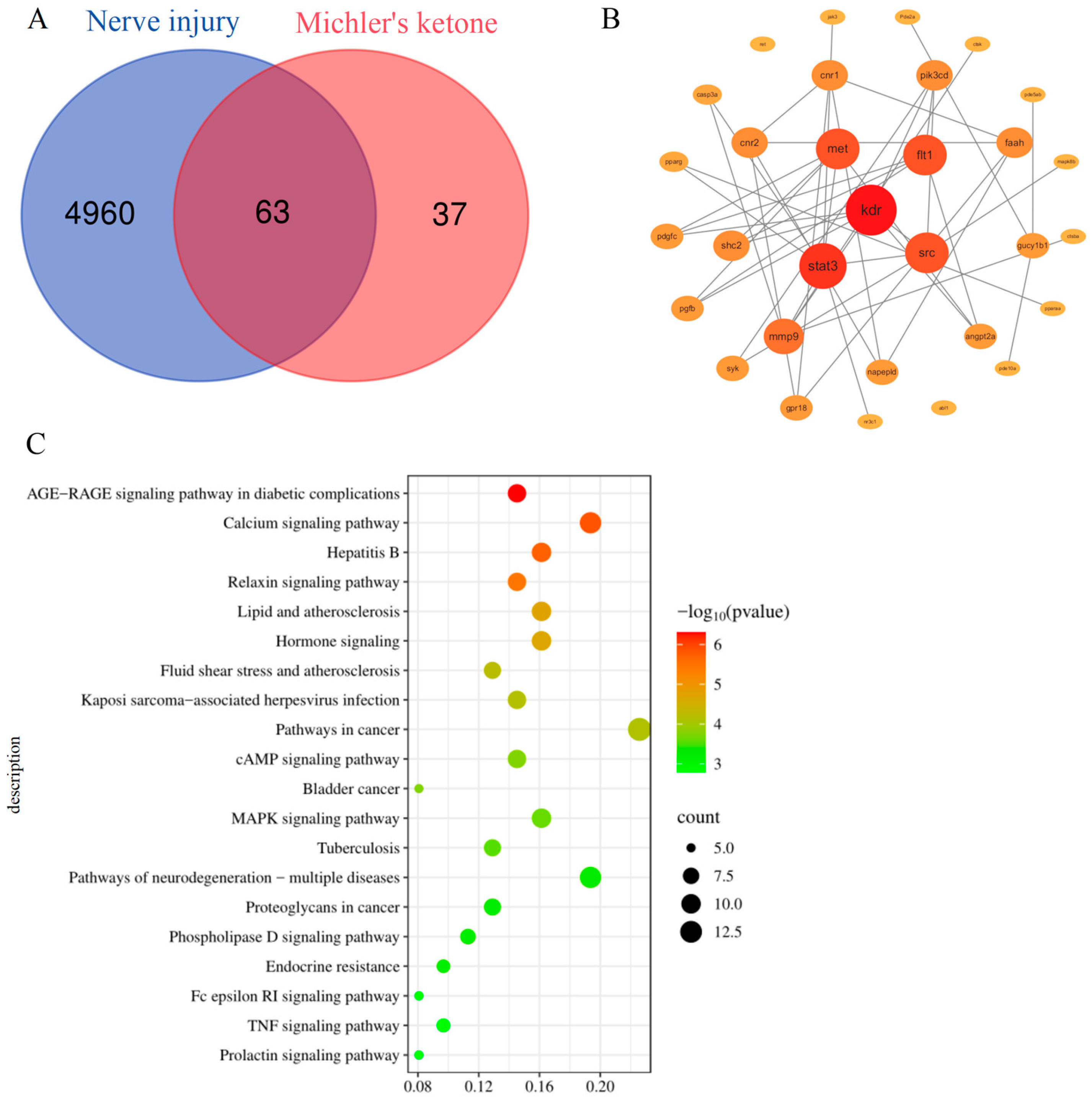
3.1. Network Toxicological Analysis of MK Neurotoxicity
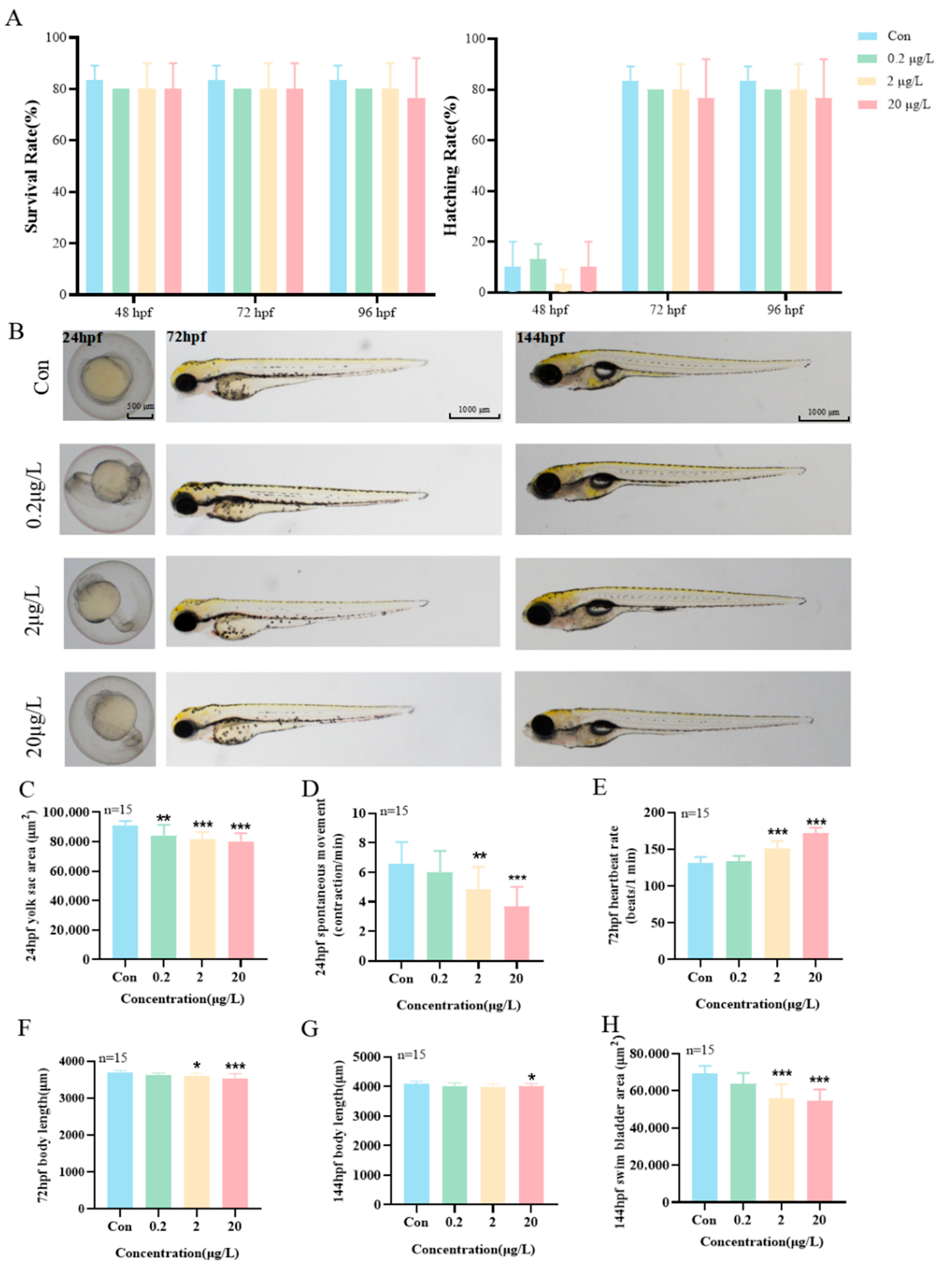
3.2. Effects of MK Exposure on the Early Development of Zebrafish Larvae
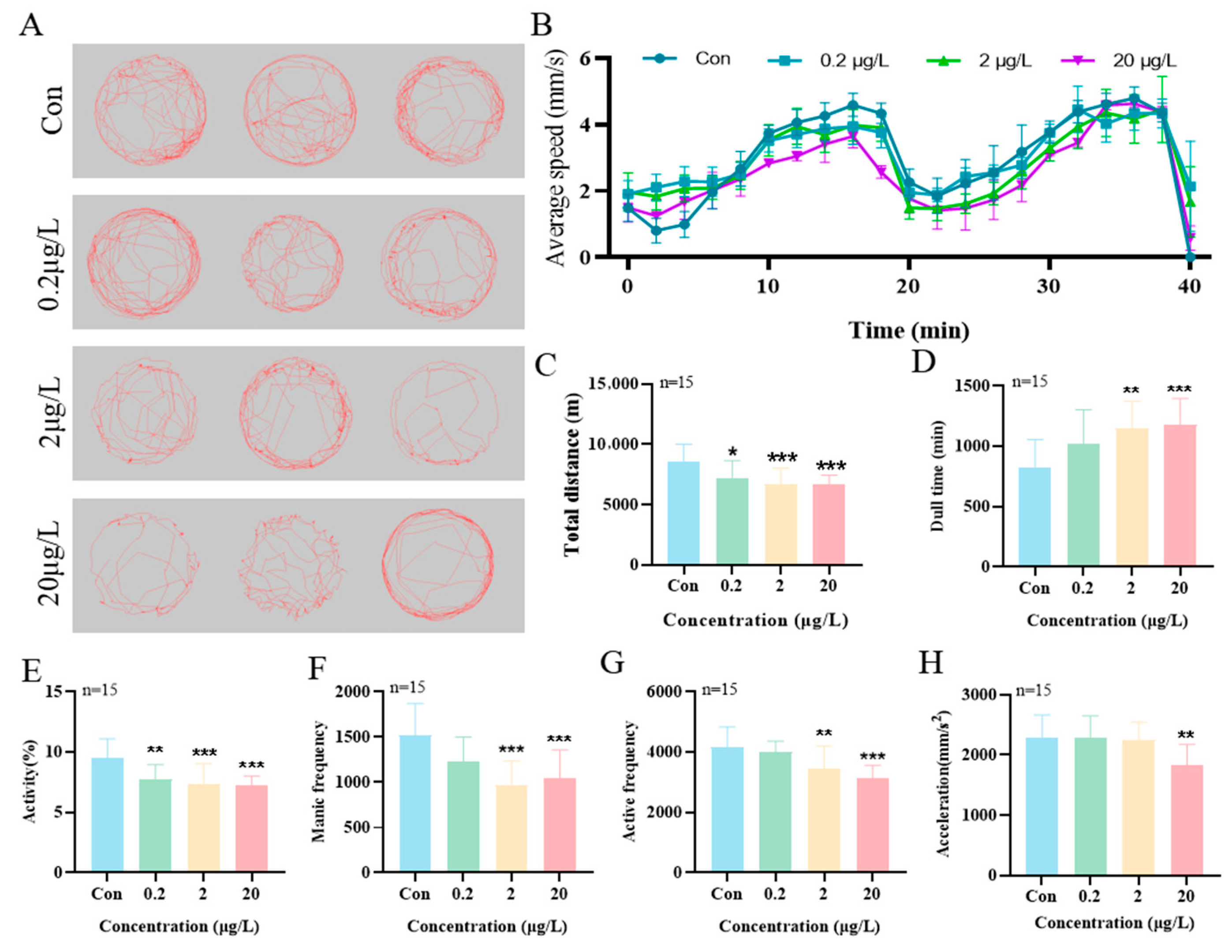
3.3. Effects of MK on Locomotor Behavior in Zebrafish Larvae
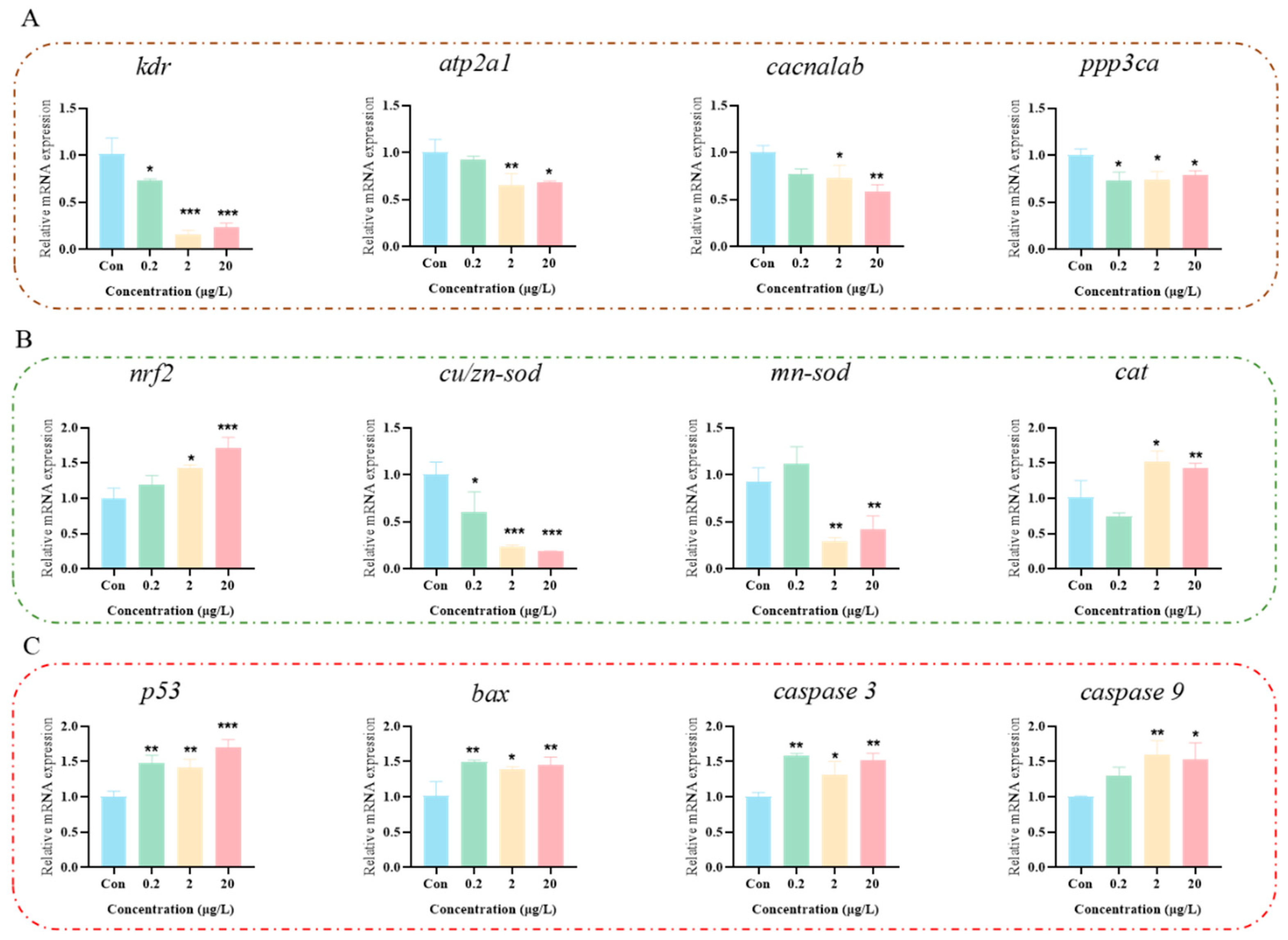
3.4. Effects of MK on the Nervous System of Zebrafish Larvae
3.5. MK Induces Oxidative Stress to Disrupt Calcium Ion Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnamoorthy, S.; Allabasha, N.; Mani, M.K.; Sarkar, A.K. Michler’s Ketone: Beta-Cyclodextrin Host-Guest Inclusion Complex for Enhancing the Ultraviolet Protection Factor of Poplin Cotton Fabric. Photochem. Photobiol. 2022, 98, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Kawasaki, C.; Kawamura, Y.; Tanamoto, K. Migration of bisphenol A and benzophenones from paper and paperboard products used in contact with food. Shokuhin Eiseigaku Zasshi 2006, 47, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Yamaguchi, Y.; Fujita, T.; Kuroda, K.; Endo, G. Chemical analysis and genotoxicological safety assessment of paper and paperboard used for food packaging. Food Chem. Toxicol. 2004, 42, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Parodi, S.; Santi, L.; Russo, P.; Albini, A.; Vecchio, D.; Pala, M.; Ottaggio, L.; Carbone, A. DNA damage induced by auramine O in liver, kidney, and bone marrow of rats and mice, and in a human cell line (alkaline elution assay and SCE induction). J. Toxicol. Environ. Health 1982, 9, 941–952. [Google Scholar] [CrossRef]
- Williams, G.M.; Laspia, M.F.; Dunkel, V.C. Reliability of the hepatocyte primary culture/DNA repair test in testing of coded carcinogens and noncarcinogens. Mutat. Res. 1982, 97, 359–370. [Google Scholar] [CrossRef]
- Lafi, A.; Parry, J.M.; Parry, E.M. The effect of Michler’s ketone on cell division, chromosome number and structure in cultured Chinese hamster cells. Mutagenesis 1986, 1, 17–20. [Google Scholar] [CrossRef]
- Han, X.; Nabb, D.L.; Mingoia, R.T.; Yang, C.H. Determination of xenobiotic intrinsic clearance in freshly isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) and rat and its application in bioaccumulation assessment. Environ. Sci. Technol. 2007, 41, 3269–3276. [Google Scholar] [CrossRef]
- Gibert, Y.; Trengove, M.C.; Ward, A.C. Zebrafish as a genetic model in pre-clinical drug testing and screening. Curr. Med. Chem. 2013, 20, 2458–2466. [Google Scholar] [CrossRef]
- Planchart, A.; Mattingly, C.J.; Allen, D.; Ceger, P.; Casey, W.; Hinton, D.; Kanungo, J.; Kullman, S.W.; Tal, T.; Bondesson, M.; et al. Advancing toxicology research using in vivo high throughput toxicology with small fish models. ALTEX 2016, 33, 435–452. [Google Scholar] [CrossRef]
- Hughes, S.; Hessel, E.V.S. Zebrafish and nematodes as whole organism models to measure developmental neurotoxicity. Crit. Rev. Toxicol. 2024, 54, 330–343. [Google Scholar] [CrossRef]
- Ravenscroft, G.; Sollis, E.; Charles, A.K.; North, K.N.; Baynam, G.; Laing, N.G. Fetal akinesia: Review of the genetics of the neuromuscular causes. J. Med. Genet. 2011, 48, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Yuan, Q.; Sun, S.; Liu, F.; Liao, X.; Lu, H.; Chen, J.; Cao, Z. Isavuconazole Induces Neurodevelopment Defects and Motor Behaviour Impairment in Zebrafish Larvae. Mol. Neurobiol. 2024, 61, 10072–10082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, S.; Shi, X.; Luo, C.; Huang, W.; Lin, H.; Peng, J.; Tan, W.; Wu, K. Neurodevelopmental toxicity of organophosphate flame retardant triphenyl phosphate (TPhP) on zebrafish (Danio rerio) at different life stages. Environ. Int. 2023, 172, 107745. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Guo, M.; Yin, X.; Huang, C.; Qian, L.; Zhou, L.; Wang, Z.; Wang, L.; Shi, L.; Ji, G. A systematic comparison of neurotoxicity of bisphenol A and its derivatives in zebrafish. Sci. Total Environ. 2022, 805, 150210. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Li, M.; Huan, F.; Gao, R.; Wang, J. Effects of exposure to 3,6-DBCZ on neurotoxicity and AhR pathway during early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2024, 270, 115892. [Google Scholar] [CrossRef]
- Verdu, E.; Ceballos, D.; Vilches, J.J.; Navarro, X. Influence of aging on peripheral nerve function and regeneration. J. Peripher. Nerv. Syst. 2000, 5, 191–208. [Google Scholar] [CrossRef]
- Mueller, T.; Wullimann, M.F. Expression domains of neuroD (nrd) in the early postembryonic zebrafish brain. Brain Res. Bull. 2002, 57, 377–379. [Google Scholar] [CrossRef]
- Kowara, R.; Menard, M.; Brown, L.; Chakravarthy, B. Co-localization and interaction of DPYSL3 and GAP43 in primary cortical neurons. Biochem. Biophys. Res. Commun. 2007, 363, 190–193. [Google Scholar] [CrossRef]
- Frey, D.; Laux, T.; Xu, L.; Schneider, C.; Caroni, P. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 2000, 149, 1443–1454. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxidative Med. Cell. Longev. 2021, 2021, 6685204. [Google Scholar] [CrossRef]
- Lal, R.; Dharavath, R.N.; Chopra, K. Nrf2 Signaling Pathway: A Potential Therapeutic Target in Combating Oxidative Stress and Neurotoxicity in Chemotherapy-Induced Cognitive Impairment. Mol. Neurobiol. 2024, 61, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fuentes, G.; Rubio-Escalante, F.J.; Norena-Barroso, E.; Escalante-Herrera, K.S.; Schlenk, D. Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 172–173, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, S.; Qian, Y.; Xu, Z.; Yu, Y.; Xu, Y.; He, Y.; Zhang, Y. The assessment of the eco-toxicological effect of gabapentin on early development of zebrafish and its antioxidant system. RSC Adv. 2018, 8, 22777–22784. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef]
- Weber, K.J.; Wenz, F. p53, apoptosis and radiosensitivity–experimental and clinical data. Onkologie 2002, 25, 136–141. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, J.; Lu, H.; Zhou, X. The ARTS of p53-dependent mitochondrial apoptosis. J. Mol. Cell Biol. 2023, 14, mjac074. [Google Scholar] [CrossRef]
- Johnson, C.R.; Jarvis, W.D. Caspase-9 regulation: An update. Apoptosis 2004, 9, 423–427. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, W.; Zhang, Y.; Hu, Y.; Liang, Z.; Chen, Z.; Lu, Z.; Zhang, W.; Wang, Z.; Jiang, Y.; et al. Neurotoxicity mechanisms of pyrethroids studied by network toxicology and molecular docking. Toxicol. Mech. Methods 2025, 35, 1533–1546. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Hu, J.; Zha, X.; Liu, X.; Jin, H.; Fan, Y.; Zhao, X.; Hu, J.; Wang, J. Investigation of the Neurotoxic Effects and Mechanisms of Michler’s Ketone as Investigated by Network Toxicology and Transcriptomics. Biology 2026, 15, 3. https://doi.org/10.3390/biology15010003
Hu J, Zha X, Liu X, Jin H, Fan Y, Zhao X, Hu J, Wang J. Investigation of the Neurotoxic Effects and Mechanisms of Michler’s Ketone as Investigated by Network Toxicology and Transcriptomics. Biology. 2026; 15(1):3. https://doi.org/10.3390/biology15010003
Chicago/Turabian StyleHu, Jun, Xianke Zha, Xin Liu, Huilin Jin, Yue Fan, Xin Zhao, Jie Hu, and Jian Wang. 2026. "Investigation of the Neurotoxic Effects and Mechanisms of Michler’s Ketone as Investigated by Network Toxicology and Transcriptomics" Biology 15, no. 1: 3. https://doi.org/10.3390/biology15010003
APA StyleHu, J., Zha, X., Liu, X., Jin, H., Fan, Y., Zhao, X., Hu, J., & Wang, J. (2026). Investigation of the Neurotoxic Effects and Mechanisms of Michler’s Ketone as Investigated by Network Toxicology and Transcriptomics. Biology, 15(1), 3. https://doi.org/10.3390/biology15010003



