2-(Methylthio) Benzothiazole (MTBT) Induces Cardiovascular Toxicity in Zebrafish Larvae and Investigates Its Mechanism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Zebrafish Culture and Embryo Acquisition
2.3. Solution Preparation and Exposure Test Design
2.4. Developmental Toxicity Assessment of Zebrafish Larvae
2.5. Assessment of Cardiac Development and Function in Zebrafish Larvae
2.6. Assessment of Vascular Development in Zebrafish Larvae
2.7. Gene Expression Measurements
2.8. Protein–Protein Interaction (PPI) Network Analysis
2.9. Statistics and Analysis of Data
3. Results
3.1. Effects of MTBT Exposure on the Early Development of Zebrafish Larvae
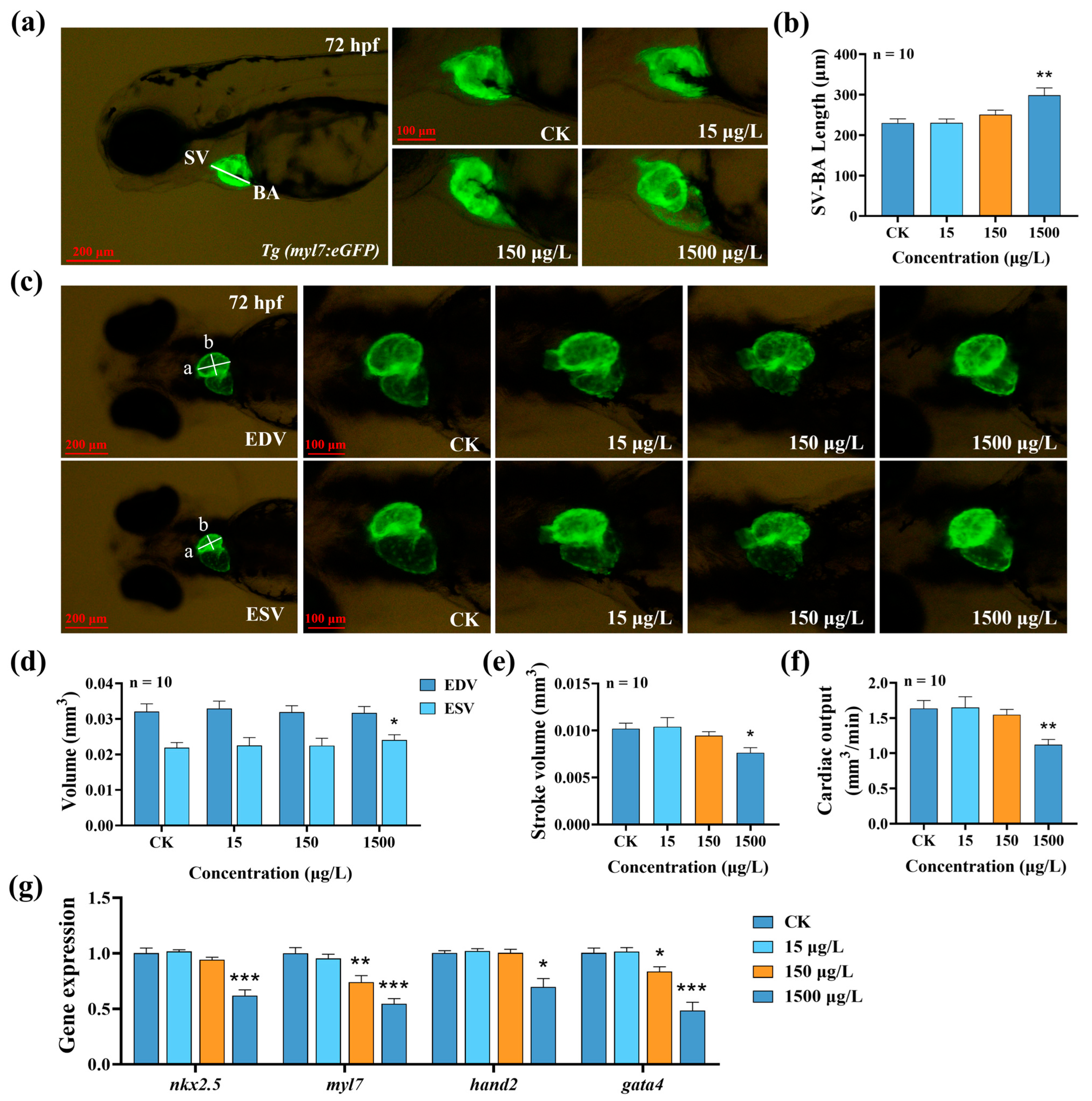
3.2. Effects of MTBT Exposure on Cardiac Development and Function in Zebrafish Larvae
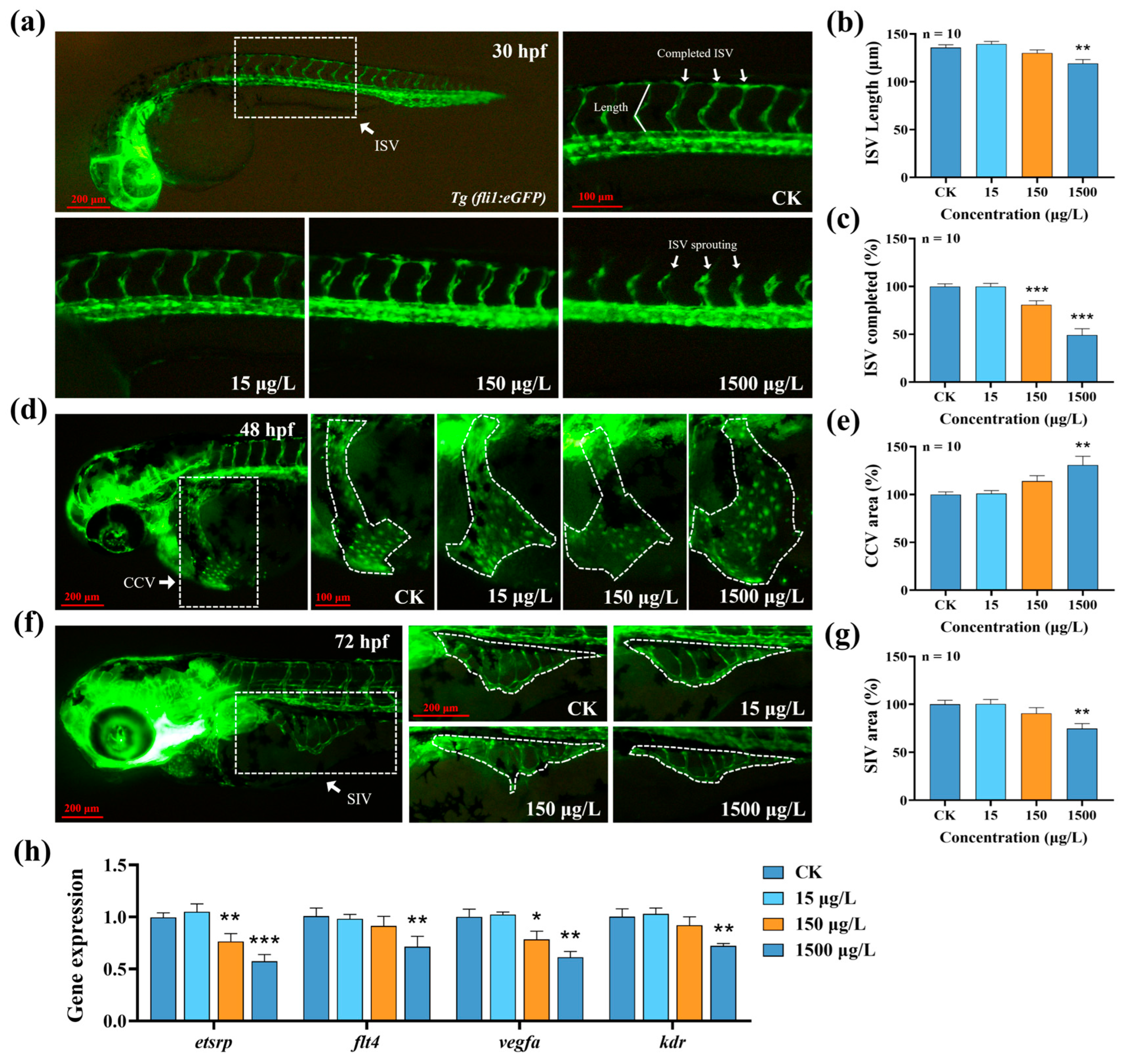
3.3. Effects of MTBT Exposure on Vascular Development in Zebrafish Larvae
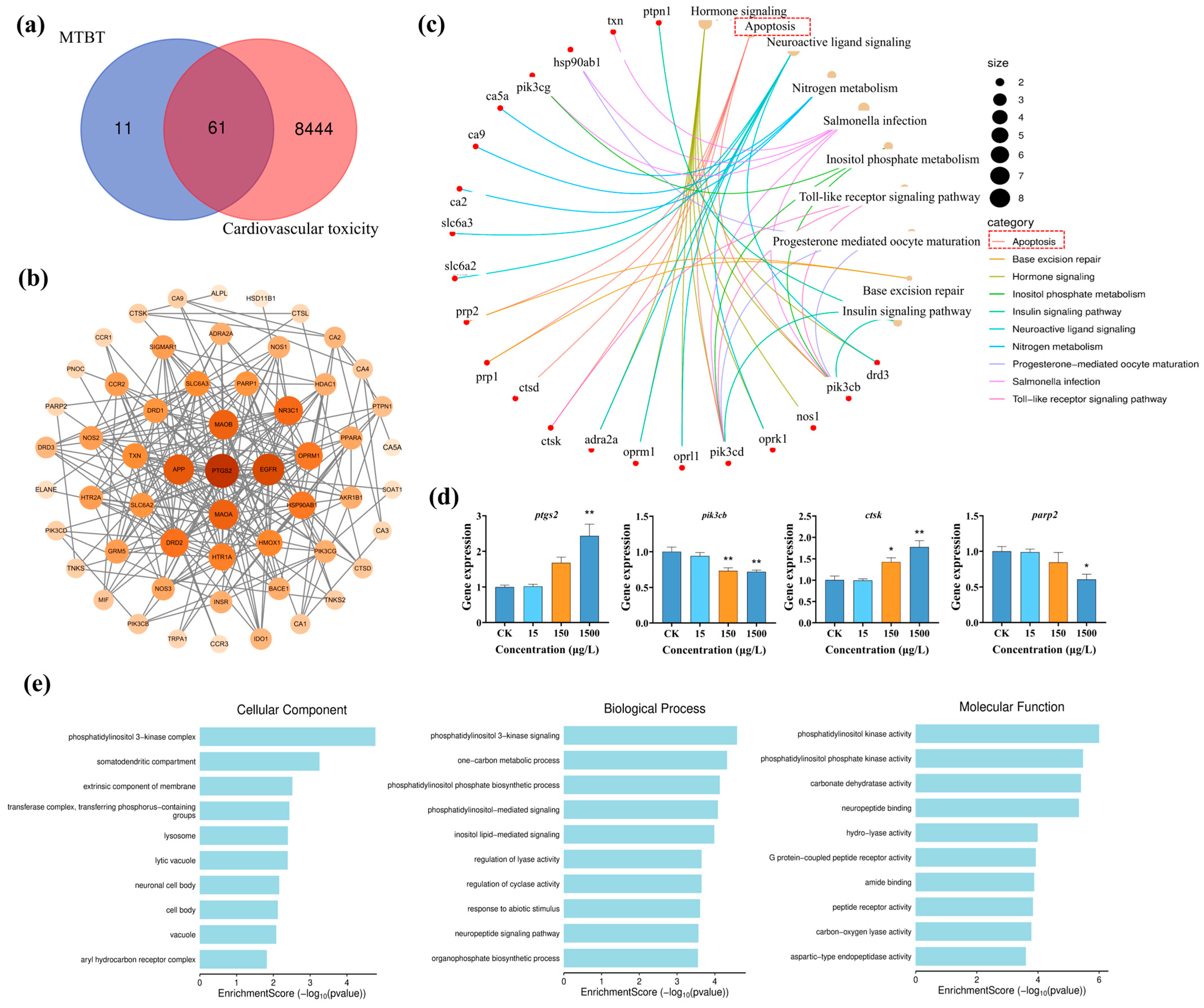
3.4. Potential Mechanism of Action of MTBT in Inducing Cardiovascular Toxicity in Zebrafish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, J.; Guo, L.; Chen, C.; Ji, G.; Wang, L. Neurobehavioral toxic effects and mechanisms of 2-aminobenzothiazole exposure on zebrafish. Sci. Total Environ. 2024, 913, 169495. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, X.; Zhang, T.; Fu, X.; Zhang, F.; Zhu, J. Discovery of novel 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives as multifunctional mao-b inhibitors for the treatment of parkinson’s disease. J. Enzyme Inhib. Med. Chem. 2023, 38, 2159957. [Google Scholar] [CrossRef]
- Liu, Y.H.; Mei, Y.X.; Liang, X.N.; Ge, Z.Y.; Huang, Z.; Zhang, H.Y.; Zhao, J.L.; Liu, A.; Shi, C.; Ying, G.G. Small-intensity rainfall triggers greater contamination of rubber-derived chemicals in road stormwater runoff from various functional areas in megalopolis cities. Environ. Sci. Technol. 2024, 58, 13056–13064. [Google Scholar] [CrossRef]
- Zeng, F.; Sherry, J.P.; Bols, N.C. Evaluating the toxic potential of benzothiazoles with the rainbow trout cell lines, rtgill-w1 and rtl-w. Chemosphere 2016, 155, 308–318. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Sun, H.; Zhou, Q. Widespread occurrence of benzotriazoles and benzothiazoles in tap water: Influencing factors and contribution to human exposure. Environ. Sci. Technol. 2016, 50, 2709–2717. [Google Scholar] [CrossRef]
- De Wever, H.; Besse, P.; Verachtert, H. Microbial transformations of 2-substituted benzothiazoles. Appl. Microbiol. Biotechnol. 2001, 57, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.H.; Elgemeie, G.H.; Abdelrazek, A.R.; Eldaly, S.M. Synthesis, antibacterial evaluation and in silico studies of novel 2-(benzo[d]thiazol-2-yl)-n-arylacetamides and their derivatives as potential dhfr inhibitors. BMC Chem. 2025, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Petzer, J.P.; Cloete, T.T.; Petzer, A. Synthesis and evaluation of 2-methylbenzothiazole derivatives as monoamine oxidase inhibitors. Med. Chem. Res. 2024, 33, 1829–1837. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Huang, Z.; Liu, Y.H.; Hu, L.X.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Ying, G.G. Occurrence and risks of 23 tire additives and their transformation products in an urban water system. Environ. Int. 2023, 171, 107715. [Google Scholar] [CrossRef]
- Kong, L.; Kadokami, K.; Wang, S.; Duong, H.T.; Chau, H.T.C. Monitoring of 1300 organic micro-pollutants in surface waters from tianjin, north china. Chemosphere 2015, 122, 125–130. [Google Scholar] [CrossRef]
- Tisler, S.; Tüchsen, P.L.; Christensen, J.H. Non-target screening of micropollutants and transformation products for assessing aop-bac treatment in groundwater. Environ. Pollut. 2022, 309, 119758. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.T.; Drake, K.D.; Watson, C.F.; Foster, G.D.; Maier, K.J. Comparative aquatic toxicity evaluation of 2-(thiocyanomethylthio)benzothiazole and selected degradation products using ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 2005, 48, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, L.; Shen, Y.; Hu, J.; Gu, J.; Ji, G. Oxidative damage and cardiotoxicity induced by 2-aminobenzothiazole in zebrafish (Danio rerio). J. Hazard. Mater. 2024, 476, 135032. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gu, J.; Yuan, W.; Hu, J.; Zhang, X.; Ji, G. Long-term exposure to emamectin benzoate impairs reproductive health in adult zebrafish and alters neurodevelopment in their offspring. J. Hazard. Mater. 2025, 486, 137007. [Google Scholar] [CrossRef]
- Planchart, A.; Mattingly, C.J.; Allen, D.; Ceger, P.; Casey, W.; Hinton, D.; Kanungo, J.; Kullman, S.W.; Tal, T.; Bondesson, M.; et al. Advancing toxicology research using in vivo high throughput toxicology with small fish models. Altex 2016, 33, 435–452. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R. Zebrafish as a model for cardiovascular and metabolic disease: The future of precision medicine. Biomedicines 2024, 12, 693. [Google Scholar] [CrossRef]
- Gu, J.; Guo, L.; Hu, J.; Ji, G.; Yin, D. Potential adverse outcome pathway (AOP) of emamectin benzoate mediated cardiovascular toxicity in zebrafish larvae (Danio rerio). Sci. Total Environ. 2023, 900, 165787. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Chen, M.; Yang, Q.; Zhuang, S.; Lv, L.; Zuo, Z.; Wang, C. Exposure to low-level metalaxyl impacts the cardiac development and function of zebrafish embryos. J. Environ. Sci. 2019, 85, 1–8. [Google Scholar] [CrossRef]
- Fuchte, H.E.; Beck, N.; Bieg, E.; Bayer, V.J.; Achten, C.; Krauss, M.; Schäffer, A.; Smith, K.E.C. A look down the drain: Identification of dissolved and particle bound organic pollutants in urban runoff waters and sediments. Environ. Pollut. 2022, 302, 119047. [Google Scholar] [CrossRef]
- Gauvrit, S.; Bossaer, J.; Lee, J.; Collins, M.M. Modeling human cardiac arrhythmias: Insights from zebrafish. J. Cardiovasc. Dev. Dis. 2022, 9, 13. [Google Scholar] [CrossRef]
- Sanders, P.; Kistler, P.M.; Morton, J.B.; Spence, S.J.; Kalman, J.M. Remodeling of sinus node function in patients with congestive heart failure: Reduction in sinus node reserve. Circulation 2004, 110, 897–903. [Google Scholar] [CrossRef]
- Yang, M.; Luan, J.; Xu, Y.; Zhao, C.; Sun, M.; Feng, X. Cardiotoxicity of zebrafish induced by 6-benzylaminopurine exposure and its mechanism. Int. J. Mol. Sci. 2022, 23, 8438. [Google Scholar] [CrossRef]
- Tu, S.; Chi, N.C. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 2012, 84, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; Waxman, J.S. Atrial and sinoatrial node development in the zebrafish heart. J. Cardiovasc. Dev. Dis. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Z.; Pan, J.; Li, X.; Feng, J.; Yang, H. An integrated microfluidic array system for evaluating toxicity and teratogenicity of drugs on embryonic zebrafish developmental dynamics. Biomicrofluidics 2011, 5, 24115. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Ueyama, T.; Wakimoto, H.; Liu, M.K.; Maguire, C.T.; Converso, K.L.; Kang, P.M.; Manning, W.J.; Lawitts, J.; Paul, D.L.; et al. Nkx2.5 homeoprotein regulates expression of gap junction protein connexin 43 and sarcomere organization in postnatal cardiomyocytes. J. Mol. Cell Cardiol. 2003, 35, 243–256. [Google Scholar] [CrossRef]
- de Sena-Tomás, C.; Aleman, A.G.; Ford, C.; Varshney, A.; Yao, D.; Harrington, J.K.; Saúde, L.; Ramialison, M.; Targoff, K.L. Activation of nkx2.5 transcriptional program is required for adult myocardial repair. Nat. Commun. 2022, 13, 2970. [Google Scholar] [CrossRef]
- Tsuchihashi, T.; Maeda, J.; Shin, C.H.; Ivey, K.N.; Black, B.L.; Olson, E.N.; Yamagishi, H.; Srivastava, D. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev. Biol. 2011, 351, 62–69. [Google Scholar] [CrossRef]
- de Soysa, T.Y.; Ranade, S.S.; Okawa, S.; Ravichandran, S.; Huang, Y.; Salunga, H.T.; Schricker, A.; Del Sol, A.; Gifford, C.A.; Srivastava, D. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature 2019, 572, 120–124. [Google Scholar] [CrossRef]
- Garg, V.; Kathiriya, I.S.; Barnes, R.; Schluterman, M.K.; King, I.N.; Butler, C.A.; Rothrock, C.R.; Eapen, R.S.; Hirayama-Yamada, K.; Joo, K.; et al. Gata4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 2003, 424, 443–447. [Google Scholar] [CrossRef]
- Miyata, S.; Minobe, W.; Bristow, M.R.; Leinwand, L.A. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ. Res. 2000, 86, 386–390. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, J.; Lian, Z.; Jia, J.; Shen, J.; Li, Q.; Wong, J.M.J.; Jin, K.; Yan, W.; Ren, X.; et al. Modified mrna treatment restores cardiac function in desmocollin-2-deficient mouse models of arrhythmogenic right ventricular cardiomyopathy. Circulation 2025, 151, 1780–1796. [Google Scholar] [CrossRef]
- Ellertsdóttir, E.; Lenard, A.; Blum, Y.; Krudewig, A.; Herwig, L.; Affolter, M.; Belting, H.G. Vascular morphogenesis in the zebrafish embryo. Dev. Biol. 2010, 341, 56–65. [Google Scholar] [CrossRef]
- Hamm, M.J.; Kirchmaier, B.C.; Herzog, W. Sema3d controls collective endothelial cell migration by distinct mechanisms via Nrp1 and PlxnD1. J. Cell. Biol. 2016, 215, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Goi, M.; Childs, S.J. Patterning mechanisms of the sub-intestinal venous plexus in zebrafish. Dev. Biol. 2016, 409, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Salanga, M.C.; Meadows, S.M.; Myers, C.T.; Krieg, P.A. Ets family protein etv2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the xenopus embryo. Dev. Dyn. 2010, 239, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, K.L.; Goldie, L.C.; Hirschi, K.K. Regulation of endothelial cell differentiation and specification. Circ. Res. 2013, 112, 1272–1287. [Google Scholar] [CrossRef]
- Sumanas, S.; Lin, S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006, 4, e10. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. Vegf in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial vegf receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (vegf) and its receptor (vegfr) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, S.; Coşkun, Ö.; Günçer, B. Studying protein-protein interactions: Latest and most popular approaches. J. Struct. Biol. 2024, 216, 108118. [Google Scholar] [CrossRef]
- Sarkar, S.; Gulati, K.; Kairamkonda, M.; Mishra, A.; Poluri, K.M. Elucidating protein-protein interactions through computational approaches and designing small molecule inhibitors against them for various diseases. Curr. Top. Med. Chem. 2018, 18, 1719–1736. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Zhang, Z.; Xu, J.; Jiang, Y. Pik3cb promotes oesophageal cancer proliferation through the pi3k/akt/mtor signalling axis. Cell Biol. Int. 2022, 46, 1399–1408. [Google Scholar] [CrossRef]
- Pu, P.; Kang, C.; Zhang, Z.; Liu, X.; Jiang, H. Downregulation of pik3cb by sirna suppresses malignant glioma cell growth in vitro and in vivo. Technol. Cancer Res. Treat. 2006, 5, 271–280. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Fan, C.; Kong, D.; Feng, X.; Sun, C.; Xu, Y.; Li, B.; Jiang, Y.; Zheng, C. Pdcd4 interacting with pik3cb and ctsz promotes the apoptosis of multiple myeloma cells. FASEB J. 2024, 38, e70024. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, P.; von Gise, A.; Gu, F.; Ma, Q.; Chen, J.; Guo, H.; van Gorp, P.R.; Wang, D.Z.; Pu, W.T. Pi3kcb links hippo-yap and pi3k-akt signaling pathways to promote cardiomyocyte proliferation and survival. Circ. Res. 2015, 116, 35–45. [Google Scholar] [CrossRef]
- Tran, H.N.; Al Subhi, M.; Ward, N.; Nguyen, P.; Sultana, M.; Ananthapavan, J.; Brown, V. How much is invested in obesity prevention in Australia? An analysis of major research and federal government funding, 2013–2022. Public Health Res. Pract. 2024, 34, 3412404. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Gao, J.; Jin, W.; Hu, J.; Sun, Y.; Zhu, H.; Xu, G. Apoptosis, MAPK signaling pathway affected in tilapia liver following nano-microplastics and sulfamethoxazole acute co-exposure. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 53, 101370. [Google Scholar] [CrossRef]
- Guo, R.; Hua, Y.; Ren, J.; Bornfeldt, K.E.; Nair, S. Cardiomyocyte-specific disruption of cathepsin k protects against doxorubicin-induced cardiotoxicity. Cell Death Dis. 2018, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Samokhin, A.O.; Wong, A.; Saftig, P.; Brömme, D. Role of cathepsin k in structural changes in brachiocephalic artery during progression of atherosclerosis in apoe-deficient mice. Atherosclerosis 2008, 200, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, C.; Chen, H.; Ren, M.; Liu, X. Cathepsins trigger cell death and regulate radioresistance in glioblastoma. Cells 2022, 11, 4108. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Formentini, L.; Gerace, E.; Camaioni, E.; Pellegrini-Giampietro, D.E.; Chiarugi, A.; Pellicciari, R. Selective parp-2 inhibitors increase apoptosis in hippocampal slices but protect cortical cells in models of post-ischaemic brain damage. Br. J. Pharmacol. 2009, 157, 854–862. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ma, W.; Fei, T.; He, C.; Pang, S. Tri-explosophoric groups driven fused energetic heterocycles featuring superior energetic and safety performances outperforms HMX. Nat. Commun. 2022, 13, 5697. [Google Scholar] [CrossRef]
- El-Ghobashy, N.M.; El-Sayed, S.M.; Shehata, I.A.; El-Ashmawy, M.B. Synthesis, biological evaluation, and molecular modeling studies of new benzoxazole derivatives as parp-2 inhibitors targeting breast cancer. Sci. Rep. 2022, 12, 16246. [Google Scholar] [CrossRef]
- Liao, X.; Zou, T.; Chen, M.; Song, Y.; Yang, C.; Qiu, B.; Chen, Z.F.; Tsang, S.Y.; Qi, Z.; Cai, Z. Contamination profiles and health impact of benzothiazole and its derivatives in pm(2.5) in typical Chinese cities. Sci. Total Environ. 2021, 755, 142617. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.N.; Wang, K.; Zhang, L.; Huang, Z.; Liu, J.; Zhang, Z.; Luo, M.; Lei, Y.; Peng, Y.; et al. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J. Hepatol. 2019, 70, 66–77. [Google Scholar] [CrossRef]
- Cai, Z.L.; Shen, B.; Yuan, Y.; Liu, C.; Xie, Q.W.; Hu, T.T.; Yao, Q.; Wu, Q.Q.; Tang, Q.Z. The effect of hmga1 in lps-induced myocardial inflammation. Int. J. Biol. Sci. 2020, 16, 1798–1810. [Google Scholar] [CrossRef]
- Hammond, B.G.; Barbee, S.J.; Wheeler, A.G.; Cascieri, T. Absence of mutagenic activity for monosodium cyanurate. Fundam. Appl. Toxicol. 1985, 5, 655–664. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, H.; Hua, L.; Hou, C.; Jia, Q.; Chen, J.; Zhang, S.; Wang, Y.; He, S.; Jia, E. Verification of ferroptosis and pyroptosis and identification of ptgs2 as the hub gene in human coronary artery atherosclerosis. Free Radic. Biol. Med. 2021, 171, 55–68. [Google Scholar] [CrossRef]

| Accession Number | Forward | Reverse | |
|---|---|---|---|
| NM_181601.5 | β-actin | 5′-ACAGGGAAAAGATGACACAGATCA-3′ | 5′-CAGCCTGGATGGCAACGTA-3′ |
| NC_133189.1 | nkx2.5 | 5′-ACAAACATCACTTTGCCGCC-3′ | 5′-ATGCCCGACTGTTAAGGCTC-3′ |
| NM_131329 | myl7 | 5′-TGAGCAATCACAAATACAGG-3′ | 5′-GCAGCAGTTACAGACAGAATA-3′ |
| NM_131626.3 | hand2 | 5′-ACGTCGACAGGGATTGGAAC-3′ | 5′-CAGACACCAACTGTCTCCCC-3′ |
| NM_131236 | gata4 | 5′-TCCAGGCGGGTGGGTTTATC-3′ | 5′-TGTCTGGTTCAGTCTTGATGGGTC-3′ |
| NM_001037375.1 | etsrp | 5′-GCAGTGGTGAAGACCTGTCC-3′ | 5′-CAGCCATCACCAGTCCAACT-3′ |
| NM_130945.2 | flt4 | 5′-ACCACTATCCCTGAAGGATTCG-3′ | 5′-GACAGCCACTGTGTTTGCAG-3′ |
| NC_133191.1 | vegfα | 5′-ATGAGAACCACACAGGACGG-3′ | 5′-ACACTCTCGCTTTGCTTCCT-3′ |
| NM_001024653.2 | kdr | 5′-TGAGAAGGACGTGTCGTTGG-3′ | 5′-CGTCCACAGAGATACGAGCC-3′ |
| NM_153657.1 | ptgs2 | 5′-GCTACAAAAGCTGGGAAGCG-3′ | 5′-GGTGAAGGCTGGTCCCTTTT-3′ |
| XM_005157466.6 | pik3cb | 5′-TGTGTTCTCTGCCATGCCTC-3′ | 5′-ACTGGTCGTAGTGCATGGTG-3′ |
| NC_133191.1 | ctsk | 5′-GACCCGTGTCAGTTGGCATA-3′ | 5′-ACCCCAGCTTCAAAAACAGAAA-3′ |
| NM_001204270.1 | parp2 | 5′-CCCCAGTTGTGCTCTGTGAT-3′ | 5′-CAGGGGCTTTGCCCTTCATA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Gu, J.; Ye, F.; Guo, L. 2-(Methylthio) Benzothiazole (MTBT) Induces Cardiovascular Toxicity in Zebrafish Larvae and Investigates Its Mechanism. Biology 2025, 14, 1398. https://doi.org/10.3390/biology14101398
Wang Y, Wang J, Gu J, Ye F, Guo L. 2-(Methylthio) Benzothiazole (MTBT) Induces Cardiovascular Toxicity in Zebrafish Larvae and Investigates Its Mechanism. Biology. 2025; 14(10):1398. https://doi.org/10.3390/biology14101398
Chicago/Turabian StyleWang, Yidi, Junjie Wang, Jie Gu, Fei Ye, and Liguo Guo. 2025. "2-(Methylthio) Benzothiazole (MTBT) Induces Cardiovascular Toxicity in Zebrafish Larvae and Investigates Its Mechanism" Biology 14, no. 10: 1398. https://doi.org/10.3390/biology14101398
APA StyleWang, Y., Wang, J., Gu, J., Ye, F., & Guo, L. (2025). 2-(Methylthio) Benzothiazole (MTBT) Induces Cardiovascular Toxicity in Zebrafish Larvae and Investigates Its Mechanism. Biology, 14(10), 1398. https://doi.org/10.3390/biology14101398






