Phytochemicals: Principles and Practice
Simple Summary
Abstract
1. Introduction
1.1. Global Healthcare Challenges and Potential Phytochemical Solutions
1.2. Botanical Functions and Distribution of Phytochemicals
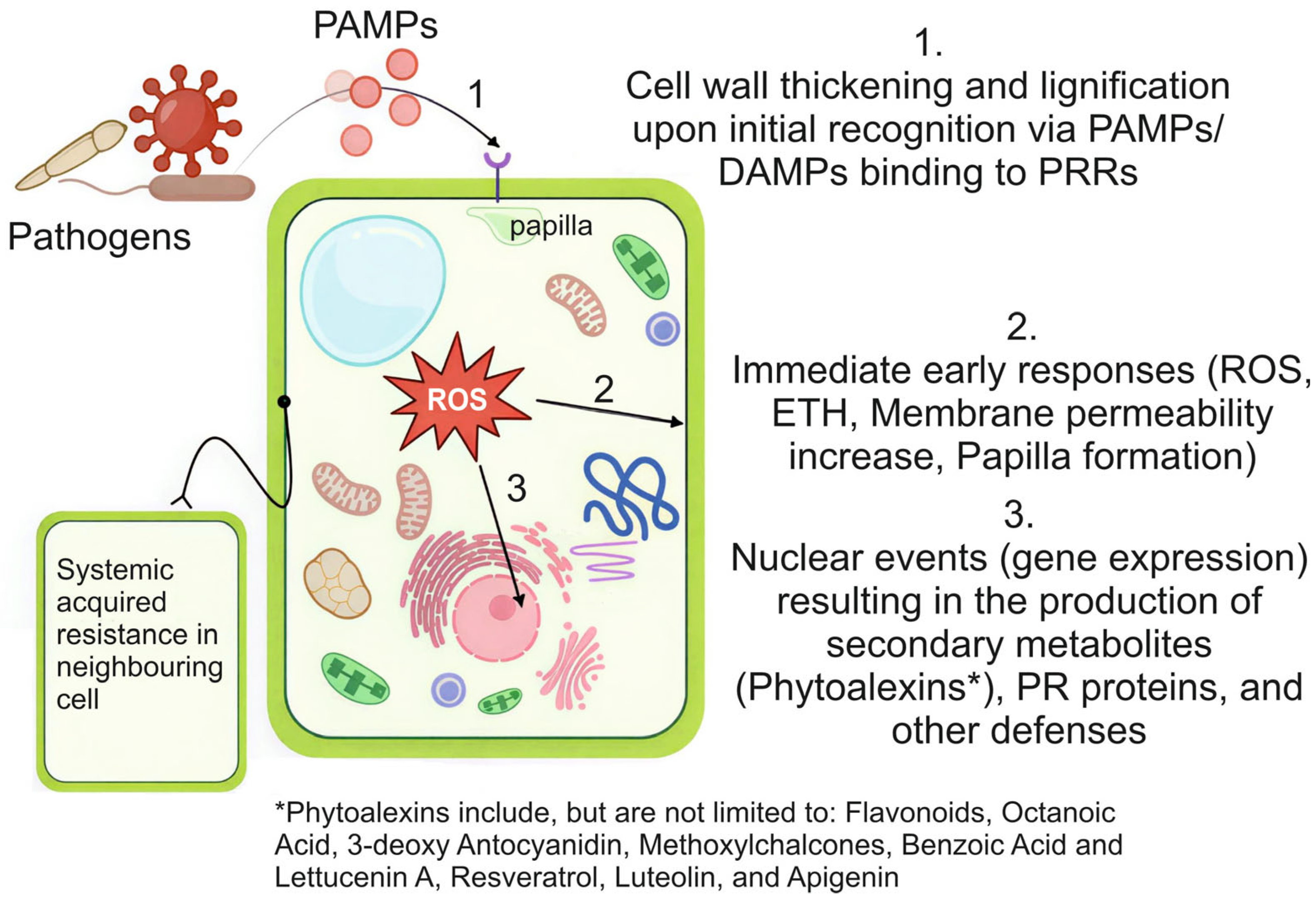
1.2.1. Plant Defence Mechanisms and Pathways
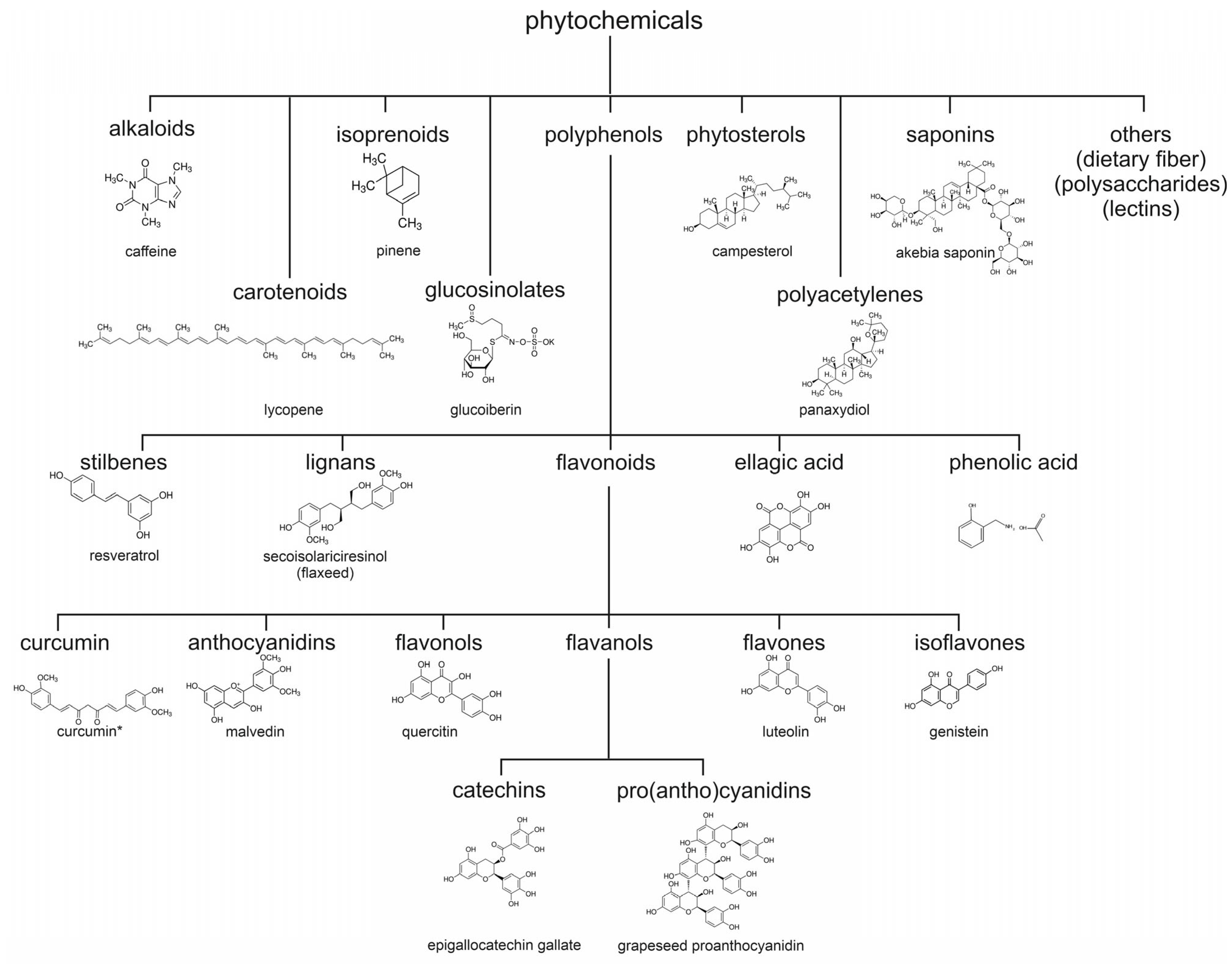
1.2.2. Structural Classification and Diversity of Phytochemicals
| Plant Species | Health Benefit | Active Phytochemical | GI Metabolite | References |
|---|---|---|---|---|
| Artemisia annua (Artemisia) | Antimalarial | Artemisinin | Dihydroartemisinin | [20] |
| Curcuma longa (Turmeric) | Anti-inflammatory | Curcumin | Tetrahydrocurcumin | [40] |
| Camellia sinensis (Green tea) | Cardioprotective | EGCG | 5-(3′,4′,5′-Trihydroxy-phenyl)-γ-valerolactone | [65] |
| Allium sativum (Garlic) | Antimicrobial | Allicin | Allyl methyl sulfide | [66] |
| Vitis vinifera (Grape) | Antioxidant | Resveratrol | Dihydroresveratrol | [67] |
| Zingiber officinale (Ginger) | Anti-nausea | 6-Gingerol | 6-gingerol glucuronide | [68] |
| Panax ginseng (Ginseng) | Adaptogenic | Ginsenosides | Compound K | [69] |
| Echinacea purpurea (Echinacea) | Immune stimulation | Alkamides | Tetradeca-8E-10E- dienoic acid isobutylamide | [70] |
| Silybum marianum (Milk thistle) | Hepatoprotective | Silymarin | Sulfated conjugates | [71,72] |
| Piper nigrum (Black pepper) | Bioenhancer | Piperine | Piperonylic acid | [73] |
2. Principles of Phytochemicals
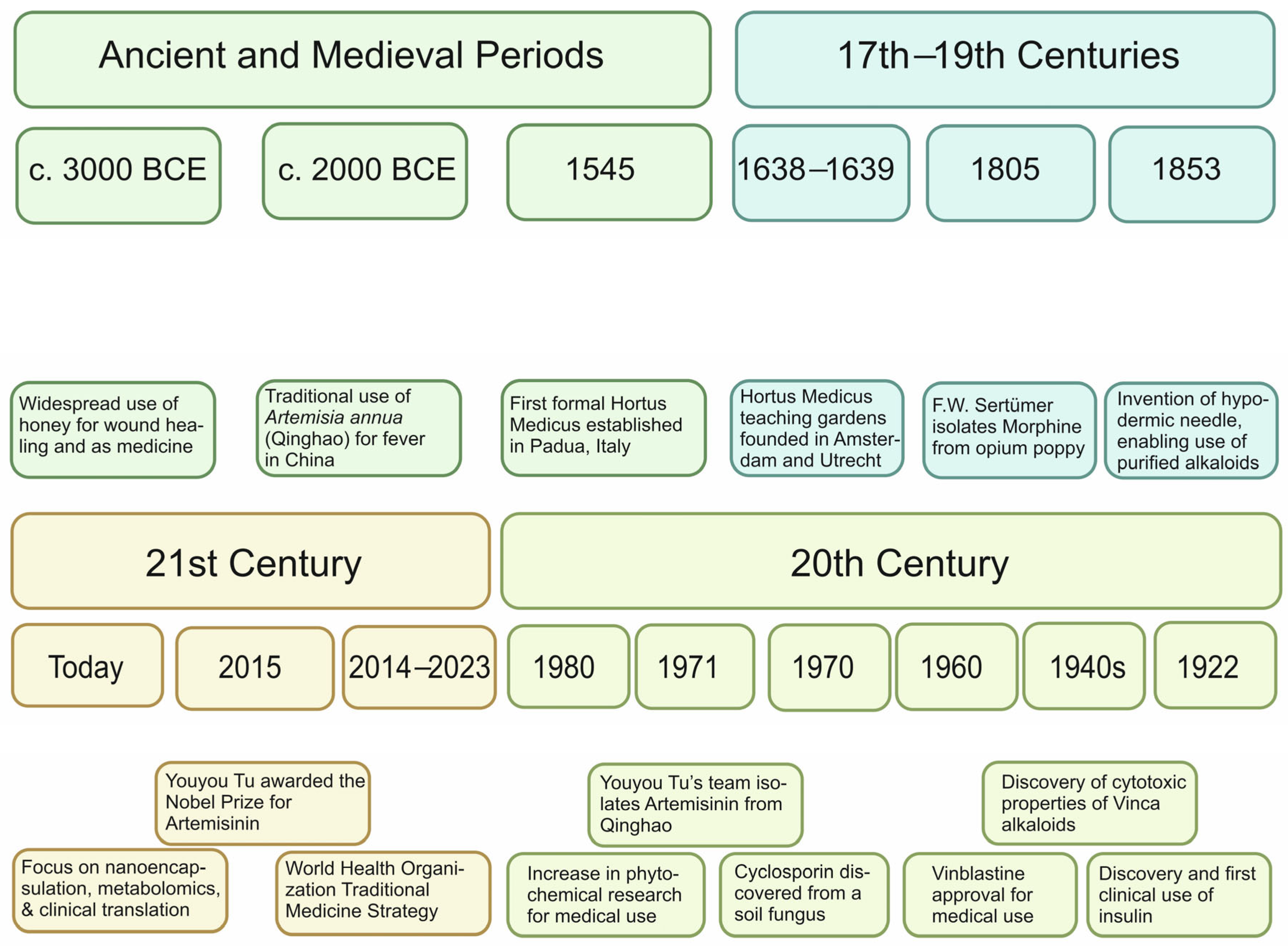
2.1. Foundations and Evolution of Phytochemistry Science
2.2. Extraction Methods and Technological Advances
| Phytochemical | Plant Sources | Extraction Method(s) | Bioavailability Profile |
|---|---|---|---|
| Curcumin | Curcuma longa | Solvent Extraction, MAE 1, SFE | Low; enhanced by nanoencapsulation and piperine co-administration |
| Resveratrol | Grapes, Berries | Solvent Extraction, SFE | Moderate; rapid metabolism limits bioactivity |
| Quercetin | Onions, Apples | Solvent Extraction, UAE | Low; increased when consumed with dietary fats |
| Catechins | Green Tea | Solvent Extraction, MAE | Moderate; stable in aqueous form, affected by intestinal metabolism |
| Lycopene | Tomatoes | Solvent Extraction, SFE | Low; better absorbed when cooked with oil |
| Genistein | Soybeans | Solvent Extraction, Enzyme-Assisted Extraction | Variable; metabolized by gut flora, enhanced by fermented products |
| Silymarin | Milk Thistle | Solvent Extraction | Poor; improved with phospholipid complexes |
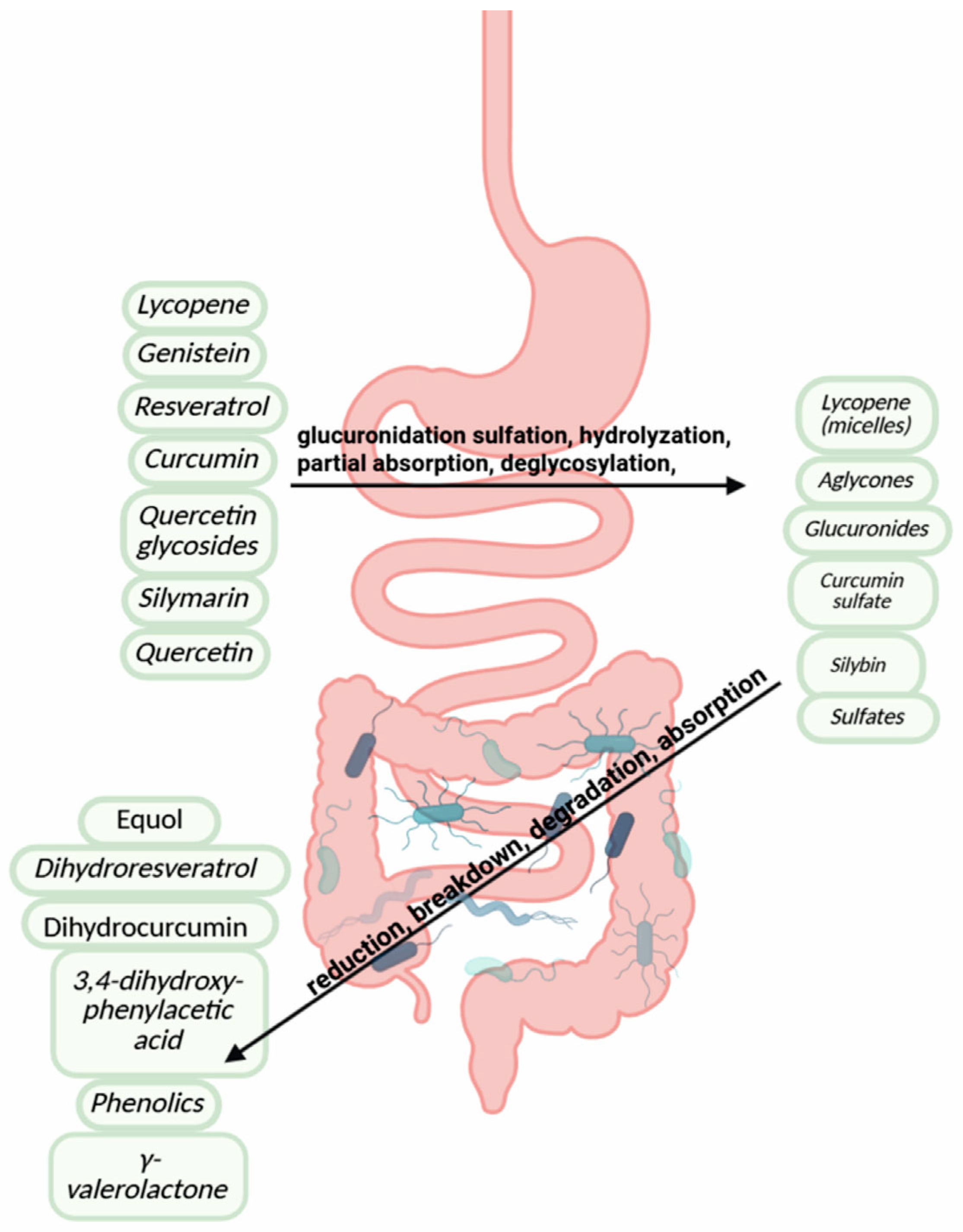
2.3. Metabolism, Bioavailability, and Clinical Translation
| Region | Phytochemicals and Metabolism | References |
|---|---|---|
| Stomach | Lycopene is released from the food matrix due to low pH and emulsified for micelle formation, facilitating absorption in the small intestine. | [102] |
| Small Intestine | Curcumin undergoes extensive first-pass metabolism via glucuronidation and sulfation, limiting systemic availability. | [56,103] |
| Resveratrol is absorbed here and conjugated into glucuronides and sulfates. Bioavailability of resveratrol is limited by rapid metabolism. | [90,104] | |
| Quercetin glycosides are hydrolyzed by enzymes like LPH 1 and cBG into aglycones that passively diffuse through enterocytes. | [91] | |
| Catechins such as EGCG are partially absorbed; unabsorbed catechins reach the colon. | [90,91,92,93,94,95,96,102,104,105,106,107] | |
| Genistein is deglycosylated into aglycones by intestinal enzymes before absorption. | [92,108] | |
| Silymarin components like silybin are absorbed inefficiently due to poor water solubility; partial metabolism occurs in enterocytes. | [109,110] | |
| Colon (Large Intestine) | Curcumin that escapes upper GI absorption is further reduced and degraded by gut microbes. | [56,111] |
| Resveratrol is converted by microbiota into dihydroresveratrol and other phenolics. | [112] | |
| Quercetin is broken down into smaller phenolic acids such as 3,4-dihydroxyphenylacetic acid by colonic bacteria. | [113,114] | |
| Catechins are degraded to γ-valerolactones like DHPV by gut microbes as well as intestinal enzymes (e.g., Flavonifractor plautii). | [98] | |
| Genistein is metabolized into equol, a more estrogenic metabolite, by bacteria like Slackia isoflavoniconvertens (only in equol producers). | [92] | |
| Silymarin constituents undergo microbial breakdown into less active phenolics and acids. | [109,110] |
3. Practice of Phytochemicals
3.1. Biomedical Applications: From Molecular Mechanisms to Global Health Impact
| Phytochemical | Role in Plant/Biosynthesis | Human Medicinal Application |
|---|---|---|
| Curcumin | Produced by Curcuma longa in response to biotic stress; antimicrobial and wound-healing defence | Anti-inflammatory, anticancer (inhibits NF-κB), antioxidant, used in trials for prostate, colorectal, and breast cancers [21,131] |
| EGCG 1 | Flavonoid in green tea; deters herbivores, protects against UV damage | Cardiovascular protection, neuroprotection, weight loss support, activates AMPK, antioxidant and anti-inflammatory [132,133] |
| Sulforaphane | Formed upon tissue damage in cruciferous vegetables; part of plant defence enzyme system | Induces phase II antioxidant [134] enzymes, epigenetic modulation [135], potential anticancer and neuroprotective roles [136] |
| Myricetin | Plant-derived flavonol; acts in UV protection and defence against microbes | Synergizes with antibiotics [137], breaks biofilm [138], used against multidrug-resistant Pseudomonas aeruginosa [139] |
| Resveratrol | Stilbene synthesized under pathogen attack or UV stress in grapes | Cardioprotective [140], neuroprotective [141], anti-ageing via SIRT1 activation [142], used in metabolic [143] and cognitive disorder trials [142] |
| Anthocyanins | Pigments for pollinator attraction and ROS scavenging in high-light environments | Antihypertensive [144], reduce LDL oxidation [108], improve vascular elasticity [145], improve cognitive function [146] |
| Flavonoids | Broad-spectrum metabolites defending against pathogens, UV, and oxidative stress | Anti-inflammatory [147], reduce CVD risk [148], modulate cytokines and endothelial markers like ICAM/VCAM [149] |
| Polyphenols | Defensive compounds for herbivore deterrence and pathogen resistance | Improve endothelial function [150], reduce oxidative stress [151], support microbiota balance [152], prevent metabolic syndrome [151] |
| Hydroxycitric acid | Derived from Garcinia cambogia rind; involved in regulation of seed germination and storage metabolism | Anti-obesity, appetite suppressant via ATP-citrate lyase inhibition, reduces fat accumulation [153] |
| Gingerol | Phenolic compound in ginger for antimicrobial defence | Antidiabetic, anti-inflammatory, improves insulin sensitivity and digestive function [154] |
| Berberine | Isoquinoline alkaloid involved in allelopathy and microbial inhibition in Berberis spp. | Improves glucose metabolism, lowers LDL-C, modulates gut microbiota, used in diabetes and metabolic syndrome [155] |
| Asiatic acid | Triterpenoid in Bacopa monnieri, involved in defence signalling and repair processes | Neuroprotective potential [156,157] |
| Loliolide | Monoterpenoid lactone from many plants; signals stress and regulates interplant interactions | Antioxidant and potential anti-ageing [158], neuroprotective properties [159] |
3.2. Dietary Synergies and Precision Delivery: Beyond Quantity
3.3. Policy Integration and Sustainable Implementation
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OMRU | Ottawa Model of Research Use |
| GC | Gas chromatography |
| MS | Mass spectrometry |
| PAL | Phenylalanine ammonia-lyase |
| CHS | Duplicated chalcone synthase |
| LDL | Low-density lipoprotein |
| PRRs | Pattern recognition receptors |
| PAMPs | Pathogen-associated molecular patterns |
| ROS | Reactive oxygen species |
| PR | Pathogenesis-Related |
| EGCG | Epigallocatechin-3-gallate |
| NMR | Nuclear magnetic resonance |
| HPLC | High-performance liquid chromatography |
| UPLC | Ultra-performance liquid chromatography |
| MAE | Microwave-assisted extraction |
| SFE | Supercritical fluid extraction |
| UAE | Ultrasound-assisted extraction |
| GI | Gastrointestinal |
| DHPV | 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone |
| GMM | Gut microbial metabolites |
| WHO | World Health Organization |
| CVD | Cardiovascular Disease |
| NCD | Noncommunicable diseases |
| TCM | Traditional and complementary medicine |
| LPH/cBG | Lactase-phlorizin hydrolase/cytosolic β-glucosidase |
| NfkB | Nuclear factor kappa-light-chain-enhancer of activated B-cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| IL-6 | Interleukin-6 |
| DAMPs | Danger-associated molecular patterns |
References
- Glazenburg, J. Rembrandt’s anatomy of Dr. Nicolaas Tulp. Arch. Chir. Neerl. 1977, 29, 83–91. [Google Scholar] [PubMed]
- Baljet, B.; Bouman, F. The Hortus Medicus in Amsterdam (1638–1665) and the catalogue of Johannes Snippendael. Stud. Hist. Gard. Des. Landsc. 2015, 35, 246–256. [Google Scholar] [CrossRef]
- Rhodes, D. The Botanical Garden of Padua. The First Hundred Years. J. Gard. Hist. 1984, IV, 327–331. [Google Scholar] [CrossRef]
- Mahara, G.; Tian, C.; Xu, X.; Zhu, J. Breakthrough of glycobiology in the 21st century. Front. Immunol. 2023, 13, 1071360. [Google Scholar] [CrossRef]
- Santesso, N.; Tugwell, P. Knowledge translation in developing countries. J. Contin. Educ. Health Prof. 2006, 26, 87–96. [Google Scholar] [CrossRef]
- Coburn, J.; Yaqub, O.; Chataway, J. Targeting research to address societal needs: What can we learn from 30 years of targeting neglected diseases? In Handbook of Public Funding of Research, 1st ed.; Lepori, B., Jongbloed, B., Hicks, D., Eds.; Edward Elgar Publishing: Cheltenham, UK, 2023; pp. 156–171. [Google Scholar]
- Schmallenbach, L.; Bley, M.; Bärnighausen, T.W.; Sugimoto, C.R.; Lerchenmüller, C.; Lerchenmueller, M.J. Global distribution of research efforts, disease burden, and impact of US public funding withdrawal. Nat. Med. 2025, 31, 3101–3109. [Google Scholar] [CrossRef]
- Ridic, G.; Gleason, S.; Ridic, O. Comparisons of health care systems in the United States, Germany and Canada. Mater. Sociomed. 2012, 24, 112–120. [Google Scholar] [CrossRef]
- Kohane, I.S.; Drazen, J.M.; Campion, E.W. A glimpse of the next 100 years in medicine. N. Engl. J. Med. 2012, 367, 2538–2539. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Richards, N.; Harrison, J.; Barnes, J. Prevalence of Use of Traditional, Complementary and Alternative Medicine by the General Population: A Systematic Review of National Studies Published from 2010 to 2019. Drug Saf. 2022, 45, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hansen, L.G.; Gudich, O.; Viehrig, K.; Lassen, L.M.M.; Schrübbers, L.; Adhikari, K.B.; Rubaszka, P.; Carrasquer-Alvarez, E.; Chen, L.; et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature 2022, 609, 341–347. [Google Scholar] [CrossRef]
- Wang, X.; Ma, T.; Zhang, W.; Chu, Q. Effectiveness and safety research of Qingfei Paidu (QFPD) in treatment of COVID-19: An up-to-date systematic review and meta-analysis. Chin. Med. 2022, 17, 122. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wu, W.Y.; Hou, J.J.; Zhang, L.L.; Li, F.F.; Gao, L.; Wu, X.D.; Shi, J.Y.; Zhang, R.; Long, H.L.; et al. Active constituents and mechanisms of Respiratory Detox Shot, a traditional Chinese medicine prescription, for COVID-19 control and prevention: Network-molecular docking-LC-MSE analysis. J. Integr. Med. 2020, 18, 229–241. [Google Scholar] [CrossRef]
- Topliss, J.G.; Clark, A.M.; Ernst, E.; Hufford, C.D.; Johnston, G.A.R.; Rimoldi, J.M.; Weimann, B.J. Natural and synthetic substances related to human health (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 1957–1985. [Google Scholar] [CrossRef]
- Borel, J.F. Ciclosporin and its future. Prog. Allergy 1986, 38, 9–18. [Google Scholar] [PubMed]
- Noble, R.L. The discovery of the vinca alkaloids--chemotherapeutic agents against cancer. Biochem. Cell. Biol. 1990, 68, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Jabali, S.H.; Yazdani, S.; Pourasghari, H.; Maleki, M. From bench to policy: A critical analysis of models for evidence-informed policymaking in healthcare. Front. Public Health 2024, 12, 1264315. [Google Scholar] [CrossRef]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.; Graham, I.D. Toward a Comprehensive Interdisciplinary Model of Health Care Research Use. Sci. Comm. 1998, 20, 227–246. [Google Scholar] [CrossRef]
- Park, J.Y. Artemisinin and the Nobel Prize in physiology or medicine 2015. Korean J. Pain. 2019, 32, 145–146. [Google Scholar] [CrossRef]
- Shahnawaz, M.K.; Shubham, P.S.; Gupta, M.; Kumar, A. A Comprehensive Review of Phytochemicals: Health Benefits and Therapeutic Applications. Intl. J. Pharm. Res. Appl. 2024, 9, 1373–1379. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, W. Health Benefits and Future Research of Phytochemicals: A Literature Review. J. Nutr. 2025, 155, 87–101. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Grel, A.; Akta, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Commisso, M.; Strazzer, P.; Toffali, K.; Stocchero, M.; Guzzo, F. Untargeted metabolomics: An emerging approach to determine the composition of herbal products. Comput. Struct. Biotechnol. J. 2013, 4, e201301007. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.X.; Sanggaard, K.W.; Kreuzer, I.; Knudsen, A.D.; Bemm, F.; Thøgersen, I.B.; Bräutigam, A.; Thomsen, L.R.; Schliesky, S.; Dyrlund, T.F.; et al. The protein composition of the digestive fluid from the venus flytrap sheds light on prey digestion mechanisms. Mol. Cell. Proteom. 2012, 11, 1306–1319. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, Y.; Lee, Y.; Kwon, O. Diverse and Synergistic Actions of Phytochemicals in a Plant-Based Multivitamin/Mineral Supplement against Oxidative Stress and Inflammation in Healthy Individuals: A Systems Biology Approach Based on a Randomized Clinical Trial. Antioxidants 2023, 13, 36. [Google Scholar] [CrossRef]
- Vanden Berghe, W.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef]
- ter Kuile, F.; White, N.J.; Holloway, P.; Pasvol, G.; Krishna, S. Plasmodium falciparum: In vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 1993, 76, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Xiang, L.; Han, X.; Zhang, Y.; Lyu, R.; Yuan, L.; Chen, S. The evolutionary advantage of artemisinin production by Artemisia annua. Trends Plant Sci. 2025, 30, 213–226. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Petrén, H.; Anaia, R.A.; Aragam, K.S.; Bräutigam, A.; Eckert, S.; Heinen, R.; Jakobs, R.; Ojeda-Prieto, L.; Popp, M.; Sasidharan, R.; et al. Understanding the chemodiversity of plants: Quantification, variation and ecological function. Ecolog. Monogr. 2024, 94, e1635. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Pawase, P.A.; Goswami, C.; Shams, R.; Pandey, V.K.; Tripathi, A.; Rustagi, S.; Darshan, G. A conceptual review on classification, extraction, bioactive potential and role of phytochemicals in human health. Future Foods 2024, 9, 100313. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Chu, Y.C.; Huang, C.C. Role of Lutein Supplements in the Management of Dry Eye Syndrome: A Systematic Review. Int. J. Vitam. Nutr. Res. 2024, 95, 36626. [Google Scholar] [CrossRef]
- Petti, C. Low Caffeine Concentrations Induce Callus and Direct Organogenesis in Tissue Cultures of Ornithogalum dubium. Plants 2025, 14, 1127. [Google Scholar] [CrossRef]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Calderone, V. Pharmacological modulation of the hydrogen sulfide (H2S) system by dietary H2S-donors: A novel promising strategy in the prevention and treatment of type 2 diabetes mellitus. Phytother. Res. 2021, 35, 1817–1846. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, Y.; Feng, J.; Wang, Y.; Lu, Y.; Chen, X. Tetrahydrocurcumin as a stable and highly active curcumin derivative: A review of synthesis, bioconversion, detection and application. Food Biosci. 2023, 53, 102591. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Barkas, F.; Bathrellou, E.; Nomikos, T.; Panagiotakos, D.; Liberopoulos, E.; Kontogianni, M.D. Plant Sterols and Plant Stanols in Cholesterol Management and Cardiovascular Prevention. Nutrients 2023, 15, 2845. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry 2022, 201, 113288. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, R.; Chen, J.; Li, S.; Huang, K.; Cao, H.; Farag, M.A.; Battino, M.; Daglia, M.; Capanoglu, E.; et al. Health benefits of saponins and its mechanisms: Perspectives from absorption, metabolism, and interaction with gut. Crit. Rev. Food Sci. Nutr. 2024, 64, 9311–9332. [Google Scholar] [CrossRef]
- Veronese, N.; Gianfredi, V.; Solmi, M.; Barbagallo, M.; Dominguez, L.J.; Mandalà, C.; Di Palermo, C.; Carruba, L.; Solimando, L.; Stubbs, B.; et al. The impact of dietary fiber consumption on human health: An umbrella review of evidence from 17,155,277 individuals. Clin. Nutr. 2025, 51, 325–333. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef]
- Plaha, N.S.; Awasthi, S.; Sharma, A.; Kaushik, N. Distribution, biosynthesis and therapeutic potential of lignans. 3 Biotech. 2022, 12, 255. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Bartoszek, A. Relationship between Chemical Structure and Biological Activity Evaluated In Vitro for Six Anthocyanidins Most Commonly Occurring in Edible Plants. Molecules 2023, 28, 6156. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological Applications and Health-Promoting Properties of Flavonols: An Updated View. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, D.; Kłósek, M.; Bronikowska, J.; Krawczyk-Łebek, A.; Perz, M.; Kostrzewa-Susłow, E.; Czuba, Z.P. Methyl Derivatives of Flavone as Potential Anti-Inflammatory Compounds. Int. J. Mol. Sci. 2025, 26, 729. [Google Scholar] [CrossRef]
- Ul Hassan, M.H.; Shahbaz, M.; Imran, M.; Momal, U.; Naeem, H.; Mujtaba, A.; Hussain, M.; Anwar, M.J.; Alsagaby, S.A.; Al Abdulmonem, W.; et al. Isoflavones: Promising Natural Agent for Cancer Prevention and Treatment. Food Sci. Nutr. 2025, 13, e70091. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Amiot, M.J.; Latgé, C.; Plumey, L.; Raynal, S. Intake Estimation of Phytochemicals in a French Well-Balanced Diet. Nutrients 2021, 13, 3628. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Maruyama, K.; Kihara-Negishi, F.; Ohkura, N.; Nakamura, Y.; Nasui, M.; Saito, M. Simultaneous Determination of Catechins and Caffeine in Green Tea-Based Beverages and Foods for Specified Health Uses. Food Nutr. Sci. 2017, 8, 316–325. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: Review of current evidence. Int. J. Prev. Med. 2013, 4, S36–S42. [Google Scholar] [PubMed]
- Wu, L.; Bae, J.; Kraus, G.; Wurtele, E.S. Diacetylenic isobutylamides of Echinacea: Synthesis and natural distribution. Phytochemistry 2004, 65, 2477–2484. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, Transport and Drug-Drug Interactions of Silymarin. Molecules 2019, 24, 3693. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Cirilli, I.; Marcheggiani, F.; Silvestri, S.; Orlando, P.; Muvhulawa, N.; Moetlediwa, M.T.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hlengwa, N.; et al. Bioactive Properties, Bioavailability Profiles, and Clinical Evidence of the Potential Benefits of Black Pepper (Piper nigrum) and Red Pepper (Capsicum annum) against Diverse Metabolic Complications. Molecules 2023, 28, 6569. [Google Scholar] [CrossRef]
- Egbuna, C.; Ifemeje, J.; Kryeziu, T.; Mukherjee, M.; Narasimha, R.; Gido, L.; Tijjani, H. Introduction to phytochemistry. In Phytochemistry, Volume 1 Fundamentals, Modern Techniques, and Applications; Egbuna, C., Ifemeje, J., Udedi, S., Kumar, S., Eds.; Apple Academic Press: Oakville, ON, Canada, 2018; pp. 1–29. [Google Scholar] [CrossRef]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Uhl, A.; Knierim, L.; Höß, T.; Flemming, M.; Schmidt, A.; Strube, J. Autonomous Hydrodistillation with a Digital Twin for Efficient and Climate Neutral Manufacturing of Phytochemicals. Processes 2024, 12, 217. [Google Scholar] [CrossRef]
- Seyyedi-Mansour, S.; Donn, P.; Carpena, M.; Chamorro, F.; Barciela, P.; Perez-Vazquez, A.; Jorge, A.; Prieto, M.A. Utilization of Ultrasonic-Assisted Extraction for Bioactive Compounds from Floral Sources. Biol. Life Sci. Forum 2024, 40, 15. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Gupta, C. Bioactive phytochemicals on humanoid endurance & advancement of herbal medicine. J. Microbial. Exp. 2023, 11, 125–135. [Google Scholar] [CrossRef]
- Carev, I.; Maravić, A.; Ilić, N.; Čikeš Čulić, V.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Aadil, R.M.; Kurup, S.S.; Maqsood, S. Comparative evaluation of ultrasound-assisted extraction with other green extraction methods for sustainable recycling and processing of date palm bioresources and by-products: A review of recent research. Ultrason. Sonochem. 2025, 114, 107252. [Google Scholar] [CrossRef] [PubMed]
- Jahnavi, P.; Indirabanu, S.; Chellappan, R.D.; Mubeen, M.I.; Bodapati, A.; Dharmamoorthy, G.; Pandiyan, B.; Teja Kumar Reddy, K. Supercritical Fluid Extraction: A Green and Sustainable Approach for the Isolation of High-Value Compounds from Natural Sources. Asian J. Green Chem. 2025, 9, 605–629. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Robledo, P.; Tanner, J.A.; Boronat, A.; Pérez-Mañá, C.; Oliver Chen, C.Y.; Tyndale, R.F.; de la Torre, R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017, 217, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Rodriguez-Morató, J.; Serreli, G.; Fitó, M.; Tyndale, R.F.; Deiana, M.; de la Torre, R. Contribution of Biotransformations Carried Out by the Microbiota, Drug-Metabolizing Enzymes, and Transport Proteins to the Biological Activities of Phytochemicals Found in the Diet. Adv. Nutr. 2021, 12, 2172–2189. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; de Jesús Ornelas-Paz, J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Ibarra-Junquera, V.; Yahia, E.M.; Gardea-Béjar, A.A. Effects of Pectin on Lipid Digestion and Possible Implications for Carotenoid Bioavailability during Pre-Absorptive Stages: A Review. Food Res. Int. 2017, 99, 917–927. [Google Scholar] [CrossRef]
- Low, D.Y.; D’Arcy, B.; Gidley, M.J. Mastication Effects on Carotenoid Bioaccessibility from Mango Fruit Tissue. Food. Res. Int. 2015, 67, 238–246. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cermak, R.; Landgraf, S.; Wolffram, S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Crespy, V.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998, 426, 331–336. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef]
- Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Catechin Bioavailability Following Consumption of a Green Tea Extract Confection Is Reduced in Obese Persons without Affecting Gut Microbial-Derived Valerolactones. Antioxidants 2022, 11, 2490. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Vélez, L.A.; Delgado, Y.; Ferrer-Acosta, Y.; Suárez-Arroyo, I.J.; Rodríguez, P.; Pérez, D. Theoretical Prediction of Gastrointestinal Absorption of Phytochemicals. Int. J. Plant Biol. 2022, 13, 163–179. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Bond, T.; Derbyshire, E. Tea Compounds and the Gut Microbiome: Findings from Trials and Mechanistic Studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Hullar, M.A.; Lancaster, S.M.; Li, F.; Tseng, E.; Beer, K.; Atkinson, C.; Wähälä, K.; Copeland, W.K.; Randolph, T.W.; Newton, K.M.; et al. Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the United States. Cancer Epidemiol. Biomark. Prev. 2015, 24, 546–554. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Crespy, V.; Oliveira, M. (+)-Catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res. 2006, 40, 1029–1034. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic Review of Anthocyanins and Markers of Cardiovascular Disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef]
- Křen, V.; Walterova, D. Silybin and silymarin—New effects and applications. Biomed Pap. 2005, 149, 29–41. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Czuczwar, S.J. Post-Ischemic Brain Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Possible Therapeutic Role of Curcumin. Nutrients 2022, 14, 248. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Liu, L.; Barber, E.; Kellow, N.J.; Williamson, G. Improving quercetin bioavailability: A systematic review and meta-analysis of human intervention studies. Food Chem. 2025, 477, 143630. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Mena, P.; Bub, A.; Ulaszewska, M.; Del Rio, D.; Kulling, S.E.; Mattivi, F. Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples. Metabolites 2019, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; He, D.; Gao, S.; Tao, X.; Dong, X. Pharmacokinetics in Pharmacometabolomics: Towards Personalized Medication. Pharmaceuticals 2023, 16, 1568. [Google Scholar] [CrossRef]
- Vuorelaa, P.; Leinonenb, M.; Saikkuc, P.; Tammelaa, P.; Rauhad, J.P.; Wennberge, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Amirkia, V.; Heinrich, M. Natural products and drug discovery: A survey of stakeholders in industry and academia. Front. Pharmacol. 2015, 6, 237. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Zhao, Y.; Peng, R.; Zhang, Z.; Xu, Z.; Simal-Gandara, J.; Yang, H.; Deng, J. Bioactive sulforaphane from cruciferous vegetables: Advances in biosynthesis, metabolism, bioavailability, delivery, health benefits, and applications. Crit. Rev. Food Sci. Nutr. 2025, 65, 3027–3047. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, Y.; Yang, W.; Li, Y.; Wang, C.; Gao, K. The covalent modification of STAT1 cysteines by sulforaphane promotes antitumor immunity via blocking IFN-γ-induced PD-L1 expression. Redox Biol. 2025, 81, 103543. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.I.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Gao, S.; Zhang, Y.; Cui, X.; Mo, W.; Xu, J.; Yao, M. A meta-analysis of resveratrol protects against cerebral ischemia/reperfusion injury: Evidence from rats studies and insight into molecular mechanisms. Front. Pharmacol. 2022, 13, 988836. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/handle/10665/92455 (accessed on 21 September 2025).
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Asante, D.B.; Wiafe, G.A. Therapeutic Benefit of Vernonia amygdalina in the Treatment of Diabetes and Its Associated Complications in Preclinical Studies. J. Diabetes Res. 2023, 2023, 3159352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Gibbs, J.; Cappuccio, F.P. Plant-Based Dietary Patterns for Human and Planetary Health. Nutrients 2022, 14, 1614. [Google Scholar] [CrossRef]
- Ratiner, K.; Ciocan, D.; Abdeen, S.K.; Elinav, E. Utilization of the microbiome in personalized medicine. Nat. Rev. Microbiol. 2024, 22, 291–308. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Antiinflamm. Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Boddupalli, S.; Mein, J.R.; Lakkanna, S.; James, D.R. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: Perspectives in maintaining the antioxidant activity of vitamins A, C, and E. Front. Genet. 2012, 3, 7. [Google Scholar] [CrossRef]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, B.; Li, Y.; Yuan, Q. Potential mechanisms of cancer prevention and treatment by sulforaphane, a natural small molecule compound of plant-derived. Mol. Med. 2024, 30, 94. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Dhake, A.S. Antibacterial principles from Myristica fragrans seeds. J. Med. Food 2006, 9, 395–399. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef]
- Kose, T.I.; Yardimci, G.B.; Kirci, D.; Polat, D.C.; Demirci, B.; Eryilmaz, M.; Kilic, C.S. Bioactivities and Chemotaxonomy of Four Heracleum Species: A Comparative Study Across Plant Parts. Pharmaceuticals 2025, 18, 576. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsieh, T.C. Resveratrol: A cardioprotective substance. Ann. N. Y. Acad. Sci. 2011, 1215, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Cao, W.; Dou, Y.; Li, A. Resveratrol Boosts Cognitive Function by Targeting SIRT1. Neurochem. Res. 2018, 43, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Xin, M.; Xu, A.; Tian, J.; Wang, L.; He, Y.; Jiang, H.; Yang, B.; Li, B.; Sun, Y. Anthocyanins as natural bioactives with anti-hypertensive and atherosclerotic potential: Health benefits and recent advances. Phytomedicine 2024, 132, 155889. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Mesnage, R.; Fernandes, F.; Abhayaratne, N.; Xu, Y.; Zhang, Z.; Bell, L.; Williams, C.; Rodriguez-Mateos, A. Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2023, 117, 1306–1319. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Tamargo, J.; Pérez-Vizcaino, F.; Duarte, J. Cardiovascular Effects of Flavonoids. Curr. Med. Chem. 2019, 26, 6991–7034. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Ghazimoradi, M.M.; Fatima, G.; Bahramsoltani, R. Citrus flavonoids and adhesion molecules: Potential role in the management of atherosclerosis. Heliyon 2023, 9, e21849. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Res. 2019, 25, 2443–2458. [Google Scholar] [CrossRef]
- Tian, C.; Hao, L.; Yi, W.; Ding, S.; Xu, F. Polyphenols, Oxidative Stress, and Metabolic Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 7398453. [Google Scholar] [CrossRef]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. Biofactors 2022, 48, 255–273. [Google Scholar] [CrossRef]
- Amini, M.R.; Salavatizadeh, M.; Kazeminejad, S.; Javadi, F.; Hajiaqaei, M.; Askari, G.; Hekmatdoost, A. The effects of Garcinia cambogia (hydroxycitric acid) on serum leptin concentrations: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2024, 84, 103060. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Nadeem, M.S.; Afzal, O.; Alzarea, S.I.; Altamimi, A.S.A.; Almalki, W.H.; Mubeen, B.; Iftikhar, S.; Shah, L.; Kazmi, I. Gingerol, a Natural Antioxidant, Attenuates Hyperglycemia and Downstream Complications. Metabolites 2022, 12, 1274. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Ding, L.; Liu, T.; Ma, J. Neuroprotective mechanisms of Asiatic acid. Heliyon 2023, 9, e15853. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.T. Bacopa monnieri and Their Bioactive Compounds Inferred Multi-Target Treatment Strategy for Neurological Diseases: A Cheminformatics and System Pharmacology Approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef]
- Cho, D.H.; Yun, J.H.; Heo, J.; Lee, I.K.; Lee, Y.J.; Bae, S.; Yun, B.S.; Kim, H.S. Identification of Loliolide with Anti-Aging Properties from Scenedesmus deserticola JD052. J. Microbiol. Biotechnol. 2023, 33, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a New Therapeutic Option for Neurological Diseases? In Vitro Neuroprotective and Anti-Inflammatory Activities of a Monoterpenoid Lactone Isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef]
- Lentjes, M.A.H. The balance between food and dietary supplements in the general population. Proc. Nutr. Soc. 2019, 78, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Azmy Harahap, I.; Suthar, P.; Wu, Y.S.; Ghosh, N.; Castro-Muñoz, R. A Comprehensive Review of Phytonutrients as a Dietary Therapy for Obesity. Foods 2023, 12, 3610. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Netzel, M.E. Introduction to the Special Issue: Foods of Plant Origin. Foods 2019, 8, 555. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Sablani, S.S.; Tang, J.; Powers, J.; Swanson, B.G. Stability of anthocyanins in frozen and freeze-dried raspberries during long-term storage: In relation to glass transition. J. Food Sci. 2011, 76, E414–E421. [Google Scholar] [CrossRef] [PubMed]
- Gamba, M.; Pano, O.; Raguindin, P.F.; Roa-Diaz, Z.M.; Muka, T.; Glisic, M.; Franco, O.H.; Marques-Vidal, P. Association between Total Dietary Phytochemical Intake and Cardiometabolic Health Outcomes-Results from a 10-Year Follow-Up on a Middle-Aged Cohort Population. Nutrients 2023, 15, 4793. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M.A.; Mathew, B.; Abdel-Daim, M.M.; Aleya, L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020, 707, 135624. [Google Scholar] [CrossRef]
- Yin, R.; Kuo, H.C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.N. Gut microbiota, dietary phytochemicals and benefits to human health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Kensler, T.W. Phytochemicals: Do They Belong on our Plate for Sustaining Healthspan? Food Front. 2021, 2, 235–239. [Google Scholar] [CrossRef]
- Van Tulleken, C. Ultraprocessed People; Penguin Cornerstone: London, UK, 2024. [Google Scholar]
- Global Traditional Medicine Strategy 2025–2034; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/publications/i/item/9789240113176 (accessed on 14 December 2025).
- Brazil Ministério da Saúde; Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, 2nd ed.; Anvisa: Brasília, Brazil, 2012.
- Shree Devi, M.S.; Sathiyarajeswaran, P.; Kanakavalli, K.; Vinod, N.P.; Baskar, V. Analysis of AYUSH studies registered in clinical trials registry of India from 2009 to 2020. J. Ayurveda Integr. Med. 2021, 12, 346–350. [Google Scholar] [CrossRef]
- Yamana, H.; Ono, S.; Michihata, N.; Jo, T.; Yasunaga, H. Outpatient Prescriptions of Kampo Formulations in Japan. Intern. Med. 2020, 59, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Yik, M.H.; Wong, V.C.T.; Wong, T.H.; Shaw, P.C. HerBChain, a blockchain-based informative platform for quality assurance and quality control of herbal products. J. Tradit. Complement. Med. 2021, 11, 598–600. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Saving Lives, Spending Less: A Strategic Response to Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/WHO-NMH-NVI-18.8 (accessed on 14 December 2025).
- Arshad, M.T.; Ali, M.K.M.; Maqsood, S.; Ikram, A.; Hossain, M.S.; Aljameel, A.I.; Al-Farga, A.; Gnedeka, K.T. Dietary Phytochemicals in Cardiovascular Disease Prevention and Management: A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70872. [Google Scholar] [CrossRef]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Lindsay, S.W.; Thomas, C.J. Global warming and risk of vivax malaria in Great Britain. Glob. Change Hum. Health 2001, 2, 80–84. [Google Scholar] [CrossRef]
- Reitera, P.; Thomas, C.J.; Atkinson, P.M.; Hay, S.I.; Randolph, S.E.; Rogers, D.J.; Shanks, G.D.; Snow, R.W.; Spielman, A. Global warming and malaria: A call for accuracy. Lancet Infect. Dis. 2004, 4, 323–324. [Google Scholar] [CrossRef]
- Wadman, M. Mosquito-borne viruses surge in a warming Europe. Science 2025, 389, 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Rossano, R.; Larocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What are the proteolytic enzymes of honey and what they do tell us? A fingerprint analysis by 2-D zymography of unifloral honeys. PLoS ONE 2012, 7, e49164. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Al-Kafaween, M.A.; Alwahsh, M.; Mohd Hilmi, A.B.; Abulebdah, D.H. Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review. Antibiotics 2023, 12, 337. [Google Scholar] [CrossRef]
- Bea Martínez, M.; Domingo Sanz, I.; Angás Pajas, J. El abrigo de Barranco Gómez (Castellote, Teruel), un nuevo conjunto con arte levantino en el núcleo rupestre del Guadalope. Trab. Prehist. 2021, 78, 164–178. [Google Scholar] [CrossRef]
- Rosales, G. Medicinal Uses of Melipona beecheii Honey, by the Ancient Maya. In Pot Honey, A Legacy of Stingless Bees; Patricia, V., Pedro, S., Roubik, D., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Molan, P.C. Potential of honey in the treatment of wounds and burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds 2015, 27, 141–151. [Google Scholar] [PubMed]
- Bansal, V.; Medhi, B.; Pandhi, P. Honey—A remedy rediscovered and its therapeutic utility. Kathmandu Univ. Med. J. 2005, 3, 305–309. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Popovic, L.; Rijkers, G.T. Phytochemicals: Principles and Practice. Biology 2026, 15, 18. https://doi.org/10.3390/biology15010018
Popovic L, Rijkers GT. Phytochemicals: Principles and Practice. Biology. 2026; 15(1):18. https://doi.org/10.3390/biology15010018
Chicago/Turabian StylePopovic, Lara, and Ger T. Rijkers. 2026. "Phytochemicals: Principles and Practice" Biology 15, no. 1: 18. https://doi.org/10.3390/biology15010018
APA StylePopovic, L., & Rijkers, G. T. (2026). Phytochemicals: Principles and Practice. Biology, 15(1), 18. https://doi.org/10.3390/biology15010018







