The Dual Role of RASSF4 in Tumorigenesis: Mechanisms and Epigenetic Targeting Strategies
Simple Summary
Abstract
1. Introduction
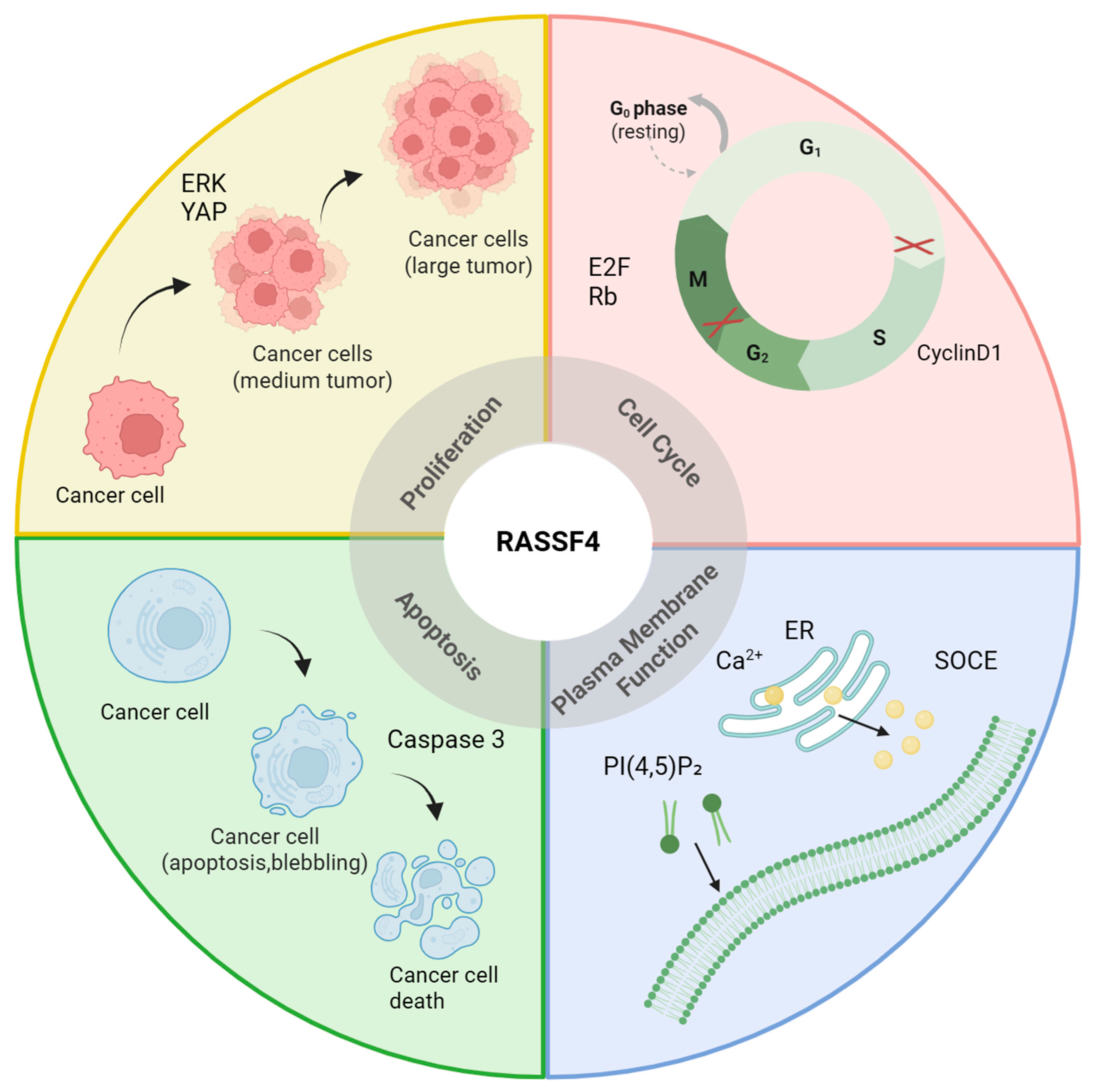
2. Cell Biological Processes Involving RASSF4
2.1. Proliferation
2.2. Apoptosis
2.3. Functional Activities of the Plasma Membrane
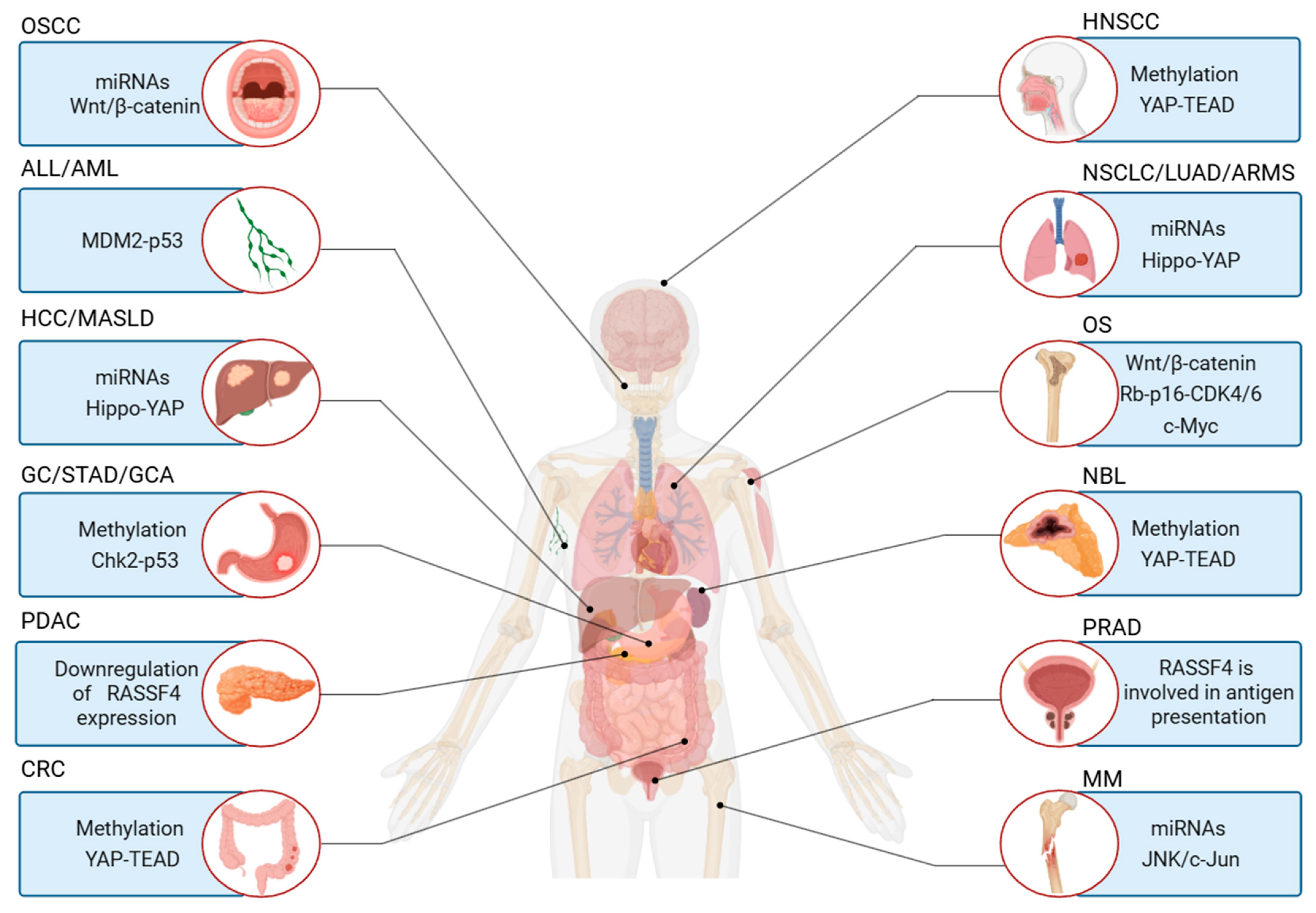
3. Clinical Significance of RASSF4
3.1. As a Diagnostic and Prognostic Biomarker
3.2. Role in Disease Progression
4. Roles of RASSF4 in Tumorigenesis and Tumor Progression
4.1. Mechanisms of How RASSF4 Inhibits Tumor Progression
4.2. Mechanisms by Which RASSF4 Promotes Tumor Progression
4.3. RASSF4 Regulation of the Cell Microenvironment and Immunity
4.4. The Potential Influence of Tumor Microenvironment Characteristics on RASSF4 Functions
4.5. The Relationship Between Genetic Changes in Tumor Cells and the Functioning of This Protein
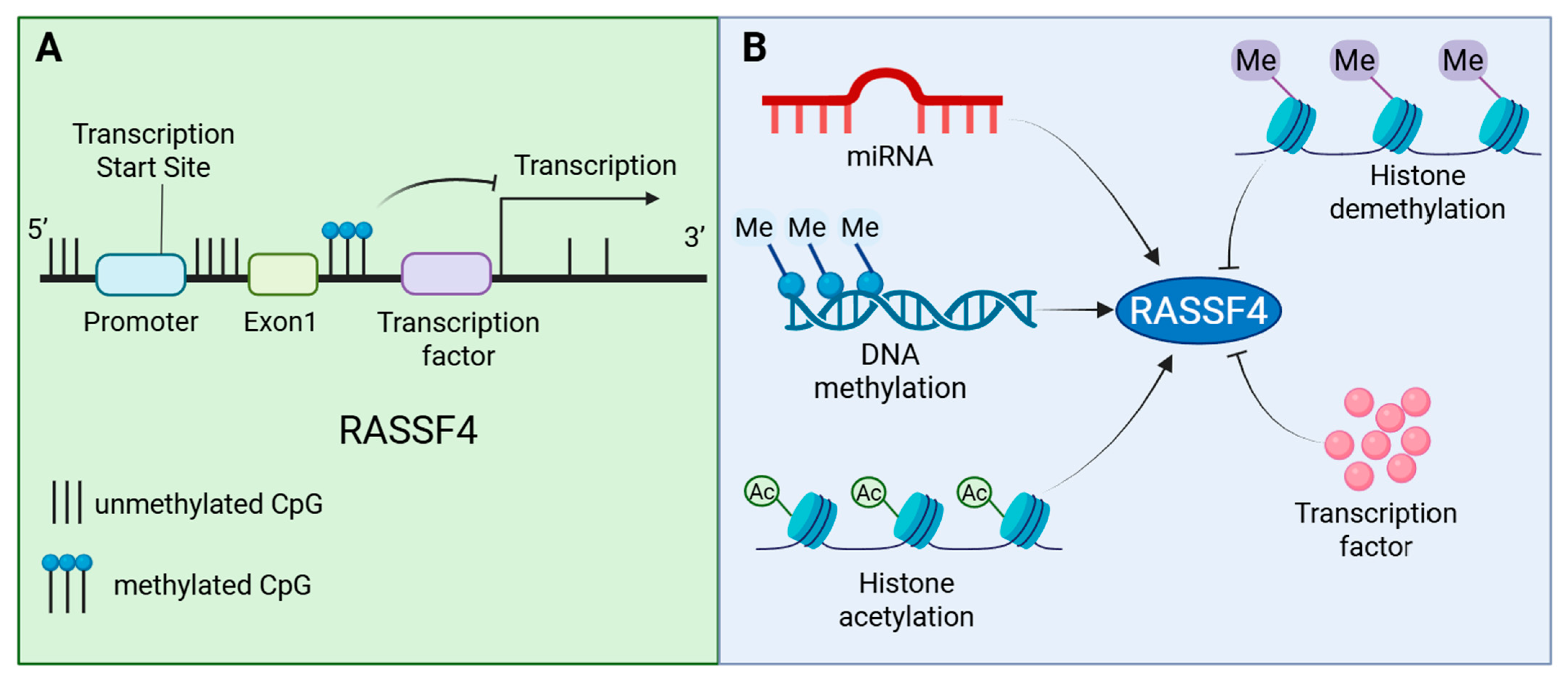
5. Regulatory Mechanisms of the RASSF4 Expression
5.1. Epigenetic Regulation
Epigenetic Regulation of RASSF4 in Cancer
5.2. Transcriptional Regulation and Subcellular Localization Regulation
6. Therapeutic Potential and Future Directions for Targeting RASSF4
6.1. Epigenetic Reactivation Strategies
6.2. miRNA-Mediated Regulation of RASSF4 and Therapeutic Prospects
6.3. Direct Gene Delivery for RASSF4 Restoration
6.4. Combination Therapy and Pathway Synergy Strategies
7. Discussion and Conclusions
7.1. Discussion
7.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Iwasa, H.; Hossain, S.; Hata, Y. Tumor suppressor C-RASSF proteins. Cell Mol. Life Sci. 2018, 75, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Guan, M.; Huo, C.; Yu, C.; Hu, B.; Cai, J. RASSF4 inhibits cell proliferation and increases drug sensitivity in colorectal cancer through YAP/Bcl-2 pathway. J. Cell Mol. Med. 2022, 26, 3538–3547. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Q.; Hao, J.; Fu, L.; Han, X.; Zheng, X.; Wang, E. RASSF4 is downregulated in nonsmall cell lung cancer and inhibits cancer cell proliferation and invasion. Tumour Biol. 2016, 37, 4865–4871. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhou, K.; Martinez, M.A.; Lopez-Torres, B.; Martinez, M.; Martinez-Larranaga, M.; Wang, X.; Anadon, A.; Ares, I. A “Janus” face of the RASSF4 signal in cell fate. J. Cell Physiol. 2022, 237, 466–479. [Google Scholar] [CrossRef]
- Richter, A.M.; Pfeifer, G.P.; Dammann, R.H. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim. Biophys. Acta 2009, 1796, 114–128. [Google Scholar] [CrossRef]
- Verma, S.K.; Ganesan, T.S.; Parker, P.J. The tumour suppressor RASSF1A is a novel substrate of PKC. Febs Lett. 2008, 582, 2270–2276. [Google Scholar] [CrossRef]
- Sherwood, V.; Recino, A.; Jeffries, A.; Ward, A.; Chalmers, A.D. The N-terminal RASSF family: A new group of Ras-association-domain-containing proteins, with emerging links to cancer formation. Biochem. J. 2009, 425, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Flatters, D.; Rodrigues-Lima, F.; Yan, J.; Thalassinos, K.; Katan, M. Comparative analysis of interactions of RASSF1-10. Adv. Biol. Regul. 2013, 53, 190–201. [Google Scholar] [CrossRef]
- Rawat, S.J.; Chernoff, J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem. Sci. 2015, 40, 149–156. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, C.; Lee, W.; Liou, J. RASSF4 controls SOCE and ER-PM junctions through regulation of PI(4,5)P(2). J. Cell Biol. 2017, 216, 2011–2025. [Google Scholar] [CrossRef]
- Sanchez-Sanz, G.; Tywoniuk, B.; Matallanas, D.; Romano, D.; Nguyen, L.K.; Kholodenko, B.N.; Rosta, E.; Kolch, W.; Buchete, N. SARAH Domain-Mediated MST2-RASSF Dimeric Interactions. PLoS Comput. Biol. 2016, 12, e1005051. [Google Scholar] [CrossRef]
- Volodko, N.; Gordon, M.; Salla, M.; Ghazaleh, H.A.; Baksh, S. RASSF tumor suppressor gene family: Biological functions and regulation. Febs Lett. 2014, 588, 2671–2684. [Google Scholar] [CrossRef]
- Mezzanotte, J.J.; Hill, V.; Schmidt, M.L.; Shinawi, T.; Tommasi, S.; Krex, D.; Schackert, G.; Pfeifer, G.P.; Latif, F.; Clark, G.J. RASSF6 exhibits promoter hypermethylation in metastatic melanoma and inhibits invasion in melanoma cells. Epigenetics-Us 2014, 9, 1496–1503. [Google Scholar] [CrossRef]
- Underhill-Day, N.; Hill, V.; Latif, F. N-terminal RASSF family: RASSF7-RASSF10. Epigenetics-Us 2011, 6, 284–292. [Google Scholar] [CrossRef]
- Chow, L.S.; Lo, K.; Kwong, J.; Wong, A.Y.; Huang, D.P. Aberrant methylation of RASSF4/AD037 in nasopharyngeal carcinoma. Oncol. Rep. 2004, 12, 781–787. [Google Scholar] [CrossRef]
- De Smedt, E.; Maes, K.; Verhulst, S.; Lui, H.; Kassambara, A.; Maes, A.; Robert, N.; Heirman, C.; Cakana, A.; Hose, D.; et al. Loss of RASSF4 Expression in Multiple Myeloma Promotes RAS-Driven Malignant Progression. Cancer Res. 2018, 78, 1155–1168. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Carneiro, F.; Oliveira, C.; Seruca, R. Colorectal cancer and RASSF family--a special emphasis on RASSF1A. Int. J. Cancer 2013, 132, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, K.; Sandner, A.; Schagdarsurengin, U.; Dammann, R.H. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol. Rep. 2009, 22, 1519–1526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crose, L.E.S.; Galindo, K.A.; Kephart, J.G.; Chen, C.; Fitamant, J.; Bardeesy, N.; Bentley, R.C.; Galindo, R.L.; Chi, J.A.; Linardic, C.M. Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J. Clin. Investig. 2014, 124, 285–296. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pang, S.W.; Revai Lechtich, E.; Shah, K.; Simon, S.E.; Ponnusamy, S.; Narayanan, R.; Poh, C.L.; Tan, K.O. Tricistronic expression of MOAP-1, Bax and RASSF1A in cancer cells enhances chemo-sensitization that requires BH3L domain of MOAP-1. J. Cancer Res. Clin. 2020, 146, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Veerasamy, T.; Eugin Simon, S.; Tan, K.O. Emerging strategies for sensitization of therapy resistant tumors toward cancer therapeutics by targeting the Bcl-2 family, TGF-beta, Wnt/beta-Catenin, RASSF and miRNA regulated signaling pathways. Int. J. Biochem. Cell B 2021, 137, 106016. [Google Scholar] [CrossRef]
- Tan, E.W.; Abdullah, A.D.I.; Ming, L.C.; Poh, C.L.; Goh, B.H.; Lau, T.P.; Tan, K.O. Adenovirus-mediated expression of MOAP-1, Bax and RASSF1A antagonizes chemo-drug resistance of human breast cancer cells expressing cancer stem cell markers. Biomed. Pharmacother. 2024, 176, 116744. [Google Scholar] [CrossRef]
- Yang, T.; Shao, Y.; Cheng, Q.; He, Y.; Qiu, Z.; Pan, D.; Zhang, H.; Jiang, Z.; Yan, M.; Ying, C.; et al. YY1/HIF-1alpha/mROS positive-feedback loop exacerbates glomerular mesangial cell proliferation in mouse early diabetic kidney disease. Acta Pharmacol. Sin. 2025, 46, 1974–1989. [Google Scholar] [CrossRef]
- Cheng, M.; Li, M.; Zhang, Y.; Gu, X.; Gao, W.; Zhang, S.; Liu, J. Exploring the mechanism of PPCPs on human metabolic diseases based on network toxicology and molecular docking. Environ. Int. 2025, 196, 109324. [Google Scholar] [CrossRef]
- Jastrzębski, M.K.; Wójcik, P.; Stępnicki, P.; Kaczor, A.A. Effects of small molecules on neurogenesis: Neuronal proliferation and differentiation. Acta Pharm. Sin. B 2024, 14, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, D.; Zhu, T.; Yin, R. RASSF4 Overexpression Inhibits the Proliferation, Invasion, EMT, and Wnt Signaling Pathway in Osteosarcoma Cells. Oncol. Res. 2017, 25, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fang, T.; Qu, J.; Miao, Y.; Tian, L.; Zhang, M.; Zhuang, H.; Sun, B.; Chen, L. RASSF4 Attenuates Metabolic Dysfunction-Associated Steatotic Liver Disease Progression via Hippo Signaling and Suppresses Hepatocarcinogenesis. Cell Mol. Gastroenter 2024, 18, 101348. [Google Scholar] [CrossRef]
- Cui, S.; Hu, X.; Yan, Y. Wnt/beta-catenin signaling pathway participates in the effect of miR-626 on oral squamous cell carcinoma by targeting RASSF4. J. Oral. Pathol. Med. 2021, 50, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, M.; Ko, K.; Chi, S. RASSF4 Suppresses Gastric Tumor Growth through Activation of Chk2-p53 Signaling Axis. Cancer Res. Treat. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Michifuri, Y.; Hirohashi, Y.; Torigoe, T.; Miyazaki, A.; Fujino, J.; Tamura, Y.; Tsukahara, T.; Kanaseki, T.; Kobayashi, J.; Sasaki, T.; et al. Small proline-rich protein-1B is overexpressed in human oral squamous cell cancer stem-like cells and is related to their growth through activation of MAP kinase signal. Biochem. Bioph Res. Co. 2013, 439, 96–102. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Z.; Mu, C.; Song, D.; Wang, J.; Meng, X.; Li, Z.; Qing, H.; Dong, Y.; Xie, H.; et al. Enhanced Proliferation of Visualizable Mesenchymal Stem Cell-Platelet Hybrid Cell for Versatile Intracerebral Hemorrhage Treatment. Acs Nano 2023, 17, 7352–7365. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, S.; Li, Y.; Liu, Z.; Mi, L.; Cai, Y.; Wang, X.; Chen, L.; Ran, H.; Xiao, D.; et al. Suppression of mitochondrial ROS by prohibitin drives glioblastoma progression and therapeutic resistance. Nat. Commun. 2021, 12, 3720. [Google Scholar] [CrossRef]
- Ullah, M.; Stich, S.; Notter, M.; Eucker, J.; Sittinger, M.; Ringe, J. Transdifferentiation of mesenchymal stem cells-derived adipogenic-differentiated cells into osteogenic- or chondrogenic-differentiated cells proceeds via dedifferentiation and have a correlation with cell cycle arresting and driving genes. Differentiation 2013, 85, 78–90. [Google Scholar] [CrossRef]
- Cheng, X.; Ferrell, J.E.J. Apoptosis propagates through the cytoplasm as trigger waves. Science 2018, 361, 607–612. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Canc Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Xu, X.; Hou, R.; Badawy, S.; Tao, Y.; Chen, D.; Ihsan, A.; Wang, X.; Wu, Q.; Yuan, Z. DNA methylation and RASSF4 expression are involved in T-2 toxin-induced hepatotoxicity. Toxicology 2019, 425, 152246. [Google Scholar] [CrossRef]
- Ikeda, M.; Kawata, A.; Nishikawa, M.; Tateishi, Y.; Yamaguchi, M.; Nakagawa, K.; Hirabayashi, S.; Bao, Y.; Hidaka, S.; Hirata, Y.; et al. Hippo pathway-dependent and -independent roles of RASSF6. Sci. Signal 2009, 2, ra59. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Matsuoka, S.; Biswas, D.; Miao, Y.; Pal, D.S.; Kamimura, Y.; Ueda, M.; Devreotes, P.N.; Iglesias, P.A. A dynamic partitioning mechanism polarizes membrane protein distribution. Nat. Commun. 2023, 14, 7909. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, U.; Mandal, T.; Chakraborty, M.; Sinha, B. The Plasma Membrane and Mechanoregulation in Cells. Acs Omega 2024, 9, 21780–21797. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Zhou, Z.; Huang, Y.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Ali, D.W.; Michalak, M.; et al. Membrane Curvature: The Inseparable Companion of Autophagy. Cells 2023, 12, 1132. [Google Scholar] [CrossRef]
- De la Cruz, L.; Traynor-Kaplan, A.; Vivas, O.; Hille, B.; Jensen, J.B. Plasma membrane processes are differentially regulated by type I phosphatidylinositol phosphate 5-kinases and RASSF4. J. Cell Sci. 2020, 133, jcs233254. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Chen, X.; Dong, L.; Zhou, B.; Cheng, Q.; Han, S.; Liu, Z.; Peng, B.; He, X.; et al. RASSF4 promotes EV71 replication to accelerate the inhibition of the phosphorylation of AKT. Biochem. Bioph Res. Co. 2015, 458, 810–815. [Google Scholar] [CrossRef]
- Guo, W.; Dong, Z.; Guo, Y.; Shen, S.; Guo, X.; Kuang, G.; Yang, Z. Decreased expression and frequent promoter hypermethylation of RASSF2 and RASSF6 correlate with malignant progression and poor prognosis of gastric cardia adenocarcinoma. Mol. Carcinogen 2016, 55, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Z.; Ni, Y.; Bian, C.; Huang, J.; Chen, L.; Xie, X.; Wang, J. Tumor-associated macrophages secret exosomal miR-155 and miR-196a-5p to promote metastasis of non-small-cell lung cancer. Transl. Lung Cancer R. 2021, 10, 1338–1354. [Google Scholar] [CrossRef]
- Alikarami, F.; Xie, H.M.; Riedel, S.S.; Goodrow, H.T.; Barrett, D.R.; Mahdavi, L.; Lenard, A.; Chen, C.; Yamauchi, T.; Danis, E.; et al. GATA2 links stemness to chemotherapy resistance in acute myeloid leukemia. Blood 2025, 145, 2179–2195. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, L.; Mo, X.; Xia, W.; Guo, Y.; Wang, M.; Zeng, K.; Wu, J.; Qiu, Y.; Lin, X.; et al. Identification of PBMC-expressed miRNAs for rheumatoid arthritis. Epigenetics-Us 2020, 15, 386–397. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Cheng, S.; Liu, Y. Expression of stearoyl coenzyme a desaturase in neuronal cells facilitates pancreatic cancer progression. Cancer Cell Int. 2025, 25, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Djos, A.; Martinsson, T.; Kogner, P.; Caren, H. The RASSF gene family members RASSF5, RASSF6 and RASSF7 show f requent DNA methylation in neuroblastoma. Mol. Cancer 2012, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, H. Prognosis related miRNAs, DNA methylation, and epigenetic interactions in lung adenocarcinoma. Neoplasma 2019, 66, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zheng, M.; Liu, Q.; Zeng, Y.; Wei, S.; Wang, Y.; Lin, Z.; Shu, C.; Zheng, Q.; Chen, L. Survival-associated alternative splicing events interact with the immune microenvironment in stomach adenocarcinoma. World J. Gastroenterol. 2021, 27, 2871–2894. [Google Scholar] [CrossRef]
- Kenawy, N.; Kalirai, H.; Sacco, J.J.; Lake, S.L.; Heegaard, S.; Larsen, A.; Finger, P.T.; Milman, T.; Chin, K.; Mosci, C.; et al. Conjunctival melanoma copy number alterations and correlation with mutation status, tumor features, and clinical outcome. Pigm Cell Melanoma R. 2019, 32, 564–575. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.; Chen, Y.; Zhang, M.; Gao, W.; Shang, Z.; Niu, Y. Identification of Neoantigens and Construction of Immune Subtypes in Prostate Adenocarcinoma. Front. Genet. 2022, 13, 886983. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Gryder, B.E.; Sinniah, R.; Peach, M.L.; Shern, J.F.; Abdelmaksoud, A.; Pomella, S.; Woldemichael, G.M.; Stanton, B.Z.; Milewski, D.; et al. KDM3B inhibitors disrupt the oncogenic activity of PAX3-FOXO1 in fusion-positive rhabdomyosarcoma. Nat. Commun. 2024, 15, 1703. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Richter, A.M.; Hornung, J.; Lange, C.; Steinmann, K.; Dammann, R.H. Frequent epigenetic inactivation of RASSF2 in thyroid cancer and functional consequences. Mol. Cancer 2010, 9, 264. [Google Scholar] [CrossRef]
- Lin, Y.; Deel, M.D.; Linardic, C.M. RASSF4 is required for skeletal muscle differentiation. Cell Biol. Int. 2020, 44, 381–390. [Google Scholar] [CrossRef]
- Jin, M.; Jin, W. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Tar. 2020, 5, 166. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Therapeut 2021, 221, 107753. [Google Scholar] [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Korz, V.; Kremslehner, C.; Malikovic, J.; Hussein, A.; Feyissa, D.D.; Nagelreiter, I.; Smidak, R.; Plasenzotti, R.; Gruber, F.; Lubec, G. Striatal Transcriptome Reveals Differences Between Cognitively Impaired and Unimpaired Aged Male Rats. Front. Aging Neurosci. 2020, 12, 611572. [Google Scholar] [CrossRef]
- Buga, A.M.; Margaritescu, C.; Scholz, C.J.; Radu, E.; Zelenak, C.; Popa-Wagner, A. Transcriptomics of post-stroke angiogenesis in the aged brain. Front. Aging Neurosci. 2014, 6, 44. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, J.; Chen, Q.; Yang, S.; Wang, Z.; Xiao, B.; Lai, Z.; Jing, Y.; Li, Y.; Li, X. Regulation of de novo and maintenance DNA methylation by DNA methyltransferases in postimplantation embryos. J. Biol. Chem. 2025, 301, 107990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hrit, J.A.; Chomiak, A.A.; Stransky, S.; Hoffman, J.R.; Tiedemann, R.L.; Wiseman, A.K.; Kariapper, L.S.; Dickson, B.M.; Worden, E.J.; et al. DNA hypomethylation promotes UHRF1-and SUV39H1/H2-dependent crosstalk between H3K18ub and H3K9me3 to reinforce heterochromatin states. Mol. Cell 2025, 85, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Huebner, A.; Shabsigh, A.; Chakravarti, A.; Lautenschlaeger, T. Combined RASSF1A and RASSF2A Promoter Methylation Analysis as Diagnostic Biomarker for Bladder Cancer. Mol. Biol. Int. 2012, 2012, 701814. [Google Scholar] [CrossRef]
- Richter, A.M.; Zimmermann, T.; Haag, T.; Walesch, S.K.; Dammann, R.H. Promoter methylation status of Ras-association domain family members in pheochromocytoma. Front. Endocrinol. 2015, 6, 21. [Google Scholar] [CrossRef]
- Al-Zyoud, W.A.; Hynson, R.M.G.; Ganuelas, L.A.; Coster, A.C.F.; Duff, A.P.; Baker, M.A.B.; Stewart, A.G.; Giannoulatou, E.; Ho, J.W.K.; Gaus, K.; et al. Binding of transcription factor GabR to DNA requires recognition of DNA shape at a location distinct from its cognate binding site. Nucleic Acids Res. 2016, 44, 1411–1420. [Google Scholar] [CrossRef]
- Lai, H.C.; Klisch, T.J.; Roberts, R.; Zoghbi, H.Y.; Johnson, J.E. In vivo neuronal subtype-specific targets of Atoh1 (Math1) in dorsal spinal cord. J. Neurosci. 2011, 31, 10859–10871. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Han, L.; Zhou, Y.; Sun, S. Green tea polyphenol EGCG reverse cisplatin resistance of A549/DDP cell line through candidate genes demethylation. Biomed. Pharmacother. 2015, 69, 285–290. [Google Scholar] [CrossRef]
- Eckfeld, K.; Hesson, L.; Vos, M.D.; Bieche, I.; Latif, F.; Clark, G.J. RASSF4/AD037 is a potential ras effector/tumor suppressor of the RASSF family. Cancer Res. 2004, 64, 8688–8693. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Gao, P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell 2022, 13, 877–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, M.; Qu, J.; Liu, G. Epigenetic Modifications in Cardiovascular Aging and Diseases. Circ. Res. 2018, 123, 773–786. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, Q.; Xie, P.; He, C.; Li, Q.; Yao, X.; Mao, Y.; Wu, X.; Zhang, T. Epigenetic modifications and emerging therapeutic targets in cardiovascular aging and diseases. Pharmacol. Res. 2025, 211, 107546. [Google Scholar] [CrossRef]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Lee, A.V.; Nestler, K.A.; Chiappinelli, K.B. Therapeutic targeting of DNA methylation alterations in cancer. Pharmacol. Therapeut 2024, 258, 108640. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2017, 169, 361. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef]
- Klutstein, M.; Nejman, D.; Greenfield, R.; Cedar, H. DNA Methylation in Cancer and Aging. Cancer Res. 2016, 76, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Hetzel, S.; Meissner, A. DNA methylation in mammalian development and disease. Nat. Rev. Genet. 2025, 26, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, H.; Yu, Q.; Xiao, W.; Wang, D.O. LncRNA-mediated DNA methylation: An emerging mechanism in cancer and beyond. J. Exp. Clin. Canc Res. 2022, 41, 100. [Google Scholar] [CrossRef]
- Minisini, M.; Mascaro, M.; Brancolini, C. HDAC-driven mechanisms in anticancer resistance: Epigenetics and beyond. Cancer Drug Resist. 2024, 7, 46. [Google Scholar] [CrossRef]
- Ischenko, I.; Petrenko, O.; Hayman, M.J. A MEK/PI3K/HDAC inhibitor combination therapy for KRAS mutant pancreatic cancer cells. Oncotarget 2015, 6, 15814–15827. [Google Scholar] [CrossRef]
- Carson, R.; Celtikci, B.; Fenning, C.; Javadi, A.; Crawford, N.; Carbonell, L.P.; Lawler, M.; Longley, D.B.; Johnston, P.G.; Van Schaeybroeck, S. HDAC Inhibition Overcomes Acute Resistance to MEK Inhibition in BRAF-Mutant Colorectal Cancer by Downregulation of c-FLIPL. Clin. Cancer Res. 2015, 21, 3230–3240. [Google Scholar] [CrossRef]
- Gallimore, F.; Fandy, T.E. Therapeutic Applications of Azanucleoside Analogs as DNA Demethylating Agents. Epigenomes 2023, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yao, H.; He, G.; Lai, S.; Deng, Y.; Zhang, S.; He, Y.; Xiong, Y.; Chang, A.H.; Su, Y.; et al. Demethylating agents in combination with CD7-targeted CAR-T for the successful treatment of a case with mixed-phenotype acute leukemia relapsed after allogeneic hematopoietic stem cell transplantation: A Case Report. Front. Immunol. 2023, 14, 1254010. [Google Scholar] [CrossRef]
- Hasegawa, N.; Benabdallah, N.S.; Smith-Fry, K.; Li, L.; McCollum, S.; Li, J.; Jones, C.A.; Wagner, L.; Dalal, V.; Golde, V.; et al. DNA demethylating agents suppress preclinical models of synovial sarcoma. J. Clin. Investig. 2025, 135, e190855. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yin, J.; Liao, Y.; Liu, J.; Zhao, Y.; Chen, X.; Liu, Y.; Wang, X.; Huang, C. The efficacy and safety of venetoclax combined with demethylating agents in elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Eur. Rev. Med. Pharmaco 2024, 28, 1837–1846. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Watanabe, T.; Ureshino, H.; Kurahashi, Y.; Fukuda-Kurahashi, Y.; Kamachi, K.; Kawasoe, K.; Kidoguchi, K.; Yamashita, S.; Hattori, N.; et al. DNA demethylating agents for chemoprevention of oncovirus-associated leukemogenesis. Leukemia 2024, 38, 1613–1616. [Google Scholar] [CrossRef]
- Kawasoe, K.; Watanabe, T.; Yoshida-Sakai, N.; Yamamoto, Y.; Kurahashi, Y.; Kidoguchi, K.; Ureshino, H.; Kamachi, K.; Fukuda-Kurahashi, Y.; Kimura, S. A Combination of Alectinib and DNA-Demethylating Agents Synergistically Inhibits Anaplastic-Lymphoma-Kinase-Positive Anaplastic Large-Cell Lymphoma Cell Proliferation. Cancers 2023, 15, 5089. [Google Scholar] [CrossRef]
- Mayyas, I.M.; Weeks, R.J.; Day, R.C.; Magrath, H.E.; O’Connor, K.M.; Kardailsky, O.; Hore, T.A.; Hampton, M.B.; Morison, I.M. Hairpin-bisulfite sequencing of cells exposed to decitabine documents the process of DNA demethylation. Epigenetics-Us 2021, 16, 1251–1259. [Google Scholar] [CrossRef]
- Taib, N.; Merhi, M.; Inchakalody, V.; Mestiri, S.; Hydrose, S.; Makni-Maalej, K.; Raza, A.; Sahir, F.; Azizi, F.; Nizamuddin, P.B.; et al. Treatment with decitabine induces the expression of stemness markers, PD-L1 and NY-ESO-1 in colorectal cancer: Potential for combined chemoimmunotherapy. J. Transl. Med. 2023, 21, 235. [Google Scholar] [CrossRef]
- Heumann, T.R.; Baretti, M.; Sugar, E.A.; Durham, J.N.; Linden, S.; Lopez-Vidal, T.Y.; Leatherman, J.; Cope, L.; Sharma, A.; Weekes, C.D.; et al. A randomized, phase II trial of oral azacitidine (CC-486) in patients with resected pancreatic adenocarcinoma at high risk for recurrence. Clin. Epigenetics 2022, 14, 166. [Google Scholar] [CrossRef]
- Bever, K.M.; Thomas, D.L.N.; Zhang, J.; Diaz Rivera, E.A.; Rosner, G.L.; Zhu, Q.; Nauroth, J.M.; Christmas, B.; Thompson, E.D.; Anders, R.A.; et al. A feasibility study of combined epigenetic and vaccine therapy in advanced colorectal cancer with pharmacodynamic endpoint. Clin. Epigenetics 2021, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Qi, Y.; Ding, L.; Ding, S.; Han, Z.; Wang, Y.; Du, P. miRNA dosage control in development and human disease. Trends Cell Biol. 2024, 34, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Condorelli, G. Epigenetic modifications and noncoding RNAs in cardiac hypertrophy and failure. Nat. Rev. Cardiol. 2015, 12, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Ott, M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015, 16, 258–264. [Google Scholar] [CrossRef]
- Ramprosand, S.; Govinden-Soulange, J.; Ranghoo-Sanmukhiya, V.M.; Sanan-Mishra, N. miRNA, phytometabolites and disease: Connecting the dots. Phytother. Res. 2024, 38, 4570–4591. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Q.; Liu, C.; Cao, J.; Zhang, S.; Cao, H.; Chen, W. MicroRNA-mediated autophagy regulation in thyroid cancer drug resistance. Cancer Drug Resist. 2025, 8, 30. [Google Scholar] [CrossRef]
- Jones, C.A.; Rees, J.M.; Dodds, W.N.; Jayson, M.I. Changes in plasma opioid concentrations after physiotherapeutic exercises for arthritic patients. Neuropeptides 1985, 5, 561–562. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, L.; Li, S.; Yang, W.; Chen, F.; Cai, Z.; Liu, X.; Xu, W.; Lehto, V.; Lachelt, U.; et al. From structural design to delivery: mRNA therapeutics for cancer immunotherapy. Exploration 2024, 4, 20210146. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Zhang, Q.; Ali, D.W.; Michalak, M.; Chen, X.; Zhou, C.; et al. Dysregulation of tRNA methylation in cancer: Mechanisms and targeting therapeutic strategies. Cell Death Discov. 2024, 10, 327. [Google Scholar] [CrossRef]
- Hong, J.; Sim, D.; Lee, B.; Sarangthem, V.; Park, R. Multifunctional elastin-like polypeptide nanocarriers for efficient miRNA delivery in cancer therapy. J. Nanobiotechnology 2024, 22, 293. [Google Scholar] [CrossRef]
- Garo, L.P.; Murugaiyan, G. The Use of MiRNA Antagonists in the Alleviation of Inflammatory Disorders. Methods Mol. Biol. 2016, 1390, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Brown, D.; Winkler, M. The promise of microRNA replacement therapy. Cancer Res. 2010, 70, 7027–7030. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.; Joshi, J.; Shah, F. Significance of metastamiR-10b in breast cancer therapeutics. J. Egypt. Natl. Cancer 2022, 34, 19. [Google Scholar] [CrossRef]
- Su, Y.; Swiderski, P.; Marcucci, G.; Kortylewski, M. Targeted Delivery of miRNA Antagonists to Myeloid Cells In Vitro and In Vivo. Methods Mol. Biol. 2019, 1974, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Avendano-Portugal, C.; Montano-Samaniego, M.; Guttman-Bazbaz, R.; Bravo-Estupinan, D.M.; Ibanez-Hernandez, M. Therapeutic Applications of Poly-miRNAs and miRNA Sponges. Int. J. Mol. Sci. 2025, 26, 4535. [Google Scholar] [CrossRef]
- Tang, M.; Li, H.; Chang, S.; Li, Y.; Nie, H.; Li, F. Dysregulated circular RNAs in rheumatoid arthritis: Cellular roles and clinical prospects. Autoimmun. Rev. 2025, 24, 103774. [Google Scholar] [CrossRef]
- Fu, S.; Sun, W.; Liu, L.; Xiao, J.; Xiong, J.; Hu, Y.; Zhou, Q.; Yin, X. Muller Cells Harboring Exosomal lncRNA OGRU Modulate Microglia Polarization in Diabetic Retinopathy by Serving as miRNA Sponges. Diabetes 2024, 73, 1919–1934. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, M.; Yang, L.; An, X.; Zhao, H.; Xia, S.; Jin, Z.; Huang, S.; Qu, Y.; Ai, J. MicroRNA-153 impairs hippocampal synaptic vesicle trafficking via downregulation of synapsin I in rats following chronic cerebral hypoperfusion. Exp. Neurol. 2020, 332, 113389. [Google Scholar] [CrossRef]
- Park, W.; Lee, H.; Sim, D.; Im, E.; Park, J.; Ahn, C.; Shim, B.; Kim, S. miR193a-5p Mediated ZNF746 and c-Myc Signaling Axis Is Critically Involved in Morusin Induced Apoptosis in Colorectal Cancer Cells. Cells 2021, 10, 2065. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Luo, C.; Wu, G.; Xiao, Z.; Hu, R.; Qiao, M.; Li, W.; Liu, C.; Li, Z.; Lan, C.; Huang, Z. Role of miRNA regulation in IGFBP-2 overexpression and neuronal ferroptosis: Insights into the Nrf2/SLC7A11/GPX4 pathway in Alzheimer’s disease. Int. J. Biol. Macromol. 2025, 287, 138537. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Duurland, C.L.; Gunst, T.D.; Boer, H.C.D.; Bosch, M.T.J.V.; Telford, B.J.; Vos, R.M.; Xie, X.; Zang, M.; Wang, F.; Shao, Y.; et al. INT-1B3, an LNP formulated miR-193a-3p mimic, promotes anti-tumor immunity by enhancing T cell mediated immune responses via modulation of the tumor microenvironment and induction of immunogenic cell death. Oncotarget 2024, 15, 470–485. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliver Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Nicoll, T.R.; Jensen, M.M. Staphylococcosis of turkeys. 5. Large-scale control programs using bacterial interference. Avian Dis. 1987, 31, 85–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, C.; Sun, T.; Wang, L.; Chen, C. Tumor Therapy Strategies Based on Microenvironment-Specific Responsive Nanomaterials. Adv. Healthc. Mater. 2023, 12, e2300153. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Di, Z.; Zhang, G.; Sun, L.; Li, L.; Yan, C. Engineering of Upconverted Metal-Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 3939–3946. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, J.; Yang, X. Deformable nanocarriers for enhanced drug delivery and cancer therapy. Exploration 2024, 4, 20230037. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A.; Shah, S.; Vataliya, J.; Mittal, A.; Chitkara, D. RNA Interference Nanotherapeutics for Treatment of Glioblastoma Multiforme. Mol. Pharm. 2020, 17, 4040–4066. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Mahabady, M.K.; Zabolian, A.; Abbaspour, A.; Fallahzadeh, P.; Noori, M.; Hashemi, F.; Hushmandi, K.; Daneshi, S.; Kumar, A.P.; et al. Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: Current status with an emphasis on delivery systems. Life Sci. 2021, 275, 119368. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro-Oncology 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Breton, C.A.; White, J.; Zhang, J.; Che, Y.; Saveliev, A.; McMenamin, D.; He, Z.; Latshaw, C.; et al. CRISPR/Cas9-mediated in vivo gene targeting corrects hemostasis in newborn and adult factor IX-knockout mice. Blood 2019, 133, 2745–2752. [Google Scholar] [CrossRef]
- VanLith, C.; Guthman, R.; Nicolas, C.T.; Allen, K.; Du, Z.; Joo, D.J.; Nyberg, S.L.; Lillegard, J.B.; Hickey, R.D. Curative Ex Vivo Hepatocyte-Directed Gene Editing in a Mouse Model of Hereditary Tyrosinemia Type 1. Hum. Gene Ther. 2018, 29, 1315–1326. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, X.; Wang, H.; Xing, D. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Triggered Isothermal Amplification for Site-Specific Nucleic Acid Detection. Anal. Chem. 2018, 90, 2193–2200. [Google Scholar] [CrossRef]
- Zhi, D.; Zhao, Y.; Cui, S.; Chen, H.; Zhang, S. Conjugates of small targeting molecules to non-viral vectors for the mediation of siRNA. Acta Biomater. 2016, 36, 21–41. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, H.; Fang, J.; Yu, X.; Su, Z.; Cheng, D.; Shuai, X. Construction of negatively charged and environment-sensitive nanomedicine for tumor-targeted efficient siRNA delivery. Chem. Commun. 2016, 52, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.H. Casualties of the health care system: Patients depressed by medicine’s “moral dilemmas”. Pharos Alpha Omega Alpha Honor. Med. Soc. 1987, 50, 19–20. [Google Scholar] [PubMed]
- Ni, J.; Zhang, Z.; Ge, M.; Chen, J.; Zhuo, W. Immune-based combination therapy to convert immunologically cold tumors into hot tumors: An update and new insights. Acta Pharmacol. Sin. 2023, 44, 288–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, M.; Li, S.; Zhang, X.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Chen, X.; Tang, J.; et al. TRPM channels in human cancers: Regulatory mechanism and therapeutic prospects. Biomark. Res. 2024, 12, 152. [Google Scholar] [CrossRef] [PubMed]



| Cancer Types | Cell Lines | Methylation Factors | Genomic Regions Involved | References |
|---|---|---|---|---|
| Head and neck squamous cell carcinoma | Hep-2, RPMI-2650, and UM-SCC-14C | MST1, RARβ, MLH1, DAPK, p16, RASSF5, MGMT | The CpG island promoter regions of RASSF2 and RASSF4 | [18] |
| Neuroblastoma | Kelly, NB69, SK-N-SH, SH SY-5Y, SK-N-AS, SK-N-BE (2), SK-N-DZ, SK-N-FI, and IMR-32 | RASSF5, RASSF6, RASSF7, RASSF2A, RASSF4, RASSF8, and RASSF10 | The CpG island promoter regions of RASSF5A, RASSF5C, RASSF6, RASSF7 | [48] |
| Gastric cancer | SNU16, SNU216, | RASSF4 | The exon 1 of RASSF4 | [29] |
| SNU484, SNU601, | ||||
| SNU620, SNU638, | ||||
| SNU719, MKN1, | ||||
| MKN28, AGS, | ||||
| MKN74, MKN45, | ||||
| KATOIII | ||||
| Stomach adenocarcinoma | MKN-45, and AGS | — | The exon regions of CD44, RASSF4, PPP2R5D, and LOH12CR1; the terminator regions of PPHLN1 and CADPS; the alternative promoter regions of KIAA1147, CDKN3, and WEE1 | [50] |
| Gastric cardia adenocarcinoma | — | RASSF, RASSF3, | The exon 1 of RASSF2, RASSF3, RASSF4, and RASSF6 | [43] |
| RASSF4, and | ||||
| RASSF6 | ||||
| Colorectal cancer | LoVo, HCT-8, HCT116, and HCT15 | RASSF4 | The promoter regions of Bcl-2 | [2] |
| Type | Potential Representative Drug | Target | Cancer | Potential Mechanism | Reference |
|---|---|---|---|---|---|
| Epigenetic regulatory drugs | Azacitidine | DNA CpG islands | OSCC HNSCC | Demethylating | [28] [18] |
| Decitabine | GCA LUAD NSCLC | [43] [49] [67] | |||
| EGCG | |||||
| Vorinostat | Histone acetyltransferase | M M | Histone deacetylase inhibitors | [16] | |
| Panobinostat | |||||
| Gene-targeted therapeutic drugs | dCas9-TET1 fusion system | GC CRC | Gene activation /editing | [29] [2] [49] | |
| SunTag-TET1 multivalent system | RASSF4 | LUAD | |||
| miRNA sponge vector | miRNA | NSCLC LUAD OSCC | miRNA antagonist | [44] [49] [28] | |
| LNA-antimiR-626 | |||||
| Cholesterol-modified antagomiR-155 | |||||
| Locked nucleic acid (LNA) or GalNAc–siRNA conjugate | RASSF4 splicing variants | STAD | siRNA | [50] | |
| Signal pathway synergistic inhibitor | Verteporfin | YAP | aRMS OSCC CRC HNSCC | Inhibition of YAP–TEAD binding | [19] [28] [2] [18] |
| XMU-MP-1 | MAT1/2 | HCC MM aRMS | Activate MST1/2 | [27] [49] [19] | |
| Trametinib | MEK | M M | RAS/MAPK | [49] | |
| Sotorasib | KRas | Inhibition of KRAS activity | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, R.; Wu, Y.; Yuan, W.; Tian, L.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Zhang, Q.; Ali, D.W.; et al. The Dual Role of RASSF4 in Tumorigenesis: Mechanisms and Epigenetic Targeting Strategies. Biology 2025, 14, 1289. https://doi.org/10.3390/biology14091289
Tian R, Wu Y, Yuan W, Tian L, Zhang R, Lyu H, Xiao S, Guo D, Zhang Q, Ali DW, et al. The Dual Role of RASSF4 in Tumorigenesis: Mechanisms and Epigenetic Targeting Strategies. Biology. 2025; 14(9):1289. https://doi.org/10.3390/biology14091289
Chicago/Turabian StyleTian, Rui, Yixin Wu, Wenbin Yuan, Lingli Tian, Rui Zhang, Hao Lyu, Shuai Xiao, Dong Guo, Qi Zhang, Declan William Ali, and et al. 2025. "The Dual Role of RASSF4 in Tumorigenesis: Mechanisms and Epigenetic Targeting Strategies" Biology 14, no. 9: 1289. https://doi.org/10.3390/biology14091289
APA StyleTian, R., Wu, Y., Yuan, W., Tian, L., Zhang, R., Lyu, H., Xiao, S., Guo, D., Zhang, Q., Ali, D. W., Michalak, M., Zhou, C., Tang, J., & Chen, X.-Z. (2025). The Dual Role of RASSF4 in Tumorigenesis: Mechanisms and Epigenetic Targeting Strategies. Biology, 14(9), 1289. https://doi.org/10.3390/biology14091289







