Welander Distal Myopathy-Associated TIA1 E384K Mutation Disrupts Stress Granule Dynamics Under Distinct Stress Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stress Induction
2.3. Western Blot Analysis
2.4. Immunofluorescence and Confocal Microscopy
2.5. Statistical Analysis
3. Results
3.1. Analysis of eIF2α Phosphorylation Dynamics in FT293-TIA1aWT/WDM Cells Under Distinct Stress Conditions
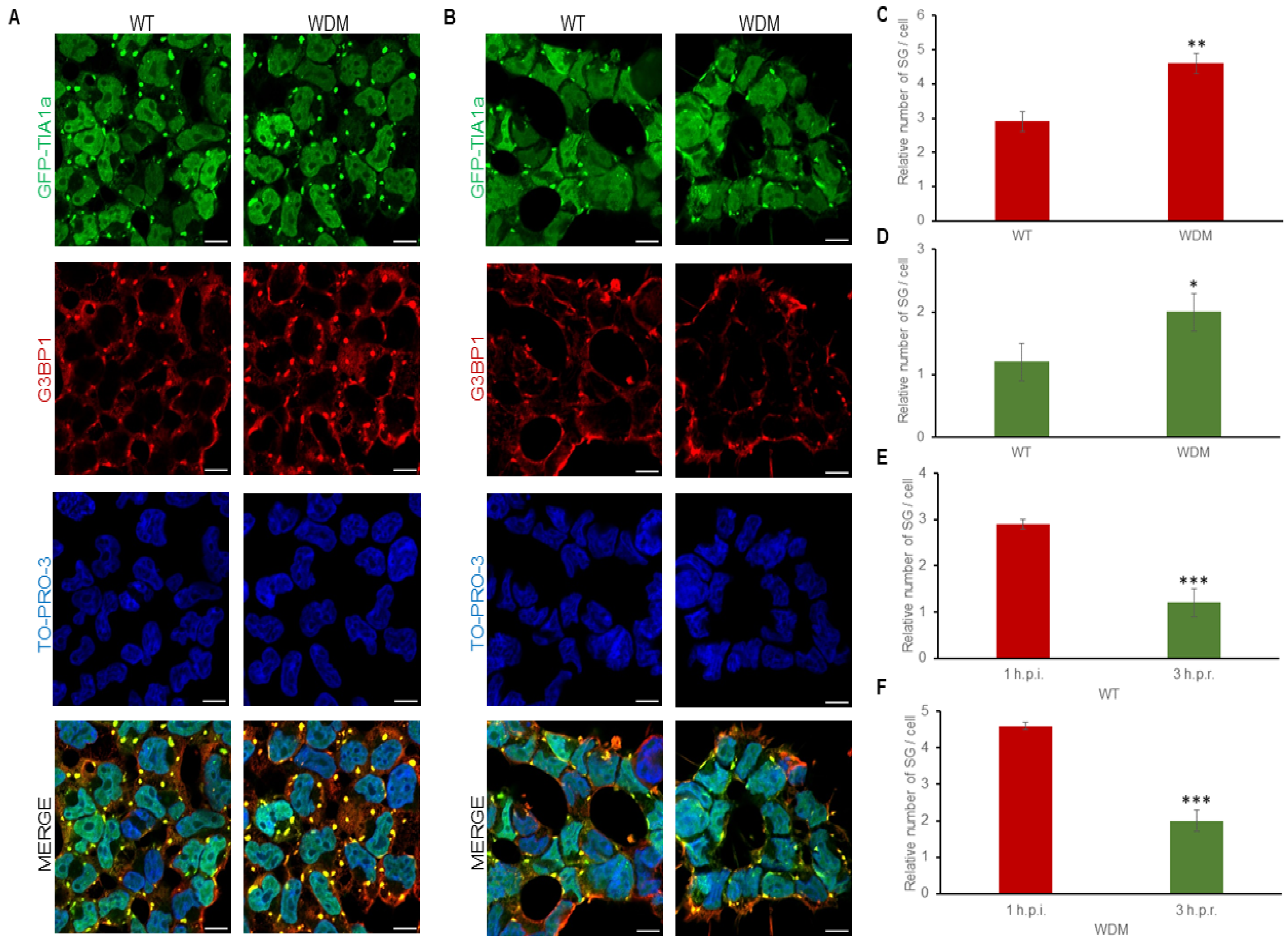
3.2. Dynamics of TIA1-Dependent SGs Under Heat Shock
3.3. Dynamics of TIA1-Dependent SGs Under Endoplasmic Reticulum-Dependent Stress
3.4. Dynamics of TIA1-Dependent SGs Under Oxidative Stress
3.5. Dynamics of TIA1-Dependent SGs Under Osmotic Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| circRNAs | Circular RNAs |
| C9orf72 | C9orf72-SMCR8 Complex Subunit |
| EIF2AK1/HRI | Eukaryotic translation initiation factor 2 alpha kinase 1/heme-regulated inhibitor |
| EIF2AK2/PKR | Eukaryotic translation initiation factor 2 alpha kinase 2/Protein kinase RNA-activated |
| EIF2AK3/PERK | Unfolded protein response-coupled eukaryotic translation initiation factor 2 alpha kinase 3/protein kinase R-like endoplasmic reticulum kinase |
| EIF2AK4/GCN2 | Eukaryotic translation initiation factor 2 alpha kinase 4/General control non-derepressible 2 (GCN2) eIF2 alpha kinase |
| eIFs | Eukaryotic translation initiation factors |
| eIF2α | Eukaryotic translation initiation factor 2 subunit alpha |
| G3BP1/2 | GTPase-activating protein (SH3 domain) binding protein 1 and 2 |
| GFP | Green fluorescence protein |
| HEK293 | Human embryonic kidney 293 |
| HSP | Heat shock protein |
| HSPA4/HSP70 | Heat shock protein family A (Hsp70) member 4 |
| HuR | Hu antigen R |
| LLPS | Liquid–liquid phase separation |
| miRNAs | MicroRNAs |
| mRNA | Protein-coding RNAs |
| NaCl | Sodium chloride |
| NaAsO2 | Sodium arsenite |
| ncRNAs | Short and long non-coding RNAs |
| PB | P-bodies |
| RBP | RNA-binding protein |
| ROS | Reactive oxygen species |
| SG | Stress granule |
| TIA1 | T-cell intracellular antigen 1 |
| TIAR | TIA1-related protein |
| TUBA | Tubulin subunit alpha |
| UPR | Unfolded protein response |
| WDM | Welander distal myopathy |
References
- Chua, B.A.; Van Der Werf, I.; Jamieson, C.; Signer, R.A.J. Post-transcriptional regulation of homeostatic, stressed, and malignant stem cells. Cell Stem Cell 2020, 26, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Scharf, K.D.; Neumann, D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Biol. 1983, 3, 1648–1655. [Google Scholar] [PubMed]
- Biancon, G.; Busarello, E.; Cheng, M.; Halene, S.; Tebaldi, T. Dissecting the stress granule RNA world: Dynamics, strategies, and data. RNA 2025, 31, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Cho, M.R.; Li, W.; Yacono, P.W.; Chen, S.; Gilks, N.; Golan, D.E.; Anderson, P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 2000, 151, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Protter, D.S.W.; Parker, R. Principles and properties of stress granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Khong, A.; Matheny, T.; Jain, S.; Mitchell, S.F.; Wheeler, J.R.; Parker, R. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell 2017, 68, 808–820.e5. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.-Y.; Dyakov, B.J.A.; Zhang, J.; Knight, J.D.R.; Vernon, R.M.; Forman-Kay, J.D.; Gingras, A.-C. Properties of stress granule and P-body proteomes. Mol. Cell 2019, 76, 286–294. [Google Scholar] [CrossRef]
- Sanders, D.W.; Kedersha, N.; Lee, D.S.W.; Strom, A.R.; Drake, V.; Riback, J.A.; Bracha, D.; Eeftens, J.M.; Iwanicki, A.; Wang, A.; et al. Competing protein-RNA interaction networks control multiphase intracellular organization. Cell 2020, 181, 306–324.e28. [Google Scholar] [CrossRef]
- Tauber, D.; Tauber, G.; Parker, R. Mechanisms and regulation of RNA condensation in RNP granule formation. Trends Biochem. Sci. 2020, 45, 764–778. [Google Scholar] [CrossRef]
- Hirose, T.; Ninomiya, K.; Nakagawa, S.; Yamazaki, T. A guide to membraneless organelles and their various roles in gene regulation. Nat. Rev. Mol. Cell Biol. 2023, 24, 288–304. [Google Scholar] [CrossRef]
- Tian, Q.; Streuli, M.; Saito, H.; Schlossman, S.F.; Anderson, P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 1991, 67, 629–639. [Google Scholar] [CrossRef] [PubMed]
- López de Silanes, I.; Galbán, S.; Martindale, J.L.; Yang, X.; Mazan-Mamczarz, K.; Indig, F.E.; Falco, G.; Zhan, M.; Gorospe, M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol. Cell. Biol. 2005, 25, 9520–9531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kayikci, M.; Briese, M.; Zarnack, K.; Luscombe, N.M.; Rot, G.; Zupan, B.; Curk, T.; Ule, J. iCLIP Predicts the dual splicing effects of TIA-RNA interactions. PLoS Biol. 2010, 8, e1000530. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, E.L.; Freese, P.; Pratt, G.A.; Wang, X.; Wei, X.; Xiao, R.; Blue, S.M.; Chen, J.Y.; Cody, N.A.L.; Dominguez, D.; et al. A large-scale binding and functional map of human RNA-binding proteins. Nature 2020, 583, 711–719. [Google Scholar] [CrossRef]
- Kedersha, N.L.; Gupta, M.; Li, W.; Miller, I.; Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 1999, 147, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maaharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 2018, 174, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, A.; Izquierdo, J.M. The multifunctional faces of T-cell intracellular antigen 1 in health and disease. Int. J. Mol. Sci. 2022, 23, 1400. [Google Scholar] [CrossRef] [PubMed]
- Welander, L. Myopathia distalis tarda hereditaria; 249 Examined cases in 72 pedigrees. Acta Med. Scand. Suppl. 1951, 265, 1–124. [Google Scholar] [PubMed]
- Borg, K.; Ählberg, G.; Anvret, M.; Edström, L. Welander distal myopathy—An overview. Neuromuscul. Disord. 1998, 8, 115–118. [Google Scholar] [CrossRef]
- Hackman, P.; Sarparanta, J.; Lehtinen, S.; Vihola, A.; Evilä, A.; Jonson, P.H.; Luque, H.; Kere, J.; Screen, M.; Chinnery, P.F.; et al. Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann. Neurol. 2013, 73, 500–509. [Google Scholar] [CrossRef]
- Klar, J.; Sobol, M.; Melberg, A.; Mäbert, K.; Ameur, A.; Johansson, A.C.; Feuk, L.; Entesarian, M.; Orlén, H.; Casar-Borota, O.; et al. Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum. Mutat. 2013, 34, 572–577. [Google Scholar]
- Mackenzie, I.R.; Nicholson, A.M.; Sarkar, M.; Messing, J.; Purice, M.D.; Pottier, C.; Annu, K.; Baker, M.; Perkerson, R.B.; Kurti, A.; et al. TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 2017, 95, 808–816.e9. [Google Scholar] [CrossRef]
- Markmiller, S.; Soltanieh, S.; Server, K.L.; Mak, R.; Jin, W.; Fang, M.; Luo, E.C.; Krach, F.; Yang, D.; Sen, A.; et al. Context-dependent and disease-specific diversity in protein interactions within stress granules. Cell 2018, 172, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Carrascoso, I.; Sánchez-Jiménez, C.; Silion, E.; Alcalde, J.; Izquierdo, J.M. A heterologous cell model for studying the role of T-cell intracellular antigen 1 in Welander distal myopathy. Mol. Cell. Biol. 2019, 39, e0029918. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, A.; Velasco, B.R.; Izquierdo, J.M. Dynamics of T-cell intracellular antigen 1-dependent stress granules in proteostasis and Welander distal myopathy under oxidative stress. Cells 2022, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Rey, I.; Ramos-Velasco, B.; Alcalde, J.; Izquierdo, J.M. Decoding the molecular grammar of TIA1-dependent stress granules in proteostasis and Welander distal myopathy under oxidative stress. Cells 2024, 13, 1961. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Velasco, B.; Alcalde, J.; Izquierdo, J.M. Welander distal myopathy-associated TIA1 mutation exacerbates P-body and stress granule dynamics concomitant with nucleolar stress under oxidative stress. Genes Dis. 2025, 12, 101543. [Google Scholar] [CrossRef]
- Purcell, N.; Manousakis, G. Diverse phenotypic presentation of the Welander distal myopathy founder mutation, with myopathy and amyotrophic lateral sclerosis in the same family. J. Clin. Neuromuscul. Dis. 2024, 26, 42–46. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, C.; Ludeña, M.D.; Izquierdo, J.M. T-cell intracellular antigens function as tumor suppressor genes. Cell Death Dis. 2015, 6, e1669. [Google Scholar] [CrossRef]
- Carrascoso, I.; Alcalde, J.; Sánchez-Jiménez, C.; González-Sánchez, P.; Izquierdo, J.M. T-cell Intracellular antigens and Hu antigen R antagonistically modulate mitochondrial activity and dynamics by regulating optic atrophy 1 gene expression. Mol. Cell. Biol. 2017, 37, e00174-17. [Google Scholar] [CrossRef]
- Farny, N.G.; Kedersha, N.L.; Silver, P.A. Metazoan stress granule assembly is mediated by P-eIF2alpha-dependent and -independent mechanisms. RNA 2009, 15, 1814–1821. [Google Scholar] [CrossRef]
- Aulas, A.; Fay, M.M.; Lyons, S.M.; Achorn, C.A.; Kedersha, N.; Anderson, P.; Ivanov, P. Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 2017, 130, 927–937. [Google Scholar] [CrossRef]
- Wippich, F.; Bodenmiller, B.; Trajkovska, M.G.; Wanka, S.; Aebersold, R.; Pelkmans, L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 2013, 152, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Bounedjah, O.; Hamon, L.; Savarin, P.; Desforges, B.; Patrick, A.; Curmi, P.A.; Pastré, D. Macromolecular crowding regulates assembly of mRNA stress granules after osmotic stress. J. Biol. Chem. 2012, 287, 2446–2458. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Li, J. IRE1α colocalizes with stress granules to regulate XBP1 mRNA splicing during ER stress. J. Cell Biol. 2024, 223, e202306123. [Google Scholar]
- Lin, Y.; Protter, D.S.W.; Rosen, M.K.; Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 2015, 60, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Chen, S.; Gilks, N.; Li, W.; Miller, I.J.; Stahl, J.; Anderson, P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 2002, 13, 195–210. [Google Scholar] [CrossRef]
- Gilks, N.; Kedersha, N.; Ayodele, M.; Shen, L.; Stoecklin, G.; Dember, L.M.; Anderson, P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 2004, 15, 5383–5398. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Sorensen, P.H. A new phase of networking: The molecular composition and regulatory dynamics of mammalian stress granules. Chem. Rev. 2023, 123, 2169–2192. [Google Scholar] [CrossRef]
- Desai, M.; Gulati, K.; Agrawal, M.; Ghumra, S.; Sahoo, P.K. Stress granules: Guardians of cellular health and triggers of disease. Neural Regen. Res. 2025, 21, 588–597. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L. Protein phase separation: A new phase in cell biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Han, T.W.; Kato, M.; Xie, S.; Wu, L.C.; Mirzaei, H.; Pei, J.; Chen, M.; Xie, Y.; Allen, J.; Xiao, G.; et al. Cell-free formation of RNA granules: Bound RNAs identify features and components of cellular assemblies. Cell 2012, 149, 768–779. [Google Scholar] [CrossRef]
- Namkoong, S.; Ho, A.; Woo, Y.M.; Kwak, H.; Lee, J.H. Systematic characterization of stress-induced RNA granulation. Mol. Cell 2018, 70, 175–187.e8. [Google Scholar] [CrossRef] [PubMed]
- Van Treeck, B.; Protter, D.S.W.; Matheny, T.; Khong, A.; Link, C.D.; Parker, R. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. USA 2018, 115, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Glauninger, H.; Hickernell, C.J.W.; Bard, J.A.M.; Drummond, D.A. Stressful steps: Progress and challenges in understanding stress-induced mRNA condensation and accumulation in stress granules. Mol. Cell 2022, 82, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Padrón, A.; Iwasaki, S.; Ingolia, N.T. Proximity RNA labeling by APEX-seq reveals the organization of translation initiation complexes and repressive RNA granules. Mol. Cell 2019, 75, 875–887.e5. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Alberti, S. Molecular determinants of condensate composition. Mol. Cell 2025, 85, 1. [Google Scholar] [CrossRef]
- Parker, D.M.; Tauber, D.; Parker, R. G3BP1 promotes intermolecular RNA-RNA interactions during RNA condensation. Mol. Cell 2025, 85, 571–584. [Google Scholar] [CrossRef]
- Wheeler, J.R.; Matheny, T.; Jain, S.; Abrisch, R.; Parker, R. Distinct stages in stress granule assembly and disassembly. Elife 2016, 5, e18413. [Google Scholar] [CrossRef]
- Ganassi, M.; Mateju, D.; Bigi, I.; Mediani, L.; Poser, I.; Lee, H.O.; Seguin, S.J.; Morelli, F.F.; Vinet, J.; Leo, G.; et al. A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity and dynamism. Mol. Cell 2016, 63, 796–810. [Google Scholar] [CrossRef]
- Mateju, D.; Franzmann, T.M.; Patel, A.; Kopach, A.; Boczek, E.E.; Maharana, S.; Lee, H.O.; Carra, S.; Hyman, A.A.; Alberti, S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017, 36, 1669–1687. [Google Scholar] [CrossRef]
- Buchan, J.R.; Kolaitis, R.-M.; Taylor, J.P.; Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 2013, 153, 1461–1474. [Google Scholar] [CrossRef]
- Seguin, S.J.; Morelli, F.F.; Vinet, J.; Amore, D.; De Biasi, S.; Poletti, A.; Rubinsztein, D.C.; Carra, S. Inhibition of autophagy, lysosome and VCP function impairs stress granule assembly. Cell Death Differ. 2014, 21, 1838–1851. [Google Scholar] [CrossRef]
- Chitiprolu, M.; Jagow, C.; Tremblay, V.; Bondy-Chorney, E.; Paris, G.; Savard, A.; Palidwor, G.; Barry, F.A.; Zinman, L.; Keith, J.; et al. A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy. Nat. Commun. 2018, 9, 2794. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B. Regulated protein aggregation: Stress granules and neurodegeneration. Mol. Neurodegener. 2012, 7, 56. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, Z.; Bai, G. Friend or foe: The role of stress granule in neurodegenerative disease. Neuron 2024, 112, 2464–2485. [Google Scholar] [CrossRef]
- Choy, M.S.; Yusoff, P.; Lee, I.C.; Newton, J.C.; Goh, C.W.; Page, R.; Shenolikar, S.; Peti, W. Structural and functional analysis of the GADD34:PP1 eIF2α phosphatase. Cell Rep. 2015, 11, 1885–1891. [Google Scholar] [CrossRef]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell. Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef]
- Kroschwald, S.; Maharana, S.; Simon, A. Hexanediol: A chemical probe to investigate the material properties of membrane-less organelles. Matters 2018, 4, e201804003. [Google Scholar]
- Yang, P.; Mathieu, C.; Kolaitis, R.-M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 2020, 181, 325–345. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, Y.; Wang, S.; Zhang, Z.; Fan, D.; Li, J.; He, X.; Zhang, Y.; Yao, Y. Liquid-liquid phase separation (LLPS) in skeletal muscle: A new frontier in muscle biology. Dev. Biol. 2025, 527, 308–317. [Google Scholar] [CrossRef]
- Vanderweyde, T.; Apicco, D.J.; Youmans-Kidder, K.; Ash, P.E.A.; Cook, C.; Lummertz da Rocha, E.; Jansen-West, K.; Frame, A.A.; Citro, A.; Leszyk, J.D.; et al. Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity. Cell Rep. 2016, 15, 1455–1466. [Google Scholar] [CrossRef]
- Apicco, D.J.; Ash, P.E.A.; Maziuk, B.; LeBlang, C.; Medalla, M.; Al Abdullatif, A.; Ferragud, A.; Botelho, E.; Balance, H.I.; Dhawan, U.; et al. Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat. Neurosci. 2018, 21, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, D.; Yang, R.; Liu, Y.; Luo, X.; Zhao, W.; Yang, H.; Chen, Z.; Shen, C.; Wang, Y.; et al. TIA1-mediated stress granules promote neurodegeneration by sequestering HSP70 mRNA in C9orf72 mice. Brain, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Lobel, J.H.; Ingolia, N.T. Deciphering disordered regions controlling mRNA decay in high-throughput. Nature 2025, 642, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Kuster, D.; Mohanty, P.; Nijssen, J.; Pombo-García, K.; Rizuan, A.; Franzmann, T.M.; Sergeeva, A.; Passos, P.M.; George, L.; et al. Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates. Cell 2025, 188, 4123–4140.e18. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Velasco, B.; Alcalde, J.; Izquierdo, J.M. Welander Distal Myopathy-Associated TIA1 E384K Mutation Disrupts Stress Granule Dynamics Under Distinct Stress Conditions. Biology 2025, 14, 1288. https://doi.org/10.3390/biology14091288
Ramos-Velasco B, Alcalde J, Izquierdo JM. Welander Distal Myopathy-Associated TIA1 E384K Mutation Disrupts Stress Granule Dynamics Under Distinct Stress Conditions. Biology. 2025; 14(9):1288. https://doi.org/10.3390/biology14091288
Chicago/Turabian StyleRamos-Velasco, Beatriz, José Alcalde, and José M. Izquierdo. 2025. "Welander Distal Myopathy-Associated TIA1 E384K Mutation Disrupts Stress Granule Dynamics Under Distinct Stress Conditions" Biology 14, no. 9: 1288. https://doi.org/10.3390/biology14091288
APA StyleRamos-Velasco, B., Alcalde, J., & Izquierdo, J. M. (2025). Welander Distal Myopathy-Associated TIA1 E384K Mutation Disrupts Stress Granule Dynamics Under Distinct Stress Conditions. Biology, 14(9), 1288. https://doi.org/10.3390/biology14091288





