Comparison of the Biological Response of a Head and Neck Carcinoma and a Glioblastoma Cell Line Under Neutron Irradiation with BPA Administration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Boron Compound Uptake
2.3. Nitrogen Content Measurement
2.4. Neutron Irradiations
2.5. Photon Irradiations at the Medical LINAC
2.6. Clonogenic Assay
2.7. Determination of CBE
3. Results
3.1. Nitrogen Content and Boron Uptake Coefficients
3.2. Photon Radiobiology Coefficients
3.3. Neutron Radiobiology Coefficients
3.4. Compound Biological Effectiveness (CBE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef]
- Hatanaka, H. Clinical Results of Boron Neutron Capture Therapy. In Neutron Beam Design, Development, and Performance for Neutron Capture Therapy; Harling, O.K., Bernard, J.A., Zamenhof, R.G., Eds.; Springer: Boston, MA, USA, 1990; Volume 54, pp. 15–21. [Google Scholar] [CrossRef]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989, 2, 388–389. [Google Scholar] [CrossRef]
- Barth, R.F.; Vicente, M.G.H.; Harling, O.K.; Kiger, W.S.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current Status of Boron Neutron Capture Therapy of High Grade Gliomas and Recurrent Head and Neck Cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zhou, D.; Wu, X.; Gao, M.; Wu, J.; Yang, Y.; Pan, X.; Wang, N. A clinician’s perspective on boron neutron capture therapy: Promising advances, ongoing trials, and future outlook. Int. J. Radiat. Biol. 2024, 100, 1126–1142. [Google Scholar] [CrossRef]
- Wittig, A.; Sauerwein, W.A.; Coderre, J.A. Mechanisms of Transport of p-Borono-Phenylalanine through the Cell Membrane In Vitro. Radiat. Res. 2000, 153, 173–180. [Google Scholar] [CrossRef]
- Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sanada, Y.; Hattori, Y.; Suzuki, M. Correlation between the expression of LAT1 in cancer cells and the potential efficacy of boron neutron capture therapy. J. Radiat. Res. 2023, 64, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Coderre, J.A.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; Liu, H.B.; Joel, D.D.; Slatkin, D.N.; Amols, H.I. Derivations of Relative Biological Effectiveness for the High-Let Radiations Produced during Boron Neutron Capture Irradiations of the 9l Rat Gliosarcoma In Vitro and In Vivo. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 1121–1129. [Google Scholar] [CrossRef]
- Coderre, J.A.; Morris, G.M. The Radiation Biology of Boron Neutron Capture Therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef]
- Chadha, M.; Capala, J.; Coderre, J.A.; Elowitz, E.H.; Iwai, J.; Joel, D.D.; Liu, H.B.; Wielopolski, L.; Chanana, A.D. Boron Neutron-Capture Therapy (BNCT) for Glioblastoma Multiforme (GBM) Using the Epithermal Neutron Beam at the Brookhaven National Laboratory. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.R.; Goorley, J.T.; Kiger, W.S.; Busse, P.M.; Riley, K.J.; Harling, O.K.; Zamenhof, R.G. Treatment Planning and Dosimetry for the Harvard-MIT Phase I Clinical Trial of Cranial Neutron Capture Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1361–1379. [Google Scholar] [CrossRef] [PubMed]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Välimäki, P.; Beule, A.; Collan, J.; Kortesniemi, M.; Uusi-Simola, J.; Kotiluoto, P.; Auterinen, I.; et al. L-Boronophenylalanine-Mediated Boron Neutron Capture Therapy for Malignant Glioma Progressing After External Beam Radiation Therapy: A Phase I Study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Capala, J.; H.-Stenstam, B.; Sköld, K.; afRosenschöld, P.M.; Giusti, V.; Persson, C.; Wallin, E.; Brun, A.; Franzen, L.; Carlsson, J.; et al. Boron Neutron Capture Therapy for Glioblastoma Multiforme: Clinical Studies in Sweden. J. Neuro-Oncol. 2003, 62, 135–144. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakai, K.; Kageji, T.; Kumada, H.; Endo, K.; Matsuda, M.; Shibata, Y.; Matsumura, A. Boron Neutron Capture Therapy for Newly Diagnosed Glioblastoma. Radiother. Oncol. 2009, 91, 80–84. [Google Scholar] [CrossRef]
- Miyatake, S.-I.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival Benefit of Boron Neutron Capture Therapy for Recurrent Malignant Gliomas. J. Neuro-Oncol. 2009, 91, 199–206. [Google Scholar] [CrossRef]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for Recurrent Head and Neck Malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakurai, Y.; Nagata, K.; Kinashi, Y.; Masunaga, S.; Ono, K.; Maruhashi, A.; Kato, I.; Fuwa, N.; Hiratsuka, J.; et al. Impact of Intra-Arterial Administration of Boron Compounds on Dose–Volume Histograms in Boron Neutron Capture Therapy for Recurrent Head-and-Neck Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head-and-Neck Cancer: Final Analysis of a Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Komori, S.; Hirose, K.; Sato, M.; Yamazaki, Y.; Takeuchi, A.; Kato, R.; Motoyanagi, T.; Narita, Y.; Kato, T.; Takai, Y. Retrospective analysis of treatment-positioning accuracy and dose error in boron neutron capture therapy using a sitting-position treatment system for head and neck cancer. Phys. Med. 2024, 126, 104818. [Google Scholar] [CrossRef]
- Takeno, S.; Yoshino, Y.; Aihara, T.; Higashino, M.; Kanai, Y.; Hu, N.; Kakino, R.; Kawata, R.; Nihei, K.; Ono, K. Preliminary Outcomes of Boron Neutron Capture Therapy for Head and Neck Cancers as a Treatment Covered by Public Health Insurance System in Japan: Real-world Experiences over a 2-year Period. Cancer Med. 2024, 13, e7250. [Google Scholar] [CrossRef]
- MedPath. Available online: https://trial.medpath.com/news/5b2177fcbd89b128/first-european-patients-receive-accelerator-based-boron-neutron-capture-therapy-for-head-and-neck-cancer (accessed on 19 June 2025).
- Marcaccio, B.; Crepaldi, M.; Postuma, I.; Simeone, E.; Guidi, C.; Fatemi, S.; Ramos, R.L.; Vercesi, V.; Ferrari, C.; Cansolino, L.; et al. Towards an adequate description of the dose-response relationship in BNCT of glioblastoma multiforme. Med. Phys. 2025, 52, 2606–2617. [Google Scholar] [CrossRef]
- Bernardini, G.F.P.; Bortolussi, S.; Koivunoro, H.; Provenzano, L.; Ferrari, C.; Cansolino, L.; Postuma, I.; Germán-Carando, D.; Kankaanranta, L.; Joensuu, H.; et al. Comparison of Photon Isoeffective Dose Models Based on In Vitro and In Vivo Radiobiological Experiments for Head and Neck Cancer Treated with BNCT. Radiat. Res. 2022, 198, 134–144. [Google Scholar] [CrossRef]
- Abele, H.; Dubbers, D.; Häse, H.; Klein, M.; Knöpfler, A.; Kreuz, M.; Lauer, T.; Märkisch, B.; Mund, D.; Nesvizhevsky, V.; et al. Characterization of a ballistic supermirror neutron guide. Nucl. Instrum. Methods Phys. Res. A 2006, 562, 407–417. [Google Scholar] [CrossRef]
- Pedrosa-Rivera, M.; Ruiz-Magaña, M.J.; Porras, I.; Praena, J.; Torres-Sánchez, P.; Sabariego, M.P.; Köster, U.; Forsyth, T.; Soldner, T.; Haertlein, M.; et al. Neutron Radiobiology Studies with a Pure Cold Neutron Beam. Nucl. Instrum. Methods Phys. Res. B 2020, 462, 24–31. [Google Scholar] [CrossRef]
- Pedrosa-Rivera, M.; Ruiz-Magaña, M.J.; Álvarez, P.; Porras, I.; Praena, J.; Sabariego, M.P.; Köster, U.; Haertlein, M.; Forsyth, V.T.; Soldner, T.; et al. Radiobiology Data of Melanoma Cells after Low-Energy Neutron Irradiation and Boron Compound Administration. Appl. Radiat. Isot. 2020, 163, 109205. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa-Rivera, M.; Praena, J.; Porras, I.; Sabariego, M.P.; Köster, U.; Haertlein, M.; Forsyth, V.T.; Ramírez, J.C.; Jover, C.; Jimena, D.; et al. Thermal Neutron Relative Biological Effectiveness Factors for Boron Neutron Capture Therapy from In Vitro Irradiations. Cells 2020, 9, 2144. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van-Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Pedrosa-Rivera, M.; Praena, J.; Porras, I.; Ruiz-Magaña, M.J.; Ruiz-Ruiz, C. A Simple Approximation for the Evaluation of the Photon Iso-Effective Dose in Boron Neutron Capture Therapy Based on Dose-Independent Weighting Factors. Appl. Radiat. Isot. 2020, 157, 109018. [Google Scholar] [CrossRef]
- Pedrosa-Rivera, M. Radiobiological Studies for Improving the Treatment Planning of Neutron Capture Therapy. Ph.D. Thesis, University of Granada, Granada, Spain, 14 July 2020. Available online: https://hdl.handle.net/10481/63920 (accessed on 19 June 2025).
- Fowler, J.F. The Linear-Quadratic Formula and Progress in Fractionated Radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Scarboro, S.B.; Followill, D.S.; Howell, R.M.; Kry, S.F. Variations in photon energy spectra of a 6 MV beam and their impact on TLD response. Med. Phys. 2011, 38, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, M.; Puskin, J.; Hertel, N.; Eckerman, K. An empirical method for deriving RBE values associated with electrons, photons and radionuclides. Radiat. Prot. Dosim. 2015, 167, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. (Eds.) Numerical Recipes: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Bauer, V.L.; Hieber, L.; Schaeffner, Q.; Weber, J.; Braselmann, H.; Huber, R.; Walch, A.; Zitzelsberger, H. Establishment and Molecular Cytogenetic Characterization of a Cell Culture Model of Head and Neck Squamous Cell Carcinoma (HNSCC). Genes 2010, 1, 388–412. [Google Scholar] [CrossRef] [PubMed]
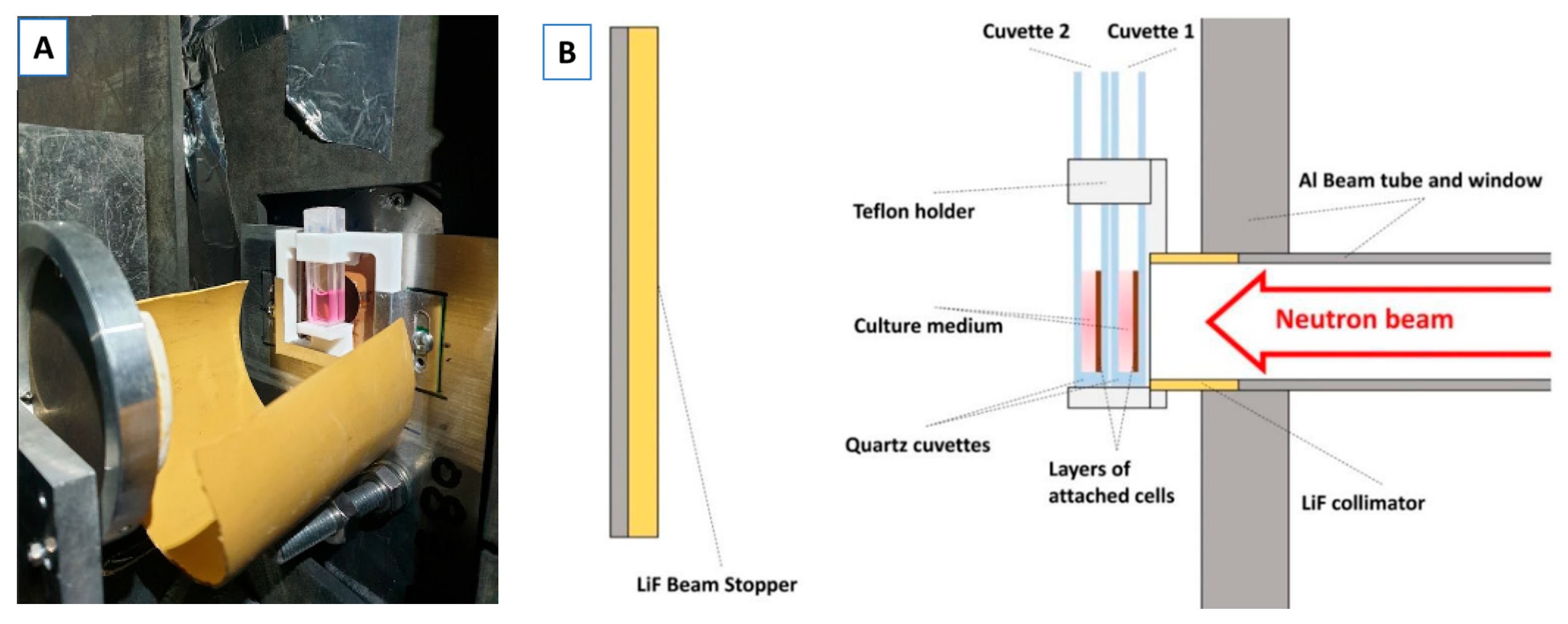


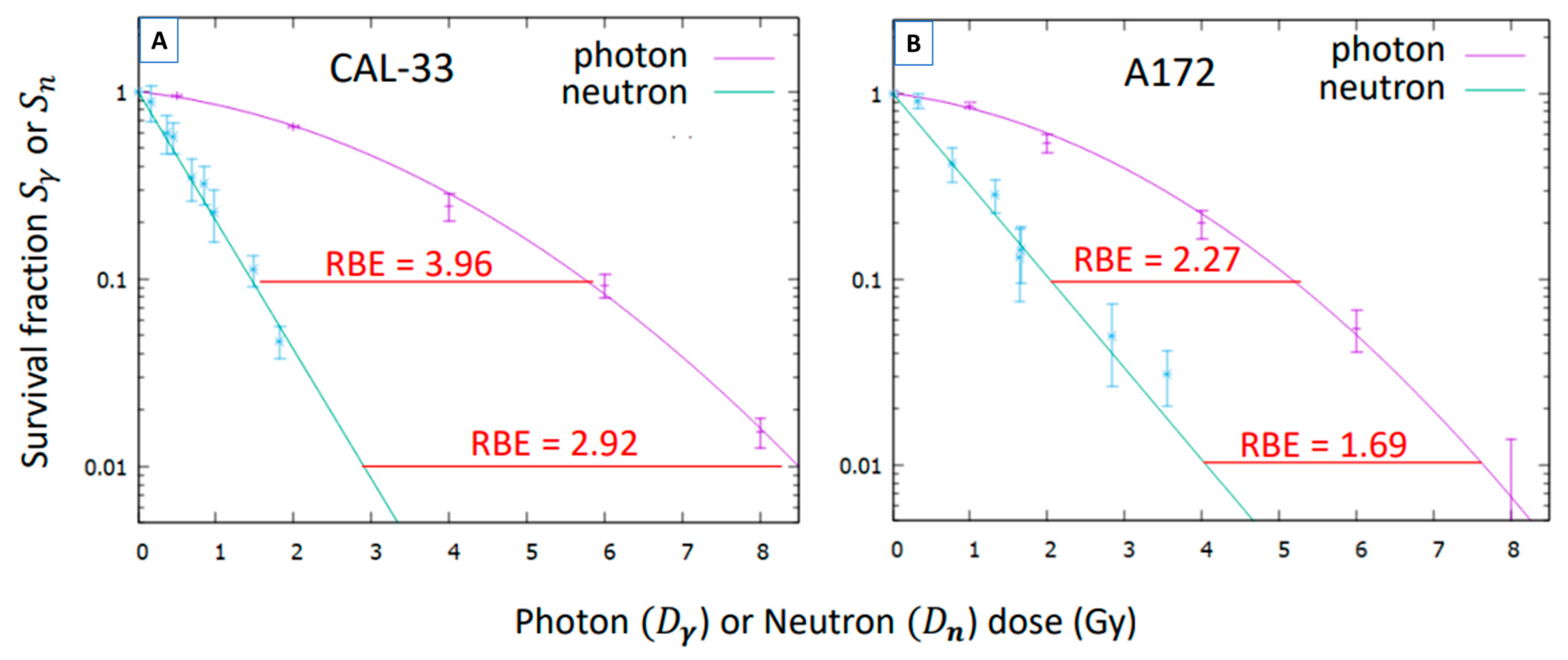
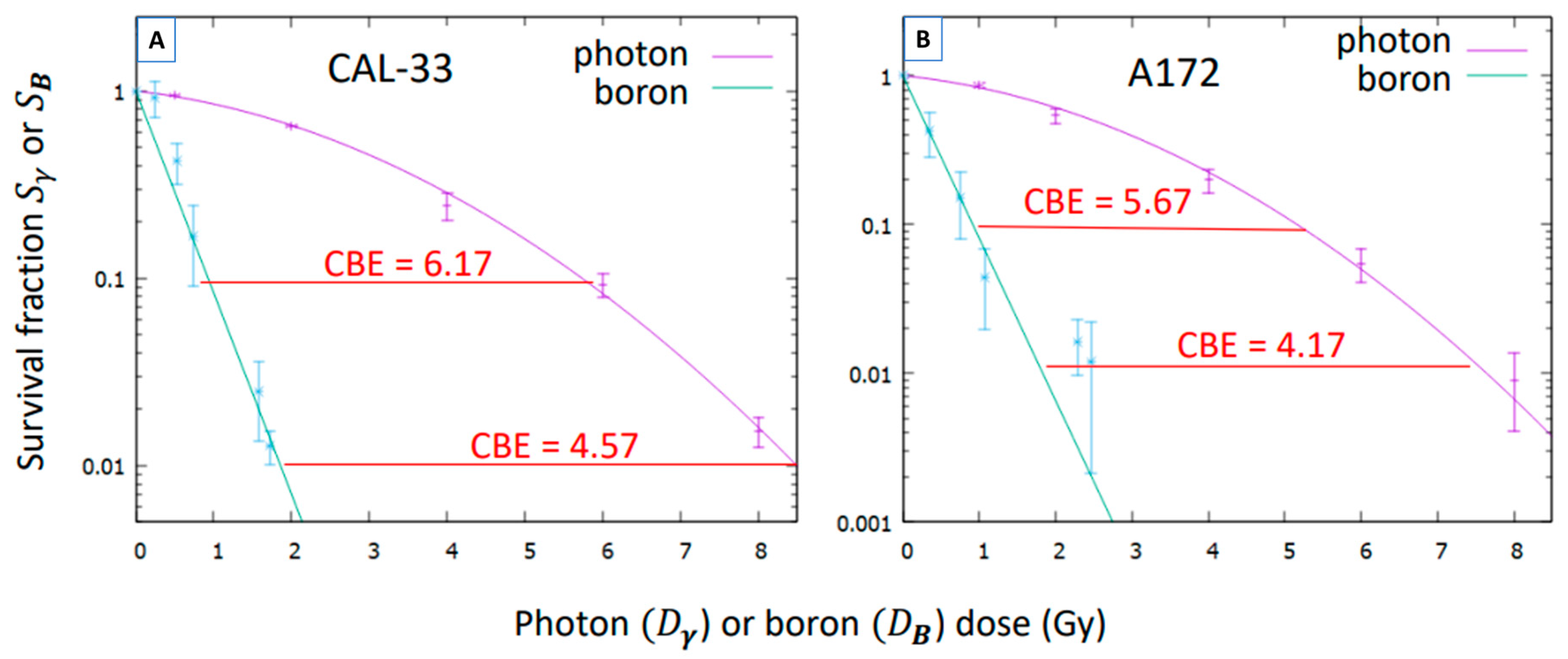
| Cell Line | %Nitrogen | B Conc (ppm) |
|---|---|---|
| CAL-33 | 2.3 ± 0.1 | 29 ± 5 |
| A172 | 3.5 ± 0.5 | 23 ± 5 |
| Cell Line | (Gy−1) | (Gy−2) | (Gy) |
|---|---|---|---|
| CAL-33 | 0.109 ± 0.009 | 0.051 ± 0.003 | 2.12 ± 0.30 |
| A172 | 0.121 ± 0.031 | 0.063 ± 0.008 | 1.92 ± 0.74 |
| Cell Line | (Gy−1) | (Gy−1) | ||
|---|---|---|---|---|
| CAL-33 | 1.59 ± 0.05 | 14.5 ± 1.6 | 2.48 ± 0.11 | 22.7 ± 2.9 |
| A172 | 1.02 ± 0.06 | 8.4 ± 2.6 | 2.51 ± 0.29 | 20.8 ± 7.7 |
| CAL-33 | A172 | |||
|---|---|---|---|---|
| CBE | CBE | |||
| 1 | 5.96 | 5.96 | 5.43 | 5.43 |
| 2 | 8.82 | 4.41 | 8.02 | 4.01 |
| 3 | 11.0 | 3.67 | 10.0 | 3.34 |
| 4 | 12.9 | 3.22 | 11.7 | 2.93 |
| 5 | 14.5 | 2.90 | 13.2 | 2.64 |
| 6 | 16.0 | 2.66 | 14.5 | 2.42 |
| 7 | 17.3 | 2.48 | 15.8 | 2.25 |
| 8 | 18.6 | 2.33 | 16.9 | 2.12 |
| 9 | 19.8 | 2.20 | 18.0 | 2.00 |
| 10 | 20.9 | 2.09 | 19.0 | 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Rodríguez, P.; Méndez-Malagón, C.; Porras-Quesada, M.; Pedrosa-Rivera, M.; Köster, U.; Porras, I.; Praena, J.; Estrada, R.; Pérez-Fuentes, L.; Osorio-Ceballos, J.L.; et al. Comparison of the Biological Response of a Head and Neck Carcinoma and a Glioblastoma Cell Line Under Neutron Irradiation with BPA Administration. Biology 2025, 14, 1252. https://doi.org/10.3390/biology14091252
Álvarez-Rodríguez P, Méndez-Malagón C, Porras-Quesada M, Pedrosa-Rivera M, Köster U, Porras I, Praena J, Estrada R, Pérez-Fuentes L, Osorio-Ceballos JL, et al. Comparison of the Biological Response of a Head and Neck Carcinoma and a Glioblastoma Cell Line Under Neutron Irradiation with BPA Administration. Biology. 2025; 14(9):1252. https://doi.org/10.3390/biology14091252
Chicago/Turabian StyleÁlvarez-Rodríguez, Patricia, Cristina Méndez-Malagón, Maribel Porras-Quesada, María Pedrosa-Rivera, Ulli Köster, Ignacio Porras, Javier Praena, Rocío Estrada, Leonor Pérez-Fuentes, Juan Luis Osorio-Ceballos, and et al. 2025. "Comparison of the Biological Response of a Head and Neck Carcinoma and a Glioblastoma Cell Line Under Neutron Irradiation with BPA Administration" Biology 14, no. 9: 1252. https://doi.org/10.3390/biology14091252
APA StyleÁlvarez-Rodríguez, P., Méndez-Malagón, C., Porras-Quesada, M., Pedrosa-Rivera, M., Köster, U., Porras, I., Praena, J., Estrada, R., Pérez-Fuentes, L., Osorio-Ceballos, J. L., Ruiz-Ruiz, C., Sancey, L., & Ruiz-Magaña, M. J. (2025). Comparison of the Biological Response of a Head and Neck Carcinoma and a Glioblastoma Cell Line Under Neutron Irradiation with BPA Administration. Biology, 14(9), 1252. https://doi.org/10.3390/biology14091252








