Mucus Secretion as a Defensive Mechanism in the Freshwater Flatworm Stenostomum sphagnetorum Against the Ciliate Predator Coleps hirtus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Culture Methods
2.2. Preparation of Secretion-Deprived S. sphagnetorum
2.3. Isolation of Toxicyst Discharge of C. hirtus
2.4. Liquid Chromatography–Mass Spectrometry
2.5. Analysis of the Content of Glandular Secretion of S. sphagnetorum
2.6. Toxicity Test
2.7. Chemicals
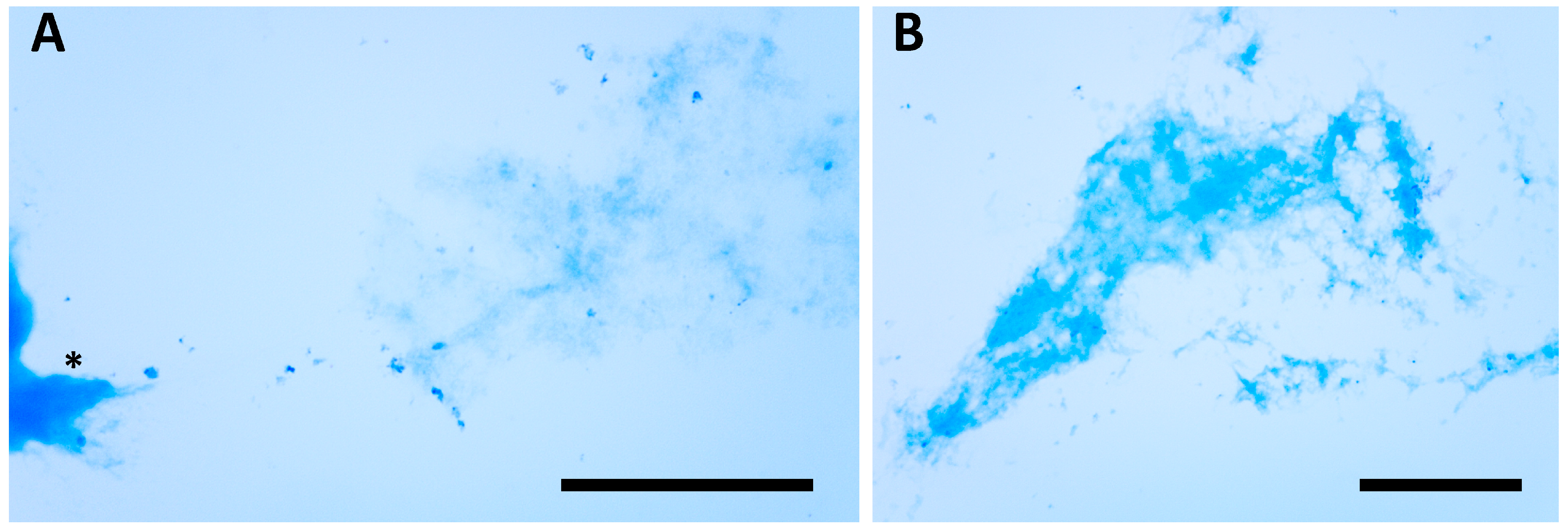
3. Results
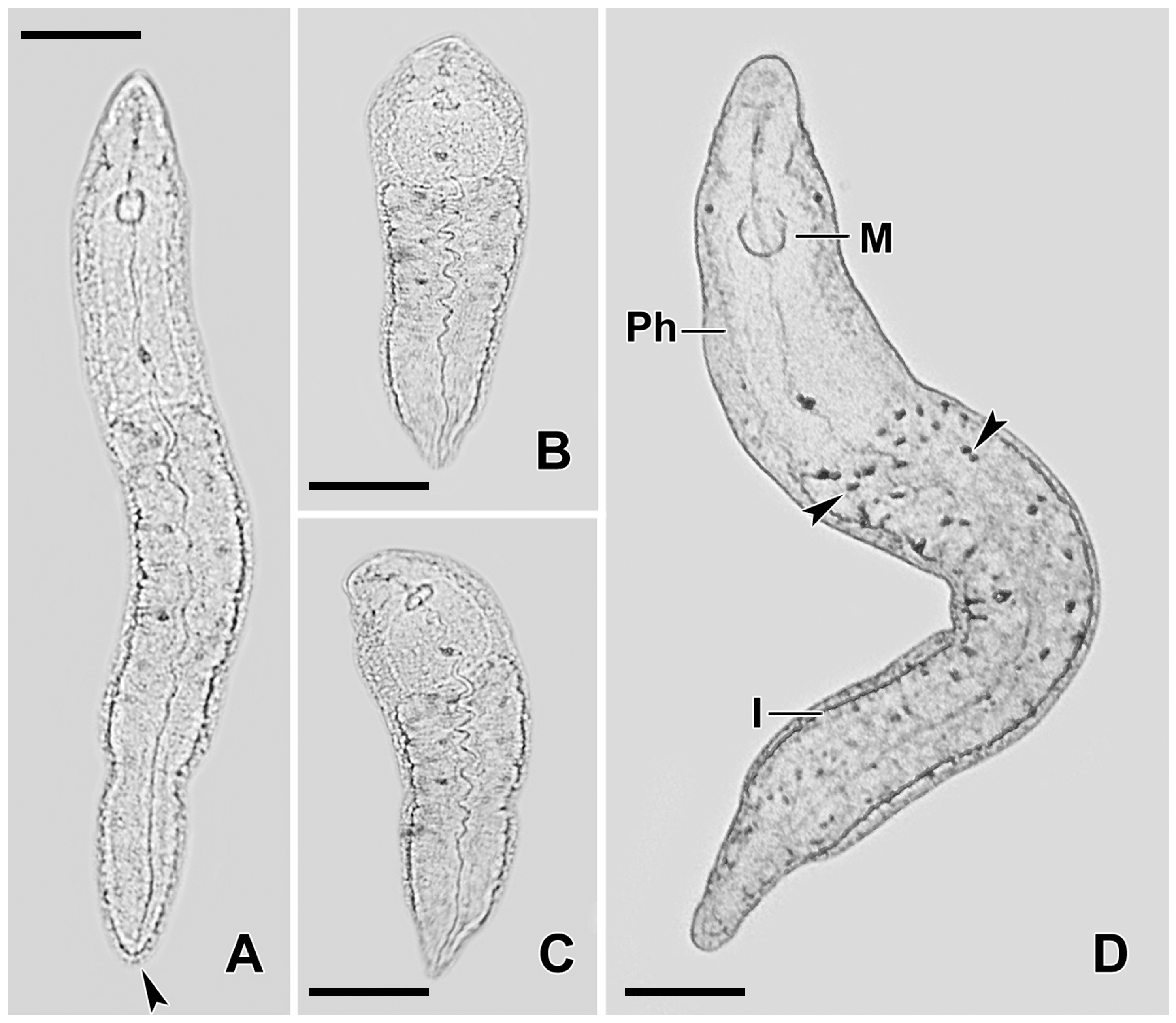
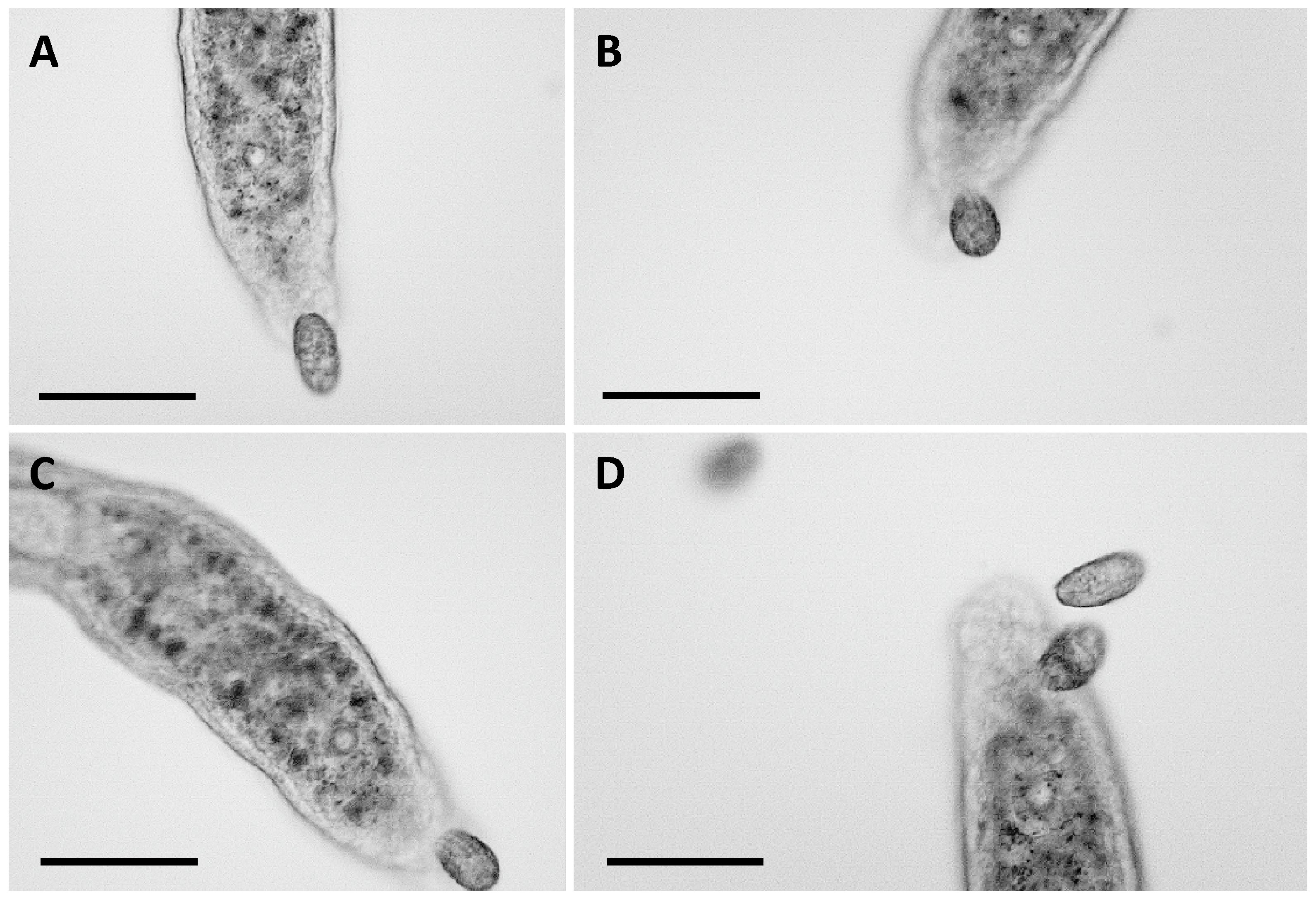
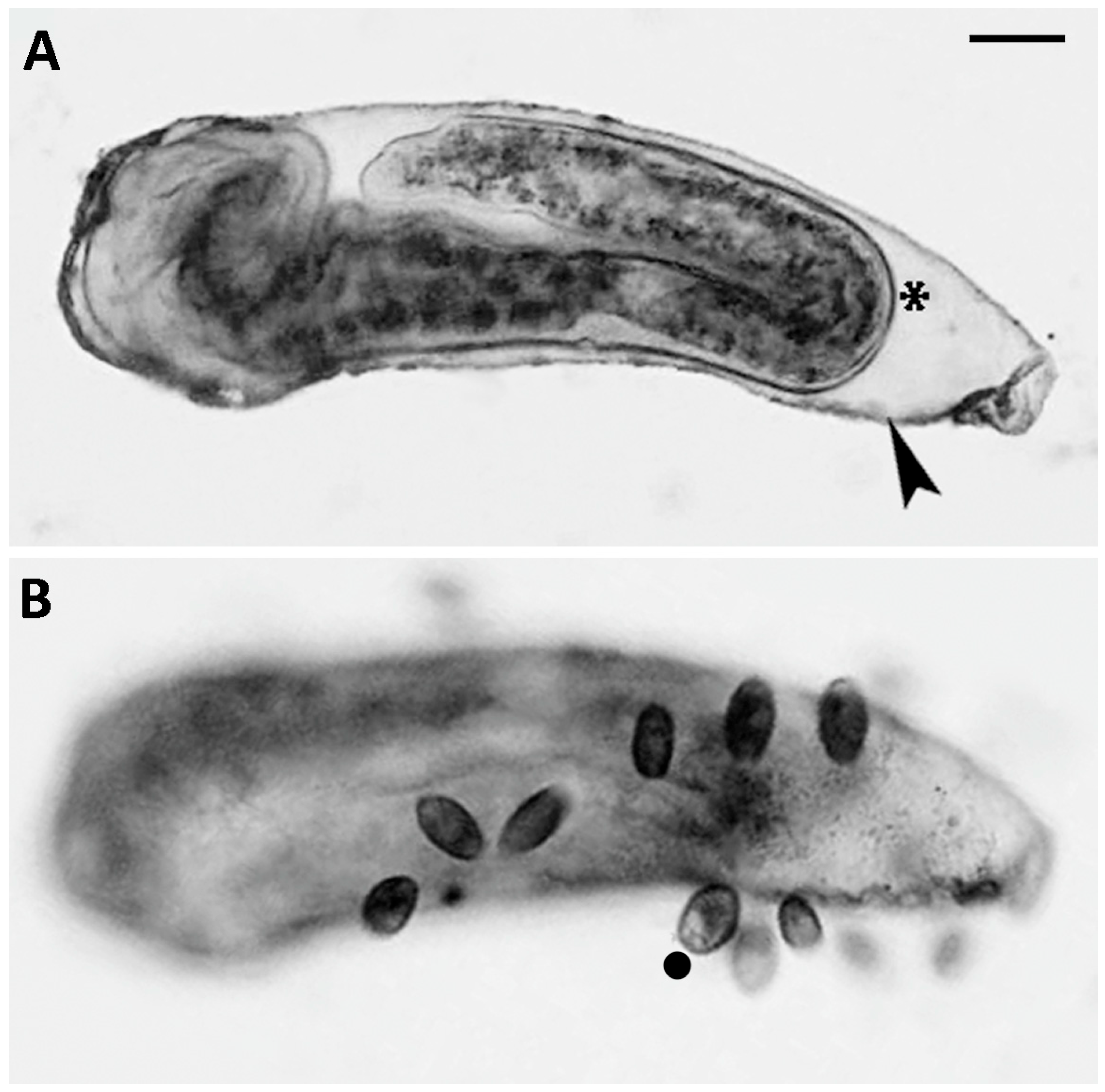
3.1. Predator–Prey Interactions Between S. sphagnetorum and C. hirtus
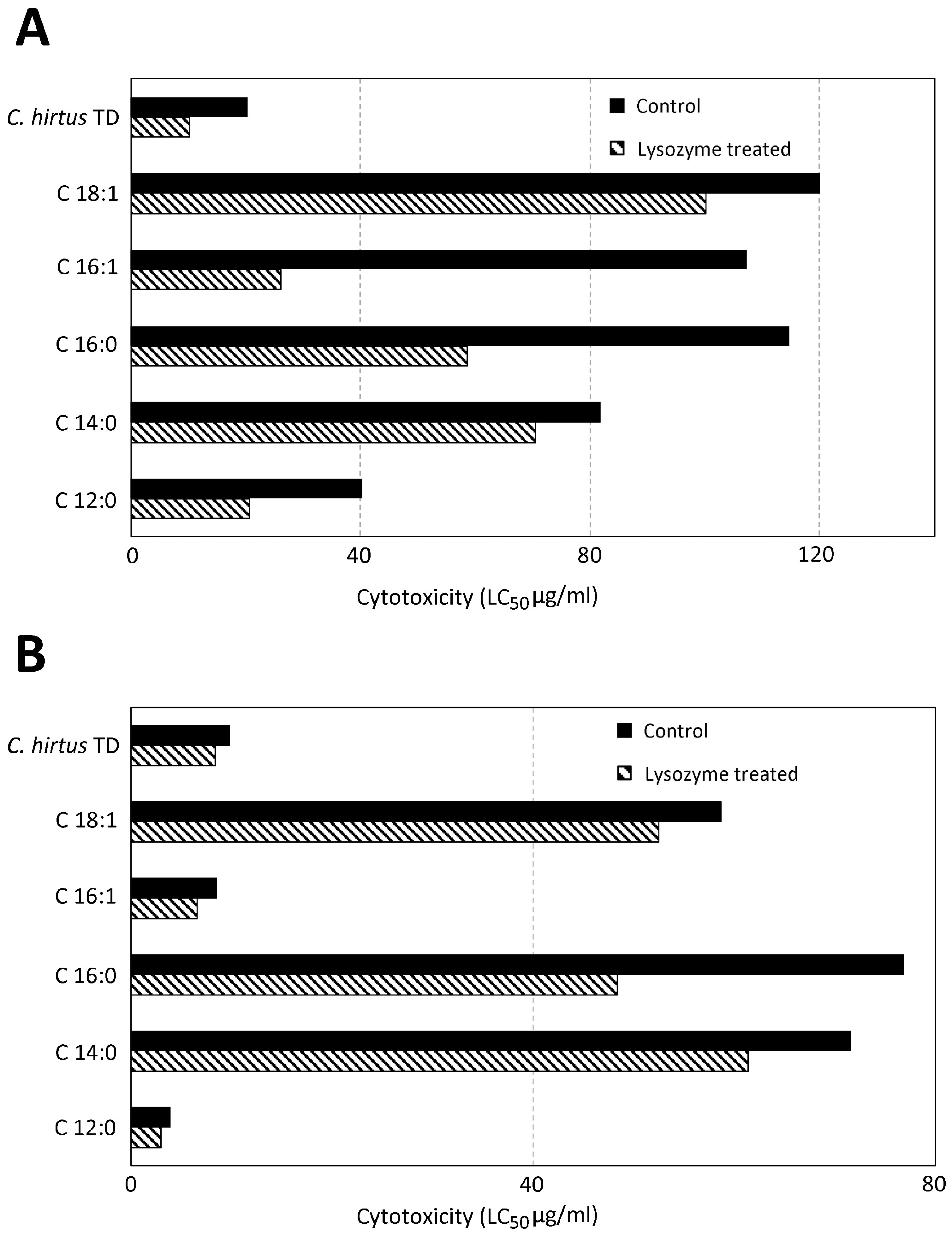
3.2. Toxicity Evaluation of TD of C. hirtus and Fatty Acids on S. sphagnetorum
3.3. Characterization of the Main Components of the Secretion of S. sphagnetorum
4. Discussion
4.1. Predator–Prey Interactions
4.2. Toxicity Tests
4.3. Chemical Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMB | Synthetic medium for Blepharisma |
| SD | Secretion-deprived |
| TD | Toxicyst discharge |
| PAS | Periodic acid–Schiff |
References
- Bharti, D.; Brusa, F.; Kumar, S.; Chandra, K. First record of Stenostomum sphagnetorum (Platyhelminthes, Catenulida) from India. Zootaxa 2020, 4816, 392–396. [Google Scholar] [CrossRef]
- Larsson, K.; Jondelius, U. Phylogeny of Catenulida and support for Platyhelminthes. Org. Divers. Evol. 2008, 8, 378–387. [Google Scholar] [CrossRef]
- Buonanno, F. Antipredator behavior of freshwater microturbellarian Stenostomum sphagnetorum against the predatory ciliate Dileptus margaritifer. Zool. Sci. 2009, 26, 443–447. [Google Scholar] [CrossRef]
- Laas, S.; Spaak, P. Chemically induced antipredator defences in plankton: A review. Hydrobiologia 2003, 491, 221–239. [Google Scholar] [CrossRef]
- Buonanno, F. The changes in the predatory behavior of the microturbellarian Stenostomum sphagnetorum on two species of toxin-secreting ciliates of the genus Spirostomum. Biologia 2011, 66, 648–653. [Google Scholar] [CrossRef]
- Kuhlmann, H.-W.; Heckmann, K. Interspecific morphogens regulating prey–predator relationships in protozoa. Science 1985, 227, 1347–1349. [Google Scholar] [CrossRef]
- Kuhlmann, H.-W.; Heckmann, K. Predation risk of typical ovoid and winged morphs of Euplotes (Protozoa, Ciliophora). Hydrobiologia 1994, 284, 219–227. [Google Scholar] [CrossRef]
- Foissner, W.; Berger, H.; Scaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Bayerisches Landesamt für Wasserwirtschaft: München, Germany, 1999; pp. 272–287. [Google Scholar]
- Buonanno, F.; Anesi, A.; Guella, G.; Kumar, S.; Bharti, D.; La Terza, A.; Quassinti, L.; Bramucci, M.; Ortenzi, C. Chemical offense by means of toxicysts in the freshwater ciliate Coleps hirtus. J. Eukaryot. Microbiol. 2014, 61, 293–304. [Google Scholar] [CrossRef]
- Auer, B.; Czioska, E.; Hartmut, A. The pelagic community of a gravel pit lake: Significance of Coleps hirtus viridis (Prostomatida) and its role as a scavenger. Limnologica 2004, 34, 187–198. [Google Scholar] [CrossRef]
- Mazanec, A.I.; Trevarrow, B. Coleps, scourge of the baby Zebrafish. Zebrafish Sci. Monit. 1998, 5, 1. [Google Scholar]
- Bharti, D.; Kumar, S.; Buonanno, F.; Ortenzi, C.; Montanari, A.; Quintela-Alonso, P.; La Terza, A. Free living ciliated protists from the chemoautotrophic cave ecosystem of Frasassi (Italy). Subterr. Biol. 2022, 44, 167–198. [Google Scholar] [CrossRef]
- Miyake, A. Cell interaction by gamones in Blepharisma. In Sexual Interactions in Eukaryotic Microbes; O’Day, D.H., Horgen, P.A., Eds.; Academic Press: New York, NY, USA, 1981; pp. 95–129. [Google Scholar]
- Belcher, H.; Swale, E. Culturing Algae; Titus Wilson & Son Ltd.: Kendal, UK, 1982; pp. 10–15. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Dettner, K. Chemical defense and toxins of lower terrestrial and freshwater animals. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Liu, H.-W., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; Volume 4, pp. 387–410. [Google Scholar]
- Liana, M.K.; Quiroga, S.Y.; Bola, D.M.; Litvaitis, M.K. Comparative morphology of the epidermis of seven species of polyclad flatworms (Platyhelminthes: Rhabditophora). Zool. Anz. 2011, 251, 206–214. [Google Scholar] [CrossRef]
- Peiris, T.H.; Hoyer, K.K.; Oviedo, N.J. Innate immune system and tissue regeneration in planarians: An area ripe for exploration. Semin. Immunol. 2014, 26, 295–302. [Google Scholar] [CrossRef]
- Silva, M.S.; Willemart, R.H.; Carbayo, F. Sticky flatworms (Platyhelminthes) kill armored harvestmen (Arachnida, Opiliones) but are not immune to the prey’s weapons. J. Zool. 2018, 306, 88–94. [Google Scholar] [CrossRef]
- Martin, G.G. A new function of rhabdites: Mucus production for ciliary gliding. Zoomorphologie 1978, 91, 235–248. [Google Scholar] [CrossRef]
- Stokes, A.N.; Ducey, P.K.; Neuman-Lee, L.N.; Hanifin, C.T.; French, S.S.; Pfrender, M.E.; Brodie, E.D., III; Brodie, E.D., Jr. Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: Two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense). PLoS ONE 2014, 9, e100718. [Google Scholar] [CrossRef]
- Bocchinfuso, D.G.; Taylor, P.; Ross, E.; Ignatchenko, A.; Ignatchenko, V.; Kislinger, T.; Pearson, B.J.; Moran, M.F. Proteomic profiling of the planarian Schmidtea mediterranea and its mucous reveals similarities with human secretions and those predicted for parasitic flatworms. Mol. Cell. Proteom. 2012, 11, 681–691. [Google Scholar] [CrossRef]
- Noreña, C.; Damborenea, C.; Brusa, F. A taxonomic revision of South American species of the genus Stenostomum O. Schmidt (Platyhelminthes: Catenulida) based on morphological characters. Zool. J. Linn. Soc. 2005, 144, 37–58. [Google Scholar] [CrossRef]
- Bataller, E.E.; Boghen, A.D.; Burt, M.D.B. Electron and light microscopic study of various glands and the secretions released into the environment by the turbellarian Urastoma cyprinae. J. Morphol. 2003, 258, 307–316. [Google Scholar] [CrossRef]
- McGee, C.; Wisdom, G.B.; Fairweather, I.; Blackshaw, R.P.; McIlroy, J.; Walker, B. Characterization of the proteins present in the mucus of the flatworm Artioposthia triangulata (Dendy). Comp. Biochem. Physiol. 1998, 119, 293–298. [Google Scholar] [CrossRef]
- Rosati, G.; Modeo, L. Extrusomes in ciliates: Diversification, distribution, and phylogenetic implications. J. Eukaryot. Microbiol. 2003, 50, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J. Sulphated glycosaminoglycans support an assortment of planarian rhabdite structures. Biol. Open 2017, 6, 571–581. [Google Scholar] [CrossRef]
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Mäkelä, S.; Aittokallio, T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Gambino, G.; Pozzo, E.D.; Salvetti, A.; Rossi, L. Planarian mucus: A novel source of pleiotropic cytotoxic and cytostatic agents against cancer cells. Biomolecules 2024, 14, 1075. [Google Scholar] [CrossRef]
- Luo, Y.; Cobb, R.E.; Zhao, H. Recent advances in natural product discovery. Curr. Opin. Biotechnol. 2014, 30, 230–237. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achille, G.; Kumar, S.; Bharti, D.; Guella, G.; Ortenzi, C.; Buonanno, F. Mucus Secretion as a Defensive Mechanism in the Freshwater Flatworm Stenostomum sphagnetorum Against the Ciliate Predator Coleps hirtus. Biology 2025, 14, 1253. https://doi.org/10.3390/biology14091253
Achille G, Kumar S, Bharti D, Guella G, Ortenzi C, Buonanno F. Mucus Secretion as a Defensive Mechanism in the Freshwater Flatworm Stenostomum sphagnetorum Against the Ciliate Predator Coleps hirtus. Biology. 2025; 14(9):1253. https://doi.org/10.3390/biology14091253
Chicago/Turabian StyleAchille, Gabriele, Santosh Kumar, Daizy Bharti, Graziano Guella, Claudio Ortenzi, and Federico Buonanno. 2025. "Mucus Secretion as a Defensive Mechanism in the Freshwater Flatworm Stenostomum sphagnetorum Against the Ciliate Predator Coleps hirtus" Biology 14, no. 9: 1253. https://doi.org/10.3390/biology14091253
APA StyleAchille, G., Kumar, S., Bharti, D., Guella, G., Ortenzi, C., & Buonanno, F. (2025). Mucus Secretion as a Defensive Mechanism in the Freshwater Flatworm Stenostomum sphagnetorum Against the Ciliate Predator Coleps hirtus. Biology, 14(9), 1253. https://doi.org/10.3390/biology14091253







