Seed Morphology of Allium L. Endemic Species from Section Schoenoprasum (Amaryllidaceae) in Eastern Kazakhstan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Sites
2.2. Biometric Analysis of Seeds
2.3. Statistics
3. Results
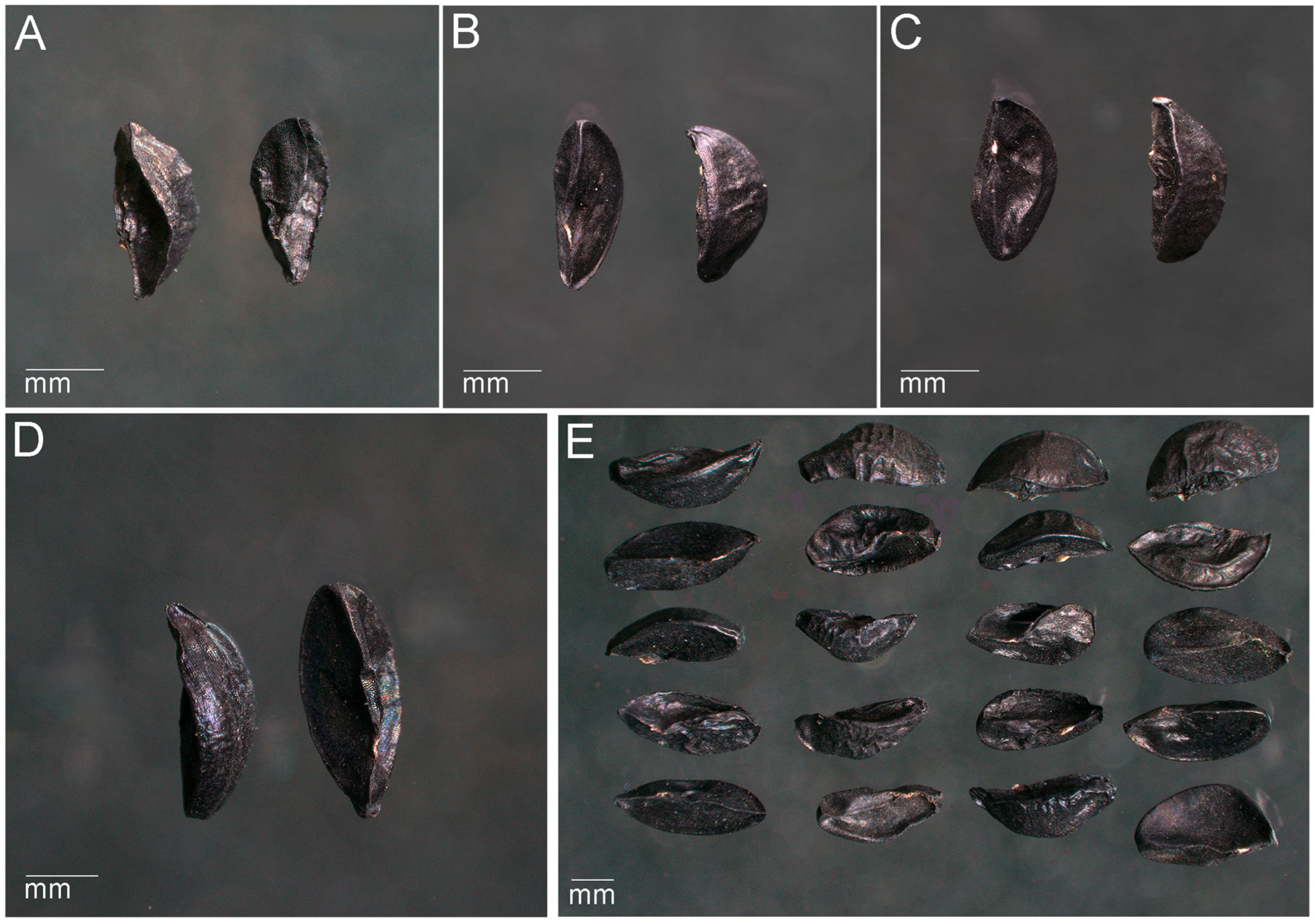
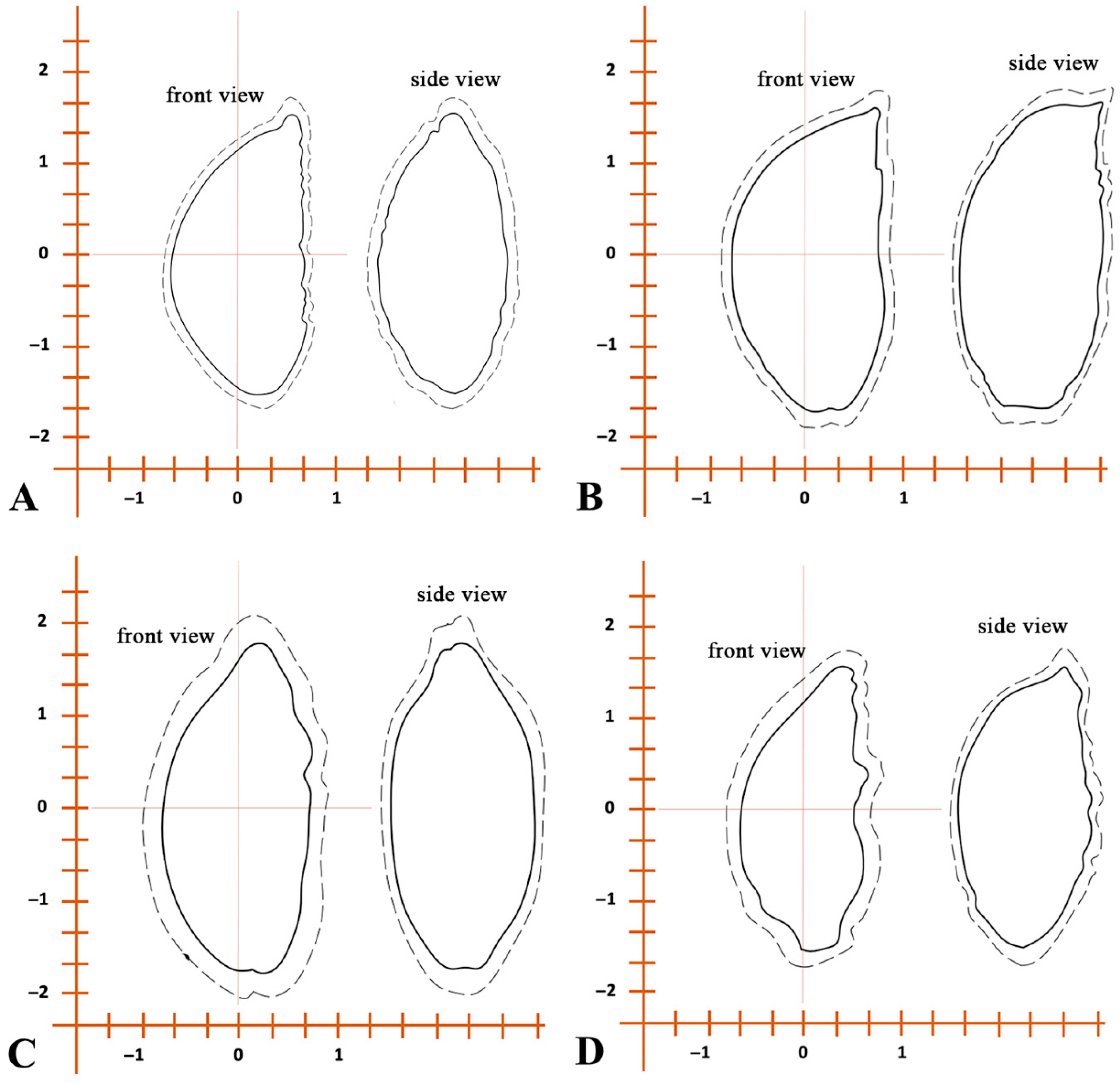
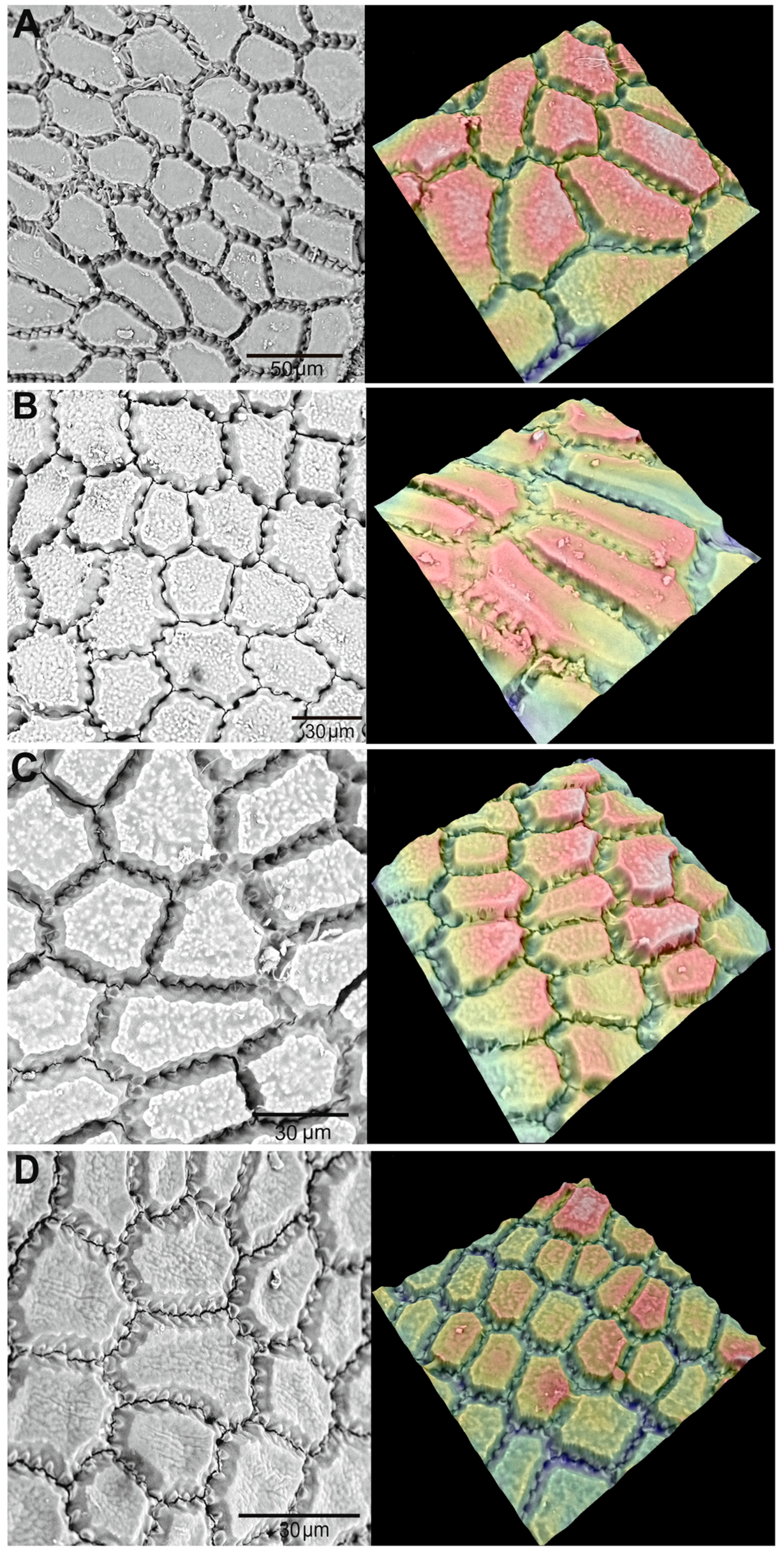
3.1. External Morphology and Seed Metrics
3.2. Analysis of Morphology of Seed Traits
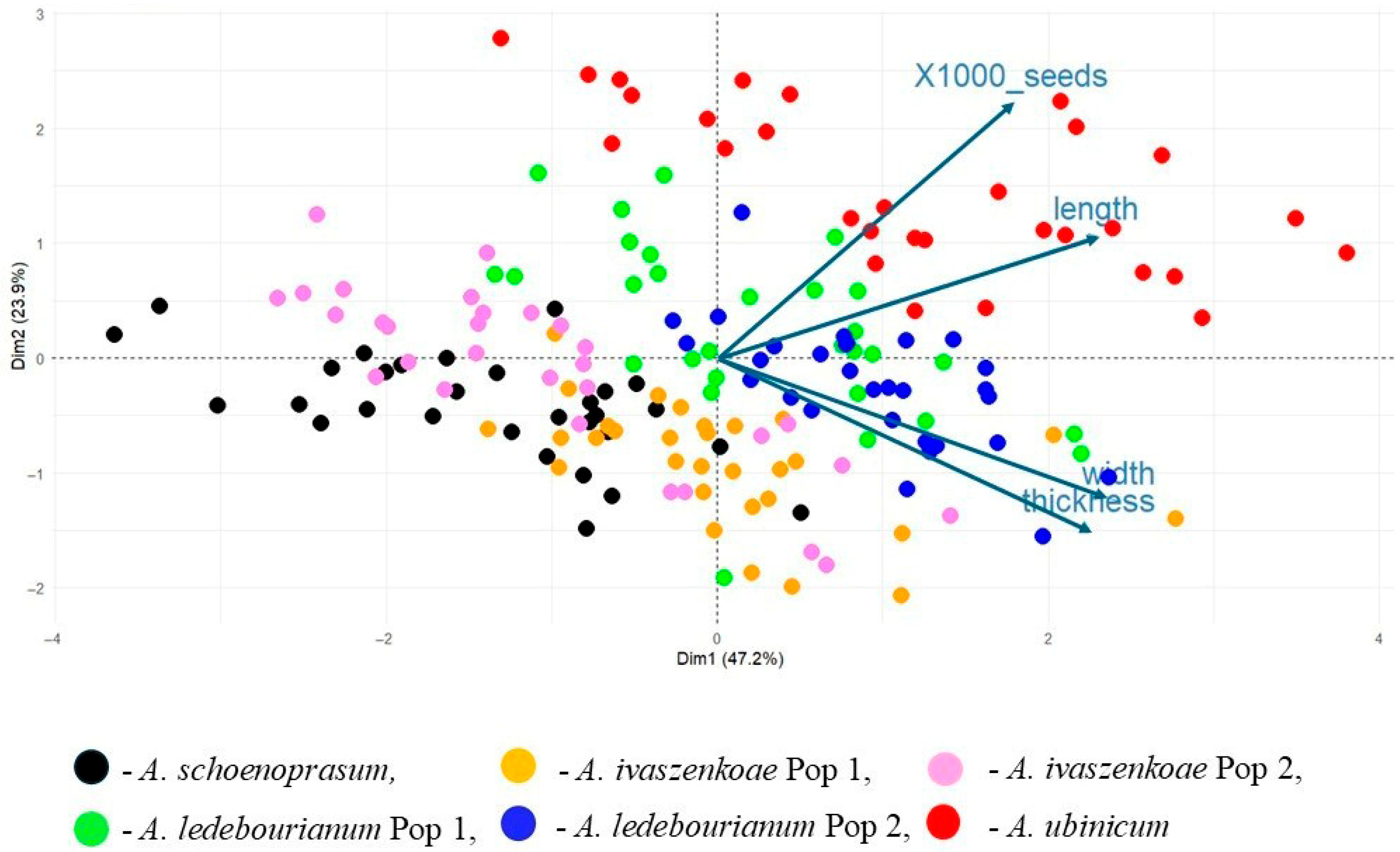
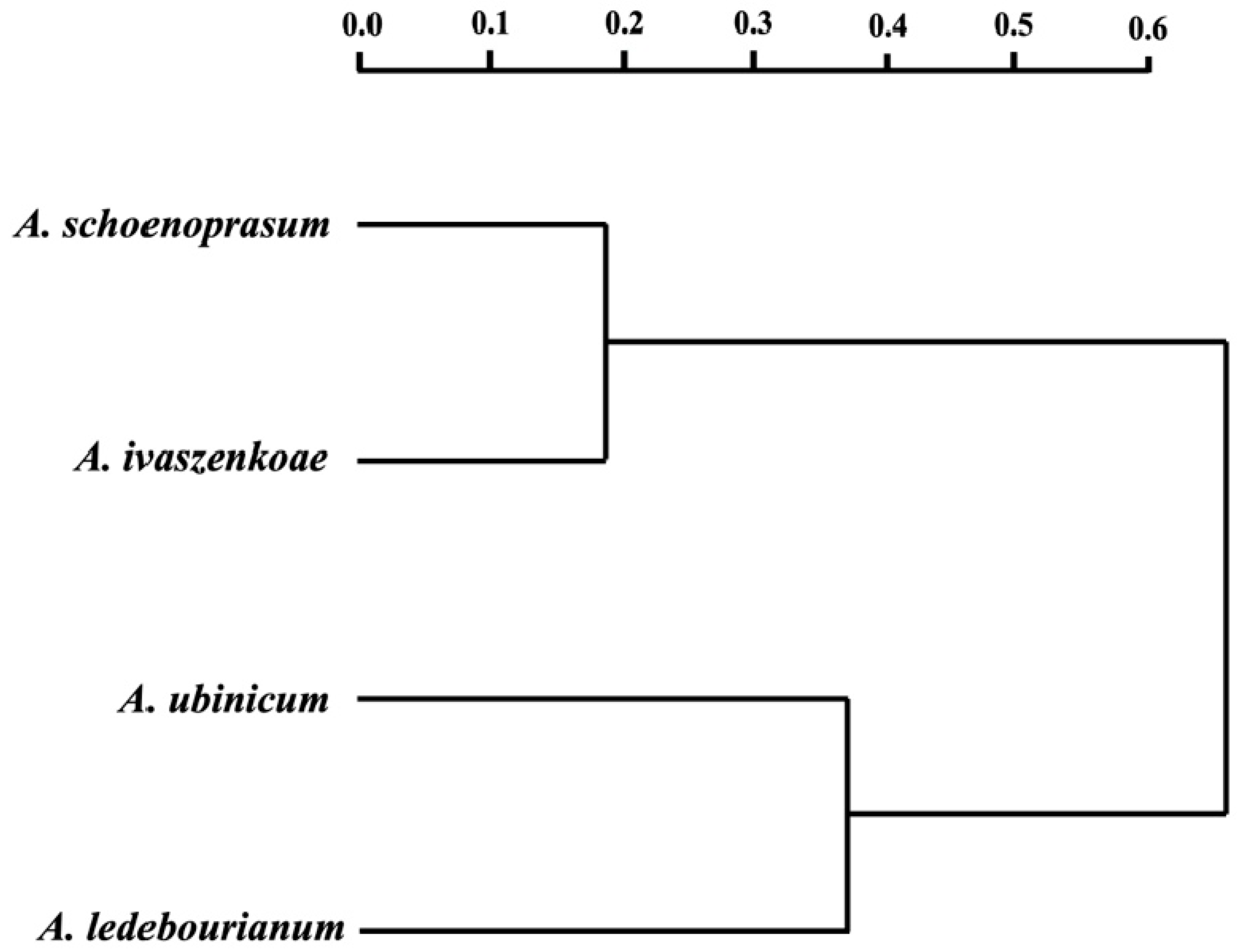
3.3. Multivariate Analysis of Seed Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscopy |
| EIVs | Ellenberg Indicator Values |
References
- POWO. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 7 March 2025).
- Friesen, N.; Fritsch, R.M.; Blattner, F.R. Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS sequences. Aliso J. Syst. Florist. Bot. 2006, 22, 372–395. [Google Scholar] [CrossRef]
- Fritsch, R.M. Taxonomy of the genus Allium: Contribution from IPK Gatersleben. Herbertia 2001, 56, 19–50. [Google Scholar]
- Sharifi-Rad, J.; Mnayer, D.; Tabanelli, G.; Stojanović-Radić, Z.Z.; Sharifi-Rad, M.; Yousaf, Z.; Iriti, M. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell. Mol. Biol. 2016, 62, 57–68. [Google Scholar] [PubMed]
- Hauenschild, F.; Favre, A.; Schnitzler, J.; Michalak, I.; Freiberg, M.; Muellner-Riehl, A.N. Spatio-temporal evolution of Allium L. in the Qinghai–Tibet-Plateau region: Immigration and in situ radiation. Plant Divers. 2017, 39, 167–179. [Google Scholar] [CrossRef]
- Khapilina, O.; Raiser, O.; Danilova, A.; Shevtsov, V.; Turzhanova, A.; Kalendar, R. DNA profiling and assessment of genetic diversity of relict species Allium altaicum Pall. on the territory of Altai. PeerJ 2021, 9, e10674. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, P. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Yan, J.K.; Zhu, J.; Liu, Y.; Chen, X.; Wang, W.; Zhang, H.; Li, L. Recent advances in research on Allium plants: Functional ingredients, physiological activities, and applications in agricultural and food sciences. Crit. Rev. Food Sci. Nutr. 2023, 63, 8107–8135. [Google Scholar] [CrossRef]
- Yusupov, Z.; Deng, T.; Volis, S.; Khassanov, F.; Makhmudjanov, D.; Tojibaev, K.; Sun, H. Phylogenomics of Allium section Cepa (Amaryllidaceae) provides new insights on domestication of onion. Plant Divers. 2021, 43, 102–110. [Google Scholar] [CrossRef]
- Munavvarov, A.; Yusupov, Z.; Ergashov, I.; Tojibaev, K.S.H.; Deng, T.; Sun, H. Complete chloroplast genomes of ten species from subgenus Allium (Allium, Amaryllidaceae). Plant Divers. Cent. Asia 2022, 1, 67–81. [Google Scholar] [CrossRef]
- Khapilina, O.; Turzhanova, A.; Danilova, A.; Tumenbayeva, A.; Shevtsov, V.; Kotukhov, Y.; Kalendar, R. Primer Binding Site (PBS) Profiling of Genetic Diversity of Natural Populations of Endemic Species Allium ledebourianum Schult. BioTech 2021, 10, 23. [Google Scholar] [CrossRef]
- Erst, A.S.; Nikulin, A.Y.; Nikulin, V.Y.; Ebel, A.L.; Zibzeev, E.V.; Sharples, M.T.; Wang, W. Distribution analysis, updated checklist, and DNA barcodes of the endemic vascular flora of the Altai mountains, a Siberian biodiversity hotspot. Syst. Biodivers. 2022, 20, 1–30. [Google Scholar] [CrossRef]
- Kechaikin, A.A.; Sinitsyna, T.A.; Shmakov, A.I.; Friesen, N.V.; Sitpaeva, G.T.; Veselova, P.V.; Bayadilov, K.O. Addition to the flora of the State National Natural Park “Altyn-Emel” (Republic of Kazakhstan). Turczaninowia 2018, 21, 73–77. (In Russian) [Google Scholar]
- Khajoei Nasab, F.; Mehrabian, A.; Mostafavi, H.; Neemati, A. The influence of climate change on the suitable habitats of Allium species endemic to Iran. Environ. Monit. Assess. 2022, 194, 169. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, C.K.; Oh, J.Y. Distribution characteristics of the four species of genus Allium at different altitudes in south Korea. Korean J. Agric. For. Meteorol. 2009, 11, 252–255. [Google Scholar] [CrossRef]
- Kadyrbayeva, G.; Zagórska, J.; Grzegorczyk, A.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Ludwiczuk, A.; Kukula-Koch, W. The phenolic compounds profile and cosmeceutical significance of two kazakh species of onions: Allium galanthum and A. turkestanicum. Molecules 2021, 26, 5491. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chauhan, G.; Krishan, P.; Shri, R. Allium schoenoprasum L.: A review of phytochemistry, pharmacology and future directions. Nat. Prod. Res. 2018, 32, 2202–2216. [Google Scholar] [CrossRef]
- Tudu, C.K.; Dutta, T.; Ghorai, M.; Biswas, P.; Samanta, D.; Oleksak, P.; Dey, A. Traditional uses, phytochemistry, pharmacology and toxicology of garlic (Allium sativum), a storehouse of diverse phytochemicals: A review of research from the last decade focusing on health and nutritional implications. Front. Nutr. 2022, 9, 949554. [Google Scholar] [CrossRef] [PubMed]
- Deniz, İ.G.; Genç, İ.; Sarı, D. Morphological and molecular data reveal a new species of Allium (Amaryllidaceae) from SW Anatolia, Turkey. Phytotaxa 2015, 212, 283–292. [Google Scholar] [CrossRef]
- Ulcay, S. Anatomy, palynology, seed and leaf micromorphology of Turkish endemic Allium brevicaule Boiss. & Balansa and Allium scorodoprasum ssp. rotundum (L.) Stearn. Acta Biol. Cracoviensia. Ser. Bot. 2022, 64, 27–38. [Google Scholar]
- Aryakia, E.; Karimi, H.R.; Naghavi, M.R.; Shahzadeh Fazeli, S.A. Morphological characterization of intra- and interspecific diversity in some Iranian wild Allium species. Euphytica 2016, 211, 185–200. [Google Scholar] [CrossRef]
- Celep, F.; Koyuncu, M.; Fritsch, R.M.; Kahraman, A.; Doğan, M. Taxonomic importance of seed morphology in Allium (Amaryllidaceae). Syst. Bot. 2012, 37, 893–912. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Hassandokht, M.; Zamani, Z.; Kashi, A.; Roldan-Ruiz, I.; Van Bockstaele, E. Seed morphogenesis and effect of pretreatments on seed germination of Persian shallot (Allium hirtifolium Boiss.), an endangered medicinal plant. Hortic. Environ. Biotechnol. 2014, 55, 19–26. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tan, D.Y. Seed testa micromorphology of thirty-eight species of Allium (Amaryllidaceae) from central Asia, and its taxonomic implications. Nord. J. Bot. 2017, 35, 189–200. [Google Scholar] [CrossRef]
- Sennikov, A.N.; Lazkov, G.A. Taxonomic revision of the Allium filidens group (Amaryllidaceae) in Kyrgyzstan. Nord. J. Bot. 2023, 2023, e04050. [Google Scholar] [CrossRef]
- Baasanmunkh, S.; Lee, J.K.; Jang, J.E.; Park, M.S.; Friesen, N.; Chung, S.; Choi, H.J. Seed morphology of Allium L. (Amaryllidaceae) from central Asian countries and its taxonomic implications. Plants 2020, 9, 1239. [Google Scholar] [CrossRef]
- Khorasani, M.; Mehrvarz, S.S.; Zarre, S. Seed morphology and testa ultrastructure in Allium stipitatum complex (Amaryllidaceae; Allioideae) and their systematic significance. Turk. J. Bot. 2020, 44, 618–632. [Google Scholar] [CrossRef]
- Abdullaeva, A.T.; Rakhimova, N.K.; Duschanova, G.M.; Temirov, E.E. Comparative study of the anatomical structure of some vegetative organs Allium caspium (Pall.) M. Bieb. and Allium tschimganicum B. Fedtsch. ex Popov growing in natural conditions of Uzbekistan. Am. J. Plant Sci. 2020, 11, 1398. [Google Scholar] [CrossRef]
- Friesen, N.; Smirnov, S.V.; Shmakov, A.I.; Herden, T.; Oyuntsetseg, B.; Hurka, H. Allium species of section Rhizomatosa, early members of the Central Asian steppe vegetation. Flora 2020, 263, 151536. [Google Scholar] [CrossRef]
- Sinitsyna, T.A.; Herden, T.; Friesen, N. Dated phylogeny and biogeography of the Eurasian Allium section Rhizirideum (Amaryllidaceae). Plant Syst. Evol. 2016, 302, 1311–1328. [Google Scholar] [CrossRef]
- Abugalieva, S.; Volkova, L.; Genievskaya, Y.; Ivaschenko, A.; Kotukhov, Y.; Sakauova, G.; Turuspekov, Y. Taxonomic assessment of Allium species from Kazakhstan based on ITS and matK markers. BMC Plant Biol. 2017, 17, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Friesen, N.; Vesselova, P.; Osmonaly, B.; Sitpayeva, G.; Luferov, A.; Shmakov, A. Allium toksanbaicum (Amaryllidaceae), a new species from Southeast Kazakhstan. Phytotaxa 2021, 494, 251–267. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Gutterman, Y. Germination strategies of some Allium species of the subgenus Melanocrommuyum from arid zone of Central Asia. J. Arid Environ. 2000, 45, 61–71. [Google Scholar] [CrossRef]
- Friesen, N.; Grützmacher, L.; Skaptsov, M.; Vesselova, P.; Dorofeyev, V.; Luferov, A.N.; Hurka, H. Allium pallasii and A. caricifolium—Surprisingly diverse old steppe species, showing a clear geographical barrier in the area of Lake Zaysan. Plants 2022, 11, 1465. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Kubentayeva, B.B. Revised checklist of endemic vascular plants of Kazakhstan. PhytoKeys 2024, 238, 241. [Google Scholar] [CrossRef] [PubMed]
- Abdulina, S.A. Checklist of Vascular Plants of Kazakhstan; Institute of Botany and Plant Introduction: Almaty, Kazakhstan, 1999. [Google Scholar]
- Kotukhov, Y.A.; Danilova, A.N.; Anufrieva, O.A. Abstract of onions (Allium L.) of the Kazakh Altai, Sauro-Manrak and Zaisan Basin; Anniversary Editorial Board: Kemerovo, Russia, 2011; Volume 171. [Google Scholar]
- Fritsch, R.M.; Friesen, N. Evolution, domestication and taxonomy. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 5–30. [Google Scholar]
- Block, E. Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Bacci, L.F.; Amorim, A.M.; Michelangeli, F.A.; Goldenberg, R. Flower morphology is correlated with distribution and phylogeny in Bertolonia (Melastomataceae), an herbaceous genus endemic to the Atlantic Forest. Mol. Phylogenetics Evol. 2020, 149, 106844. [Google Scholar] [CrossRef]
- Sumbembayev, A.A.; Abugalieva, S.I.; Danilova, A.N.; Matveyeva, E.V.; Szlachetko, D.L. A flower morphometry of members of the genus Dactylorhiza Necker ex Nevski (Orchidaceae) from the Altai Mountains of Kazakhstan. Biodiversitas 2021, 22, 8. [Google Scholar] [CrossRef]
- Žarković, L.D.; Hinić, S.S.; Matejić, J.S.; Veljić, M.M.; Marin, P.D.; Džamić, A.M. Fruit micromorphology and morphometry of eight wild-growing roses in Serbia. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2024, 158, 823–835. [Google Scholar] [CrossRef]
- Punja, Z.K.; Holmes, J.E. Hermaphroditism in marijuana (Cannabis sativa L.) inflorescences–impact on floral morphology, seed formation, progeny sex ratios, and genetic variation. Front. Plant Sci. 2020, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Sumbembayev, A.A.; Lagus, O.A.; Nowak, S. Seed morphometry of Rheum L. (Polygonaceae) species from Kazakhstan and its implications in taxonomy and species identification. Biodiversitas J. Biol. Divers. 2023, 24, 4677–4692. [Google Scholar] [CrossRef]
- Sumbembayev, A.A.; Lagus, O.; Danilova, A.N.; Rewicz, A.; Nowak, S. Morphometric parameters of seeds as a practical method for identifying rare species of the genus Tulipa L. (Liliaceae) from East Kazakhstan region. PhytoKeys 2025, 251, 67. [Google Scholar] [CrossRef]
- Kotukhov, Y.A. New species of the genus Allium L. (Alliaceae J. Agardh) from Eastern Kazakhstan. Turczaninowia 2003, 6, 5–10. (In Russian) [Google Scholar]
- Martin, A.C. The comparative internal morphology of seeds. Am. Midl. Nat. 1946, 36, 513–660. [Google Scholar] [CrossRef]
- MacAdam, D.L. Uniform color scales. J. Opt. Soc. Am. 1974, 64, 1691–1702. [Google Scholar] [CrossRef]
- Kamelin, R.V. Florogenetic Analysis of the Natural Flora of Mountainous Central Asia; Science Publishers: Saint Petersburg, Russia, 1973. (In Russian) [Google Scholar]
- Di Biase, L.; Tsafack, N.; Pace, L.; Fattorini, S. Ellenberg Indicator Values Disclose Complex Environmental Filtering Processes in Plant Communities along an Elevational Gradient. Biology 2023, 12, 161. [Google Scholar] [CrossRef]
- Mazur, M.; Marcysiak, K.; Dunajska, A.; Gawlak, M.; Kałuski, T. Taxonomic significance of seed morphology in Veronica L. (Plantaginaceae) species from central Europe. Plants 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Guo, Q.; Li, H.; Li, J.; Zuo, W.; Long, C. Estimation of morphological variation in seed traits of Sophora moorcroftiana using digital image analysis. Front. Plant Sci. 2023, 14, 1185393. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, Z.; Ergashov, I.; Volis, S.; Makhmudjanov, D.; Dekhkonov, D.; Khassanov, F.; Sun, H. Seed macro- and micromorphology in Allium (Amaryllidaceae) and its phylogenetic significance. Ann. Bot. 2022, 129, 869–911. [Google Scholar] [CrossRef] [PubMed]
- Shukherdorj, B.; Jae, C.H.; Batlai, O. Seed testa sculpture of species of Allium L. (Amaryllidaceae) and its taxonomic implications. Turczaninowia 2021, 24, 154–161. [Google Scholar]
- Vatandaşlar, Ö.; Koçyiğit Avcı, M. Scorodon ve Codonoprasum Seksiyonlarına ait 8 Allium türünün karşılaştırmalı tohum morfolojisi. Turk. J. Biosci. Collect. 2024, 8, 124–129. (In Turkish) [Google Scholar] [CrossRef]
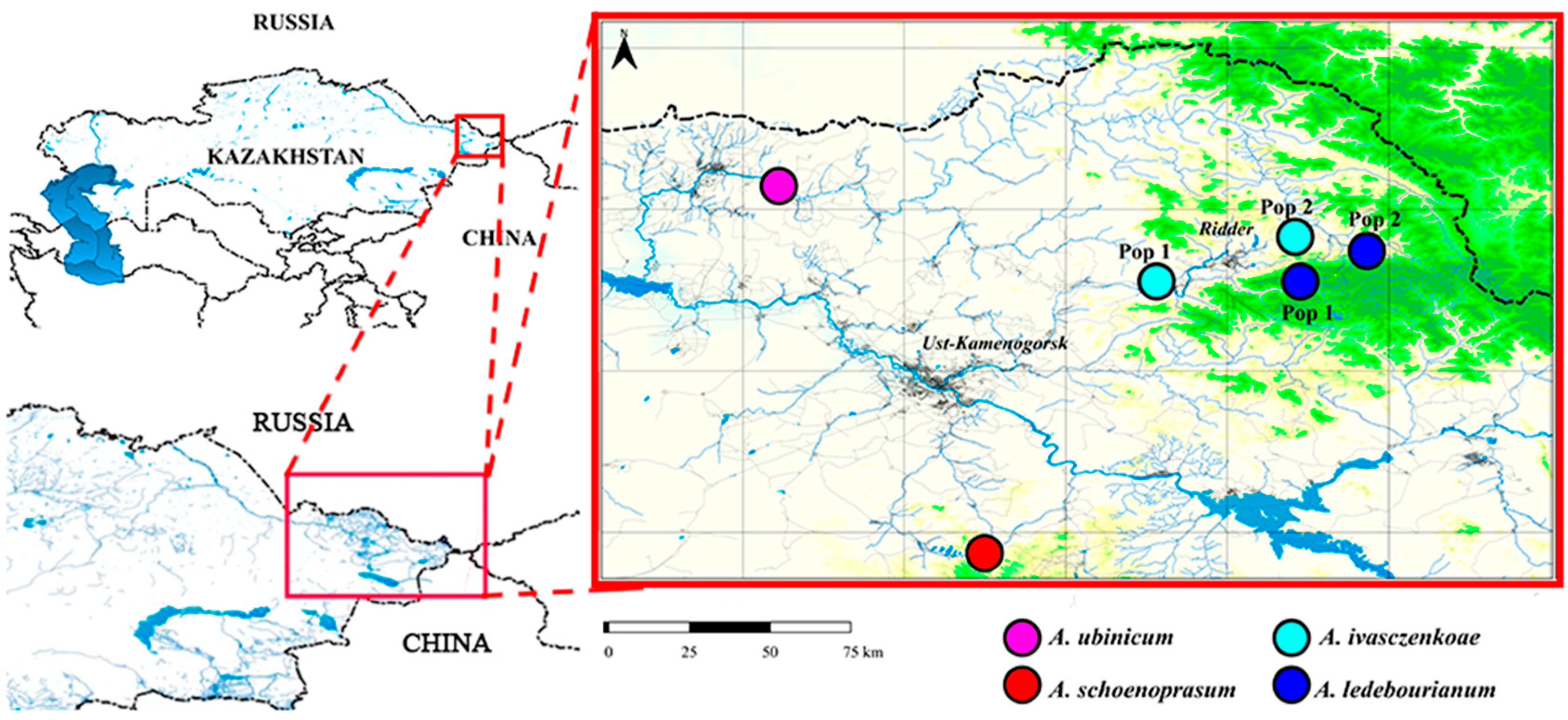



| Population | Coordinates | Collection Site and Habitat Description | Area, m2 |
|---|---|---|---|
| A. ledebourianum Schult. et Schult. | |||
| Pop 1 | 50.3422410 83.7344190 | Kazakhstan, East Kazakhstan region, Ivanovsky ridge, foothills of Listvyazhnaya mountain, hummocky bogs | 300 |
| Pop 2 | 50.358797 83.901374 | Kazakhstan, East Kazakhstan region, Ivanovsky ridge, Bolshaya Poperechka river valley, Gray Lug tract, grassy meadow | 150 |
| A. ivasczenkoae Kotuch. | |||
| Pop 1 | 50.287624 83.304463 | Kazakhstan, East Kazakhstan region, Ivanovsky ridge, Kozlushka mountain, swampy clearing in willow | 50 |
| Pop 2 | 50.379005 83.791850 | Kazakhstan, East Kazakhstan region, Ivanovsky ridge, foothills of Listvyazhnaya mountain, hummocky bogs | 250 |
| A. ubinicum Kotuch. | |||
| Pop 1 | 50.567472 82.109523 | Kazakhstan, East Kazakhstan region, Ubinsky ridge, Uba River valley, along wet banks | 200 |
| A. schoenoprasum L. | |||
| Pop 1 | 49.443428 82.723457 | Kazakhstan, East Kazakhstan region, Kalbinsky ridge, Sibinskaya depression, flood meadows | 1000 |
| Population | Length, mm | Width, mm | Thickness, mm | Weight of 1000 Seeds, g | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M ± SE Min–Max | Cv, % | P% | M ± SE Min–Max | Cv, % | P% | M ± SE Min–Max | Cv, % | P% | ||
| Allium ledebourianum | ||||||||||
| Pop 1 | 3.036 ± 0.093 2.25–3.50 | 7.932 | 1.448 | 1.539 ± 0.055 1.30–1.80 | 9.345 | 1.706 | 1.508 ± 0.037 1.30–1.70 | 6.442 | 1.175 | 1.201 |
| Pop 2 | 3.268 ± 0.055 3.10–3.57 | 4.448 | 0.812 | 1.605 ± 0.041 1.35–1.82 | 6.651 | 1.214 | 1.558 ± 0.035 1.35–1.70 | 5.898 | 1.070 | 1.116 |
| Allium ivasczenkoae | ||||||||||
| Pop 1 | 3.271 ± 0.081 2.95–3.90 | 6.499 | 1.187 | 1.537 ± 0.049 1.25–1.90 | 8.464 | 1.545 | 1.534 ± 0.036 1.35–1.75 | 6.109 | 1.115 | 0.602 |
| Pop 2 | 3.059 ± 0.063 2.75–3.35 | 5.377 | 0.981 | 1.466 ± 0.048 1.25–1.65 | 8.515 | 1.555 | 1.386 ± 0.059 1.20–1.70 | 11.098 | 2.026 | 0.955 |
| Allium ubinicum | ||||||||||
| Pop 1 | 3.438 ± 0.137 3.05–4.00 | 10.427 | 1.904 | 1.487 ± 0.062 1.17–1.85 | 10.870 | 1.985 | 1.505 ± 0.049 1.27–1.70 | 8.544 | 1.559 | 1.350 |
| Allium schoenoprasum | ||||||||||
| Pop 1 | 3.025 ± 0.084 2.60–3.40 | 7.299 | 1.333 | 1.382 ± 0.051 1.15–1.65 | 9.701 | 1.771 | 1.434 ± 0.038 1.20–1.62 | 6.999 | 1.278 | 0.883 |
| Morphological Traits | Species | |||
|---|---|---|---|---|
| A. ivasczenkoae | A. ledebourianum | A. schoenoprasum | A. ubinicum | |
| Size, shape | Small (parvum), triangular, straight | Small (parvum), triangular, straight | Small (parvum), triangular, straight | Small (parvum), triangular, straight |
| Length, mm | 2.95–3.90 (mean 3.27) | 2.25–3.50 (mean 3.04) | 2.60–3.40 (mean 3.025) | 3.05–4.00 (mean 3.438) |
| Width, mm | 1.25–1.90 (mean 1.54) | 1.30–1.80 (mean 1.53) | 1.15–1.65 (mean 1.382) | 1.17–1.85 (mean 1.487) |
| Thickness, mm | 1.35–1.75 (mean 1.53) | 1.30–1.70 (mean 1.51) | 1.20–1.62 (mean 1.434) | 1.27–1.70 (mean 1.505) |
| Weight of 1000 seeds, g | 0.602 | 1.201 | 0.883 | 1.350 |
| Seed color | Black, solid, matte | Black, solid, matte | Black, solid, matte | Black, solid, matte |
| Seed shape | Noticeably flattened. Triangular, with clearly defined curved edges. The base is significantly concave inward into the seed. One end of the seed is usually rounded; the other end is pointed. | Triangular, elongated. One end of the seed is more rounded and widened; the opposite end is sharply pointed and narrowed. The back is convex, the base is flat, slightly concave inward into the seed. | Triangular, has a flat base, slightly indented into the seed. The lateral edges are also slightly indented into the endosperm. The edges are curved and sinuous. Both ends of the seed are pointed. | Triangular, has a strongly elongated pointed end. The other end of the seed is slightly rounded. The lateral edges are pressed inward. The edges are slightly curved and pointed. |
| Seed surface | Rough, dry, leathery, slightly wrinkled, fine-grained. | Rough, dry, leathery, slightly wrinkled on the back and smooth at the base. | Rough, dry, leathery, wrinkled on the back, and smoother at the base. | Rough, dry, leathery, heavily wrinkled. |
| Micropyle | Overgrown, barely visible, triangular in shape, located at the narrow end of the seed. | Overgrown, distinct, triangular in shape, located at the narrow end of the seed. | Overgrown, barely visible, dot-shaped, located at the narrow end of the seed. | Overgrown, poorly distinguishable, dot-shaped, located at the narrow end of the seed. |
| Chalaza | Noticeably thickened, strongly ribbed, located in the basal part. | Thickened, convex, ribbed, located in the basal part. | Slightly thickened, significantly ribbed, located in the basal part. | Slightly thickened, ribbed, located in the basal part. |
| Seed hilum | Small, linear, protruding, longitudinally slit-shaped, located along the rib in the middle part. | Small, linear, longitudinally slit-shaped, slightly protruding, close to the micropyle, located on the edge. | Small, linear, oval-shaped, slightly protruding, close to the micropyle, located on the edge. | Small, linear, longitudinally slit-shaped, slightly protruding, close to the micropyle, located on the edge. |
| Raphe (seed suture) | Barely noticeable, smooth, shortened, located on the lateral edge. | Faintly visible, slightly rough, located on the lateral edge. | Hardly visible, shortened, located on the lateral edge. | Hardly visible, shortened, located on the lateral edge. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumbembayev, A.; Lagus, O.; Danilova, A.; Aimenova, Z.; Seilkhan, A.; Takiyeva, Z.; Rewicz, A.; Nowak, S. Seed Morphology of Allium L. Endemic Species from Section Schoenoprasum (Amaryllidaceae) in Eastern Kazakhstan. Biology 2025, 14, 1230. https://doi.org/10.3390/biology14091230
Sumbembayev A, Lagus O, Danilova A, Aimenova Z, Seilkhan A, Takiyeva Z, Rewicz A, Nowak S. Seed Morphology of Allium L. Endemic Species from Section Schoenoprasum (Amaryllidaceae) in Eastern Kazakhstan. Biology. 2025; 14(9):1230. https://doi.org/10.3390/biology14091230
Chicago/Turabian StyleSumbembayev, Aidar, Olga Lagus, Alevtina Danilova, Zhanar Aimenova, Ainur Seilkhan, Zhanar Takiyeva, Agnieszka Rewicz, and Sławomir Nowak. 2025. "Seed Morphology of Allium L. Endemic Species from Section Schoenoprasum (Amaryllidaceae) in Eastern Kazakhstan" Biology 14, no. 9: 1230. https://doi.org/10.3390/biology14091230
APA StyleSumbembayev, A., Lagus, O., Danilova, A., Aimenova, Z., Seilkhan, A., Takiyeva, Z., Rewicz, A., & Nowak, S. (2025). Seed Morphology of Allium L. Endemic Species from Section Schoenoprasum (Amaryllidaceae) in Eastern Kazakhstan. Biology, 14(9), 1230. https://doi.org/10.3390/biology14091230






