Physiological Response Mechanisms of Triplophysa strauchii Under Salinity Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Experimental Design
2.3. Plasma and Tissue Sample Collection
2.4. Enzyme Activity Assays
2.5. Data Processing
2.6. Formatting of Mathematical Components
3. Results
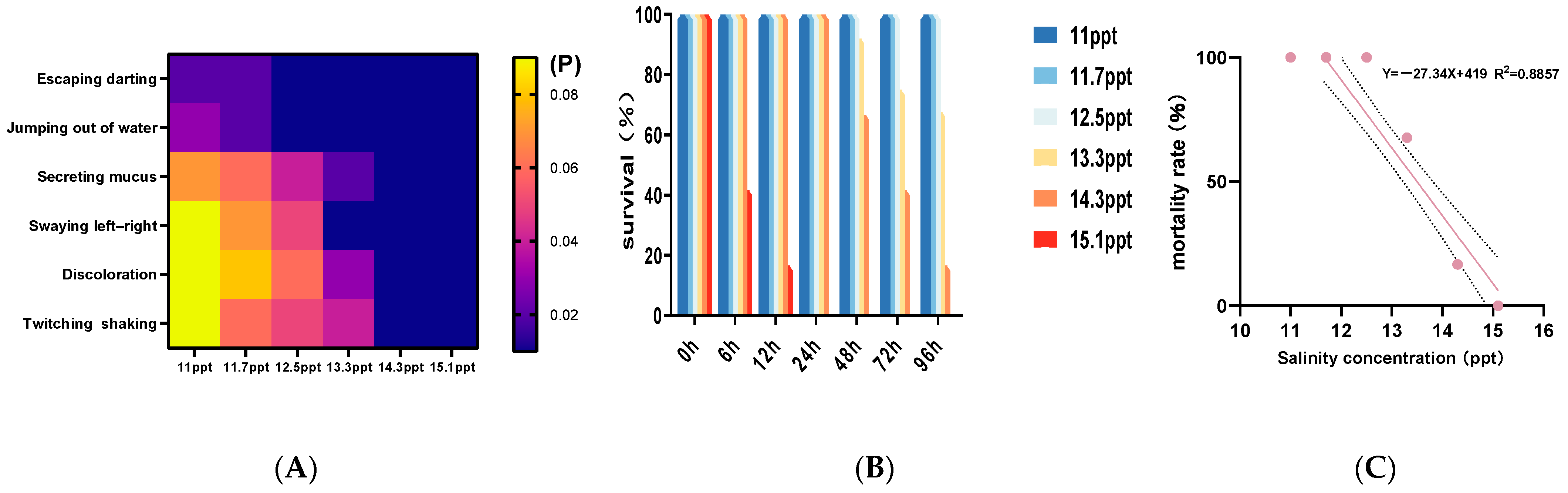
3.1. Behavioral Responses, Mortality, and Correlation Analysis Under Different Salinities
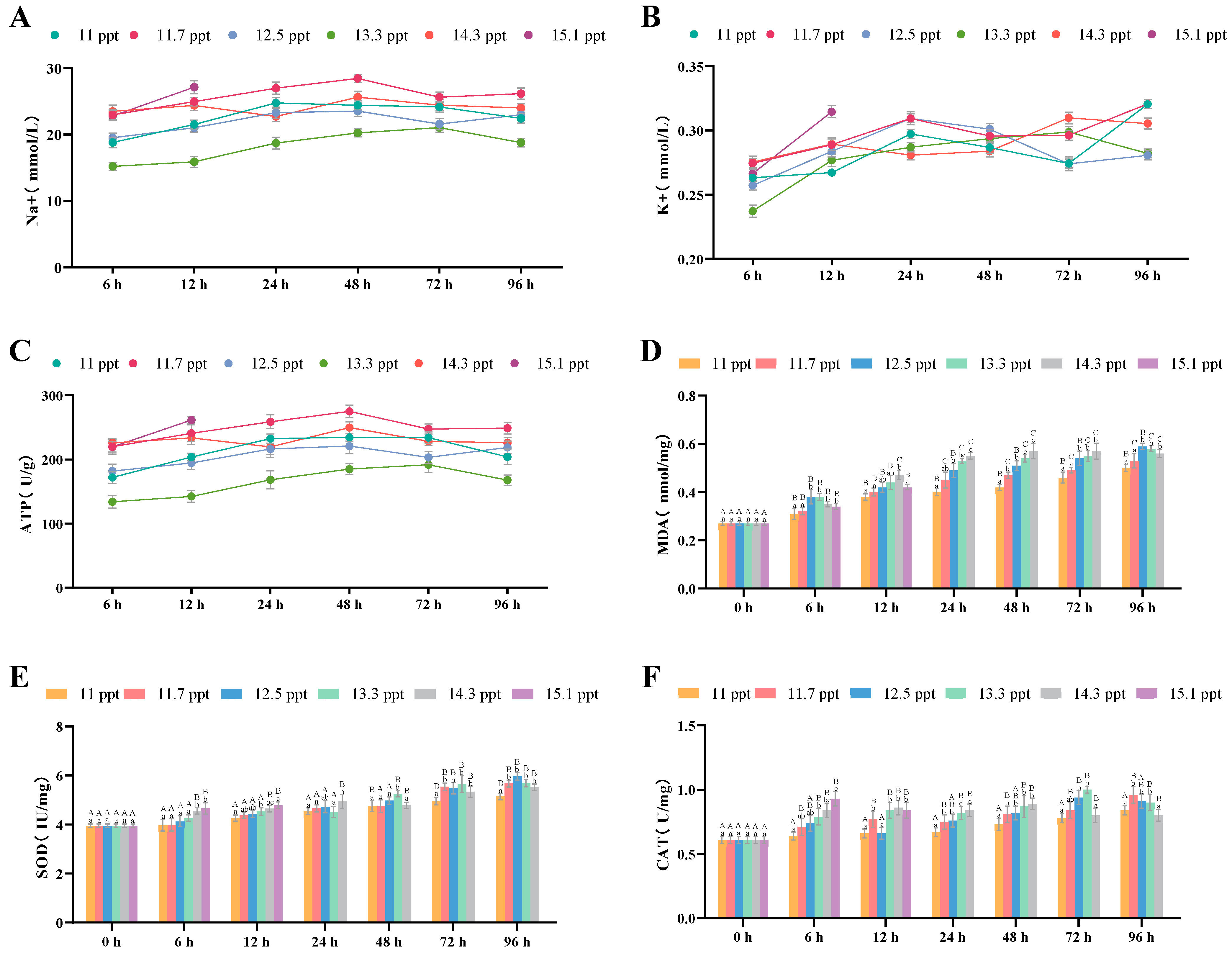
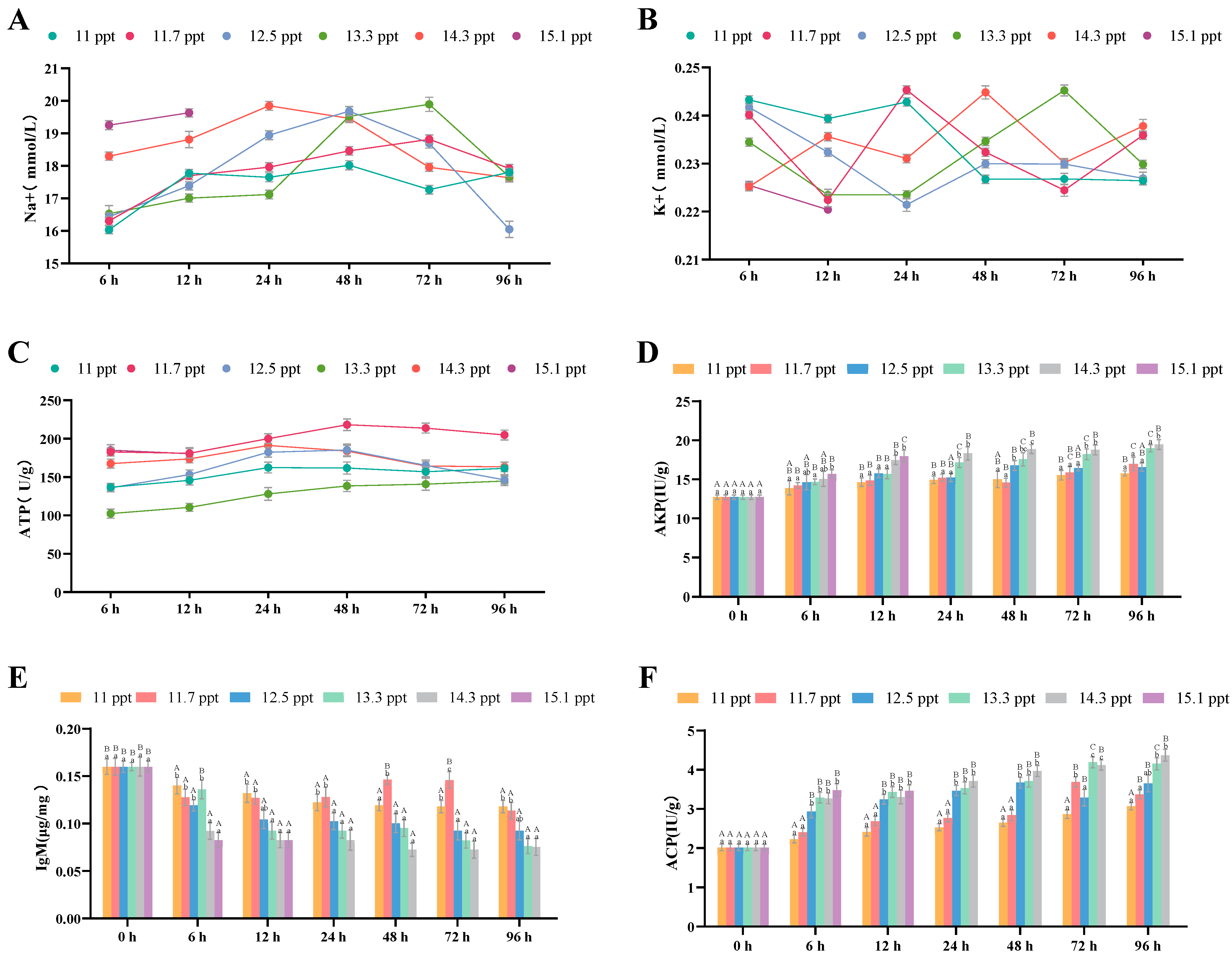
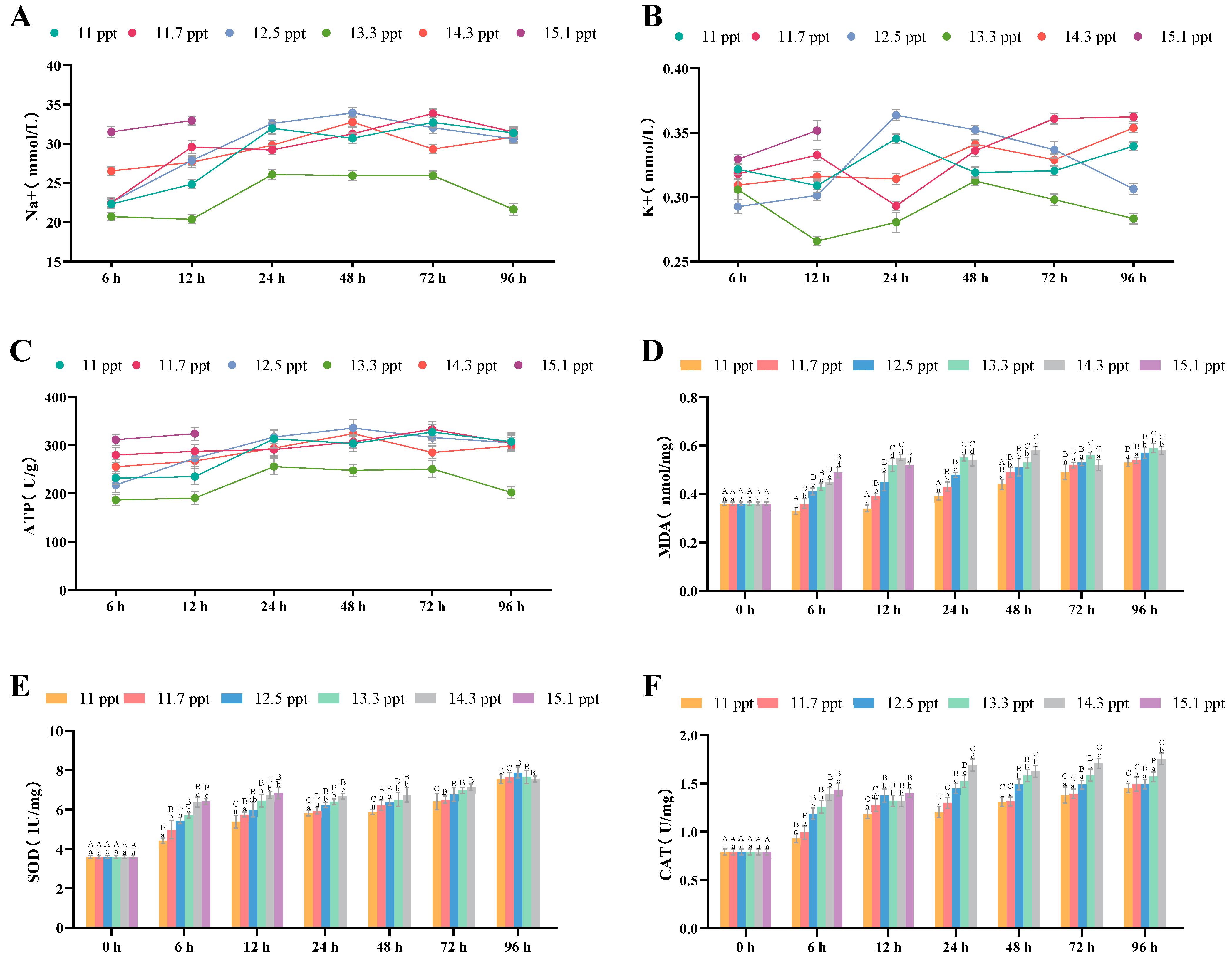
3.2. Trends in Enzyme Activities of Four Tissues and Plasma Under Different Salinities
3.2.1. Effects of Salinity Stress on Gill Osmoregulation and Antioxidation
3.2.2. Effects of Salinity Stress on Kidney Osmoregulation and Antioxidation
3.2.3. Effects of Salinity Stress on Liver Osmoregulation and Immune Function
3.2.4. Effects of Salinity Stress on Intestinal Osmoregulation and Antioxidation
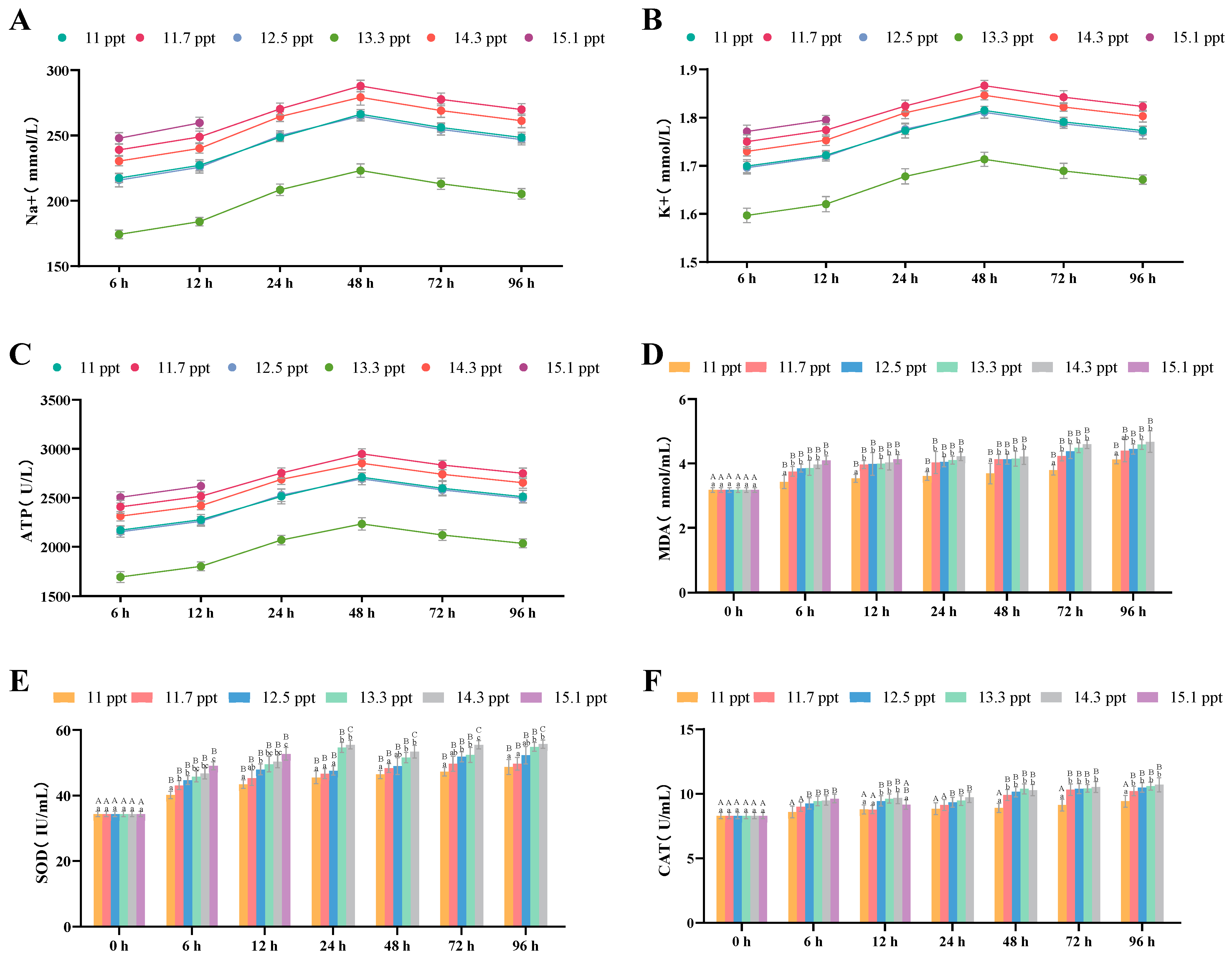
3.2.5. Effects of Salinity Stress on Plasma Osmoregulation and Antioxidation
4. Discussion
4.1. Effects of Salinity on Fish Behavior and Survival
4.2. The Impact of Salinity on Osmoregulation, Immune Response, and Oxidative Stress in Fish
4.2.1. The Impact of Salinity on Osmoregulation in Fish
4.2.2. The Impact of Salinity on the Antioxidant System in Fish
4.2.3. The Impact of Salinity on Fish Immune System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| IgM | Immunoglobulin M |
| AKP | Alkaline phosphatase |
| ACP | Acid phosphatase |
| ROS | Reactive oxygen species |
References
- Korus, J.; Filgueira, R.; Grant, J. Influence of temperature on the behaviour and physiology of Atlantic salmon (Salmo Salar) on a commercial farm. Aquaculture 2024, 589, 740978. [Google Scholar] [CrossRef]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef]
- Sundby, S.; Kristiansen, T. The principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. PLoS ONE 2015, 10, e0138821. [Google Scholar] [CrossRef]
- Hieu, D.Q.; Hang, B.T.B.; Lokesh, J.; Garigliany, M.M.; Huong, D.T.T.; Yen, D.T.; Liem, P.T.; Tam, B.M.; Hai, D.M.; Son, V.N.; et al. Salinity significantly affects intestinal microbiota and gene expression in striped catfish juveniles. Appl. Microbiol. Biotechnol. 2022, 106, 3245–3264. [Google Scholar] [CrossRef]
- Alford, S.B.; Husband, S.A.; Martin, C.W. Resilience to changes in salinity and predator diversity in representative Gulf of Mexico estuarine fish predator-prey interactions. J. Exp. Mar. Biol. Ecol. 2024, 580, 152053. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Feng, W.; Liu, S.; Zhuang, Z. Analyses of the molecular mechanisms associated with salinity adaption of Trachidermus fasciatus through combined iTRAQ-based proteomics and RNA sequencing-based transcriptomics. Prog. Biophys. Mol. Biol. 2018, 136, 40–53. [Google Scholar] [CrossRef]
- Greenwell, M.G.; Sherrill, J.; Clayton, L.A. Osmoregulation in fish. Mechanisms and clinical implications. Vet. Clin. N. Am. Exot. Anim. Pract. 2003, 6, 169–189. [Google Scholar] [CrossRef]
- Maraschi, A.C.; Faria, S.C.; McNamara, J.C. Salt transport by the gill Na+-K+-2Cl− symporter in palaemonid shrimps: Exploring physiological, molecular and evolutionary landscapes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 257, 110968. [Google Scholar] [CrossRef]
- Avella, M.; Ducoudret, O.; Pisani, D.F.; Poujeol, P. Swelling-activated transport of taurine in cultured gill cells of sea bass: Physiological adaptation and pavement cell plasticity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1149–R1160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Resende, A.D.; Lobo-da-Cunha, A.; Malhão, F.; Franquinho, F.; Monteiro, R.A.; Rocha, E. Histological and stereological characterization of brown trout (Salmo trutta f. fario) trunk kidney. Microsc. Microanal. 2010, 16, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Duan, Y.; Xie, B.; Liu, H.; Zhong, L.; Wang, M.; Liu, J.; Su, C.; Chen, X.; Zhang, S. Effects of salinity stress on the growth performance, histological characteristics, and expression of genes related to apoptosis and immunity in channel catfish (Ictalurus punctatus). J. Fish Biol. 2025, 106, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Thebault, M.-T.; Raffin, J.-P. Properties of the lysosomes from liver and gill of rainbow trout, Salmo gairdnerii R.: Effect of starvation, salinity and 2, 4, 5-T. Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 79, 541–547. [Google Scholar] [CrossRef]
- Lee, D.-W.; Choi, Y.-U.; Park, H.-S.; Park, Y.-S.; Choi, C.Y. Effect of low pH and salinity conditions on the antioxidant response and hepatocyte damage in juvenile olive flounder Paralichthys olivaceus. Mar. Environ. Res. 2022, 175, 105562. [Google Scholar] [CrossRef]
- Sundell, K.S.; Sundh, H. Intestinal fluid absorption in anadromous salmonids: Importance of tight junctions and aquaporins. Front. Physiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Chourasia, T.K.; D’Cotta, H.; Baroiller, J.F.; Slosman, T.; Cnaani, A. Effects of the acclimation to high salinity on intestinal ion and peptide transporters in two tilapia species that differ in their salinity tolerance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 218, 16–23. [Google Scholar] [CrossRef]
- Banh, S.; Wiens, L.; Sotiri, E.; Treberg, J.R. Mitochondrial reactive oxygen species production by fish muscle mitochondria: Potential role in acute heat-induced oxidative stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 191, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, K.; Jayaraman, J. Salinity adaptation in fish: Effect of thyroxine on mitochondrial status. Arch. Biochem. Biophys. 1984, 233, 728–735. [Google Scholar] [CrossRef]
- Vahdatiraad, L.; Heidari, B.; Zarei, S.; Sohrabi, T.; Ghafouri, H. Biological responses of stellate sturgeon fingerlings (Acipenser stellatus) immersed in HSP inducer to salinity changes. Mar. Environ. Res. 2023, 191, 106145. [Google Scholar] [CrossRef]
- Schmitz, M.; Ziv, T.; Admon, A.; Baekelandt, S.; Mandiki, S.N.M.; L’Hoir, M.; Kestemont, P. Salinity stress, enhancing basal and induced immune responses in striped catfish Pangasianodon hypophthalmus (Sauvage). J. Proteom. 2017, 167, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Aiyixia, X.; Xin, J. Hydrological Characteristics of the Emin River Basin. J. China Hydrol. 2002, 22, 51–53. [Google Scholar]
- Gao, J.; Jing, L.; Li, H.; Miao, Y.; Jing, L. Variation Characteristics of Precipitation and Meteorological Drought in Xinjiang Tacheng Prefecture during Recent 60 Years. Meteorol. Res. Plateau Mt. Areas 2024, 44, 134–142. [Google Scholar]
- Chen, H.T.; Tang, Y. Variation Characteristics of Precipitation in Xinjiang Tacheng Prefecture during Recent 55 Years. J. Water Resour. Water Eng. 2010, 21, 65–69. [Google Scholar]
- Jiang, Z.; Jiang, W. Analysis of Social Benefits and Ecological Effects from Comprehensive Development of Emin River Basin. Jilin Water Resour. Hydropower 2015, 8, 32–36+40. [Google Scholar] [CrossRef]
- Zongxia, W.; Suxia, L. Estimation of Groundwater Storage and Its Spatiotemporal Evolution Patterns in the Northern Slope of Tian Shan during 1990–2020. Acta Geogr. Sin. 2023, 78, 1744–1763. [Google Scholar]
- Aynu, R.L.Z.H.Z. Impacts of Climate Change on Hydrological Regime in Baiyang River Basin, Tacheng, Xinjiang during 1962–2007. J. Glaciol. Geocryol. 2010, 32, 921–926. [Google Scholar]
- Jiang, Z.; Jiang, J.; Wang, Y.; Zhang, E.; Zhang, Y.; Li, L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.; et al. Red List of Vertebrates in China. Biodiversity 2016, 24, 501–551+615. [Google Scholar]
- Wang, Y.; Chen, P.; Cui, R.; Si, W.; Zhang, Y.; Ji, W. Heavy metal concentrations in water, sediment, and tissues of two fish species (Triplohysa pappenheimi, Gobio hwanghensis) from the Lanzhou section of the Yellow River, China. Environ. Monit. Assess. 2009, 165, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, M.; Wong, H. Comparison of Three Calculation Methods for Median Lethal Concentration (LC50). Prog. Vet. Med. 2012, 33, 89–92. [Google Scholar] [CrossRef]
- Leite, T.; Branco, P.; Ferreira, M.T.; Santos, J.M. Activity, boldness and schooling in freshwater fish are affected by river salinization. Sci. Total Environ. 2022, 819, 153046. [Google Scholar] [CrossRef]
- Bertolini, F.; Rohtla, M.; Parzanini, C.; Tomkiewicz, J.; Durif, C.M.F. Blood-based gene expression as non-lethal tool for inferring salinity-habitat history of European eel (Anguilla anguilla). Sci. Rep. 2022, 12, 22142. [Google Scholar] [CrossRef]
- Islam, M.J.; Slater, M.J.; Kunzmann, A. What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: The case of European seabass, Dicentrarchus labrax. Sci. Total. Environ. 2020, 749, 141458. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, H.; Qiang, Z.; Nie, Z.; Wei, J.; Shen, J. Effects of Photoperiod and Light Intensity on Survival, Feeding, Growth, and Saline-Alkali Tolerance in Juveniles of Tarim Schizothorax. South China Fish. Sci. 2021, 17, 54–63. [Google Scholar]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Blood biochemical variables, antioxidative status, and histological features of intestinal, gill, and liver tissues of African catfish (Clarias gariepinus) exposed to high salinity and high-temperature stress. Environ. Sci. Pollut. Res. Int. 2022, 29, 56357–56369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Z.; Zhang, M. Study on Salinity and Alkalinity Tolerance in Juvenile Pengze Crucian Carp (Carassius auratus var. Pengze). J. Jimei Univ. Nat. Sci. Ed. 2004, 2, 127–130. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Geng, L.; Jiang, H.; Cao, D.; Li, C.; Xu, W. Effect of NaCl Salinity and NaHCO3 Alkalinity on Hatching and Larval Survival of Songpu Mirror Carp (Cyprinus carpio var. Songpu) and Fangzheng Crucian Carp (Carassius auratus gibelio). Chin. J. Zool. 2015, 50, 643–648. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Zhang, D.-m.; Lei, X.-y.; Wang, S.; Wan, J.-w.; Liu, H.-j.; Chen, Y.-k.; Zhao, Y.-l.; Wang, G.-q.; et al. Effects of acute salinity stress on osmoregulation, antioxidant capacity and physiological metabolism of female Chinese mitten crabs (Eriocheir sinensis). Aquaculture 2022, 552, 737989. [Google Scholar] [CrossRef]
- Sackville, M.A.; Cameron, C.B.; Gillis, J.A.; Brauner, C.J. Ion regulation at gills precedes gas exchange and the origin of vertebrates. Nature 2022, 610, 699–703. [Google Scholar] [CrossRef]
- Prunet, P.; Bornancin, M. Physiology of salinity tolerance in tilapia: An update of basic and applied aspects. Aquat. Living Resour. 1989, 2, 91–97. [Google Scholar] [CrossRef]
- Edwards, S.L.; Marshall, W.S. Principles and Patterns of Osmoregulation and Euryhalinity in Fishes. In Fish Physiology; McCormick, S.D., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 32, pp. 1–44. [Google Scholar]
- Tao, Y.F.; Qiang, J.; Dagoudo, M.; Zhu, H.J.; Bao, J.W.; Ma, J.L.; Li, M.X.; Xu, P. Transcriptome profiling reveals differential expression of immune-related genes in gills of hybrid yellow catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂) under hypoxic stress: Potential NLR-mediated immune response. Fish Shellfish Immunol. 2021, 119, 409–419. [Google Scholar] [CrossRef]
- FE, H. The gill arch of the mullet, Mugil cephalus. II. Modifigation in surface ultrastructure and Na, K-ATPase content during adaptation to various salinities. J. Exp. Zool. 1979, 208, 399–406. [Google Scholar]
- Al-Jandal, N.J.; Wilson, R.W. A comparison of osmoregulatory responses in plasma and tissues of rainbow trout (Oncorhynchus mykiss) following acute salinity challenges. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 175–181. [Google Scholar] [CrossRef]
- Geng, L.; Tong, G.; Jiang, H.; Xu, W. Effect of salinity and alkalinity on luciobarbus capito Gill Na+/K+-ATPase enzyme activity, plasma ion concentration, and osmotic pressure. BioMed Res. Int. 2016, 2016, 4605839. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z. Ontogenetic Development of Gill and Na+/K+ ATPase in the Air-Breathing Loach. Fishes 2023, 8, 23. [Google Scholar] [CrossRef]
- Chu, T.J.; Jin, Z.; Liu, K. The mitochondrial genome of Sinobdella sinensis (Synbranchiformes: Mastacembelidae) from China’s Qiantang River. Mitochondrial DNA B 2021, 6, 2741–2742. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef]
- Zhou, W.; Song, X.; Chen, G. Salinity and alkalinity tolerance of Monopterus albus. Freshw. Fish. Sci. 2014, 44, 95–99. [Google Scholar] [CrossRef]
- Zidan, E.M.; Goma, A.A.; Tohamy, H.G.; Soliman, M.M.; Shukry, M. Insight study on the impact of different salinity levels on behavioural responses, biochemical stress parameters and growth performance of African catfish (Clarias gariepinus). Aquac. Res. 2022, 53, 2750–2759. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, C.; Qi, M.; Liu, Q.; Hu, Z. The Effect of Salinity Stress on Enzyme Activities, Histology, and Transcriptome of Silver Carp (Hypophthalmichthys molitrix). Biology 2022, 11, 1580. [Google Scholar] [CrossRef]
- Li, P.; Liu, W.; Lu, W.; Wang, J. Biochemical indices, gene expression, and SNPs associated with salinity adaptation in juvenile chum salmon (Oncorhynchus keta) as determined by comparative transcriptome analysis. PeerJ 2022, 10, e13585. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Alkafafy, M.; Sewilam, H. The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol. Biochem. 2022, 48, 397–408. [Google Scholar] [CrossRef]
- Wang, M.; Xu, W.; Zou, J.; Li, S.; Song, Z.; Zheng, F.; Ji, W.; Xu, Z.; Wang, Q. The Programming of Antioxidant Capacity, Immunity, and Lipid Metabolism in Dojo Loach (Misgurnus anguillicaudatus) Larvae Linked to Sodium Chloride and Hydrogen Peroxide Pre-treatment During Egg Hatching. Front. Physiol. 2021, 12, 768907. [Google Scholar] [CrossRef] [PubMed]
- van Muilekom, D.R.; Mueller, J.; Lindemeyer, J.; Schultheiß, T.; Maser, E.; Seibel, H.; Rebl, A.; Schulz, C.; Goldammer, T. Salinity change evokes stress and immune responses in Atlantic salmon with microalgae showing limited potential for dietary mitigation. Front. Physiol. 2024, 15, 1338858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Wei, J.; Hong, K.; Zhou, Q.; Liu, X.; Hong, X.; Li, W.; Liu, C.; Zhu, X.; et al. Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii. Antioxidants 2023, 12, 1836. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal Alkaline Phosphatase Detoxifies Lipopolysaccharide and Prevents Inflammation in Zebrafish in Response to the Gut Microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar] [CrossRef]
- Zhao, F.; Qu, L.; Zhuang, P.; Zhang, L.; Liu, J.; Zhang, T. Salinity tolerance as well as osmotic and ionic regulation in juvenile Chinese sturgeon (Acipenser sinensis Gray, 1835) exposed to different salinities. J. Appl. Ichthyol. 2011, 27, 231–234. [Google Scholar] [CrossRef]


| Index Category | Specific Index | Tissue | Trend with Increasing Salinity | Trend with Prolonged Stress (0–96 h) | Key Time Points |
|---|---|---|---|---|---|
| Ion Regulation | Na+ Concentration | Gill | Low-salinity groups (≤13.3 ppt): first increase then recovery; high-salinity groups (≥14.3 ppt): persistently high | Peaks at 24–48 h; low-salinity groups recover after 72 h | 24–48 h |
| K+ Concentration | Gill | Overall increase; 14.3 ppt group decreases after 24 h | Most groups show continuous increase; 14.3 ppt group decreases after 24 h | Most groups show continuous increase; 14.3 ppt group decreases after 24 h | |
| Na+/K+-ATPase Activity | Gill | No significant regular fluctuation within 96 h | No significant regular fluctuation | 48 h (turning point) | |
| Na+ Concentration | Kidney | increase | Increases by 48 h, then decreases significantly after 48 h | 48 h (peak) | |
| K+ Concentration | Kidney | Continuous increase | Continuous increase | 96 h (highest value) | |
| Na+/K+-ATPase Activity | Kidney | 11 ppt group: no significant fluctuation; 11.7, 12.5, 14.3 ppt groups: first increase then decrease | Increases by 48 h, then decreases significantly after 48 h | 48 h (peak) | |
| Na+ Concentration | Liver | increase | trend of increasing first and then decreasing | 48 h (turning point) | |
| K+ Concentration | Liver | Irregular fluctuations over time | Irregular fluctuations over time | / | |
| Na+/K+-ATPase Activity | Liver | No significant fluctuation | No significant fluctuation | / | |
| Na+ Concentration | Intestine | 11.7, 12.5, 14.3 ppt groups: increase with stress time | Increase with stress time | 96 h (highest value) | |
| K+ Concentration | Intestine | Irregular fluctuations over time | Irregular fluctuations over time | 24 h (turning point) | |
| Na+/K+-ATPase Activity | Intestine | No significant fluctuation | No significant fluctuation | / | |
| Na+ Concentration | Plasma | increase | First increase then decrease | 48 h (peak) | |
| K+ Concentration | Plasma | increase | First increase then decrease | 48 h (peak) | |
| Na+/K+-ATPase Activity | Plasma | increase | First increase then decrease | 48 h (peak) | |
| Antioxidant System | MDA Content | Gill | <14.3 ppt: increase; ≥14.3 ppt: decrease | Continuous increase | 96 h (highest value) |
| SOD Activity | Gill | Significant increase | Continuous significant increase | 96 h (highest value) | |
| CAT Activity | Gill | Significant increase | Continuous increase | 96 h (highest value) | |
| MDA Content | Kidney | Significant increase | Continuous significant increase | 96 h (highest value) | |
| SOD Activity | Kidney | Significant increase | Continuous increase | 96 h (highest value) | |
| CAT Activity | Kidney | Significant increase | Continuous significant increase | 96 h (highest value) | |
| MDA Content | Intestine | Significant increase | Continuous increase | 96 h (highest value) | |
| SOD Activity | Intestine | Significant increase | Continuous significant increase | 96 h (highest value) | |
| CAT Activity | Intestine | Significant increase | Continuous increase | 96 h (highest value) | |
| MDA Content | Plasma | Significant increase | Continuous significant increase | 96 h (highest value) | |
| SOD Activity | Plasma | Significant increase | Continuous increase | 96 h (highest value) | |
| CAT Activity | Plasma | Significant increase | Continuous significant increase | 96 h (highest value) | |
| Immune Indicators | AKP Content | Liver | Significant increase | Continuous significant increase | 96 h (highest value) |
| IgM Content | Liver | Significant decrease | Continuous decrease | 96 h (lowest value) | |
| ACP Content | Liver | Significant increase | Continuous significant increase | 96 h (highest value) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wang, J.; Zhang, K.; Xing, G.; Tan, Y.; Chen, L.; Ai, T.; Zhang, S.; Chen, Y.; Nie, Z.; et al. Physiological Response Mechanisms of Triplophysa strauchii Under Salinity Stress. Biology 2025, 14, 1202. https://doi.org/10.3390/biology14091202
Gao S, Wang J, Zhang K, Xing G, Tan Y, Chen L, Ai T, Zhang S, Chen Y, Nie Z, et al. Physiological Response Mechanisms of Triplophysa strauchii Under Salinity Stress. Biology. 2025; 14(9):1202. https://doi.org/10.3390/biology14091202
Chicago/Turabian StyleGao, Shixin, Jinqiu Wang, Kaipeng Zhang, Guanping Xing, Yunhong Tan, Lulu Chen, Tao Ai, Shijing Zhang, Yumeng Chen, Zhulan Nie, and et al. 2025. "Physiological Response Mechanisms of Triplophysa strauchii Under Salinity Stress" Biology 14, no. 9: 1202. https://doi.org/10.3390/biology14091202
APA StyleGao, S., Wang, J., Zhang, K., Xing, G., Tan, Y., Chen, L., Ai, T., Zhang, S., Chen, Y., Nie, Z., & Wei, J. (2025). Physiological Response Mechanisms of Triplophysa strauchii Under Salinity Stress. Biology, 14(9), 1202. https://doi.org/10.3390/biology14091202








