Investigation of Anticancer Properties of Newly Synthesized Pyridazine-Based Inhibitors in Mouse and Human Breast Cancer Cell Line
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
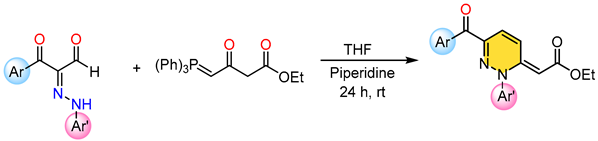
2.1. Synthesis of 2S-Series Molecules
2.2. Cell Culture
2.3. Dissolving 2S-Series Molecules
2.4. MTT Tests
2.4.1. Determination of Anticancer Activities of 2S-Series Molecules
2.4.2. Determination of Anticancer Activities of 2S-Series Molecules
2.4.3. Doxorubicin (DOX) Application
2.5. RT-QPCR Array
2.6. Cell Cycle Analysis
2.7. Apoptosis Analysis
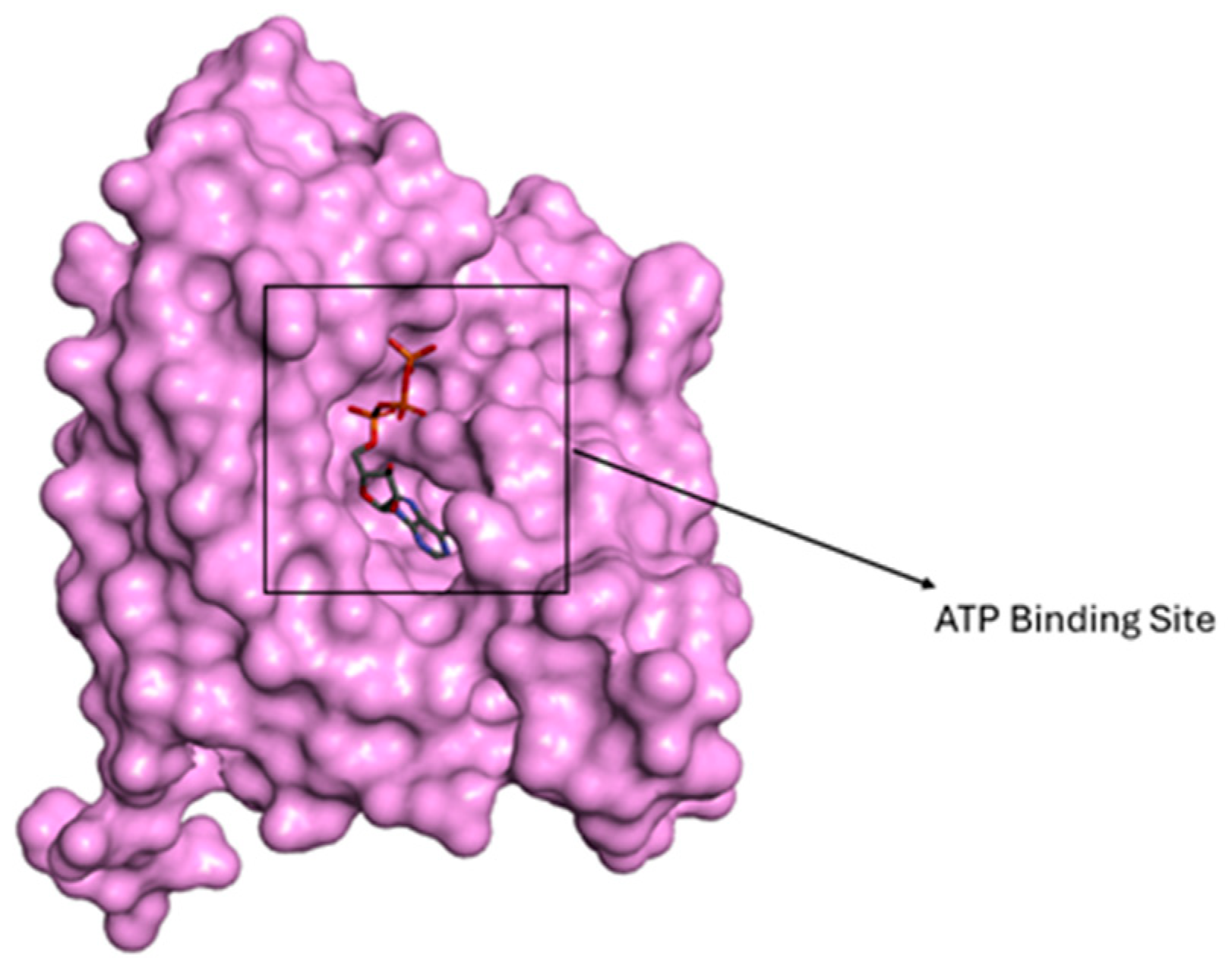
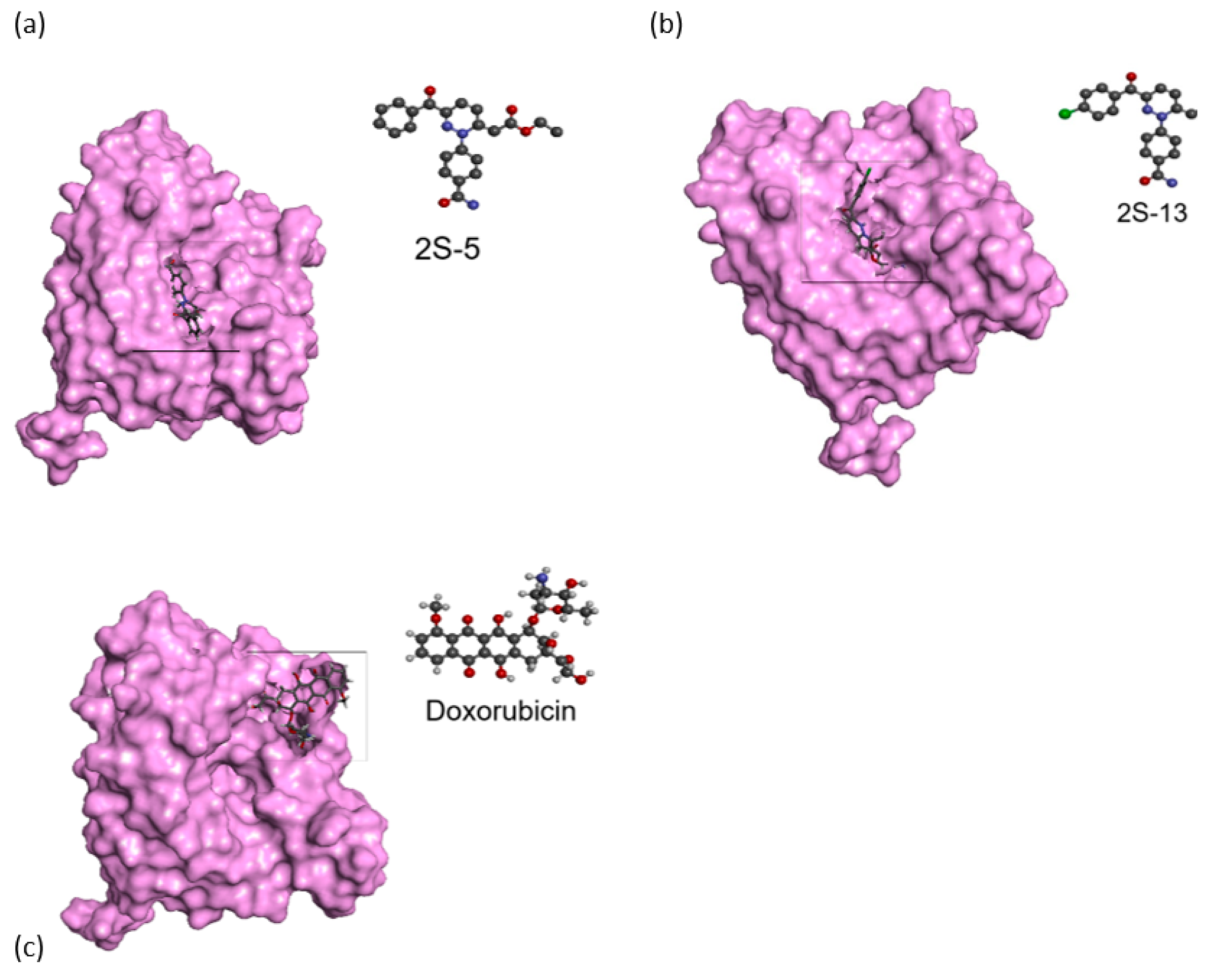
2.8. Molecular Docking Analysis
3. Results
3.1. Synthesis and Characterization of 2S-Series
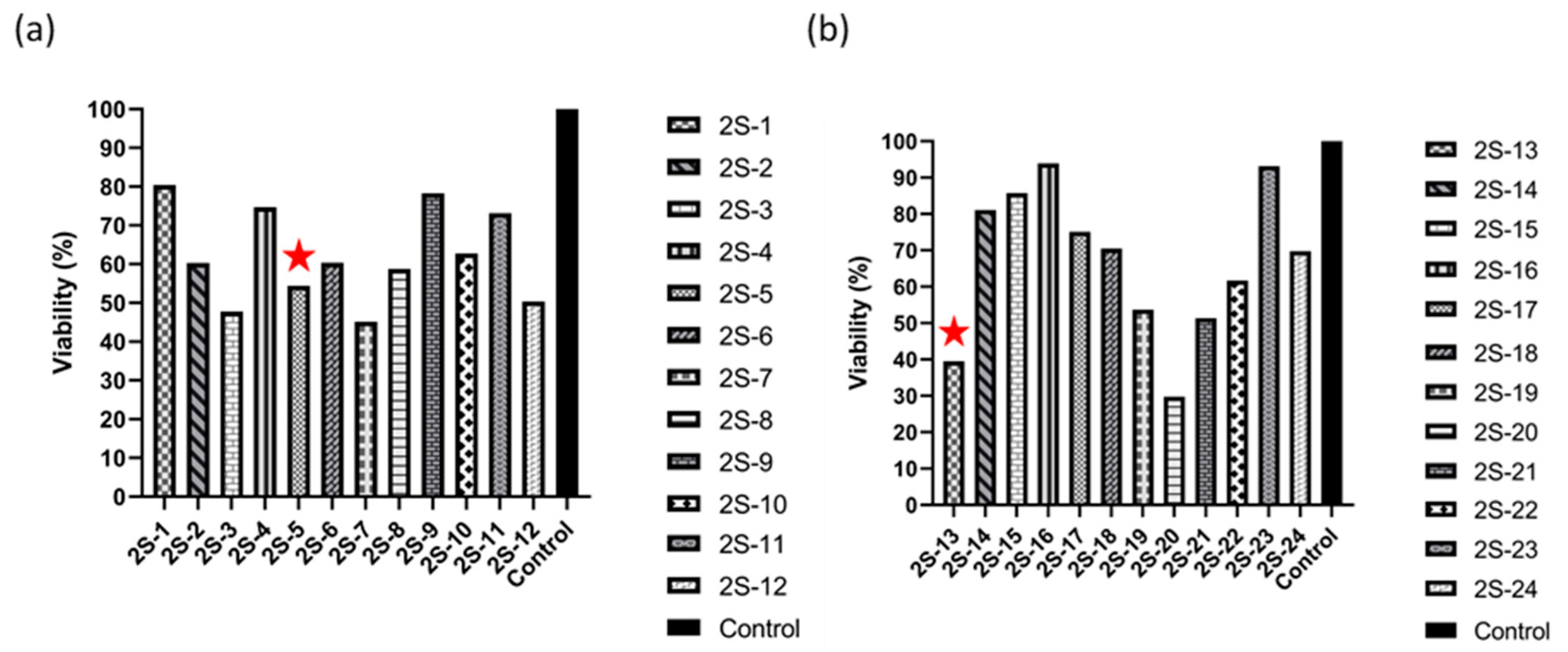
3.2. Effect of 2S-Series Molecules on 4T1 and MDA-MB-231 Cell Line
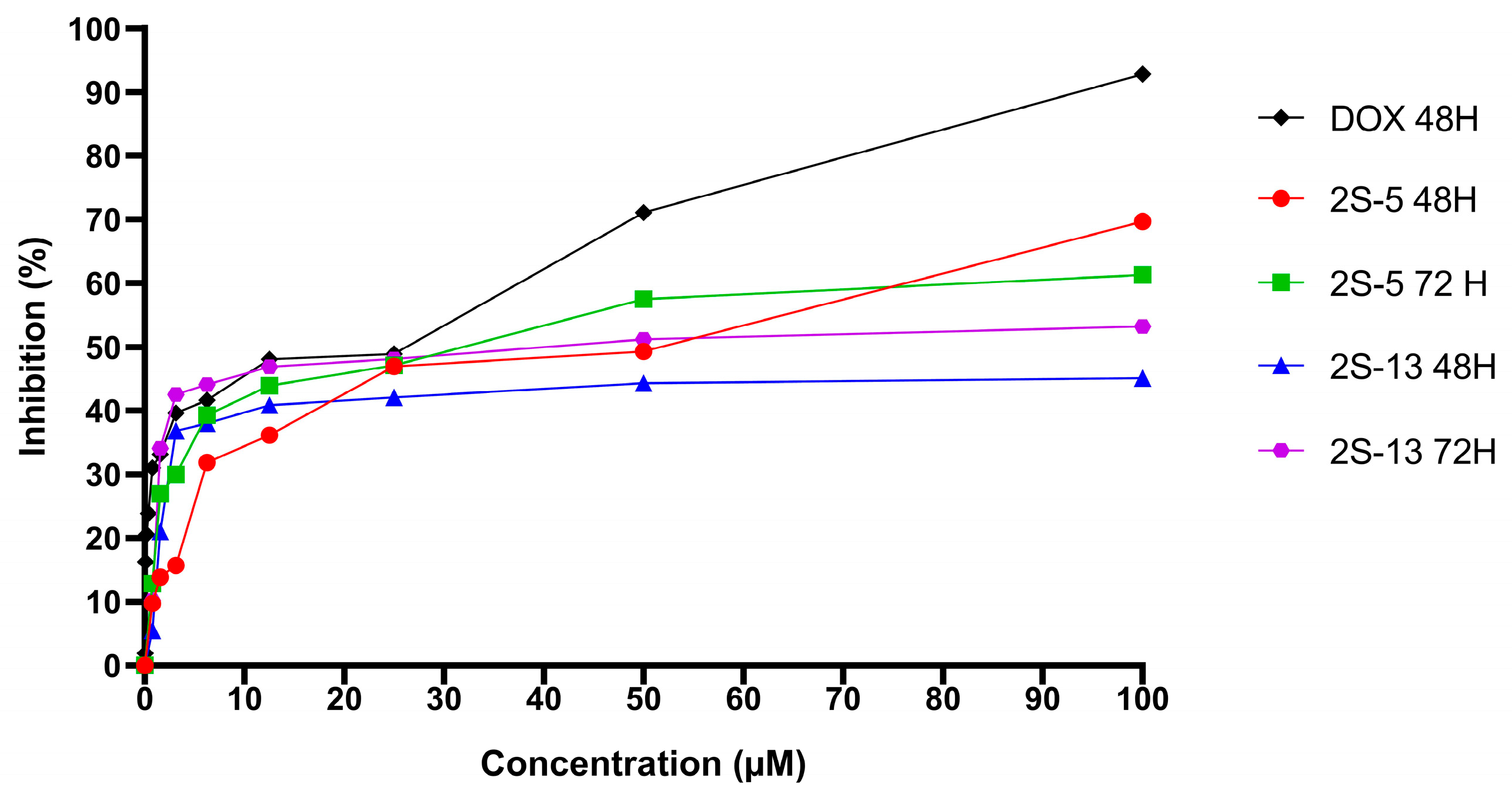
3.3. Cytotoxic Effect of 2S-5 and 2S-13 Molecules on Cell Lines
3.4. Cytotoxic Effect of DOX on Cell Lines
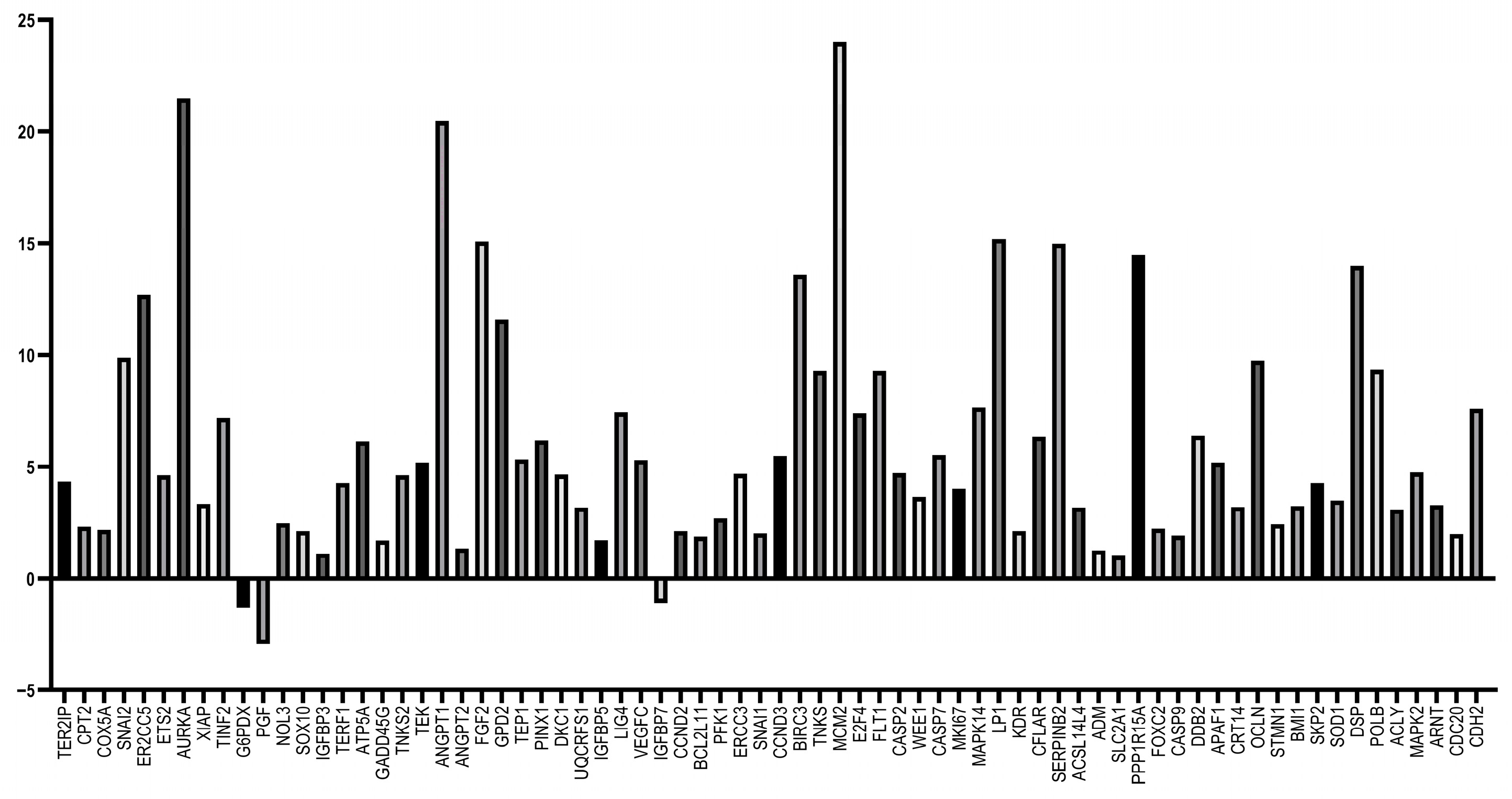
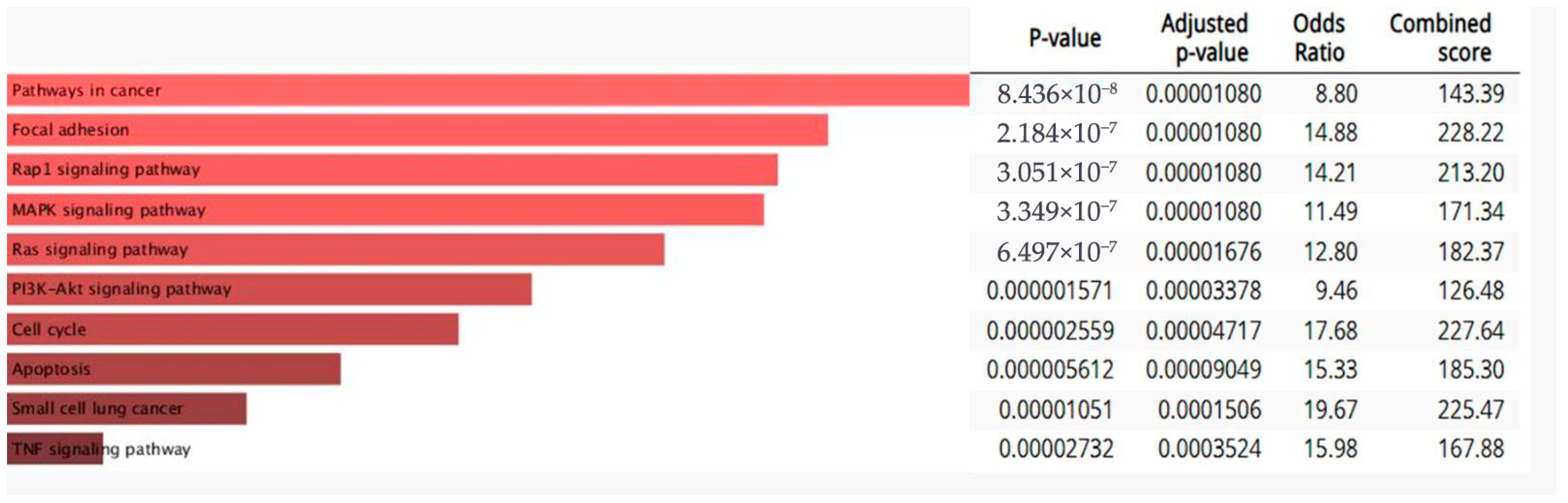
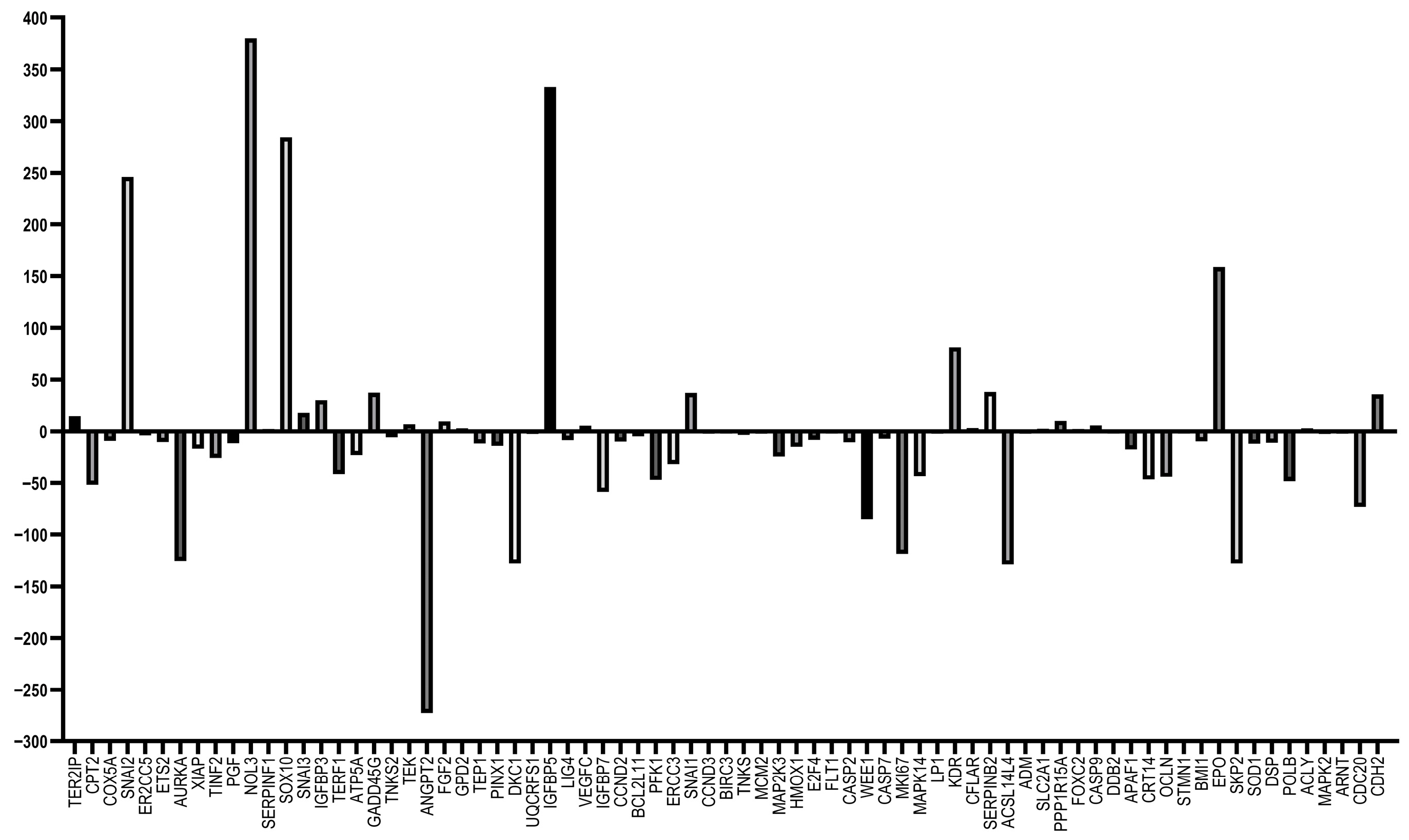
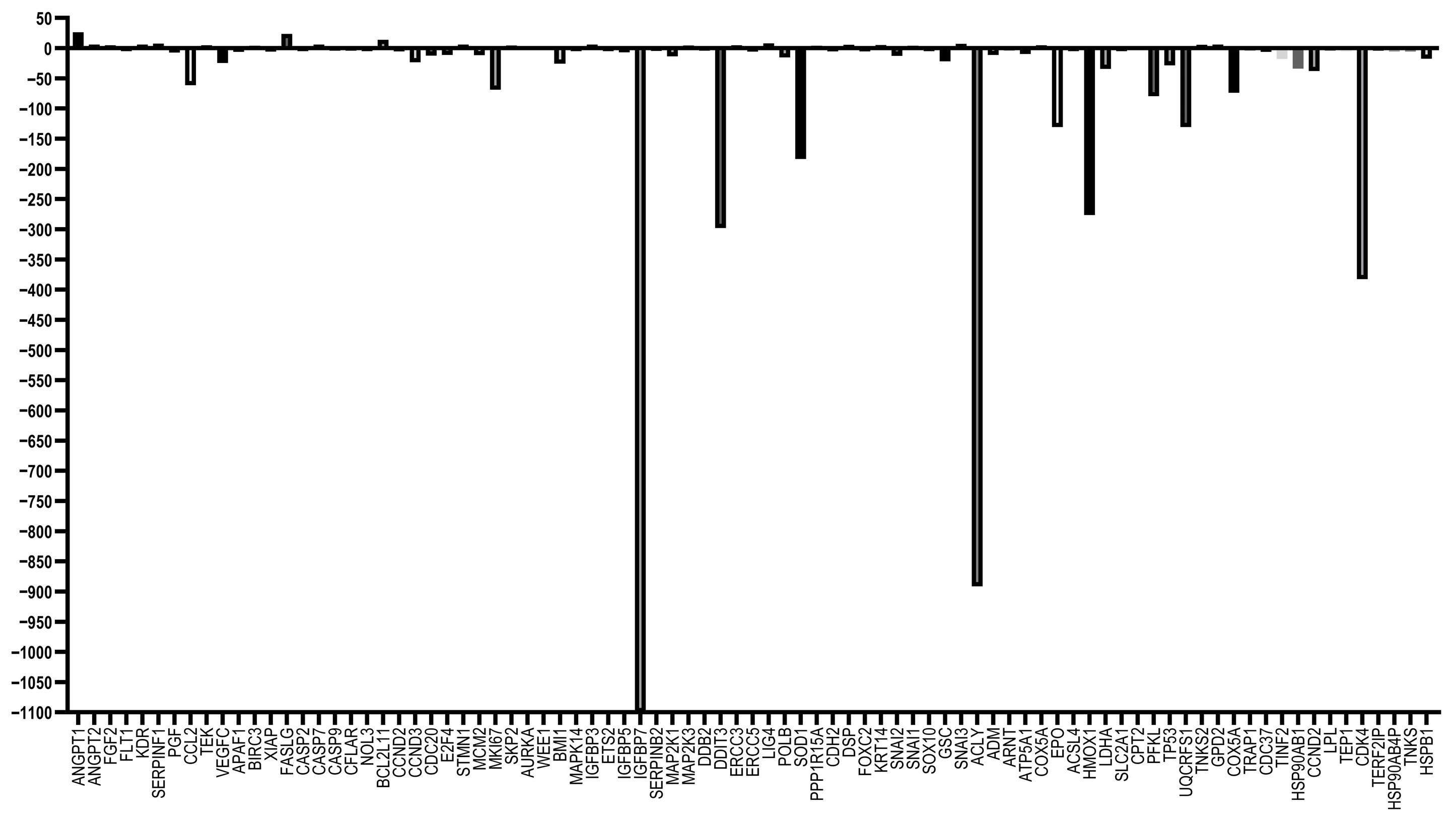
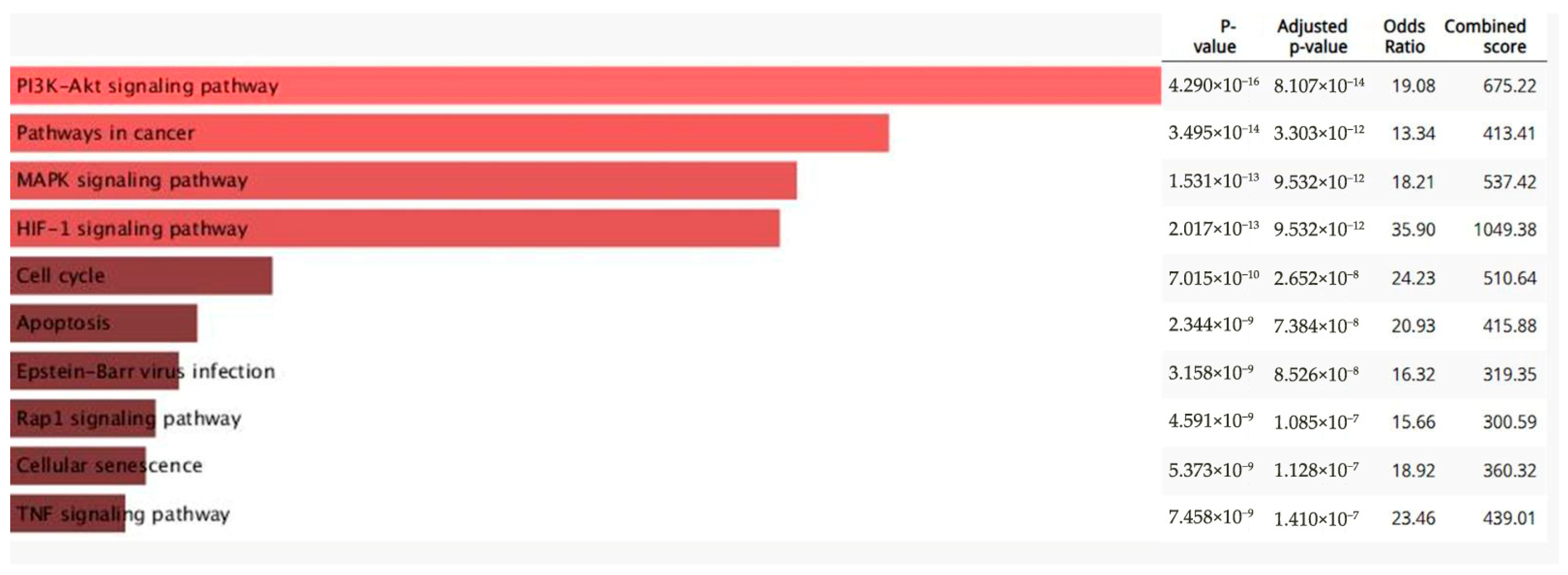
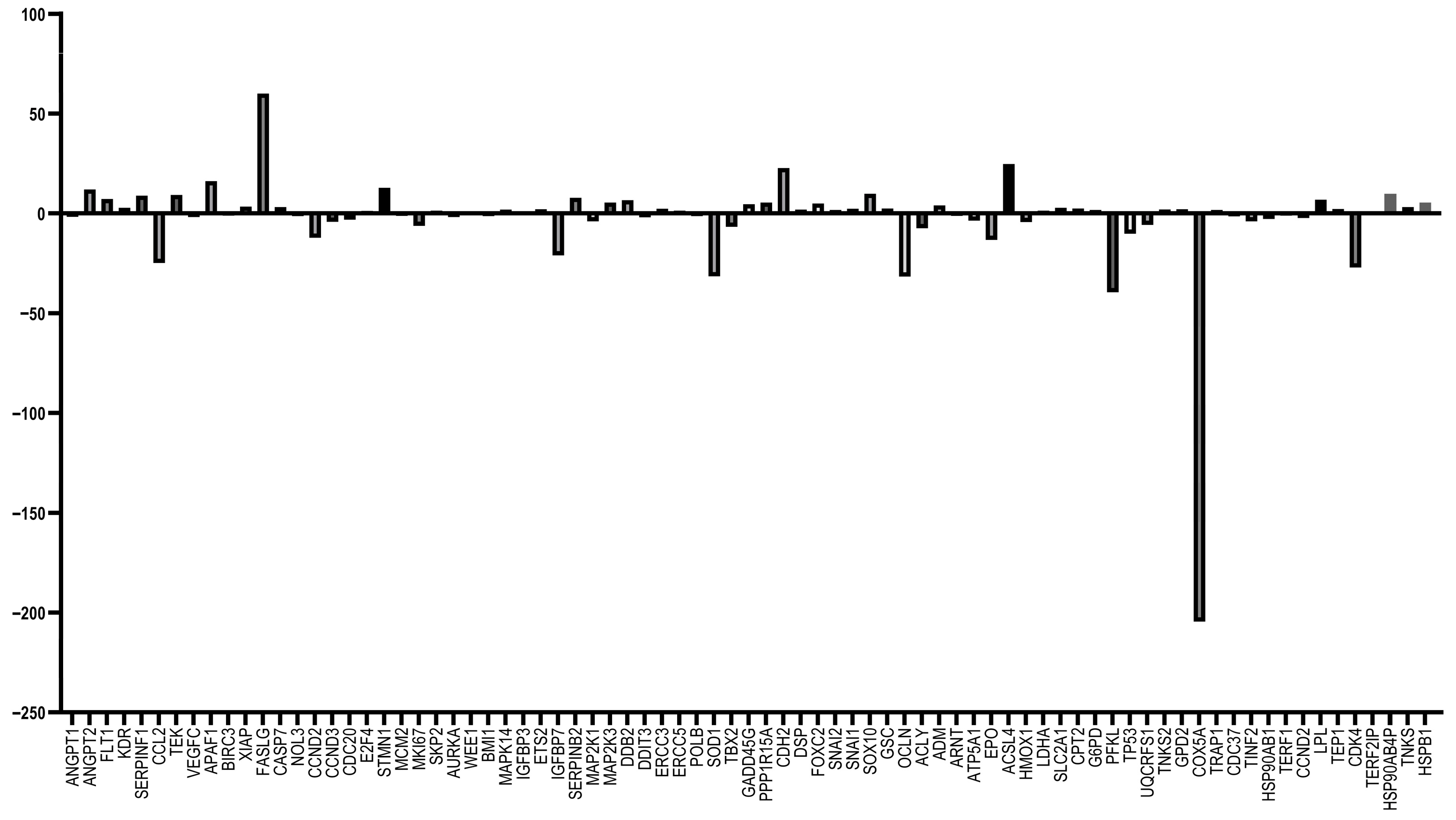
3.5. RT-qPCR Array Experiments
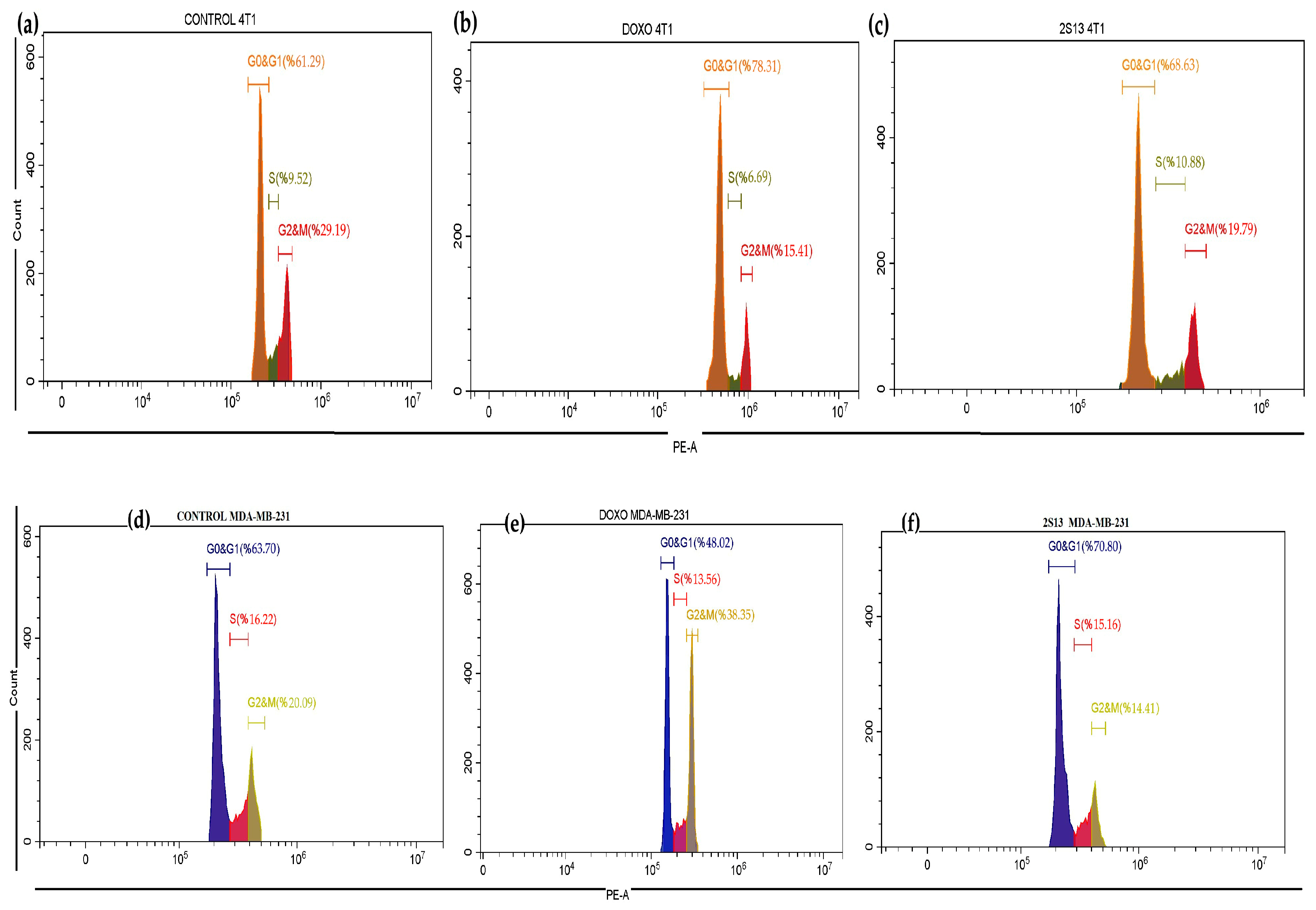
3.6. Cell Cycle Analysis with Flow Cytometry
3.7. Apoptosis Analysis with Flow Cytometry
3.8. Docking Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSP | Heat shock protein |
| TNBC | Triple-negative breast cancer |
| DOX | Doxorubicin |
| ER+ | Estrogen receptor |
| PR+ | Progesterone receptor |
| HER2+ | Human epidermal receptor 2-expressing breast cancer |
| TOP2 | Topoisomerase II |
| DSBs | Double-strand DNA breaks |
| NTD | Amino terminal part of Hsp |
| SI | Selectivity index |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphatidylinositol-3-kinase |
| AKT | Protein kinase B |
References
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Liu, Y.; Zhao, K.; Wei, S.; Sugarman, E.T.; Liu, L.; Zhang, G. Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance. Cells 2022, 11, 2778. [Google Scholar] [CrossRef]
- Rastogi, S.; Joshi, A.; Sato, N.; Lee, S.; Lee, M.-J.; Trepel, J.B.; Neckers, L. An Update on the Status of HSP90 Inhibitors in Cancer Clinical Trials. Cell Stress Chaperones 2024, 29, 519–539. [Google Scholar] [CrossRef]
- Ren, X.; Li, T.; Zhang, W.; Yang, X. Targeting Heat-Shock Protein 90 in Cancer: An Update on Combination Therapy. Cells 2022, 11, 2556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zhang, Q.; Liu, Y.L.; Yu, X.Q.; Chui, R.H.; Zhang, L.L.; Zhao, B.; Ma, L.Y. Recent contributions of pyridazine as a privileged scaffold of anticancer agents in medicinal chemistry: An updated review. Bioorganic Med. Chem. 2024, 111, 117847. [Google Scholar] [CrossRef]
- Khalil, N.A.; Ahmed, E.M.; Mohamed, K.O.; Nissan, Y.M.; Zaitone, S.A.B. Synthesis and biological evaluation of new pyrazolone–pyridazine conjugates as anti-inflammatory and analgesic agents. Bioorganic Med. Chem. 2014, 22, 2080–2089. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Hassan, M.S.; El-Malah, A.A.; Kassab, A.E. New pyridazine derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents; design, synthesis and biological evaluation. Bioorganic Chem. 2020, 95, 103497. [Google Scholar] [CrossRef] [PubMed]
- Daoui, S.; Direkel, Ş.; Ibrahim, M.M.; Tüzün, B.; Chelfi, T.; Al-Ghorbani, M.; Bouatia, M.; Karbane, M.; Doukkali, A.; Benchat, N.; et al. Synthesis, spectroscopic characterization, antibacterial activity, and computational studies of novel pyridazinone derivatives. Molecules 2023, 28, 678. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, A.A.; Almehmadi, M.M.; Asif, M. Diverse pharmacological potential of pyridazine analogs against various diseases. Med. Chem. 2024, 20, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.W.; Mohamed, K.O.; Farag, D.B.; Khalifa, A.K.; Mahmoud, Z. Synthesis of potent vasodilating agents: In silico and in vitro evaluation of 6-(4-substitutedphenyl)-3-pyridazinone derivatives as potential hydralazine analogues. Sci. Rep. 2024, 14, 29514. [Google Scholar] [CrossRef]
- Asif, M.; Singh, A.; Siddiqui, A.A. The effect of pyridazine compounds on the cardiovascular system. Med. Chem. Res. 2012, 21, 3336–3346. [Google Scholar] [CrossRef]
- Boukharsa, Y.; Alhaji Isa, M.; Sayah, K.; Alsalme, A.; Oulmidi, A.; Shehzadi, S.; Faouzi, M.E.A.; Karrouchi, K.; Ansar, M.H. Pyridazine Derivatives as New Antidiabetic Agents: Synthesis, In-Vitro α-Amylase Inhibitory Activity, Molecular Docking and Molecular Dynamics Simulations Studies. ChemistrySelect 2024, 9, e202401557. [Google Scholar] [CrossRef]
- Kalhor, H.R.; Nazari Khodadadi, A. Synthesis and structure activity relationship of pyridazine-based inhibitors for elucidating the mechanism of amyloid inhibition. Chem. Res. Toxicol. 2018, 31, 1092–1104. [Google Scholar] [CrossRef]
- Jaballah, M.Y.; Serya, R.T.; Abouzid, K. Pyridazine based scaffolds as privileged structures in anti-cancer therapy. Drug Res. 2017, 67, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Santali, E.Y.; El-Deen, E.M.M.; Naguib, I.A.; El-Haggar, R. Development of pyridazine derivatives as potential EGFR inhibitors and apoptosis inducers: Design, synthesis, anticancer evaluation, and molecular modeling studies. Bioorganic Chem. 2021, 106, 104473. [Google Scholar] [CrossRef]
- Lu, G.; Nie, W.; Xin, M.; Meng, Y.; Jiang, J.; Gu, J.; Cheng, X.; Chan, A.S.; Zou, Y. Discovery of novel benzamide derivatives bearing benzamidophenyl and phenylacetamidophenyl scaffolds as potential antitumor agents via targeting PARP-1. Eur. J. Med. Chem. 2023, 251, 115243. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, Y.; Xue, T.; Wu, J.; Song, H.; Zhou, S.; Li, Y.; Gong, J.; Wei, M.; Ji, X.; et al. Identification of selenium-containing benzamides as potent microtubule-targeting antitumor agents. Bioorganic Chem. 2025, 159, 108355. [Google Scholar] [CrossRef]
- Jiang, H.; Lan, N.; Ma, W.; Zhang, Z.; Zhao, Z.; Hu, Y.; Su, Y.; Huang, Y.; Wang, Y.; Xu, D.; et al. Synthesis and evaluation of the antitumor activity of 2-amino-4-tetrahydroindazole-substituted benzamide derivatives as HSP90 inhibitors. J. Mol. Struct. 2024, 1300, 137266. [Google Scholar] [CrossRef]
- Song, E.J.; Kim, W.S.; Han, Y.; Lee, C.; Moon, E.J.; Kim, H.J.; Kang, N.S. Discovery of a transforming growth factor-β1 inhibitory peptide, Charis 1000 to enhance the therapeutic efficacy of paclitaxel in triple-negative breast cancer. Int. J. Biol. Macromol. 2025, 314, 144216. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Ma, S.; Lin, Y.; Li, W.; Gong, Y.; Zhang, C. Combination of AURKA Inhibitor and HSP90 Inhibitor to Treat Breast Cancer with AURKA Overexpression and TP53 Mutations. Med. Oncol. 2022, 39, 180. [Google Scholar] [CrossRef]
- Liu, Z.M.; Bao, Y.; Li, T.K.; Di, Y.B.; Song, W.J. MKI67: A Potential Oncogene of Oral Squamous Cell Carcinoma via High Throughput Technology. Medicine 2022, 101, e32595. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Fu, Q.; Xu, J.; Gu, X.; Zhou, H.; Zhi, C. Transcription Factor E2F4 Is an Indicator of Poor Prognosis and Is Related to Immune Infiltration in Hepatocellular Carcinoma. J. Cancer 2021, 12, 1792–1803. [Google Scholar] [CrossRef]
- Zeng, L.-P.; Wu, Z.-Y.; Zhou, W.; Gao, Y.-X.; Du, Y.-T.; Lu, C.-Y.; Song, D.-G. Identify GADD45G as a Potential Target of 4-Methoxydalbergione in Treatment of Liver Cancer: Bioinformatics Analysis and In Vivo Experiment. World J. Surg. Oncol. 2023, 21, 324. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wang, C.; Wang, Z.; Jiang, Y.; Li, X.; Lu, H.; Deng, H. Gadd45g Initiates Embryonic Stem Cell Differentiation and Inhibits Breast Cell Carcinogenesis. Cell Death Discov. 2021, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, P.; Li, Z.; Li, J.; Hou, D.; Chen, X.; Zhang, J.; Wang, H.; Yang, J.; Qiu, J.; et al. An Allosteric Mechanism for Potent Inhibition of Human ATP-Citrate Lyase. Nature 2019, 568, 566–570. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Zhang, H.; Zhang, F.; Zhou, B.; Ning, F.; Wang, H.; Zhang, Y. Hsp90ab1 Stabilizes LRP5 to Promote Epithelial–Mesenchymal Transition via Activation of AKT and Wnt/β-Catenin Signaling Pathways in Gastric Cancer Progression. Oncogene 2019, 38, 1489–1507. [Google Scholar] [CrossRef]







 | |||||
|---|---|---|---|---|---|
| Compound |  |  | Melting Point (°C) Yield | Molecular Formula | HRMS Calculated (Found) |
| 2S-1 | 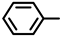 | 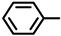 | 182–183 %74 | C21H18N2O3 | (M+H): 347.1390 (347.1393) |
| 2S-2 |  | 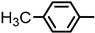 | 149–150 %76 | C22H20N2O3 | (M+H): 361.1547 (361.1548) |
| 2S-3 | 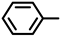 | 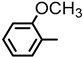 | 184–185 %78 | C22H20N2O4 | (M+H): 377.1496 (377.1507) |
| 2S-4 | 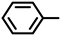 | 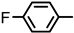 | 193–194 %68 | C21H17FN2O3 | (M+H): 365.1296 (365.1301) |
| 2S-5 | 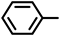 | 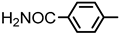 | 222–223 %72 | C22H19N3O4 | (M+H): 390.1448 (390.1452) |
| 2S-6 |  | 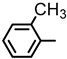 | 190–191 %63 | C22H20N2O3 | (M+H): 361.1547 (361.1544) |
| 2S-7 | 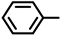 | 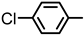 | 155–156 %69 | C21H17ClN2O3 | (M+H): 381.1006 (381.1001) |
| 2S-8 | 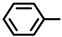 |  | 191–192 %71 | C21H17BrN2O3 | (M-): 424.0428 (424.0391) |
| 2S-9 | 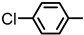 | 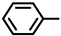 | 219–220 %79 | C21H17ClN2O3 | (M+H): 381.1000 (381.1006) |
| 2S-10 |  | 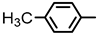 | 305–306 %71 | C22H19ClN2O3 | (M+H): 395.1157 (395.1154) |
| 2S-11 |  | 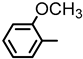 | 205–206 %71 | C22H19ClN2O4 | (M+H): 411.1106 (411.1113) |
| 2S-12 | 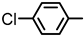 | 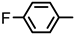 | 193–194 %68 | C21H16ClFN2O3 | (M+H): 399.0906 (399.0906) |
| 2S-13 |  | 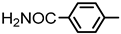 | 310–311 %70 | C22H18ClN3O4 | (M+H): 424.1059 (424.1056) |
| 2S-14 |  | 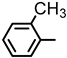 | 199–200 %68 | C22H19ClN2O3 | (M+H): 395.1157 (395.1159) |
| 2S-15 |  |  | 206–207 %77 | C21H16Cl2N2O3 | (M+H): 415.0611 (415.0611) |
| 2S-16 | 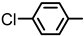 | 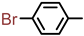 | 172–173 %69 | C21H16BrClN2O3 | (M+H): 459.0111 (-) |
| 2S-17 | 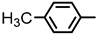 |  | 193–194 %69 | C22H20N2O3 | (M+H): 361.1547 (361.1548) |
| 2S-18 | 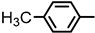 |  | 178–179 %73 | C23H22N2O3 | (M+H): 375.1703 (375.1709) |
| 2S-19 | 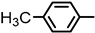 |  | 171–172 %72 | C23H22N2O4 | (M+H): 391.1613 (391.1648) |
| 2S-20 | 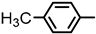 | 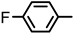 | 191–192 %75 | C22H19FN2O3 | (M+H): 379.1452 (379.1449) |
| 2S-21 | 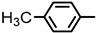 | 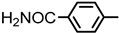 | 237–238 %73 | C23H21N3O4 | (M+H): 404.1605 (404.1606) |
| 2S-22 | 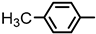 | 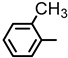 | 166–167 %64 | C23H22N2O3 | (M+H): 375.1703 (375.1709) |
| 2S-23 | 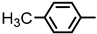 | 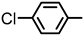 | 174–175 %76 | C22H19ClN2O3 | (M+H): 395.1157 (395.1163) |
| 2S-24 | 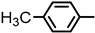 | 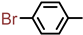 | 193–194 %72 | C22H19BrN2O3 | (M+H): 439.0652 (439.0652) |
| Cell Lines | Inhibitors (IC50 (µM)) 48 h | Inhibitors (IC50 (µM)) 72 h | Selectivity Index (SI) 48 h | Selectivity Index (SI) 72 h | ||
|---|---|---|---|---|---|---|
| 2S-13 | DOX | 2S-13 | 2S-13 | DOX | 2S-13 | |
| hTERT | 98 | 1.5 | 100 | |||
| 4T1 | 12.4 | 12.7 | 7.1 | 7.90 | 0.118 | 14.08 |
| MDA-MB-231 | 3.83 | 6.8 | 2.85 | 25.45 | 0.220 | 35.09 |
| G0 & G1 | S | G2 & M | |
|---|---|---|---|
| 4T1-CONTROL | % 61.29 | % 9.52 | % 29.19 |
| MDA-MB-231-CONTROL | % 63.70 | % 16.22 | % 20.19 |
| 4T1-2S-13 | % 68.63 | % 10.88 | % 19.79 |
| MDA-MB-231-2S-13 | % 70.80 | % 15.16 | % 14.41 |
| 4T1-DOX | % 78.31 | % 6.69 | % 15.41 |
| MDA-MB-231-DOX | % 48.02 | % 13.56 | % 38.35 |
| Live | Early Apoptotic | Late Apoptotic | Necrotic | |
|---|---|---|---|---|
| 4T1-CONTROL | 97.74% | 0.96% | 0.73% | 0.56% |
| MDA-MB-231-CONTROL | 92.81% | 3.59% | 2.35% | 1.25% |
| 4T1-DOX | 53.62% | 30.31% | 8.91% | 7.16% |
| MDA-MB-231-DOX | 51.25% | 22.00% | 21.54% | 5.21% |
| 4T1-2S-13 | 86.35% | 5.10% | 6.44% | 2.11% |
| MDA-MB-231-2S-13 | 84.61% | 9.33% | 4.11% | 1.96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coskun, K.A.; Abay, E.C.; Gumus, M.; Çelik, A.B.; Gulum, L.; Koca, I.; Tutar, Y. Investigation of Anticancer Properties of Newly Synthesized Pyridazine-Based Inhibitors in Mouse and Human Breast Cancer Cell Line. Biology 2025, 14, 1193. https://doi.org/10.3390/biology14091193
Coskun KA, Abay EC, Gumus M, Çelik AB, Gulum L, Koca I, Tutar Y. Investigation of Anticancer Properties of Newly Synthesized Pyridazine-Based Inhibitors in Mouse and Human Breast Cancer Cell Line. Biology. 2025; 14(9):1193. https://doi.org/10.3390/biology14091193
Chicago/Turabian StyleCoskun, Kübra Acikalin, Elif Cansu Abay, Mehmet Gumus, Ayşe Büşranur Çelik, Levent Gulum, Irfan Koca, and Yusuf Tutar. 2025. "Investigation of Anticancer Properties of Newly Synthesized Pyridazine-Based Inhibitors in Mouse and Human Breast Cancer Cell Line" Biology 14, no. 9: 1193. https://doi.org/10.3390/biology14091193
APA StyleCoskun, K. A., Abay, E. C., Gumus, M., Çelik, A. B., Gulum, L., Koca, I., & Tutar, Y. (2025). Investigation of Anticancer Properties of Newly Synthesized Pyridazine-Based Inhibitors in Mouse and Human Breast Cancer Cell Line. Biology, 14(9), 1193. https://doi.org/10.3390/biology14091193








