Monitoring Fish Biodiversity in the Pelagic Zone of the Western Indian Ocean Using Environmental DNA Metabarcoding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
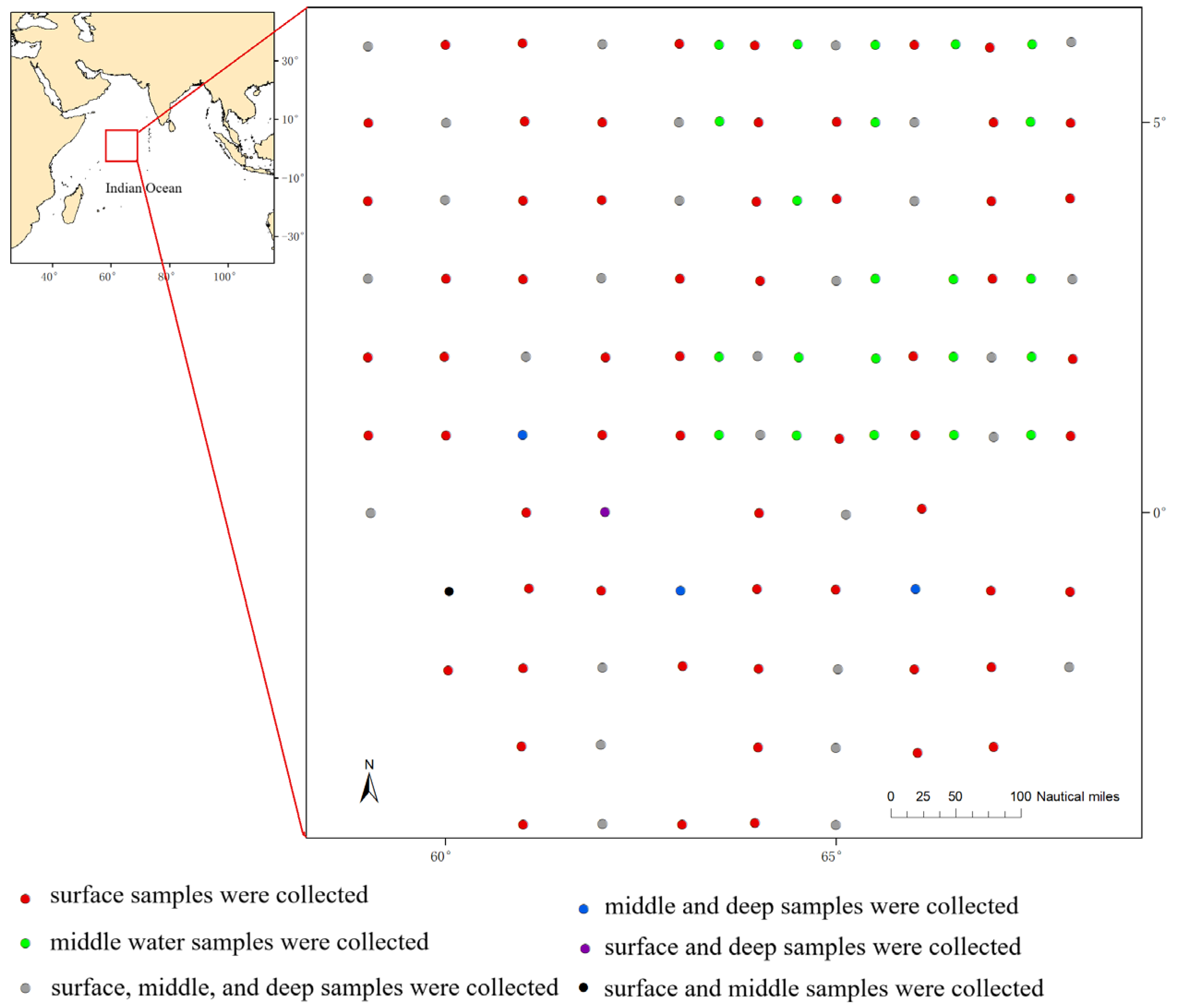
2.1. Study Area and Sample Collection
2.2. PCR Amplification and High-Throughput Sequencing
3. Results
3.1. High-Throughput Sequencing
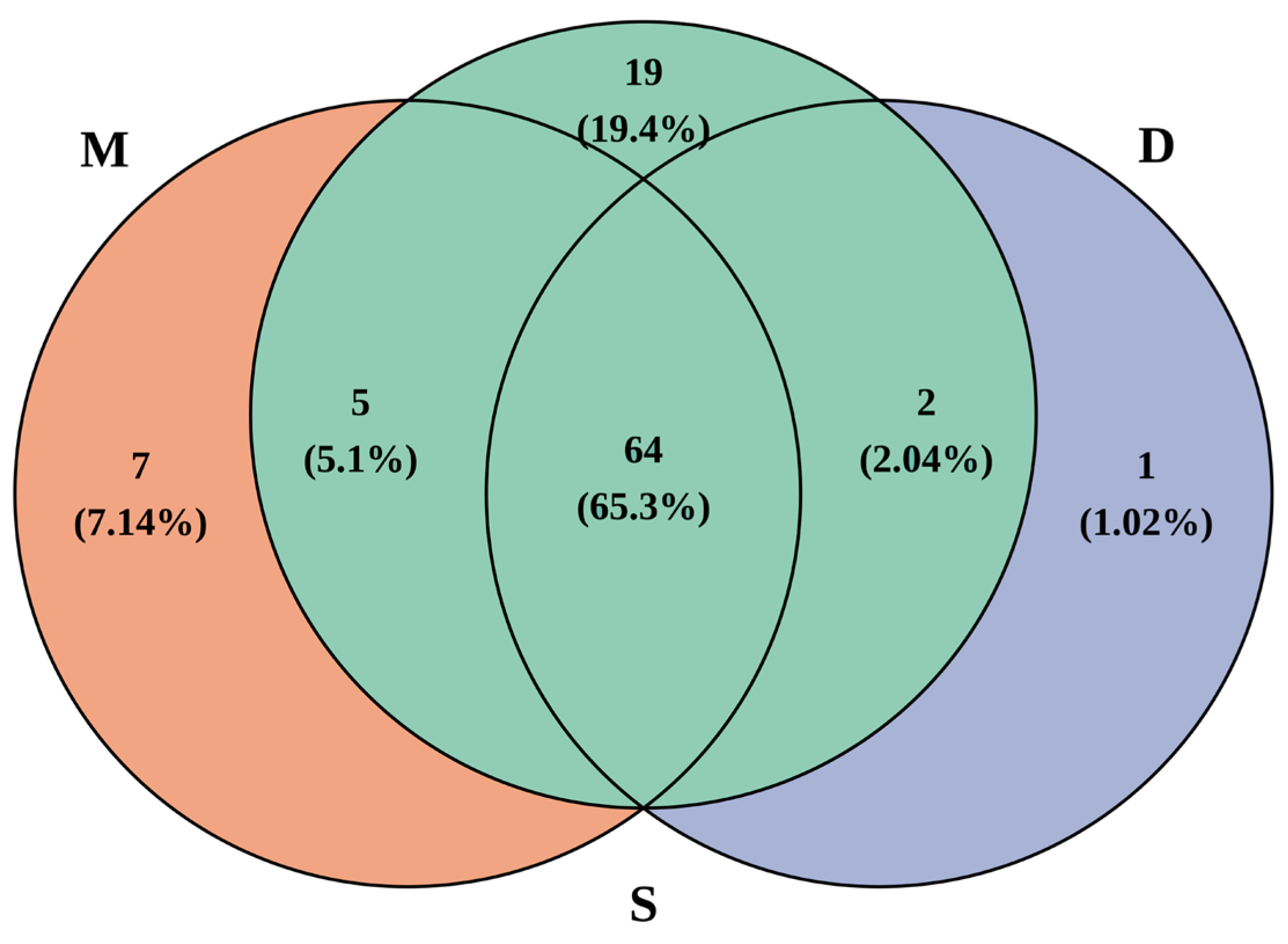
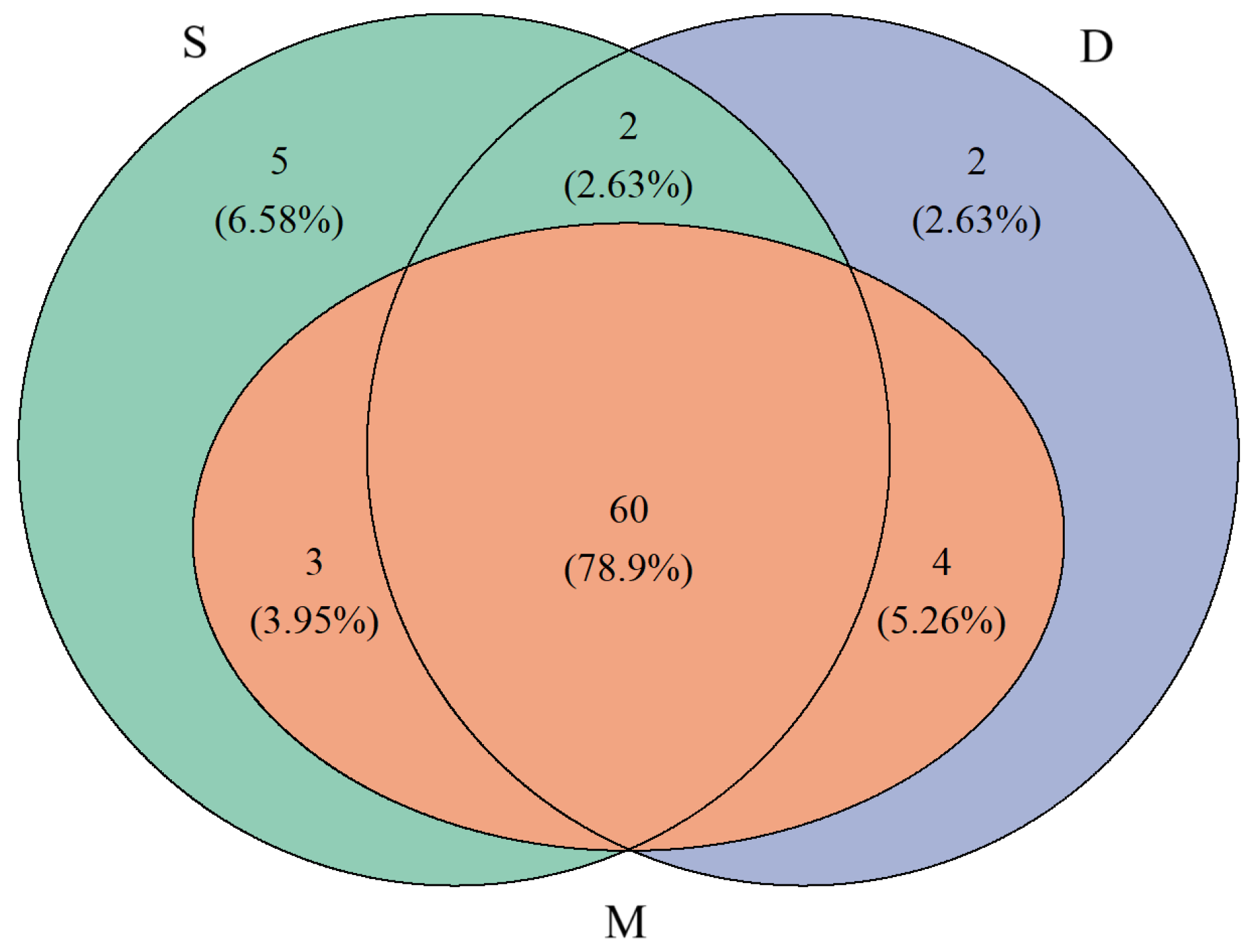
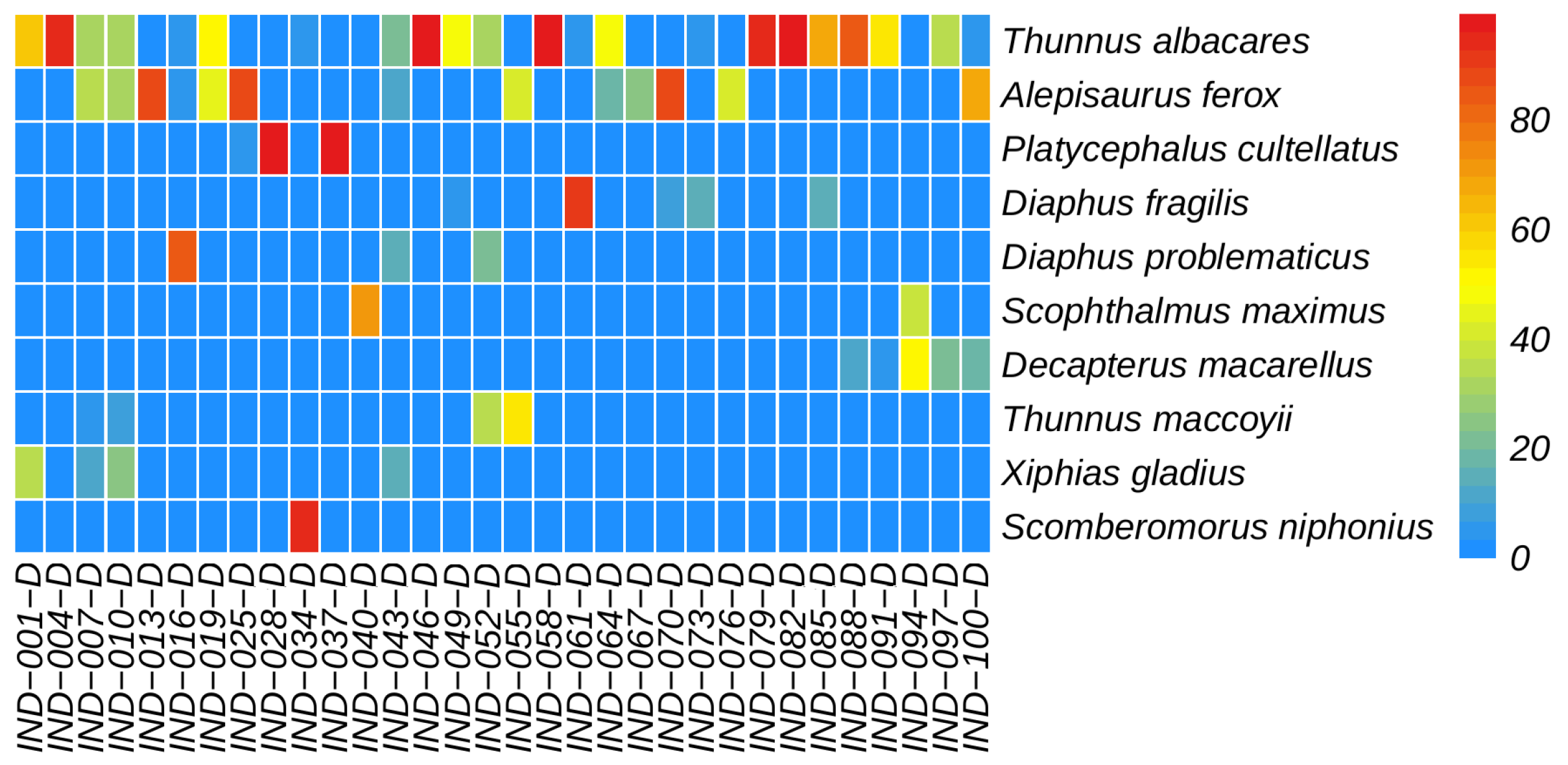
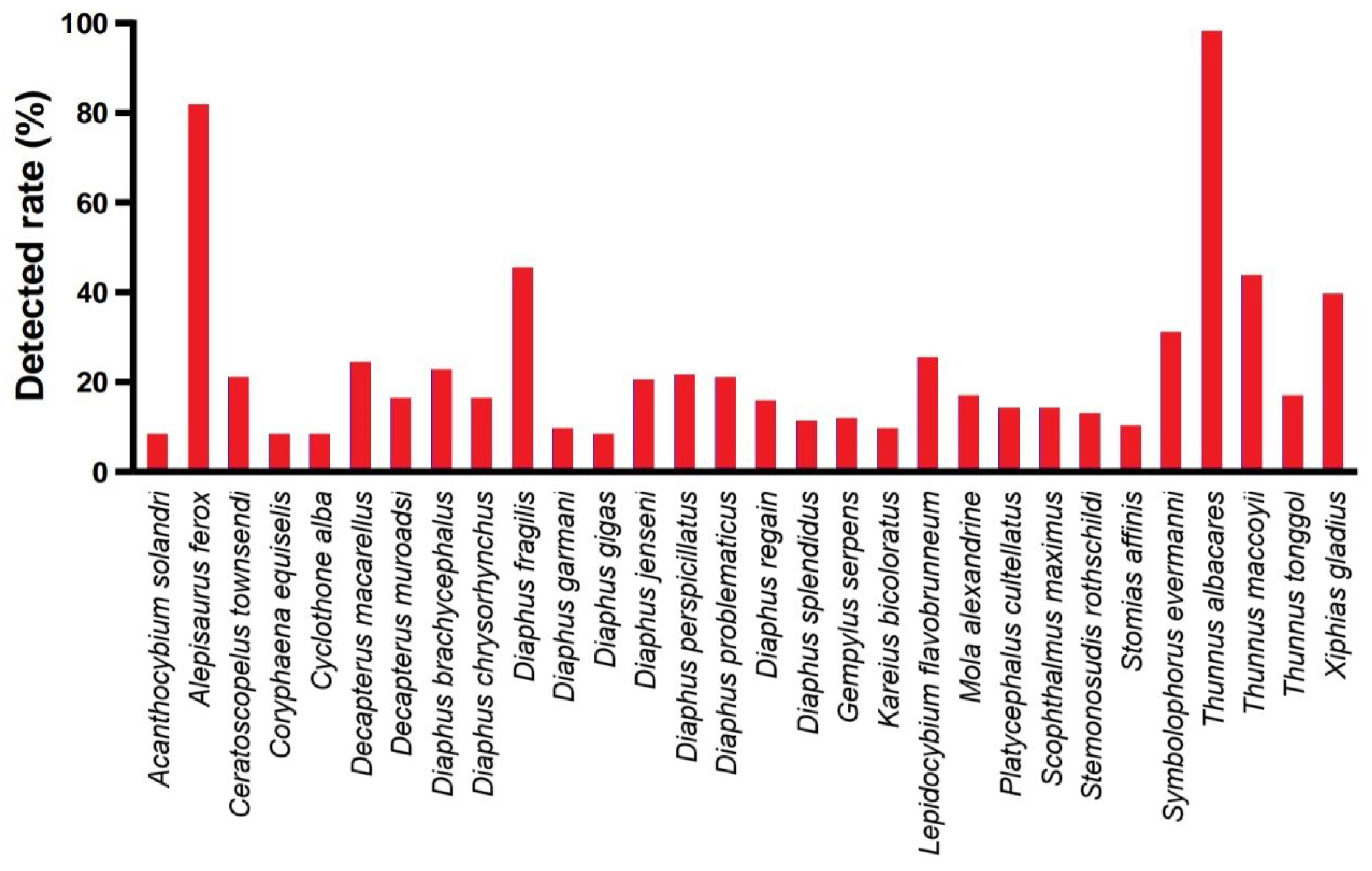
3.2. Annotation Species Statistics
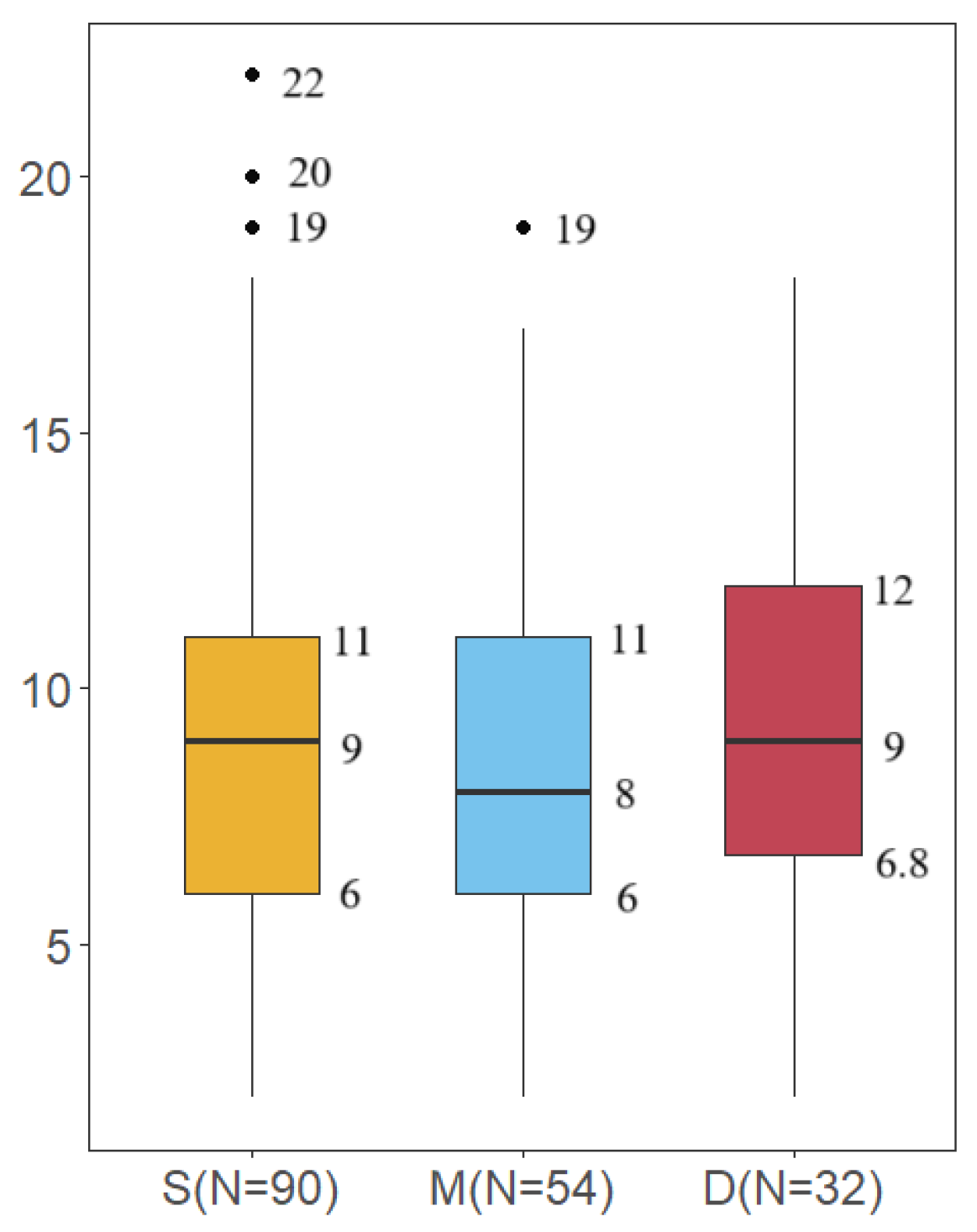
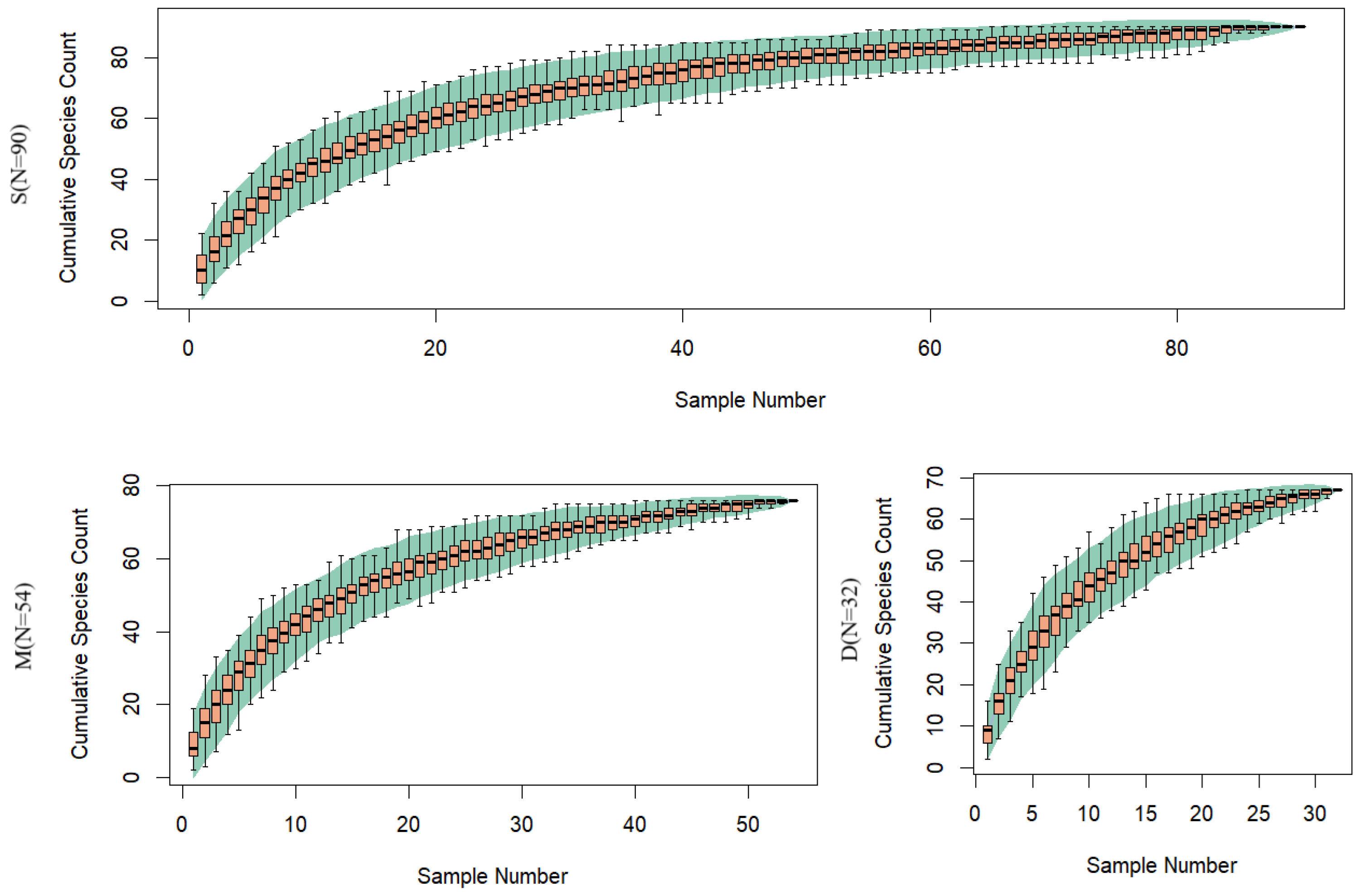
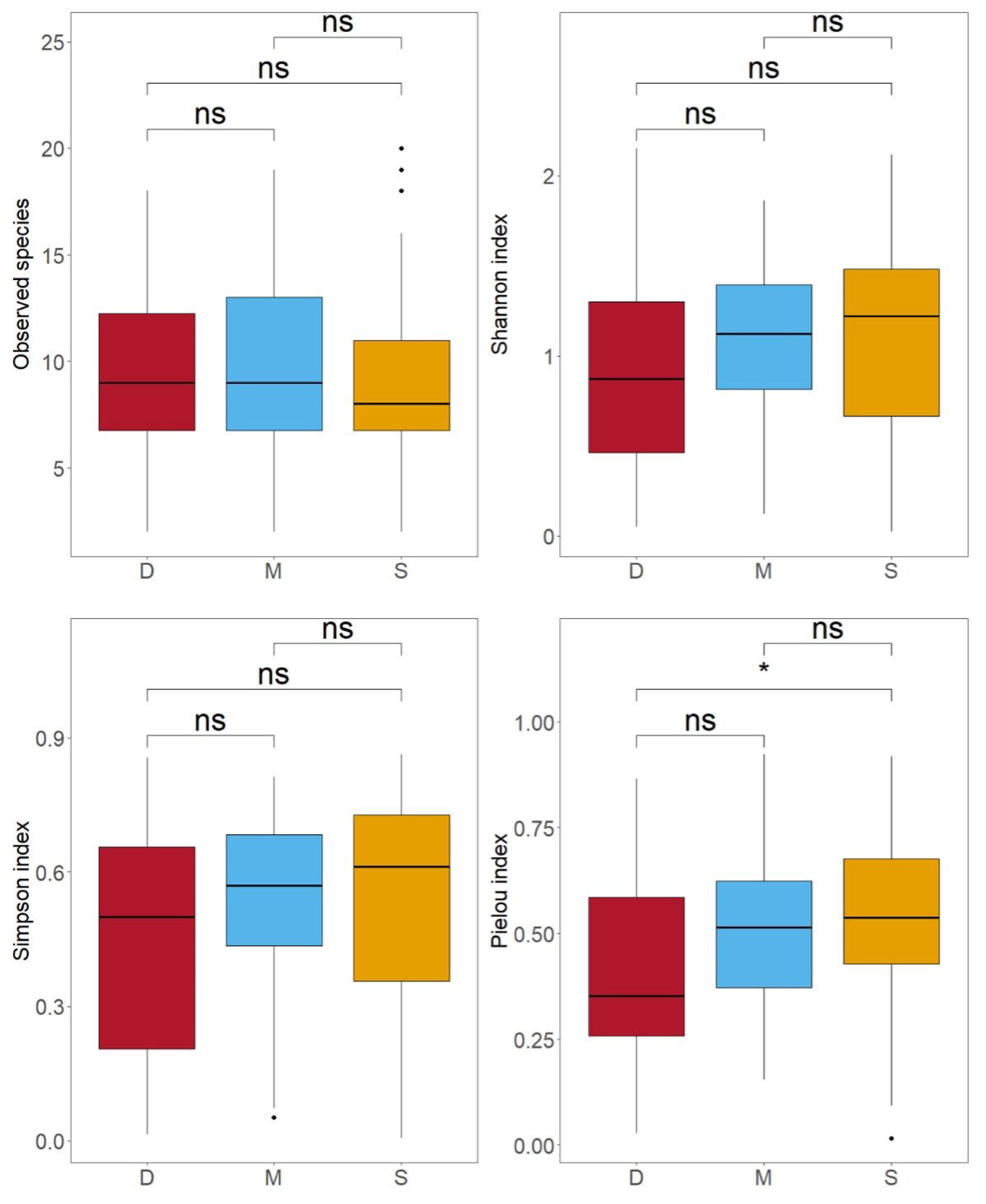
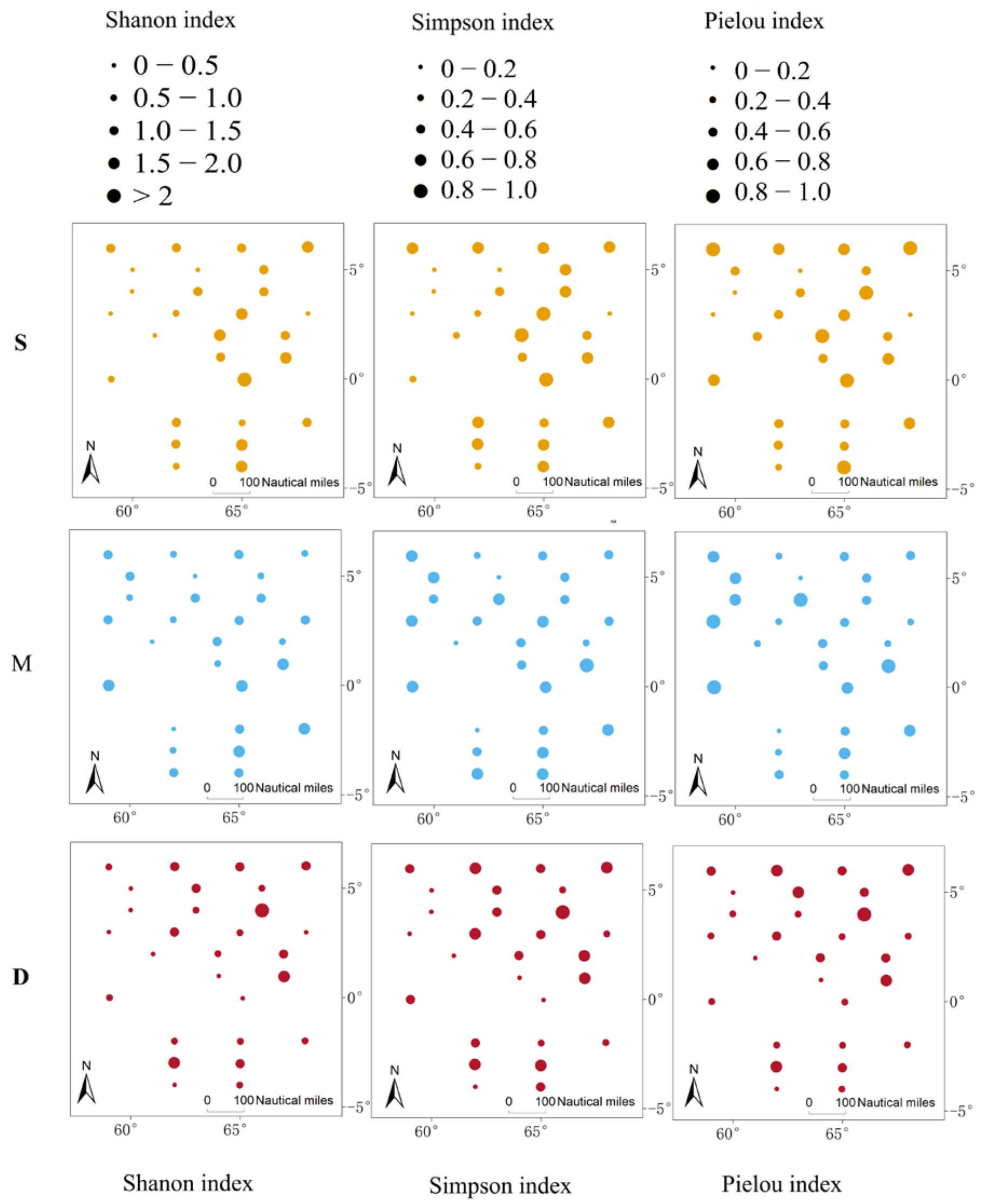
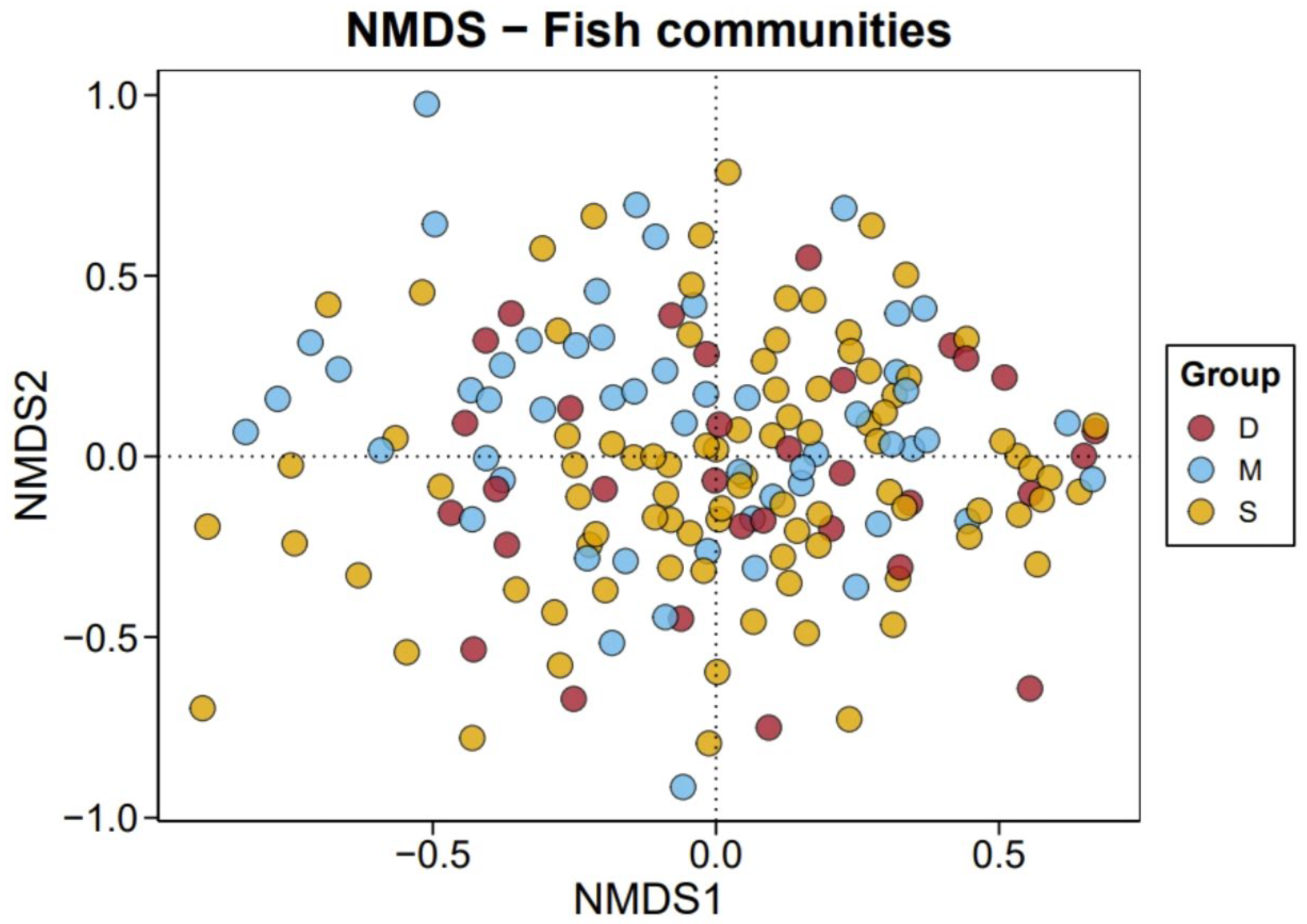
3.3. Comparison of Species Diversity Across Different Water Layers
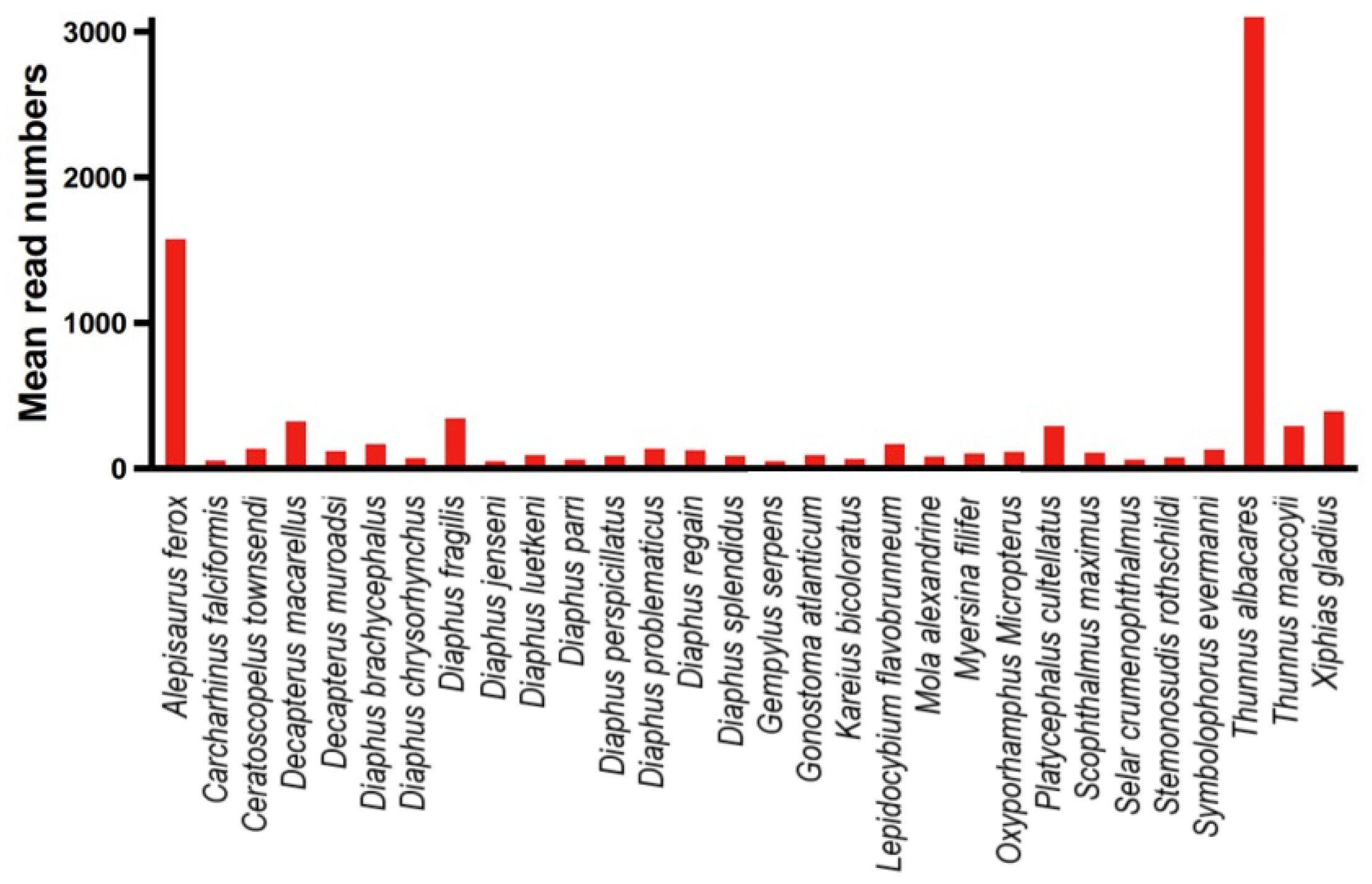
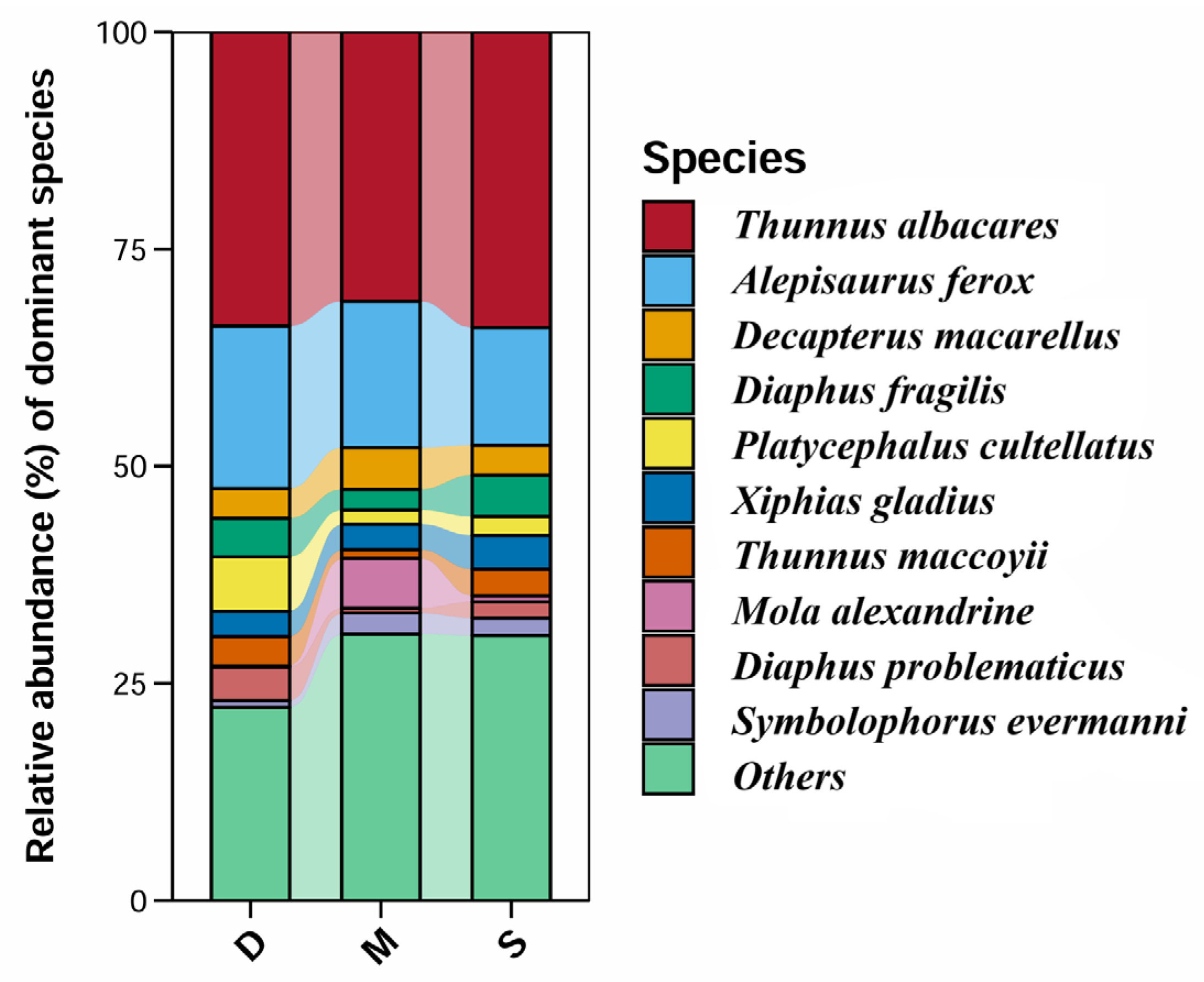
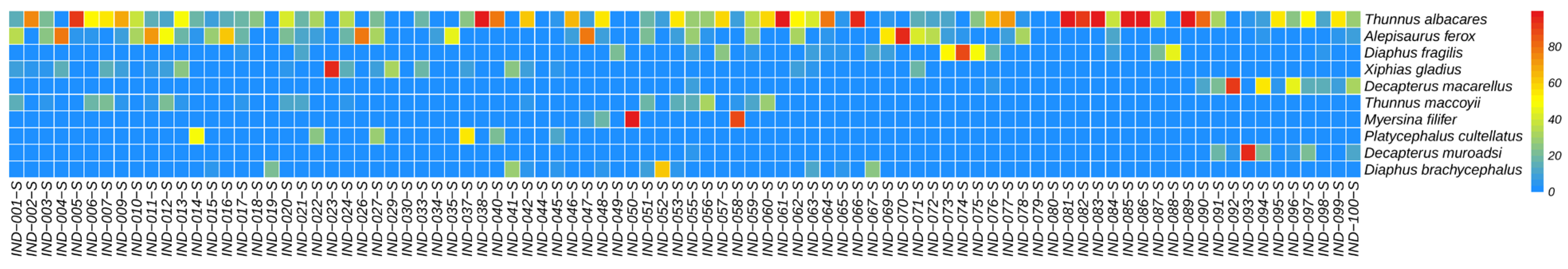
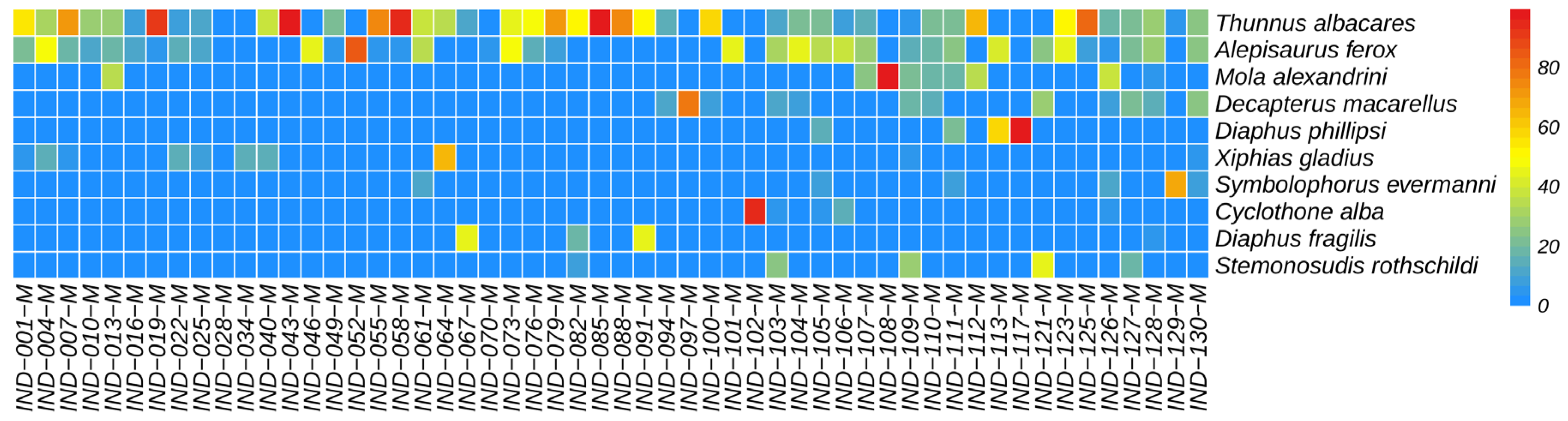
3.4. Relative Abundance of Species
4. Discussion
4.1. Application Potential of eDNA Metabarcoding in Pelagic Fish Diversity Survey
4.2. Detected Relative Abundance of Species
4.3. The Improvement Direction of Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wafar, M.; Venkataraman, K.; Ingole, B.; Khan, S.A.; LokaBharathi, P.; Hansson, L.-A. State of Knowledge of Coastal and Marine Biodiversity of Indian Ocean Countries. PLoS ONE 2011, 6, e14613. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Warmuth, L.M.; Kelly, S.; A Samoilys, M.; Popova, E.; E I Head, C.; Bonsall, M.B.; Hidalgo, M. Environmental change and connectivity drive coral reef fish abundance in the Western Indian Ocean. ICES J. Mar. Sci. 2024, 81, 1785–1795. [Google Scholar] [CrossRef]
- Mondal, S.; Punt, A.E.; Mendes, D.; Osuka, K.E.; Lee, M. Teleconnection Impacts of Climatic Variability on Tuna and Billfish Fisheries of the South Atlantic and Indian Ocean: A Study Towards Sustainable Fisheries Management. Fish Fish. 2024, 26, 240–256. [Google Scholar] [CrossRef]
- Olds, B.P.; Jerde, C.L.; Renshaw, M.A.; Li, Y.; Evans, N.T.; Turner, C.R.; Deiner, K.; Mahon, A.R.; Brueseke, M.A.; Shirey, P.D.; et al. Estimating species richness using environmental DNA. Ecol. Evol. 2016, 6, 4214–4226. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.F.; Rimet, F.; Vasselon, V.; Vautier, M.; Domaizon, I.; Bouchez, A. Fish eDNA metabarcoding from aquatic biofilm samples: Methodological aspects. Mol. Ecol. Resour. 2021, 22, 1440–1453. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Jiao, M.; Xie, T.; Xie, M.; Li, H.; Suo, A.; Yue, W.; Ding, D.; He, W. Use of passive sampling in environmental DNA metabarcoding technology: Monitoring of fish diversity in the Jiangmen coastal waters. Sci. Total. Environ. 2023, 908, 168298. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, K.N.; Meeuwig, J.J.; Zeller, D. Reconstructing past fisheries catches for large pelagic species in the Indian Ocean. Front. Mar. Sci. 2023, 10, 1177872. [Google Scholar] [CrossRef]
- Andruszkiewicz, E.A.; Starks, H.A.; Chavez, F.P.; Sassoubre, L.M.; Block, B.A.; Boehm, A.B.; Doi, H. Biomonitoring of marine vertebrates in Monterey Bay using eDNA metabarcoding. PLoS ONE 2017, 12, e0176343. [Google Scholar] [CrossRef] [PubMed]
- Sigsgaard, E.E.; Nielsen, I.B.; Carl, H.; Krag, M.A.; Knudsen, S.W.; Xing, Y.; Holm-Hansen, T.H.; Møller, P.R.; Thomsen, P.F. Seawater environmental DNA reflects seasonality of a coastal fish community. Mar. Biol. 2017, 164, 128. [Google Scholar] [CrossRef]
- Takahashi, M.; Saccò, M.; Kestel, J.H.; Nester, G.; Campbell, M.A.; van der Heyde, M.; Heydenrych, M.J.; Juszkiewicz, D.J.; Nevill, P.; Dawkins, K.L.; et al. Aquatic environmental DNA: A review of the macro-organismal biomonitoring revolution. Sci. Total. Environ. 2023, 873, 162322. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, H.; Xian, W. Fish Diversity Monitored by Environmental DNA in the Yangtze River Mainstream. Fishes 2022, 7, 1. [Google Scholar] [CrossRef]
- Bohmann, K.; Evans, A.; Gilbert, M.T.P.; Carvalho, G.R.; Creer, S.; Knapp, M.; Yu, D.W.; de Bruyn, M. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol. Evol. 2014, 29, 358–367. [Google Scholar] [CrossRef]
- Adams, C.I.; Knapp, M.; Gemmell, N.J.; Jeunen, G.-J.; Bunce, M.; Lamare, M.D.; Taylor, H.R. Beyond biodiversity: Can environmental DNA (eDNA) cut it as a population genetics tool? Genes 2019, 10, 192. [Google Scholar] [CrossRef]
- Williams, M.; O’GRady, J.; Ball, B.; Carlsson, J.; de Eyto, E.; McGinnity, P.; Jennings, E.; Regan, F.; Parle-McDermott, A. The application of CRISPR-cas for single identification from environmental DNA. Mol. Ecol. Resour. 2019, 19, 1106–1114. [Google Scholar] [CrossRef]
- Angeles, I.B.; Lejzerowicz, F.; Cordier, T.; Scheplitz, J.; Kucera, M.; Ariztegui, D.; Pawlowski, J.; Morard, R. Planktonic foraminifera eDNA signature deposited on the seafloor remains preserved after burial in marine sediments. Sci. Rep. 2020, 10, 20351. [Google Scholar] [CrossRef]
- Miya, M. Environmental DNA metabarcoding: A novel method for biodiversity monitoring of marine fish communities. Annu. Rev. Mar. Sci. 2022, 14, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: Detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, M.Y.; Adolf, J.; Charlop-Powers, Z.; Dunton, K.J.; Hinks, G.; VanMorter, S.M.; Bradbury, I. Trawl and eDNA assessment of marine fish diversity, seasonality, and relative abundance in coastal new Jersey, USA. ICES J. Mar. Sci. 2020, 78, 293–304. [Google Scholar] [CrossRef]
- Lyu, D.; Qian, T.; Li, F.; Sun, S.; Wang, W.; Shan, X. Monitoring and assessing the species diversity and abundance of marine teleost around the Yellow River estuary in June using environmental DNA. Front. Mar. Sci. 2023, 10, 1123831. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic. Acids. Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Chen, J.; Ruan, H.; Li, Z.; Guo, W.; Li, M.; Liu, L. eDNA metabarcoding as a promising conservation tool for monitoring fish diversity in a coastal wetland of the Pearl River Estuary compared to bottom trawling. Sci. Total. Environ. 2020, 702, 134704. [Google Scholar] [CrossRef]
- Letessier, T.B.; Bouchet, P.J.; Meeuwig, J.J. Sampling mobile oceanic fishes and sharks: Implications for fisheries and conservation planning. Biol. Rev. 2015, 92, 627–646. [Google Scholar] [CrossRef]
- Diao, C.; Jia, H.; Guo, S.; Hou, G.; Xian, W.; Zhang, H. Biodiversity exploration in autumn using environmental DNA in the South China sea. Environ. Res. 2022, 204, 112357. [Google Scholar] [CrossRef] [PubMed]
- Lamb, P.D.; Fonseca, V.G.; Maxwell, D.L.; Nnanatu, C.C. Systematic review and meta-analysis: Water type and temperature affect environmental DNA decay. Mol. Ecol. Resour. 2022, 22, 2494–2505. [Google Scholar] [CrossRef]
- Power, H.; Takahashi, M.; Jarman, S.; Berry, O. What is environmental DNA? Environ. DNA 2023, 5, 1743–1758. [Google Scholar] [CrossRef]
- Dowell, R.; Dunn, N.; Head, C.; Yesson, C.; Williams, J.; Ransome, E. Environmental DNA captures diurnal fluctuations of surface eukaryotes on a tropical coral reef. Environ. DNA 2024, 6, e512. [Google Scholar] [CrossRef]
- Mayne, S.R.; Manning, J.A.; Henderson, S.M.; Parsley, M.B.; Strickler, K.M.; Nielson, J.R.; Goldberg, C.S. Hydrodynamics and Aquatic Vegetation Drive Spatial Patterns of Environmental DNA in Ponds. Environ. DNA 2024, 6, e70036. [Google Scholar] [CrossRef]
- Sukumaran, S.; Sebastian, W.; Gopalakrishnan, A. Population genetic structure of Indian oil sardine, Sardinella longiceps along Indian coast. Gene 2016, 576, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Collette, B.B.; Nauen, C.E. Scombrids of the world. In An Annotated and Illustrated Catalogue of Tunas, Mackerels, Bonitos and Related Species Known to Date; FAO: Rome, Italy, 1983. [Google Scholar]
- Bhendarkar, M.; Rodriguez-Ezpeleta, N. Exploring uncharted territory: New frontiers in environmental DNA for tropical fisheries management. Environ. Monit. Assess. 2024, 196, 617. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ferguson, E.E.; Moyer, G.R. History, applications, methodological issues and perspectives for the use environmental DNA (eDNA) in marine and freshwater environments. Rev. De Biol. Trop. 2014, 62, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Pont, D. Predicting downstream transport distance of fish eDNA in lotic environments. Mol. Ecol. Resour. 2024, 24, e13934. [Google Scholar] [CrossRef]
- Laroche, O.; Kersten, O.; Smith, C.R.; Goetze, E. From sea surface to seafloor: A benthic allochthonous eDNA survey for the abyssal ocean. bioRxiv 2020. bioRxiv:2020.05.07.082602. [Google Scholar] [CrossRef]
- McCartin, L.J.; Vohsen, S.A.; Ambrose, S.W.; Layden, M.; McFadden, C.S.; Cordes, E.E.; McDermott, J.M.; Herrera, S. Temperature Controls eDNA Persistence across Physicochemical Conditions in Seawater. Environ. Sci. Technol. 2022, 56, 8629–8639. [Google Scholar] [CrossRef]
| Class | Order | Family | Species | Authority |
|---|---|---|---|---|
| Actinopterygii | Acanthuriformes | Acanthuridae | Acanthurus leucosternon | Bennett, 1833 |
| Actinopterygii | Actinopteri_norank | Pseudochromidae | Pseudoplesiops revellei | Schultz, 1953 |
| Actinopterygii | Actinopteri_norank | Sciaenidae | Johnius trewavasae | Sasaki, 1992 |
| Actinopterygii | Anguilliformes | Nemichthyidae | Nemichthys scolopaceus | Richardson, 1848 |
| Actinopterygii | Anguilliformes | Ophichthidae | Muraenichthys gymnopterus | Bleeker, 1853 |
| Actinopterygii | Aulopiformes | Alepisauridae | Alepisaurus ferox | Lowe, 1833 |
| Actinopterygii | Aulopiformes | Chlorophthalmidae | Chlorophthalmus pectoralis | Okamura & Doi, 1984 |
| Actinopterygii | Aulopiformes | Paralepididae | Lestrolepis intermedia | Poey, 1868 |
| Actinopterygii | Aulopiformes | Paralepididae | Stemonosudis miscella | Ege, 1933 |
| Actinopterygii | Aulopiformes | Paralepididae | Stemonosudis rothschildi | Richards, 1967 |
| Actinopterygii | Beloniformes | Exocoetidae | Cheilopogon abei | Parin, 1996 |
| Actinopterygii | Beloniformes | Exocoetidae | Cheilopogon atrisignis | Günther, 1866 |
| Actinopterygii | Beloniformes | Exocoetidae | Cheilopogon intermedius | Parin, 1961 |
| Actinopterygii | Beloniformes | Exocoetidae | Cheilopogon pinnatibarbatus | Bennett, 1831 |
| Actinopterygii | Beloniformes | Exocoetidae | Exocoetus monocirrhus | Richardson, 1846 |
| Actinopterygii | Beloniformes | Exocoetidae | Hirundichthys affinis | Günther, 1866 |
| Actinopterygii | Beloniformes | Exocoetidae | Hirundichthys rondeletii | Valenciennes, 1847 |
| Actinopterygii | Beloniformes | Hemiramphidae | Hemiramphus convexus | Weber, 1914 |
| Actinopterygii | Beloniformes | Hemiramphidae | Oxyporhamphus micropterus | Valenciennes, 1847 |
| Actinopterygii | Carangiformes | Carangidae | Decapterus macarellus | Cuvier, 1833 |
| Actinopterygii | Carangiformes | Carangidae | Decapterus muroadsi | Temminck & Schlegel, 1844 |
| Actinopterygii | Carangiformes | Carangidae | Elagatis bipinnulata | Quoy & Gaimard, 1825 |
| Actinopterygii | Carangiformes | Carangidae | Selar crumenophthalmus | Bloch, 1793 |
| Actinopterygii | Carangiformes | Coryphaenidae | Coryphaena equiselis | Linnaeus, 1758 |
| Actinopterygii | Carangiformes | Coryphaenidae | Coryphaena hippurus | Linnaeus, 1758 |
| Actinopterygii | Chaetodontiformes | Leiognathidae | Nuchequula nuchalis | Temminck & Schlegel, 1845 |
| Actinopterygii | Clupeiformes | Clupeidae | Clupanodon thrissa | Linnaeus, 1758 |
| Actinopterygii | Clupeiformes | Engraulidae | Coilia nasus | Temminck & Schlegel, 1846 |
| Actinopterygii | Gobiiformes | Gobiidae | Acanthogobius flavimanus | Temminck & Schlegel, 1845 |
| Actinopterygii | Gobiiformes | Gobiidae | Chaeturichthys stigmatias | Richardson, 1844 |
| Actinopterygii | Gobiiformes | Gobiidae | Myersina filifer | Valenciennes, 1837 |
| Actinopterygii | Holocentriformes | Holocentridae | Myripristis pralinia | Cuvier, 1829 |
| Actinopterygii | Istiophoriformes | Istiophoridae | Tetrapturus angustirostris | Tanaka, 1915 |
| Actinopterygii | Istiophoriformes | Xiphiidae | Xiphias gladius | Linnaeus, 1758 |
| Actinopterygii | Lampriformes | Regalecidae | Regalecus glesne | Ascanius, 1772 |
| Actinopterygii | Mugiliformes | Mugilidae | Planiliza haematocheilus | Temminck & Schlegel, 1845 |
| Actinopterygii | Mugiliformes | Mugilidae | Planiliza macrolepis | Smith, 1846 |
| Actinopterygii | Myctophiformes | Myctophidae | Bolinichthys longipes | Brauer, 1906 |
| Actinopterygii | Myctophiformes | Myctophidae | Ceratoscopelus townsendi | Eigenmann & Eigenmann, 1889 |
| Actinopterygii | Myctophiformes | Myctophidae | Ceratoscopelus warmingii | Lütken, 1892 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus brachycephalus | Tåning, 1928 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus chrysorhynchus | Gilbert & Cramer, 1897 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus fragilis | Tåning, 1928 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus fulgens | Brauer, 1904 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus garmani | Gilbert, 1906 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus gigas | Gilbert, 1913 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus jenseni | Tåning, 1932 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus luetkeni | Brauer, 1904 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus parri | Tåning, 1932 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus perspicillatus | Ogilby, 1898 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus phillipsi | Fowler, 1934 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus problematicus | Parr, 1928 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus regani | Tåning, 1932 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus splendidus | Brauer, 1904 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus suborbitalis | Weber, 1913 |
| Actinopterygii | Myctophiformes | Myctophidae | Diaphus termophilus | Tåning, 1928 |
| Actinopterygii | Myctophiformes | Myctophidae | Lampadena anomala | Parr, 1928 |
| Actinopterygii | Myctophiformes | Myctophidae | Lampadena atlantica | Maul, 1969 |
| Actinopterygii | Myctophiformes | Myctophidae | Lampadena luminosa | Garman, 1899 |
| Actinopterygii | Myctophiformes | Myctophidae | Lampadena yaquinae | Coleman & Nafpaktitis, 1972 |
| Actinopterygii | Myctophiformes | Myctophidae | Myctophum lychnobium | Bolin, 1946 |
| Actinopterygii | Myctophiformes | Myctophidae | Notolychnus valdiviae | Brauer, 1904 |
| Actinopterygii | Myctophiformes | Myctophidae | Symbolophorus californiensis | Eigenmann & Eigenmann, 1889 |
| Actinopterygii | Myctophiformes | Myctophidae | Symbolophorus evermanni | Gilbert, 1905 |
| Actinopterygii | Perciformes | Platycephalidae | Platycephalus cultellatus | Richardson, 1846 |
| Actinopterygii | Perciformes | Sebastidae | Sebastes constellatus | Jordan & Gilbert, 1880 |
| Actinopterygii | Perciformes | Sebastidae | Sebastes goodei | Eigenmann & Eigenmann, 1890 |
| Actinopterygii | Perciformes | Sebastidae | Sebastes koreanus | Kim & Lee, 1994 |
| Actinopterygii | Perciformes | Sebastidae | Sebastes melanosema | Lea, 1911 |
| Actinopterygii | Perciformes | Sebastidae | Sebastes rosenblatti | Chen, 1971 |
| Actinopterygii | Pleuronectiformes | Pleuronectidae | Kareius bicoloratus | Basilewsky, 1855 |
| Actinopterygii | Pleuronectiformes | Pleuronectidae | Pleuronectes platessa | Linnaeus, 1758 |
| Actinopterygii | Pleuronectiformes | Scophthalmidae | Scophthalmus maximus | Walbaum, 1792 |
| Actinopterygii | Scombriformes | Bramidae | Brama dussumieri | Cuvier, 1831 |
| Actinopterygii | Scombriformes | Gempylidae | Gempylus serpens | Cuvier, 1829 |
| Actinopterygii | Scombriformes | Gempylidae | Lepidocybium flavobrunneum | Smith, 1849 |
| Actinopterygii | Scombriformes | Nomeidae | Psenes pellucidus | Lütken, 1880 |
| Actinopterygii | Scombriformes | Scombridae | Acanthocybium solandri | Cuvier, 1832 |
| Actinopterygii | Scombriformes | Scombridae | Allothunnus fallai | Serventy, 1948 |
| Actinopterygii | Scombriformes | Scombridae | Auxis rochei | Risso, 1810 |
| Actinopterygii | Scombriformes | Scombridae | Auxis thazard | Lacepède, 1800 |
| Actinopterygii | Scombriformes | Scombridae | Katsuwonus pelamis | Linnaeus, 1758 |
| Actinopterygii | Scombriformes | Scombridae | Scomberomorus niphonius | Cuvier, 1832 |
| Actinopterygii | Scombriformes | Scombridae | Thunnus alalunga | Bonnaterre, 1788 |
| Actinopterygii | Scombriformes | Scombridae | Thunnus albacares | Bonnaterre, 1788 |
| Actinopterygii | Scombriformes | Scombridae | Thunnus maccoyii | Castelnau, 1872 |
| Actinopterygii | Scombriformes | Scombridae | Thunnus orientalis | Temminck & Schlegel, 1844 |
| Actinopterygii | Scombriformes | Scombridae | Thunnus thynnus | Linnaeus, 1758 |
| Actinopterygii | Scombriformes | Scombridae | Thunnus tonggol | Bleeker, 1851 |
| Actinopterygii | Scombriformes | Scombrolabracidae | Scombrolabrax heterolepis | Roule, 1921 |
| Actinopterygii | Stomiiformes | Gonostomatidae | Cyclothone alba | Brauer, 1906 |
| Actinopterygii | Stomiiformes | Gonostomatidae | Gonostoma atlanticum | Norman, 1930 |
| Actinopterygii | Stomiiformes | Stomiidae | Borostomias pacificus | Imai, 1941 |
| Actinopterygii | Stomiiformes | Stomiidae | Stomias affinis | Günther, 1887 |
| Actinopterygii | Tetraodontiformes | Molidae | Mola alexandrini | Ranzani, 1839 |
| Chondrichthyes | Carcharhiniformes | Carcharhinidae | Carcharhinus falciformis | Müller & Henle, 1839 |
| Chondrichthyes | Carcharhiniformes | Carcharhinidae | Prionace glauca | Linnaeus, 1758 |
| Chondrichthyes | Myliobatiformes | Dasyatidae | Pteroplatytrygon violacea | Bonaparte, 1832 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, D.; Xu, R.; Jin, Y.; Hu, Y.; Liu, M.; Lyu, G.; Shan, X.; Wang, W. Monitoring Fish Biodiversity in the Pelagic Zone of the Western Indian Ocean Using Environmental DNA Metabarcoding. Biology 2025, 14, 1194. https://doi.org/10.3390/biology14091194
Lyu D, Xu R, Jin Y, Hu Y, Liu M, Lyu G, Shan X, Wang W. Monitoring Fish Biodiversity in the Pelagic Zone of the Western Indian Ocean Using Environmental DNA Metabarcoding. Biology. 2025; 14(9):1194. https://doi.org/10.3390/biology14091194
Chicago/Turabian StyleLyu, Ding, Rihong Xu, Yue Jin, Yulong Hu, Mianyu Liu, Guanzheng Lyu, Xiujuan Shan, and Weiji Wang. 2025. "Monitoring Fish Biodiversity in the Pelagic Zone of the Western Indian Ocean Using Environmental DNA Metabarcoding" Biology 14, no. 9: 1194. https://doi.org/10.3390/biology14091194
APA StyleLyu, D., Xu, R., Jin, Y., Hu, Y., Liu, M., Lyu, G., Shan, X., & Wang, W. (2025). Monitoring Fish Biodiversity in the Pelagic Zone of the Western Indian Ocean Using Environmental DNA Metabarcoding. Biology, 14(9), 1194. https://doi.org/10.3390/biology14091194







