Carvacrol as a Therapeutic Candidate in Breast Cancer: Insights into Subtype-Specific Cellular Modulation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Genes and Public Database-Based Evaluation of Genes Expression and Survival Effects in Breast Cancer Patients
2.2. Cell Lines and Cell Culture
2.3. Carvacrol Preparations
2.4. Cell Viability
2.5. Wound Healing Assay
2.6. Evaluation of Apoptosis with Annexin-V/PI
2.7. Reactive Oxygen Species (ROS) Activity
2.8. Analysis of CD44/CD133 (Cancer Stem Cell Marker) by Flow Cytometry
2.9. Quantitative Real Time Polymerase Chain Reaction (qPCR)
2.10. Statistical Analysis
3. Results
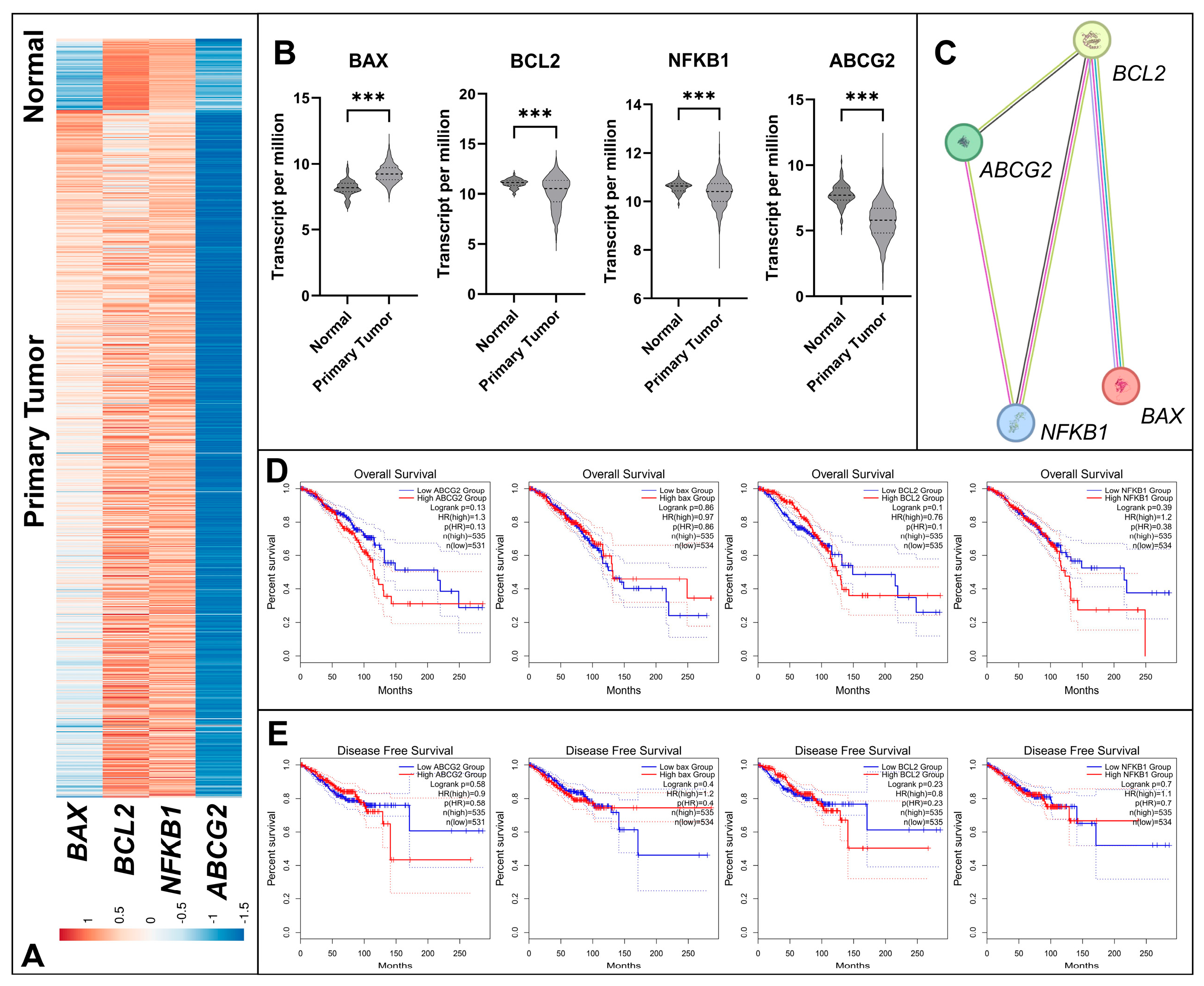
3.1. Differential Expression and Survival Analysis of Apoptosis and Resistance Related Genes in BC
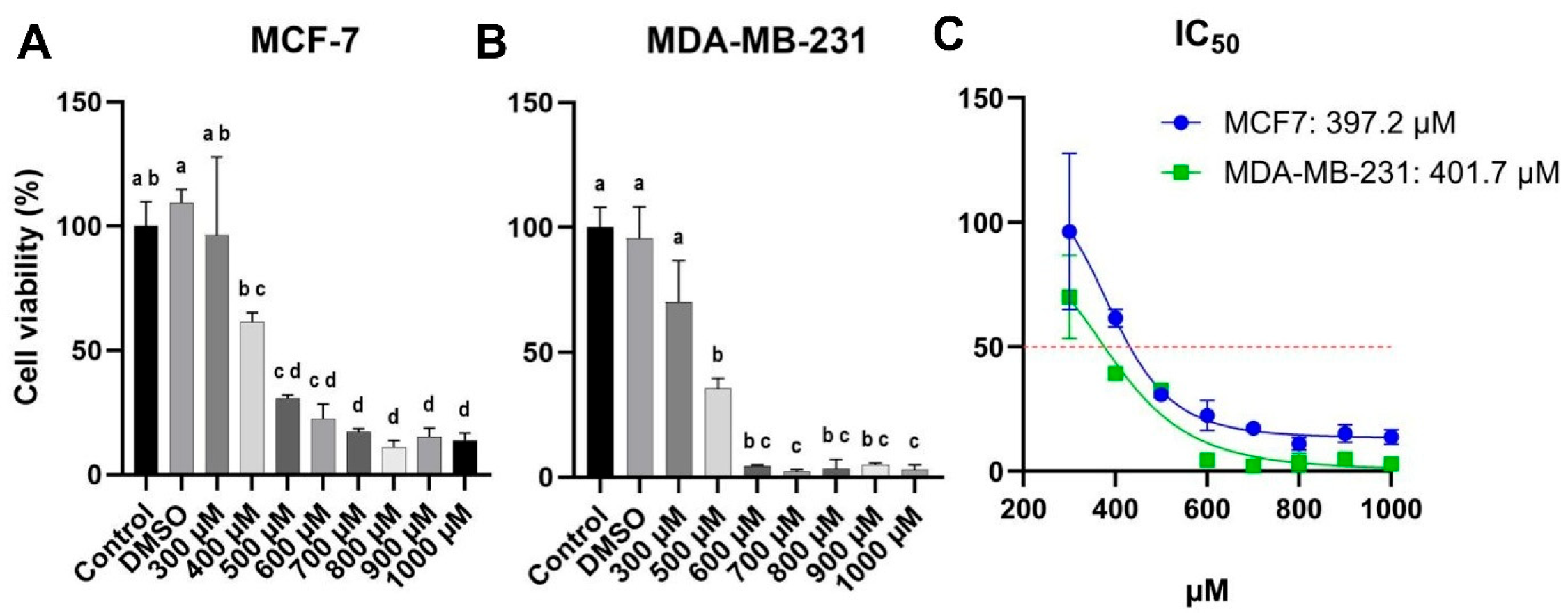
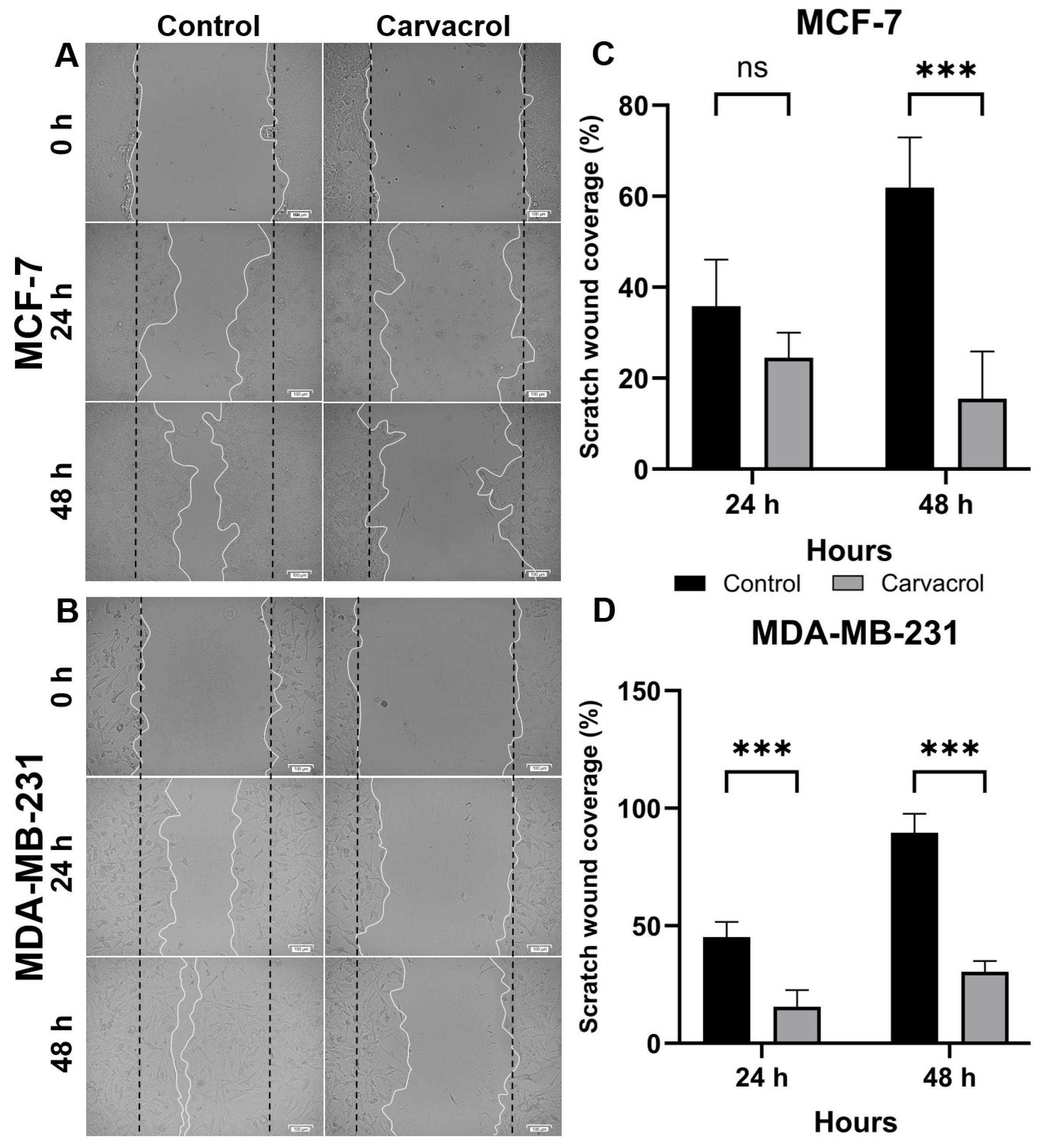
3.2. Carvacrol Suppresses Proliferation and Migration in Both HR+ and TNBC Cells
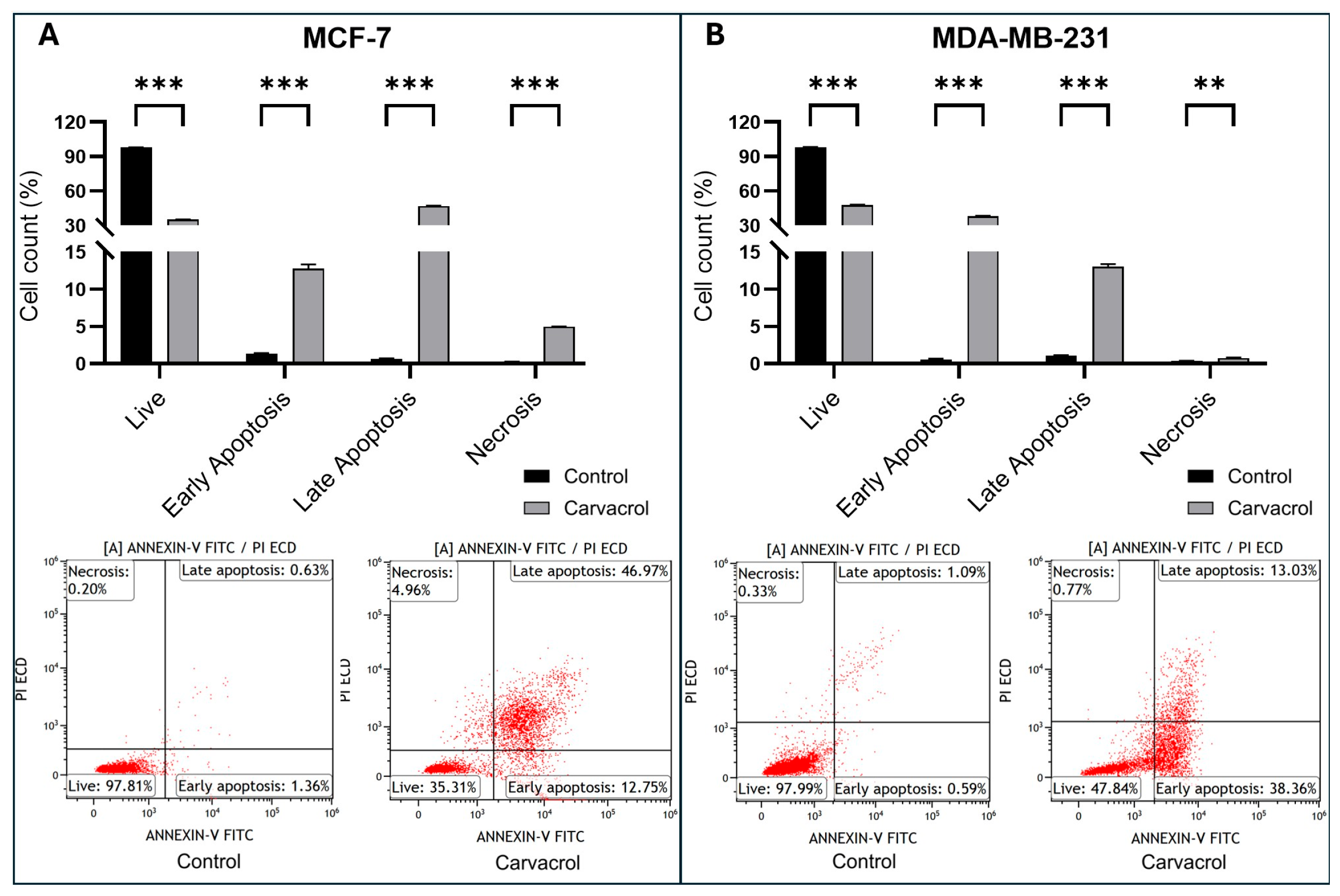
3.3. Carvacrol Induces Apoptosis via Modulation of BAX/BCL2 Ratio
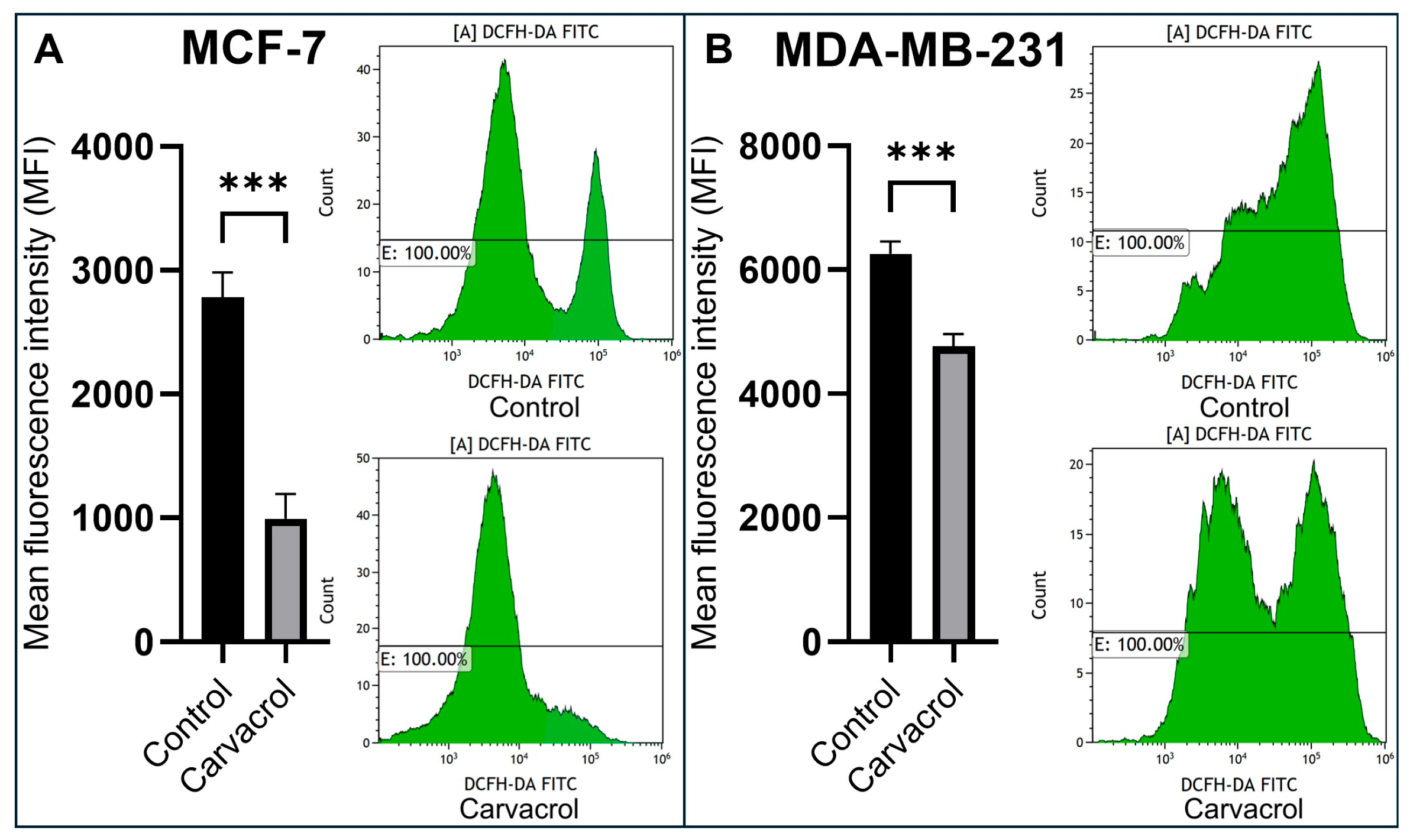
3.4. Carvacrol Reduces Intracellular ROS Levels in a Subtype-Dependent Manner
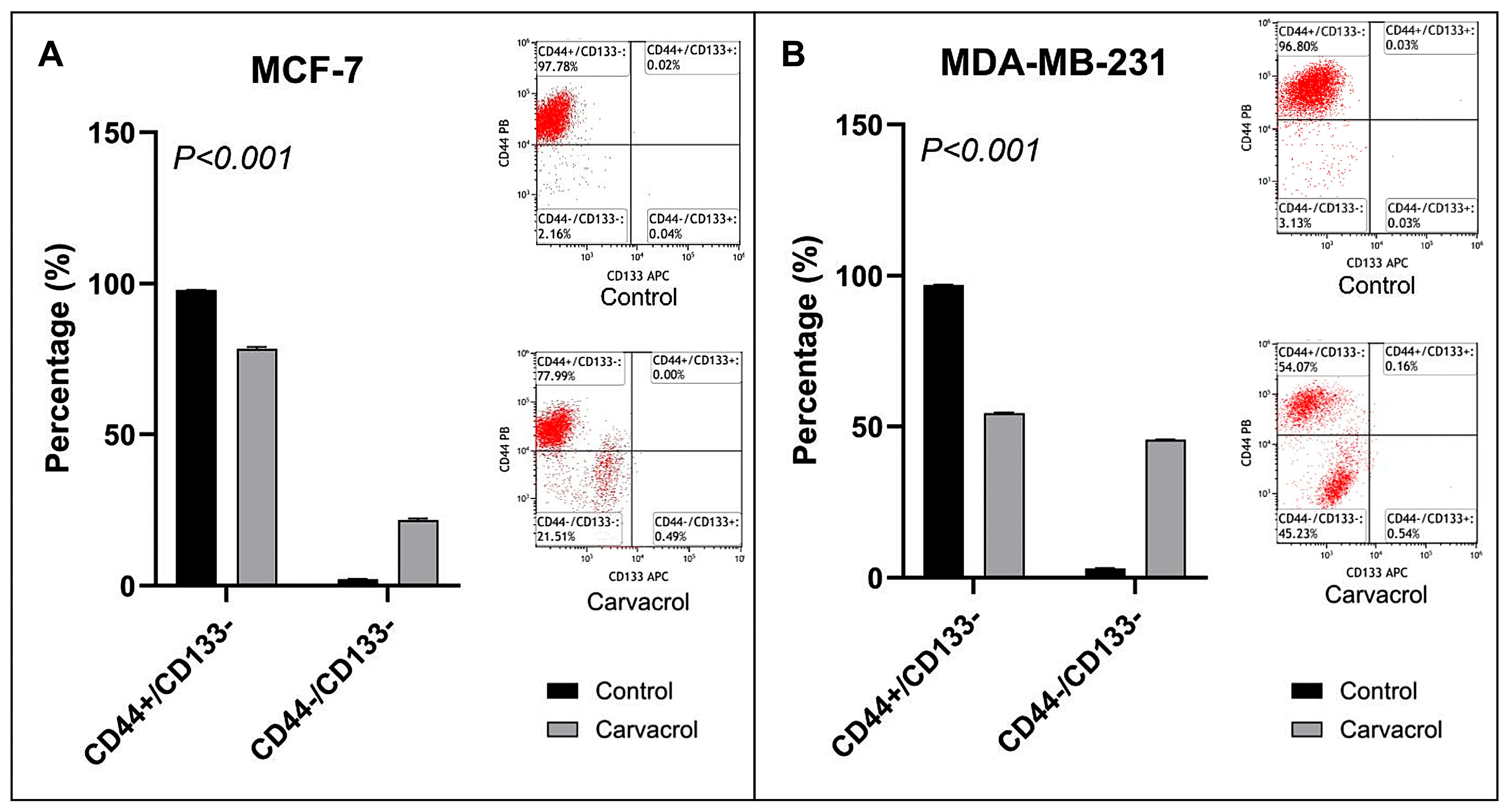
3.5. Carvacrol Downregulates CD44+ Stemness Marker in Both Subtypes
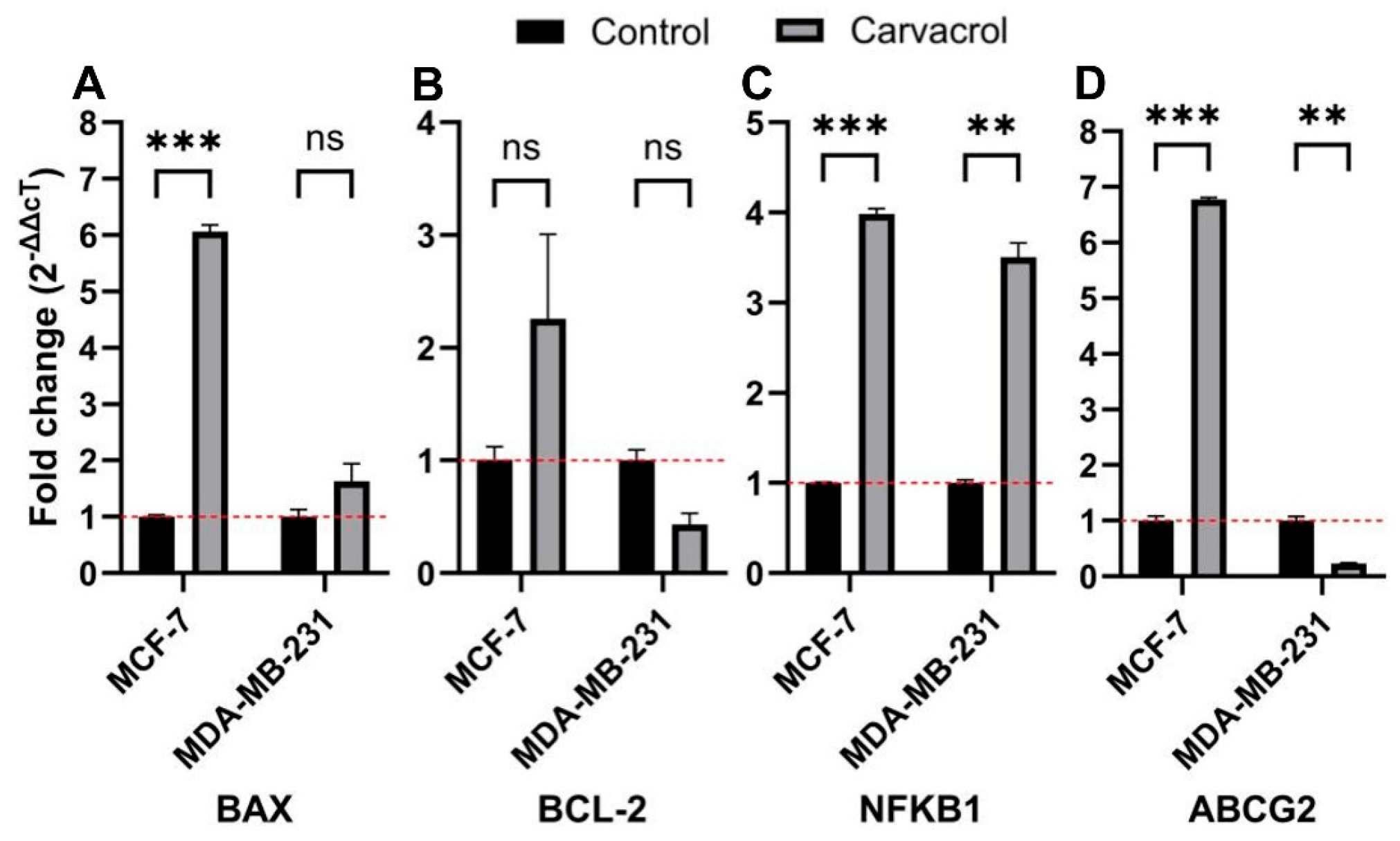
3.6. Subtype-Specific Modulation of BAX, BCL2, NFKB1, and ABCG2 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| TNBC | Triple-Negative Breast Cancer |
| BAX | Bcl-2 Associated X-protein |
| BCL2 | B-Cell Lymphoma 2 |
| NFKB | Nuclear Factor Kappa B Subunit 1 |
| ABCG | ATP-Binding Cassette Sub-Family G Member 2 |
| ABC | ATP-Binding Cassette |
| TCGA | The Cancer Genome Atlas |
| DMSO | Dimethyl Sulfoxide |
| CVDK8 | Cell Viability Detection Kit 8 |
| PI | Propidium Iodide |
| DCFH-DA | The 2′-7′-Dichlorodihydrofluorescein Diacetate Assay |
| ROS | Reactive Oxygen Species |
| qPCR | Quantitative Real Time Polymerase Chain Reaction |
| SD | Standard Deviation |
| OS | Overall Survival |
| DFS | Disease-Free Survival |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhang, X. Molecular Classification of Breast Cancer: Relevance and Challenges. Arch. Pathol. Lab. Med. 2023, 147, 46–51. [Google Scholar] [CrossRef]
- Qi, Y.-J.; Su, G.-H.; You, C.; Zhang, X.; Xiao, Y.; Jiang, Y.-Z.; Shao, Z.-M. Radiomics in breast cancer: Current advances and future directions. Cell Rep. Med. 2024, 5, 101719. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; Zhan, L.; Luo, L.; Zheng, W.; Zheng, Q.; Chen, C.; Sun, S. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp. Hematol. Oncol. 2023, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Barone, D.; Cito, L.; Tommonaro, G.; Abate, A.A.; Penon, D.; De Prisco, R.; Penon, A.; Forte, I.M.; Benedetti, E.; Cimini, A.; et al. Antitumoral potential, antioxidant activity and carotenoid content of two Southern Italy tomato cultivars extracts: San Marzano and Corbarino. J. Cell. Physiol. 2018, 233, 1266–1277. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanomedicine: A road to cancer therapeutics. Curr. Pharm. Des. 2013, 19, 1994–2010. [Google Scholar]
- D’Andrea, G.M. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J. Clin. 2005, 55, 319–321. [Google Scholar] [CrossRef]
- Jung, A.Y.; Cai, X.; Thoene, K.; Obi, N.; Jaskulski, S.; Behrens, S.; Flesch-Janys, D.; Chang-Claude, J. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am. J. Clin. Nutr. 2019, 109, 69–78. [Google Scholar] [CrossRef]
- Patuzzo, S.; Ciliberti, R. Non-conventional practice versus evidence-based medicine. A scientific and ethical analysis of the Italian regulation. Acta Biomed. 2017, 88, 143–150. [Google Scholar]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. Plant-Derived Anticancer Agents: Lessons from the Pharmacology of Geniposide and Its Aglycone, Genipin. Biomedicines 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology-An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Luqman, S.; Meena, A. Carvacrol as a Prospective Regulator of Cancer Targets/Signalling Pathways. Curr. Mol. Pharmacol. 2023, 16, 542–558. [Google Scholar] [CrossRef]
- Khan, F.; Pandey, P.; Maqsood, R.; Upadhyay, T.K. Anticancer effects of carvacrol in in vitro and in vivo models: A comprehensive review. Biointerface Res. Appl. Chem. 2023, 13, 290–303. [Google Scholar]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef]
- Mari, A.; Mani, G.; Nagabhishek, S.N.; Balaraman, G.; Subramanian, N.; Mirza, F.B.; Sundaram, J.; Thiruvengadam, D. Carvacrol promotes cell cycle arrest and apoptosis through PI3K/AKT signaling pathway in MCF-7 breast cancer cells. Chin. J. Integr. Med. 2021, 27, 680–687. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Estrada-Pérez, A.R.; Bakalara, N.; García-Vázquez, J.B.; Rosales-Hernández, M.C.; Fernández-Pomares, C.; Correa-Basurto, J. LC–MS Based Lipidomics Depict Phosphatidylethanolamine as Biomarkers of TNBC MDA-MB-231 over nTNBC MCF-7 Cells. Int. J. Mol. Sci. 2022, 23, 12074. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast cancer—Epidemiology, classification, pathogenesis and treatment (review of literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Martin, V. (Ed.) Overview of paclitaxel (Taxol®). In Seminars in Oncology Nursing; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Ferguson, P.J.; Phillips, J.R.; Selner, M.; Cass, C.E. Differential activity of vincristine and vinblastine against cultured cells. Cancer Res. 1984, 44, 3307–3312. [Google Scholar]
- Schultz, A.G. Camptothecin. Chem. Rev. 1973, 73, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Ranjitkar, S.; Zhang, D.; Sun, F.; Salman, S.; He, W.; Venkitanarayanan, K.; Tulman, E.R.; Tian, X. Cytotoxic effects on cancerous and non-cancerous cells of trans-cinnamaldehyde, carvacrol, and eugenol. Sci. Rep. 2021, 11, 16281. [Google Scholar] [CrossRef] [PubMed]
- Moradipour, A.; Dariushnejad, H.; Ahmadizadeh, C.; Lashgarian, H.E. Dietary flavonoid carvacrol triggers the apoptosis of human breast cancer MCF-7 cells via the p53/Bax/Bcl-2 axis. Med. Oncol. 2022, 40, 46. [Google Scholar] [CrossRef]
- Lim, W.; Ham, J.; Bazer, F.W.; Song, G. Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. J. Cell. Physiol. 2019, 234, 1803–1815. [Google Scholar] [CrossRef]
- Arunasree, K. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef]
- Singh, P.; Lim, B. Targeting apoptosis in cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W. Mitochondria and cell death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef]
- Ali, D.; Tripathi, A.; Alali, H.; Shahi, Y.; Mishra, K.K.; Alarifi, S.; Alkahtane, A.; Manohardas, S. ROS-dependent Bax/Bcl2 and caspase 3 pathway-mediated apoptosis induced by zineb in human keratinocyte cells. OncoTargets Ther. 2018, 11, 489–497. [Google Scholar] [CrossRef]
- Agarwal, M.K.; Agarwal, M.L.; Athar, M.; Gupta, S. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle 2004, 3, 200–299. [Google Scholar] [CrossRef]
- Vucicevic, K.; Jakovljevic, V.; Colovic, N.; Tosic, N.; Kostic, T.; Glumac, I.; Pavlovic, S.; Karan-Djurasevic, T.; Colovic, M. Association of Bax expression and Bcl2/Bax ratio with clinical and molecular prognostic markers in chronic lymphocytic leukemia. J. Med. Biochem. 2016, 35, 150. [Google Scholar] [CrossRef] [PubMed]
- Hamivand, Z.; Haddadi, G.; Fardid, R. Expression of Bax and Bcl2 genes in peripheral blood lymphocytes of patients with differentiated thyroid cancer. J. Med. Phys. 2018, 43, 41–45. [Google Scholar] [PubMed]
- Tripathi, G.; Guha, L.; Kumar, H. Seeing the unseen: The role of bioimaging techniques for the diagnostic interventions in intervertebral disc degeneration. Bone Rep. 2024, 22, 101784. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhuang, S.; Guan, H.; Li, F.; Zou, H.; Li, D. New insights into the anti-apoptotic mechanism of natural polyphenols in complex with Bax protein. J. Biomol. Struct. Dyn. 2024, 42, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Lashgarian, H.E.; Ghorbanzadeh, V.; Moradipour, A.; Pirzeh, L.; Dariushnejad, H. 5-FU and the dietary flavonoid carvacrol: A synergistic combination that induces apoptosis in MCF-7 breast cancer cells. Med. Oncol. 2022, 39, 253. [Google Scholar] [CrossRef] [PubMed]
- Dariushnejad, H.; Garavand, S.; Lashgarian, H.E.; Ahmadizadeh, C.; Ahmadi, T.; Moradipour, A. Carvacrol and paclitaxel: Synergistic effect on apoptosis in breast cancer cell lines. Biomed. Adv. 2025, 2, 137–145. [Google Scholar] [CrossRef]
- Chen, H.-H.; Fong, S.Y. Carvacrol induces apoptosis in human breast cancer cells via Bcl-2/CytC signaling pathway. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Ou-Yahia, D.; Bellaouchi, R.; Mothana, R.A.; Al-Yousef, H.M.; Asehraou, A.; Addi, M.; et al. Evaluation of the interaction between carvacrol and thymol, major compounds of Ptychotis Verticillata essential oil: Antioxidant, anti-inflammatory and anticancer activities against breast cancer lines. Life 2024, 14, 1037. [Google Scholar] [CrossRef]
- Roy, A. Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer. Molecules 2022, 27, 4597. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Guo, J.; Manning, H.C.; Gore, J.C.; Guo, N. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol. Rep. 2012, 28, 1301–1308. [Google Scholar] [CrossRef]
- Brugnoli, F.; Grassilli, S.; Al-Qassab, Y.; Capitani, S.; Bertagnolo, V. CD133 in breast cancer cells: More than a stem cell marker. J. Oncol. 2019, 2019, 7512632. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. 1999, 52, 189–196. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Ji, X.-C.; Zhang, J.-N.; Teng, W.-F.; Zhou, S.-C. A novel approach for transforming breast cancer stem cells into endothelial cells. Exp. Ther. Med. 2024, 27, 74. [Google Scholar] [CrossRef]
- Jaksch, M.; Múnera, J.; Bajpai, R.; Terskikh, A.; Oshima, R.G. Cell cycle–dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008, 68, 7882–7886. [Google Scholar] [CrossRef]
- Bidlingmaier, S.; Zhu, X.; Liu, B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J. Mol. Med. 2008, 86, 1025–1032. [Google Scholar] [CrossRef]
- Khongthong, P.; Roseweir, A.K.; Edwards, J. The NF-KB pathway and endocrine therapy resistance in breast cancer. Endocr.-Relat. Cancer 2019, 26, R369–R380. [Google Scholar] [CrossRef]
- Gholijani, N.; Gharagozloo, M.; Farjadian, S.; Amirghofran, Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 2016, 13, 157–164. [Google Scholar] [CrossRef]
- Srinivasan, M.K.; Aruchamy, M.; Namasivayam, N.; Muthupandian, S. Protective effect of pH-sensitive carvacrol zinc oxide quantum dots on Nrf-2, MAPK and NF-κB pathways and protein-bound carbohydrates in DMBA-induced mammary carcinogenesis. Pathol.-Res. Pract. 2025, 272, 156117. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; Araújo, A.A.d.S.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Roy, D.; Sharma, S.; Vishnoi, J.R.; Pareek, P.; Elhence, P.; Sharma, P.; Purohit, P. ABC transporters in breast cancer: Their roles in multidrug resistance and beyond. J. Drug Target. 2022, 30, 927–947. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yang, C.; Han, L.; Liu, L.; Liu, Y.J. Expression of BCRP/ABCG2 protein in invasive breast cancer and response to neoadjuvant chemotherapy. Oncol. Res. Treat. 2022, 45, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Tanić, N.; Prvanović, M.; Milovanović, Z.; Tanić, N. Friend or foe: ABCG2, ABCC1 and ABCB1 expression in triple-negative breast cancer. Breast Cancer 2021, 28, 727–736. [Google Scholar] [CrossRef]
- Karkabounas, S.; Kostoula, O.K.; Daskalou, T.; Veltsistas, P.; Karamouzis, M.; Zelovitis, I.; Metsios, A.; Lekkas, P.; Evangelou, A.M.; Kotsis, N.; et al. Anticarcinogenic and antiplatelet effects of carvacrol. Exp. Oncol. 2006, 28, 121–125. [Google Scholar] [PubMed]
- Subramaniyan, J.; Krishnan, G.; Balan, R.; Mgj, D.; Ramasamy, E.; Ramalingam, S.; Veerabathiran, R.; Thandavamoorthy, P.; Mani, G.K.; Thiruvengadam, D. Carvacrol modulates instability of xenobiotic metabolizing enzymes and downregulates the expressions of PCNA, MMP-2, and MMP-9 during diethylnitrosamine-induced hepatocarcinogenesis in rats. Mol. Cell. Biochem. 2014, 395, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Herrera-Calderón, O.; Ortiz-Sánchez, J.M.; Rojas-Armas, A.; Calva, J.; Castro-Luna, A.; Hilario-Vargas, J. The essential oil of Cymbopogon citratus stapt and carvacrol: An approach of the antitumor effect on 7, 12-dimethylbenz-[α]-anthracene (DMBA)-induced breast cancer in female rats. Molecules 2020, 25, 3284. [Google Scholar] [CrossRef]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Carvacrol-fabricated chitosan nanoparticle synergistic potential with topoisomerase inhibitors on breast and cervical cancer cells. ACS Omega 2023, 8, 31826–31838. [Google Scholar] [CrossRef]
- Srinivasan, M.K.; Premnath, B.J.; Parimelazhagan, R.; Namasivayam, N. Synthesis, characterization, and evaluation of the anticancer properties of pH-responsive carvacrol-zinc oxide quantum dots on breast cancer cell line (MDA-MB-231). Cell Biochem. Funct. 2024, 42, e4062. [Google Scholar] [CrossRef]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| BAX1 | 5′-CTACAGGGTTTCATCCAG-3′ | 5′-CCAGGAGAAATCAAACAGAG-3′ |
| BCL2 | 5′-GTGGATGACTGAGTACCT-3′ | 5′-CCAGGAGAAATCAAACAGAG-3′ |
| NFKB1 | 5′-TACGATGGAACCACACCCCTG-3′ | 5′-TCTGCTCCTGCTGCTTTGAGA-3′ |
| ABCG2 | 5′-AGCAGCAGGTCAGAGTGTGG-3′ | 5′-GATCGATGCCCTGCTTTACC-3′ |
| GAPDH | 5′-CCACCCATGGCAAATTCC-3′ | 5′-TGGGATTTCCATTGATGACAAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuaisha, A.; Nekay, E.; Yilmaz, O.; Yildiz, B.; Mecit, T.; Yavas, C.; Papila, B.; Arslan, H.I.; Gumus, A.H.; Nazligul, E.; et al. Carvacrol as a Therapeutic Candidate in Breast Cancer: Insights into Subtype-Specific Cellular Modulation. Biology 2025, 14, 1443. https://doi.org/10.3390/biology14101443
Abuaisha A, Nekay E, Yilmaz O, Yildiz B, Mecit T, Yavas C, Papila B, Arslan HI, Gumus AH, Nazligul E, et al. Carvacrol as a Therapeutic Candidate in Breast Cancer: Insights into Subtype-Specific Cellular Modulation. Biology. 2025; 14(10):1443. https://doi.org/10.3390/biology14101443
Chicago/Turabian StyleAbuaisha, Asmaa, Emir Nekay, Ozgur Yilmaz, Baris Yildiz, Tarik Mecit, Cuneyd Yavas, Berrin Papila, Halil Ibrahim Arslan, Aybuke Hilal Gumus, Esra Nazligul, and et al. 2025. "Carvacrol as a Therapeutic Candidate in Breast Cancer: Insights into Subtype-Specific Cellular Modulation" Biology 14, no. 10: 1443. https://doi.org/10.3390/biology14101443
APA StyleAbuaisha, A., Nekay, E., Yilmaz, O., Yildiz, B., Mecit, T., Yavas, C., Papila, B., Arslan, H. I., Gumus, A. H., Nazligul, E., Akbas, S., & Emiroglu, S. (2025). Carvacrol as a Therapeutic Candidate in Breast Cancer: Insights into Subtype-Specific Cellular Modulation. Biology, 14(10), 1443. https://doi.org/10.3390/biology14101443









