The Role of Nidogen in Reproductive Fitness in Drosophila
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
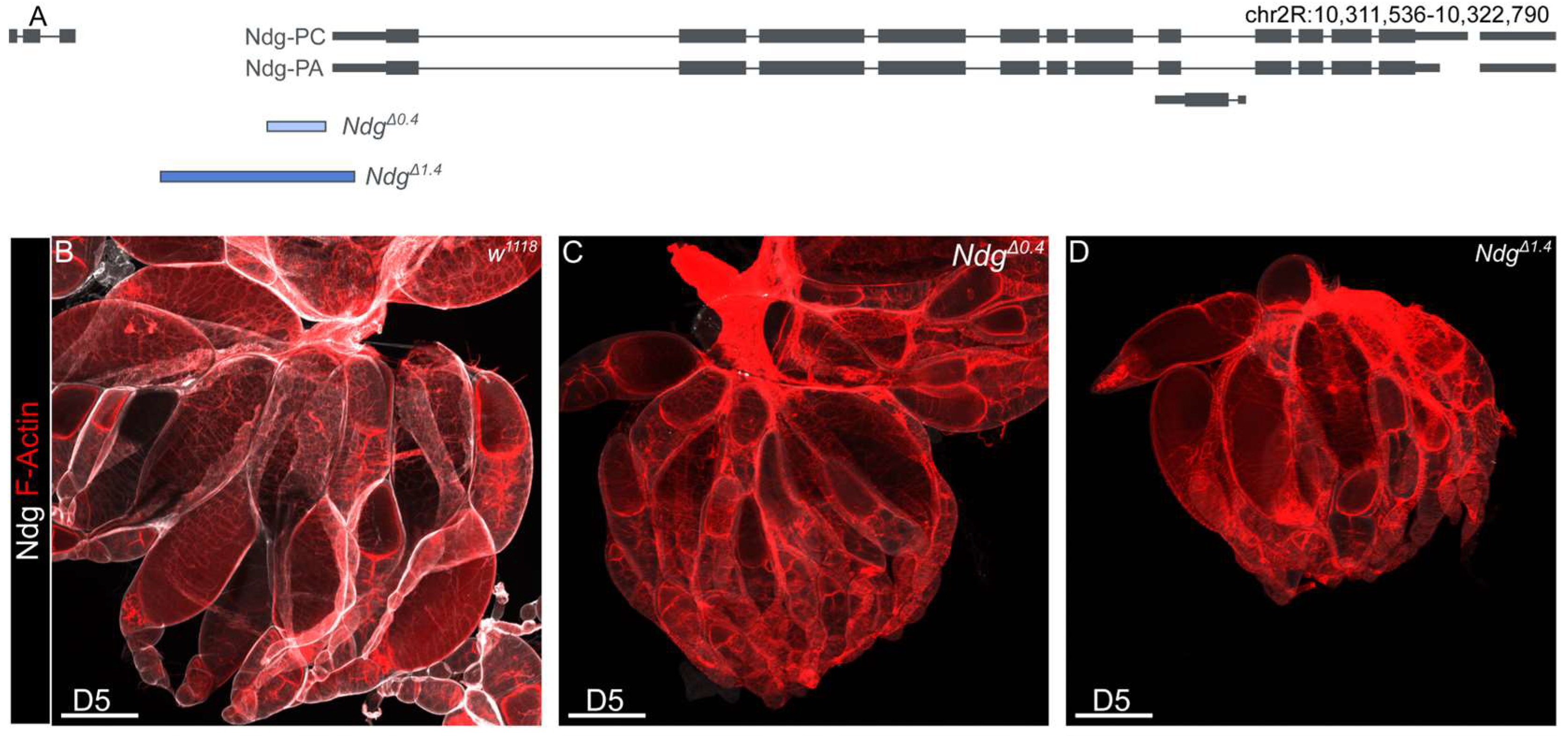
2.1. Fly Stocks and Genetics
2.2. Immunohistochemistry
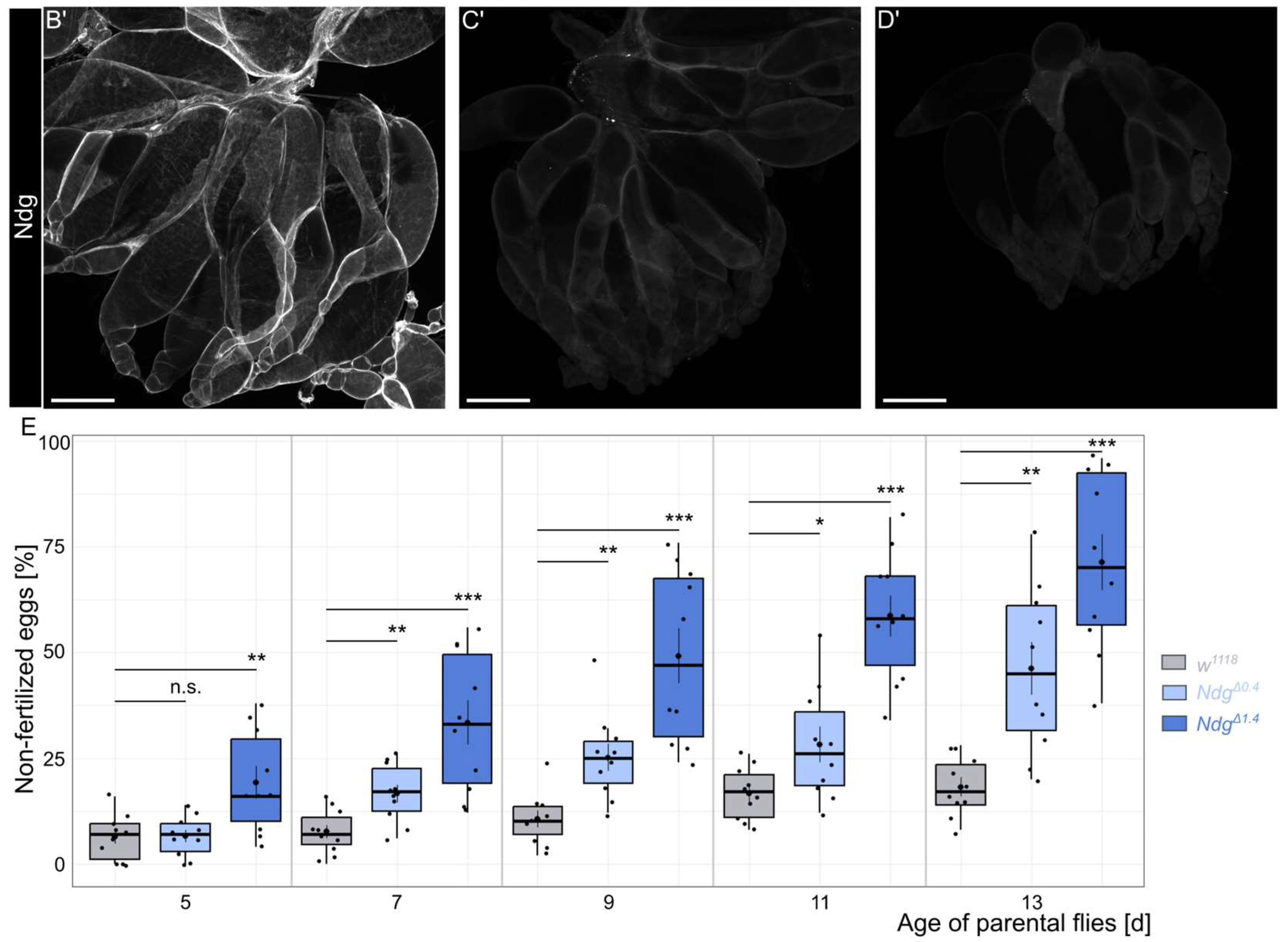
2.3. Fertility Test
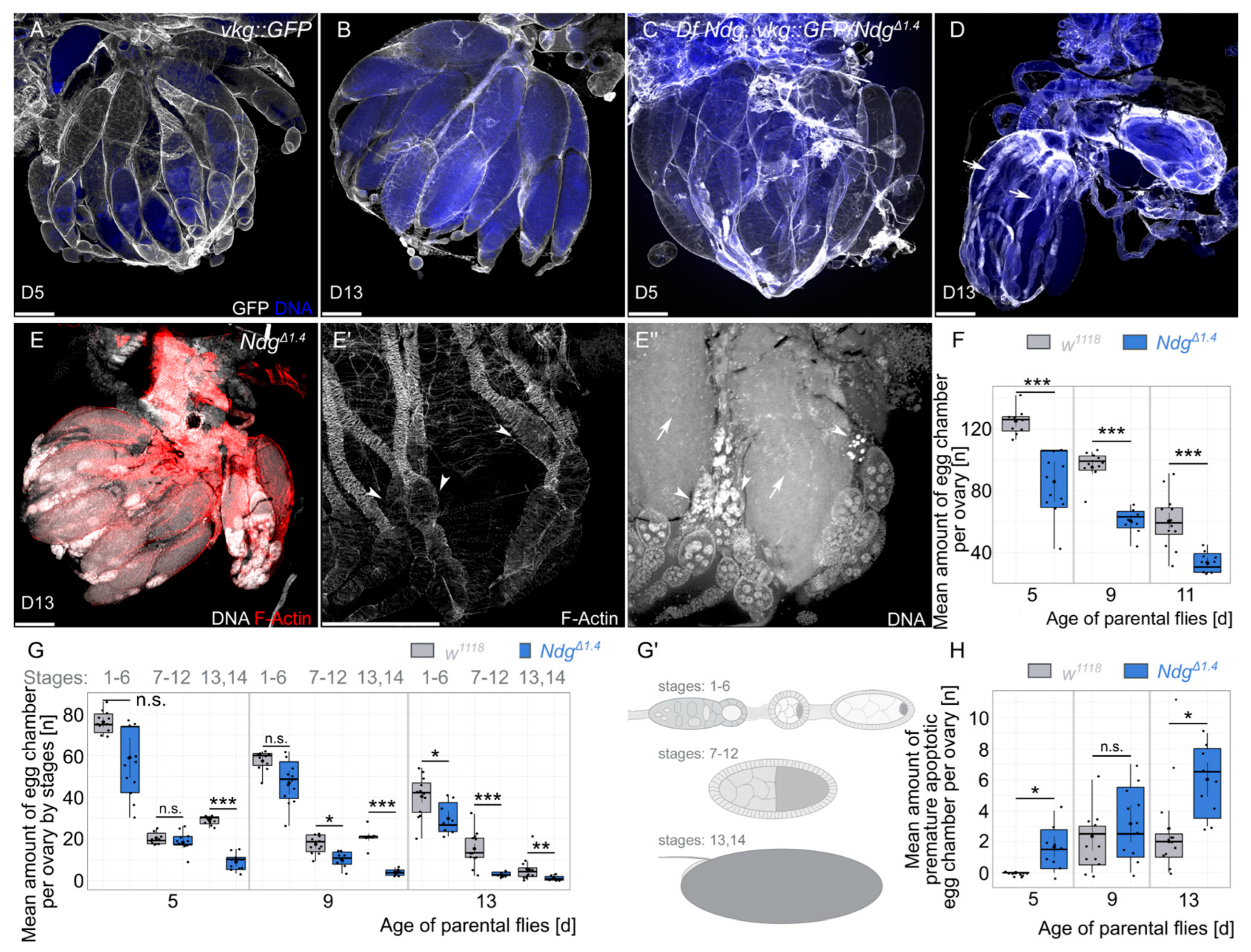
2.4. Quantification of Egg Chamber Amount and Premature Apoptotic Egg Chambers
2.5. Statistical Analysis
3. Results
3.1. Nidogen Is Required for Reproductive Fitness in Aging Flies
3.2. Premature Apoptosis in Egg Chambers of Ndg Mutant Flies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Töpfer, U. Basement Membrane Dynamics and Mechanics in Tissue Morphogenesis. Biol. Open 2023, 12, bio059980. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 130, pp. 143–191. ISBN 978-0-12-809802-8. [Google Scholar]
- Yurchenco, P.D. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef]
- Hohenester, E.; Yurchenco, P.D. Laminins in Basement Membrane Assembly. Cell Adhes. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef]
- Bader, B.L.; Smyth, N.; Nedbal, S.; Miosge, N.; Baranowsky, A.; Mokkapati, S.; Murshed, M.; Nischt, R. Compound Genetic Ablation of Nidogen 1 and 2 Causes Basement Membrane Defects and Perinatal Lethality in Mice. Mol. Cell. Biol. 2005, 25, 6846–6856. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Estrada, B.; Jacobs, S.; Sánchez-Sánchez, B.J.; Tang, J.; Ma, M.; Magadán-Corpas, P.; Pastor-Pareja, J.C.; Martín-Bermudo, M.D. Dissection of Nidogen Function in Drosophila Reveals Tissue-Specific Mechanisms of Basement Membrane Assembly. PLoS Genet. 2018, 14, e1007483. [Google Scholar] [CrossRef] [PubMed]
- Wolfstetter, G.; Dahlitz, I.; Pfeifer, K.; Töpfer, U.; Alt, J.A.; Pfeifer, D.C.; Lakes-Harlan, R.; Baumgartner, S.; Palmer, R.H.; Holz, A. Characterization of Drosophila Nidogen/Entactin Reveals Roles in Basement Membrane Stability, Barrier Function and Nervous System Patterning. Development 2019, 146, dev168948. [Google Scholar] [CrossRef]
- Töpfer, U.; Holz, A. Nidogen in Development and Disease. Front. Cell Dev. Biol. 2024, 12, 1380542. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Y.; Lewis, M.; Hsieh, J.-C.; Reing, J.; Chaillet, J.R.; Howell, C.Y.; Melhem, M.; Inoue, S.; Kuszak, J.R.; et al. Neurologic Defects and Selective Disruption of Basement Membranes in Mice Lacking Entactin-1/Nidogen-1. Lab. Investig. 2002, 82, 1617–1630. [Google Scholar] [CrossRef]
- Murshed, M.; Smyth, N.; Miosge, N.; Karolat, J.; Krieg, T.; Paulsson, M.; Nischt, R. The Absence of Nidogen 1 Does Not Affect Murine Basement Membrane Formation. Mol. Cell. Biol. 2000, 20, 7007–7012. [Google Scholar] [CrossRef] [PubMed]
- Schymeinsky, J.; Nedbal, S.; Miosge, N.; Pöschl, E.; Rao, C.; Beier, D.R.; Skarnes, W.C.; Timpl, R.; Bader, B.L. Gene Structure and Functional Analysis of the Mouse Nidogen-2 Gene: Nidogen-2 Is Not Essential for Basement Membrane Formation in Mice. Mol. Cell. Biol. 2002, 22, 6820–6830. [Google Scholar] [CrossRef]
- Böse, K.; Nischt, R.; Page, A.; Bader, B.L.; Paulsson, M.; Smyth, N. Loss of Nidogen-1 and -2 Results in Syndactyly and Changes in Limb Development. J. Biol. Chem. 2006, 281, 39620–39629. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kramer, J.M. Nidogen Is Nonessential and Not Required for Normal Type IV Collagen Localization in Caenorhabditis elegans. Mol. Biol. Cell 2000, 11, 3911–3923. [Google Scholar] [CrossRef]
- Ackley, B.D.; Kang, S.H.; Crew, J.R.; Suh, C.; Jin, Y.; Kramer, J.M. The Basement Membrane Components Nidogen and Type XVIII Collagen Regulate Organization of Neuromuscular Junctions in Caenorhabditis elegans. J. Neurosci. 2003, 23, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Hobert, O.; Bülow, H. Development and Maintenance of Neuronal Architecture at the Ventral Midline of C. elegans. Curr. Opin. Neurobiol. 2003, 13, 70–78. [Google Scholar] [CrossRef]
- Kim, S.; Wadsworth, W.G. Positioning of Longitudinal Nerves in C. elegans by Nidogen. Science 2000, 288, 150–154. [Google Scholar] [CrossRef]
- Zhu, P.; Ma, Z.; Guo, L.; Zhang, W.; Zhang, Q.; Zhao, T.; Jiang, K.; Peng, J.; Chen, J. Short Body Length Phenotype Is Compensated by the Upregulation of Nidogen Family Members in a Deleterious Nid1a Mutation of Zebrafish. J. Genet. Genom. 2017, 44, 553–556. [Google Scholar] [CrossRef]
- Dedhar, S.; Jewell, K.; Rojiani, M.; Gray, V. The Receptor for the Basement Membrane Glycoprotein Entactin Is the Integrin Alpha 3/Beta 1. J. Biol. Chem. 1992, 267, 18908–18914. [Google Scholar] [CrossRef]
- Dong, L.-J.; Hsieh, J.-C.; Chung, A.E. Two Distinct Cell Attachment Sites in Entactin Are Revealed by Amino Acid Substitutions and Deletion of the RGD Sequence in the Cysteine-Rich Epidermal Growth Factor Repeat 2. J. Biol. Chem. 1995, 270, 15838–15843. [Google Scholar] [CrossRef]
- Caracuel-Peramos, R.; Rodríguez-Baena, F.J.; Redondo-García, S.; Villatoro-García, J.A.; García-Muñoz, A.; Peris-Torres, C.; Plaza-Calonge, M.C.; Rubio-Gayarre, A.; López-Millán, B.; Ricciardelli, C.; et al. Loss of the Extracellular Protease ADAMTS1 Reveals an Antitumorigenic Program Involving the Action of NIDOGEN-1 on Macrophage Polarization. OncoImmunology 2025, 14, 2508057. [Google Scholar] [CrossRef]
- Muffler, S.; Stark, H.-J.; Amoros, M.; Falkowska-Hansen, B.; Boehnke, K.; Bühring, H.-J.; Marmé, A.; Bickenbach, J.R.; Boukamp, P. A Stable Niche Supports Long-Term Maintenance of Human Epidermal Stem Cells in Organotypic Cultures. Stem Cells 2008, 26, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, J.; Deneweth, L.; Pagliaro, L.; Ren, Z.; Giaimo, M.; Zamponi, R.; Roti, G.; Depreter, B.; Hofmans, M.; De Moerloose, B.; et al. Nidogen-1, a Player in KMT2A-Rearranged Pediatric Acute Myeloid Leukemia. Int. J. Mol. Sci. 2025, 26, 3011. [Google Scholar] [CrossRef]
- Lan, Z.; Xiao, Y.; Liao, Y.; Li, X.; Zhang, Y.; Wang, H.; Zhang, W. NID2 Affects Prognosis of Glioma via Activating the Akt Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 3859. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, I.; Prasad, A.; Sharma, B.K.; Aithabathula, R.V.; Ofosu-Boateng, M.; Gyamfi, M.A.; Jiang, J.; Park, F.; Singh, U.P.; Singla, B. Nidogen 2 Overexpression Promotes Hepatosteatosis and Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 12782. [Google Scholar] [CrossRef]
- Öztürk-Çolak, A.; Marygold, S.J.; Antonazzo, G.; Attrill, H.; Goutte-Gattat, D.; Jenkins, V.K.; Matthews, B.B.; Millburn, G.; Dos Santos, G.; Tabone, C.J.; et al. FlyBase: Updates to the Drosophila Genes and Genomes Database. Genetics 2024, 227, iyad211. [Google Scholar] [CrossRef]
- Spradling, A.C. Developmental Genetics of Oogenesis. In The Development of Drosophila Melanogaster; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 1993; pp. 1–70. [Google Scholar]
- Hudson, A.M.; Petrella, L.N.; Tanaka, A.J.; Cooley, L. Mononuclear Muscle Cells in Drosophila Ovaries Revealed by GFP Protein Traps. Dev. Biol. 2008, 314, 329–340. [Google Scholar] [CrossRef]
- Töpfer, U.; Guerra Santillán, K.Y.; Fischer-Friedrich, E.; Dahmann, C. Distinct Contributions of ECM Proteins to Basement Membrane Mechanical Properties in Drosophila. Development 2022, 149, dev200456. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.K.; Christensen, S.J.; Deal, J.A.; Coburn, R.A.; Deal, M.E.; Gresens, J.M.; Kaufman, T.C.; Cook, K.R. The Generation of Chromosomal Deletions to Provide Extensive Coverage and Subdivision of the Drosophila melanogaster Genome. Genome Biol. 2012, 13, R21. [Google Scholar] [CrossRef] [PubMed]
- Morin, X.; Daneman, R.; Zavortink, M.; Chia, W. A Protein Trap Strategy to Detect GFP-Tagged Proteins Expressed from Their Endogenous Loci in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 15050–15055. [Google Scholar] [CrossRef]
- Ashburner, M. Drosophila: A Laboratory Handbook; Cold Spring Harbor Laboratory: Woodbury, NY, USA, 1989; ISBN 0-87969-321-5. [Google Scholar]
- Wolfstetter, G.; Shirinian, M.; Stute, C.; Grabbe, C.; Hummel, T.; Baumgartner, S.; Palmer, R.H.; Holz, A. Fusion of Circular and Longitudinal Muscles in Drosophila Is Independent of the Endoderm but Further Visceral Muscle Differentiation Requires a Close Contact between Mesoderm and Endoderm. Mech. Dev. 2009, 126, 721–736. [Google Scholar] [CrossRef]
- Lebo, D.P.V.; McCall, K. Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary. Cells 2021, 10, 1454. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Töpfer, U. Analysen zur Regulation und Komposition der Extrazellulären Matrix von Drosophila melanogaster. Ph.D. Thesis, University of Gießen, Gießen, Germany, 2019. [Google Scholar] [CrossRef]
- Aumailley, M.; Wiedemann, H.; Mann, K.; Timpl, R. Binding of Nidogen and the Laminin-Nidogen Complex to Basement Membrane Collagen Type IV. Eur. J. Biochem. 1989, 184, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Deutzmann, R.; Aumailley, M.; Timpl, R.; Raimondi, L.; Yamada, Y.; Pan, T.C.; Conway, D.; Chu, M.L. Amino Acid Sequence of Mouse Nidogen, a Multidomain Basement Membrane Protein with Binding Activity for Laminin, Collagen IV and Cells. EMBO J. 1989, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hopf, M.; Göhring, W.; Mann, K.; Timpl, R. Mapping of Binding Sites for Nidogens, Fibulin-2, Fibronectin and Heparin to Different IG Modules of Perlecan1. J. Mol. Biol. 2001, 311, 529–541. [Google Scholar] [CrossRef]
- Santillán, K.Y.G.; Dahmann, C.; Fischer-Friedrich, E. ECM Proteins Shape Topographical Patterns in the Basement Membrane of Drosophila Wing Discs. Matrix Biol. 2025, 140, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Sánchez-Sánchez, B.J.; Marcotti, S.; Serna-Morales, E.; Dragu, A.; Díaz-de-la-Loza, M.-C.; Vizcay-Barrena, G.; Fleck, R.A.; Stramer, B.M. Rapid Homeostatic Turnover of Embryonic ECM during Tissue Morphogenesis. Dev. Cell 2020, 54, 33–42.e9. [Google Scholar] [CrossRef]
- Keeley, D.P.; Hastie, E.; Jayadev, R.; Kelley, L.C.; Chi, Q.; Payne, S.G.; Jeger, J.L.; Hoffman, B.D.; Sherwood, D.R. Comprehensive Endogenous Tagging of Basement Membrane Components Reveals Dynamic Movement within the Matrix Scaffolding. Dev. Cell 2020, 54, 60–74.e7. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Van De Bor, V.; Zimniak, G.; Papone, L.; Cerezo, D.; Malbouyres, M.; Juan, T.; Ruggiero, F.; Noselli, S. Companion Blood Cells Control Ovarian Stem Cell Niche Microenvironment and Homeostasis. Cell Rep. 2015, 13, 546–560. [Google Scholar] [CrossRef]
- Papagiannouli, F.; Schardt, L.; Grajcarek, J.; Ha, N.; Lohmann, I. The Hox Gene Abd-B Controls Stem Cell Niche Function in the Drosophila Testis. Dev. Cell 2014, 28, 189–202. [Google Scholar] [CrossRef][Green Version]
- Pearson, J.R.; Zurita, F.; Tomás-Gallardo, L.; Díaz-Torres, A.; Loza, M.D.C.D.; Franze, K.; Martín-Bermudo, M.D.; González-Reyes, A. ECM-Regulator Timp Is Required for Stem Cell Niche Organization and Cyst Production in the Drosophila Ovary. PLoS Genet. 2016, 12, e1005763. [Google Scholar] [CrossRef]
- Tanentzapf, G.; Devenport, D.; Godt, D.; Brown, N.H. Integrin-Dependent Anchoring of a Stem-Cell Niche. Nat. Cell Biol. 2007, 9, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Pagán, E.E.; Orr-Weaver, T.L. Activating Embryonic Development in Drosophila. Semin. Cell Dev. Biol. 2018, 84, 100–110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Töpfer, U.; Holz, A. The Role of Nidogen in Reproductive Fitness in Drosophila. Biology 2025, 14, 1192. https://doi.org/10.3390/biology14091192
Töpfer U, Holz A. The Role of Nidogen in Reproductive Fitness in Drosophila. Biology. 2025; 14(9):1192. https://doi.org/10.3390/biology14091192
Chicago/Turabian StyleTöpfer, Uwe, and Anne Holz. 2025. "The Role of Nidogen in Reproductive Fitness in Drosophila" Biology 14, no. 9: 1192. https://doi.org/10.3390/biology14091192
APA StyleTöpfer, U., & Holz, A. (2025). The Role of Nidogen in Reproductive Fitness in Drosophila. Biology, 14(9), 1192. https://doi.org/10.3390/biology14091192





