Phylogeography of Scarturus williamsi and Climate Change Impacts: Genetic Diversity and Projected Habitat Loss in Anatolia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data and Study Area
2.2. Genetic Analysis
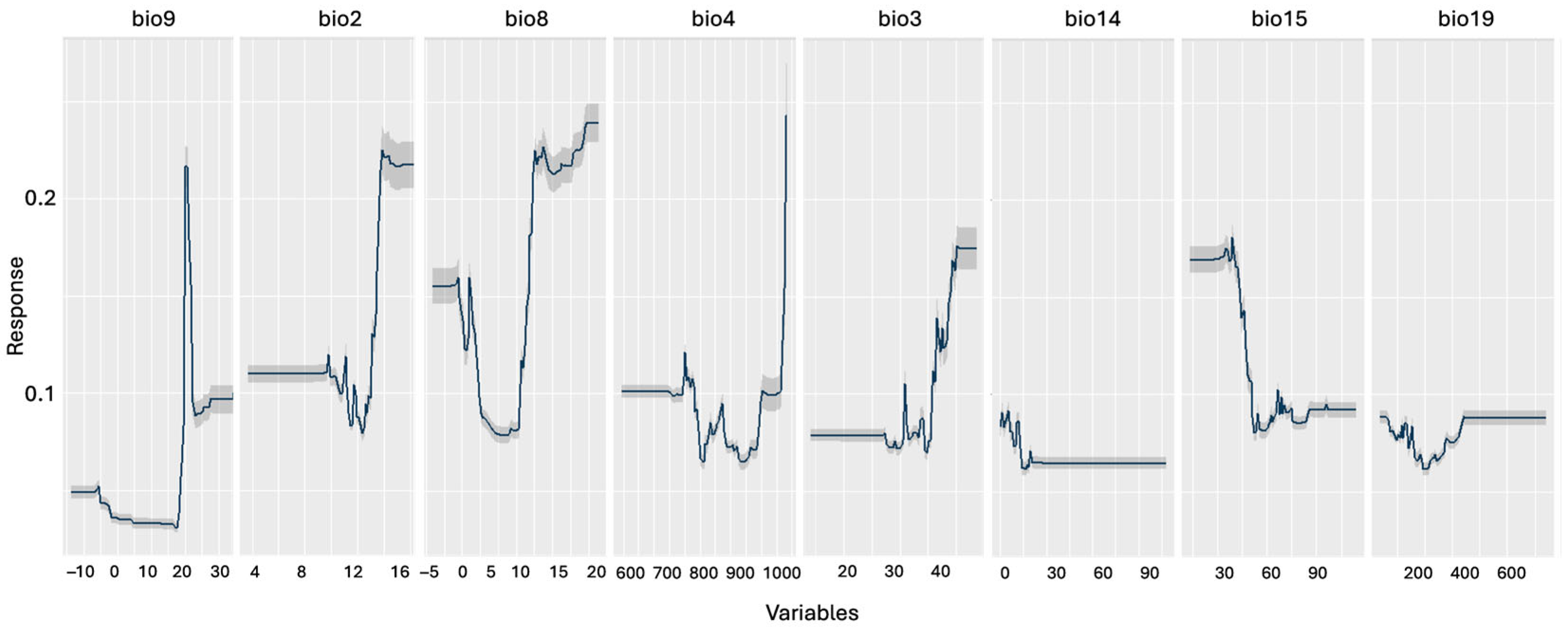
2.3. Species Distribution Modeling, Future Projections, and Climate Impact Assessment
3. Results
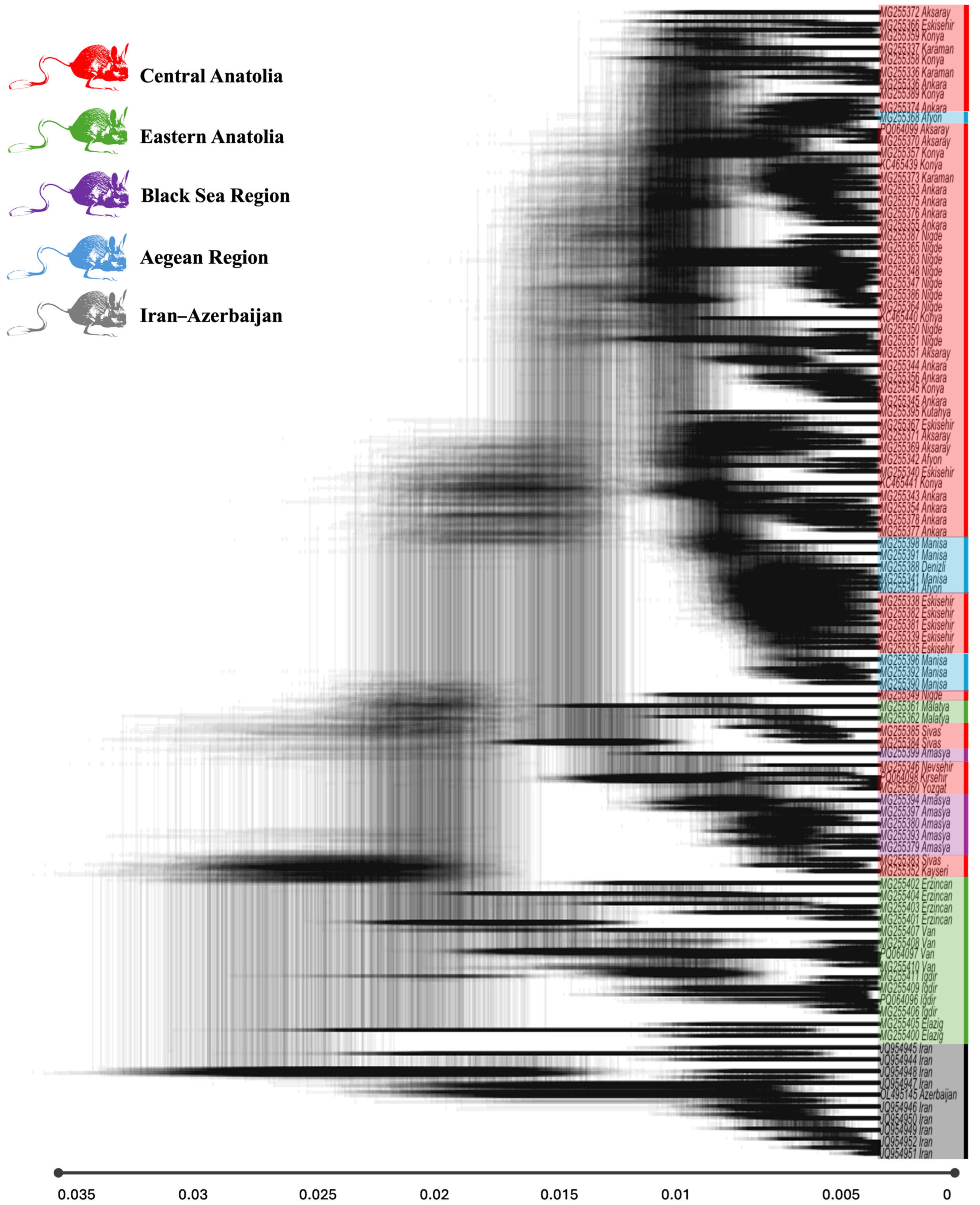
3.1. Genetic Diversity and Phylogeographic Structure
3.2. Species Distribution Modeling
3.3. Current Habitat Suitability and Species Distribution
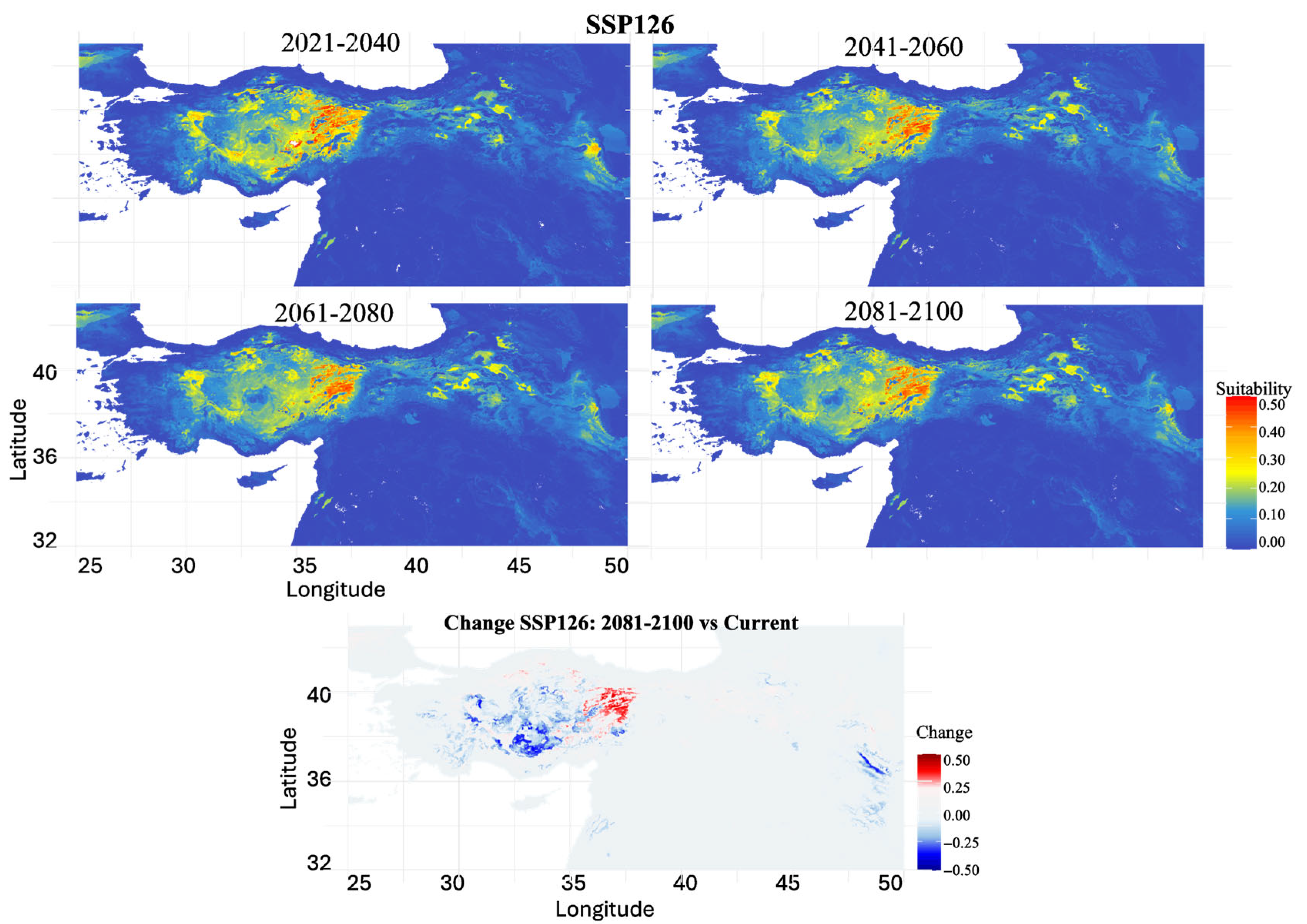
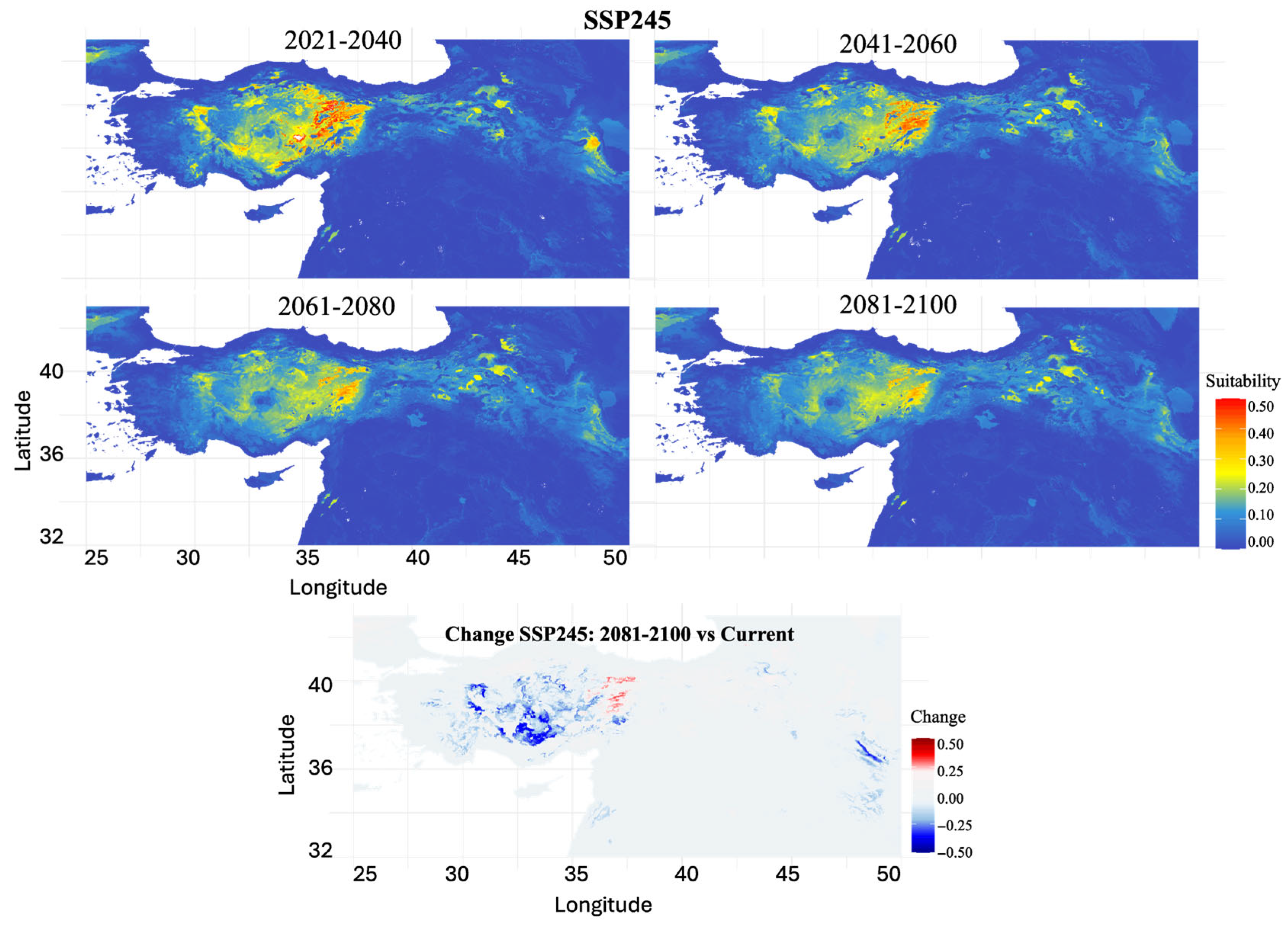
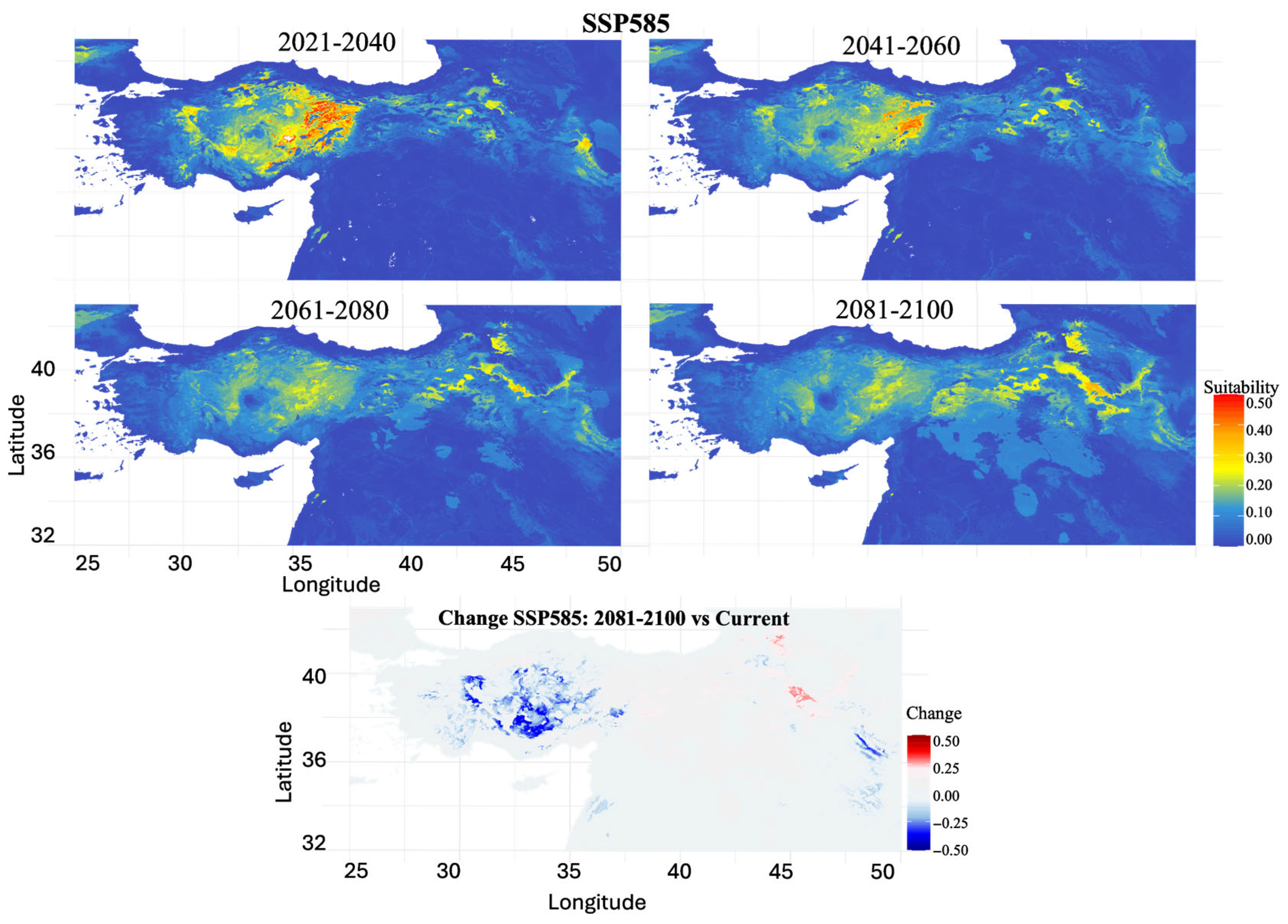
3.4. Future Habitat Projections Under Climate Scenarios
4. Discussion
4.1. Phylogeographic Structure and Evolutionary History
4.2. Refugial Dynamics and Phylogenetic Uncertainty
4.3. Integration of Genetic and Ecological Niche Data
4.4. Biogeographic Barriers and Isolation by Distance
4.5. Climate Change Vulnerability and Range Dynamics
4.6. Comparative Phylogeography and Regional Patterns
4.7. Implications for Steppe Ecosystem Understanding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gür, H. The Anatolian diagonal revisited: Testing the ecological basis of a biogeographic boundary. Zool. Middle East 2016, 62, 189–199. [Google Scholar] [CrossRef]
- Noroozi, J.; Zare, G.; Sherafati, M.; Mahmoodi, M.; Moser, D.; Asgarpour, Z.; Schneeweiss, G.M. Patterns of Endemism in Turkey, the Meeting Point of Three Global Biodiversity Hotspots, Based on Three Diverse Families of Vascular Plants. Front. Ecol. Evol. 2019, 7, 159. [Google Scholar] [CrossRef]
- Database, M.D. Mammal Diversity Database, Version 2.0; 2025. Available online: https://zenodo.org/records/15007505 (accessed on 26 August 2025).
- Karl, R.; Kiefer, C.; Ansell, S.W.; Koch, M.A. Systematics and evolution of arctic-alpine Arabis alpina (Brassicaceae) and its closest relatives in the eastern Mediterranean. Am. J. Bot. 2012, 99, 778–794. [Google Scholar] [CrossRef]
- Mutun, S. Review of oak gall wasps phylogeographic patterns in Turkey suggests a main role of the Anatolian diagonal. Turk. J. For. 2016, 17, 1–6. [Google Scholar] [CrossRef]
- Ekim, T.; Güner, A. The Anatolian Diagonal: Fact or fiction? Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1986, 89, 69–77. [Google Scholar] [CrossRef]
- Veith, M.; Schmidtler, J.F.; Kosuch, J.; Baran, I.; Seitz, A. Palaeoclimatic changes explain Anatolian mountain frog evolution: A test for alternating vicariance and dispersal events. Mol. Ecol. 2003, 12, 185–199. [Google Scholar] [CrossRef] [PubMed]
- van Riemsdijk, I.; Arntzen, J.W.; Bogaerts, S.; Franzen, M.; Litvinchuk, S.N.; Olgun, K.; Wielstra, B. The Near East as a cradle of biodiversity: A phylogeography of banded newts (genus Ommatotriton) reveals extensive inter-and intraspecific genetic differentiation. Mol. Phylogenetics Evol. 2017, 114, 73–81. [Google Scholar] [CrossRef]
- Yiğit, N.; Çetintürk, D.; Çolak, E. Phylogenetic assessment of voles of the Guentheri Group (Mammalia: Microtus) in Turkish Thrace and Western Anatolia. Eur. Zool. J. 2017, 84, 252–260. [Google Scholar] [CrossRef]
- Korkmaz, E.M.; Lunt, D.H.; Çıplak, B.; Değerli, N.; Başıbüyük, H.H.; McGeoch, M. The contribution of Anatolia to European phylogeography: The centre of origin of the meadow grasshopper, Chorthippus parallelus. J. Biogeogr. 2014, 41, 1793–1805. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Liu, Y.; Hao, H.; Zeng, B.; Chen, S.; Peng, H.; Yue, B.; Zhang, X. Phylogeography of the Large White-Bellied Rat Niviventer excelsior Suggests the Influence of Pleistocene Glaciations in the Hengduan Mountains. Zool. Sci. 2010, 27, 487–493. [Google Scholar] [CrossRef]
- Çolak, E.; Kivanç, E.; Yiğit, N. A study on taxonomic status of Allactaga euphratica Thomas, 1881 and Allactaga williamsi Thomas, 1897 (Rodentia: Dipodidae) in Turkey. Mammalia 1994, 58, 591–600. [Google Scholar] [CrossRef]
- Krystufek, B.; Vohralík, V. Mammals of Turkey and Cyprus: Rodentia I: Sciuridae, Dipodidae, Gliridae, Arvicolinae. 2005. Available online: https://www.researchgate.net/profile/Vladimir-Vohralik/publication/325973437_Mammals_of_Turkey_and_Cyprus_Rodentia_II_Cricetinae_Muridae_Spalacidae_Calomyscidae_Capromydae_Hystricidae_Castoridae/links/5b3107384585150d23d032d0/Mammals-of-Turkey-and-Cyprus-Rodentia-II-Cricetinae-Muridae-Spalacidae-Calomyscidae-Capromydae-Hystricidae-Castoridae.pdf (accessed on 20 May 2024).
- Lebedev, V.S.; Shenbrot, G.I.; Krystufek, B.; Mahmoudi, A.; Melnikova, M.N.; Solovyeva, E.N.; Lisenkova, A.A.; Undrakhbayar, E.; Rogovin, K.A.; Surov, A.V. Phylogenetic relations and range history of jerboas of the Allactaginae subfamily (Dipodidae, Rodentia). Sci. Rep. 2022, 12, 842. [Google Scholar] [CrossRef]
- Beck, M.J.; Vander Wall, S.B. Seed dispersal by scatter-hoarding rodents in arid environments. J. Ecol. 2010, 98, 1300–1309. [Google Scholar] [CrossRef]
- Olgun Karacan, G.; Çolak, R.; Yiğit, N.; Kandemir, İ.; Özkurt, Ş.Ö.; Çolak, E. Phylogeographic Structure of Five-Toed Jerboas of the Genus Scarturus (Dipodoidea and Allactaginae) With Taxonomic Clarification in Türkiye. J. Zool. Syst. Evol. Res. 2024, 2024, 8123686. [Google Scholar] [CrossRef]
- İbiş, O. Whole mitochondrial genome sequence and phylogenetic relationships of Williams’s jerboa (Scarturus williamsi) from Turkey. PeerJ 2020, 8, e9569. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K. Climate Change 2023: Synthesis Report. In Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; The Australian National University: Canberra, Australien, 2023. [Google Scholar]
- McCain, C.M.; King, S.R. Body size and activity times mediate mammalian responses to climate change. Glob. Change Biol. 2014, 20, 1760–1769. [Google Scholar] [CrossRef]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.W.; Riddell, E.A.; Beissinger, S.R.; Wolf, B.O. Keeping your cool: Thermoregulatory performance and plasticity in desert cricetid rodents. J. Exp. Biol. 2022, 225, jeb243131. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Change 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat. Commun. 2017, 8, 14196. [Google Scholar] [CrossRef]
- Ventura-Rojas, P.D.; González-Romero, A.; Moreno, C.E.; Sosa, V.J. Effect of rainfall, temperature and climate change on the ecology of the rodents of arid zones: A review. Mammal Rev. 2025, 55, e12372. [Google Scholar] [CrossRef]
- Bombi, P.; Salvi, D.; Shuuya, T.; Vignoli, L.; Wassenaar, T. Climate change effects on desert ecosystems: A case study on the keystone species of the Namib Desert Welwitschia mirabilis. PLoS ONE 2021, 16, e0259767. [Google Scholar] [CrossRef]
- Riddell, E.A.; Iknayan, K.J.; Hargrove, L.; Tremor, S.; Patton, J.L.; Ramirez, R.; Wolf, B.O.; Beissinger, S.R. Exposure to climate change drives stability or collapse of desert mammal and bird communities. Science 2021, 371, 633–636. [Google Scholar] [CrossRef]
- Kazakov, D.V.; Kruskop, S.V.; Kawai, K.; Gorban, A.A.; Gorobeyko, U.V. Phylogeography, morphometry and the species distribution modelling in Myotis longicaudatus (Chiroptera, Vespertilionidae) in the Eastern Palaearctic. Mammal Res. 2025, 70, 127–140. [Google Scholar] [CrossRef]
- Kabasakal, B.; Doğru, H.; Erdoğan, A.; Kaya, S. Transcontinental evolutionary dynamics and phylogeography of Alectoris (Aves: Galliformes): Identifying refugia, dispersal corridors, and cryptic diversity in the Palearctic region. Avian Res. 2025, 16, 100262. [Google Scholar] [CrossRef]
- Milano, F.; Casazza, G.; Galimberti, A.; Maggioni, D.; Isaia, M. Combining distribution modelling and phylogeography to understand present, past and future of an endangered spider. BMC Ecol. Evol. 2024, 24, 106. [Google Scholar] [CrossRef] [PubMed]
- Gavin, D.G.; Fitzpatrick, M.C.; Gugger, P.F.; Heath, K.D.; Rodríguez-Sánchez, F.; Dobrowski, S.Z.; Hampe, A.; Hu, F.S.; Ashcroft, M.B.; Bartlein, P.J. Climate refugia: Joint inference from fossil records, species distribution models and phylogeography. New Phytol. 2014, 204, 37–54. [Google Scholar] [CrossRef]
- Sales, L.P.; Ribeiro, B.R.; Hayward, M.W.; Paglia, A.; Passamani, M.; Loyola, R. Niche conservatism and the invasive potential of the wild boar. J. Anim. Ecol. 2017, 86, 1214–1223. [Google Scholar] [CrossRef]
- Wielstra, B.; Crnobrnja-Isailović, J.; Litvinchuk, S.N.; Reijnen, B.T.; Skidmore, A.K.; Sotiropoulos, K.; Toxopeus, A.G.; Tzankov, N.; Vukov, T.; Arntzen, J.W. Tracing glacial refugia of Triturus newts based on mitochondrial DNA phylogeography and species distribution modeling. Front. Zool. 2013, 10, 13. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- GBIF.org. GBIF Home Page. 2022. Available online: https://www.gbif.org (accessed on 17 February 2019).
- IUCN. The IUCN Red List of Threatened Species. Version 2025-1. Available online: https://www.iucnredlist.org (accessed on 18 May 2025).
- South, A.; South, M.A. R Package: Rnaturalearth. World Map Data from Natural Earth, Version 0.1.0. 2017. Available online: https://cran.r-project.org/web/packages/rnaturalearth/rnaturalearth.pdf (accessed on 12 November 2024).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2016, 2, 1–189. [Google Scholar]
- Paradis, E. pegas: An R package for population genetics with an integrated–modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
- Watterson, G. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 1975, 7, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Bilderbeek, R.J.; Etienne, R.S. babette: BEAUti 2, BEAST2 and Tracer for R. bioRxiv 2018, 271866. [Google Scholar] [CrossRef]
- Bouckaert, R.R. DensiTree: Making sense of sets of phylogenetic trees. Bioinformatics 2010, 26, 1372–1373. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Hijmans, R.J. geosphere: Spherical Trigonometry, in CRAN. R-project. R Package. 2024. Available online: https://cran.r-project.org/web/packages/geosphere/geosphere.pdf (accessed on 9 February 2024).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’HAra, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package. R package. 2022. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 30 May 2023).
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Legendre, P.; Fortin, M.J. Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol. Ecol. Resour. 2010, 10, 831–844. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: Retrospect and prospect. J. Biogeogr. 2009, 36, 3–15. [Google Scholar] [CrossRef]
- Taberlet, P.; Fumagalli, L.; WUST-SAUCY, A.G.; COSSON, J.F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef]
- Schmitt, T. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Front. Zool. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Templeton, A.R. Nested clade analyses of phylogeographic data: Testing hypotheses about gene flow and population history. Mol. Ecol. 1998, 7, 381–397. [Google Scholar] [CrossRef]
- Rogers, A.R.; Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992, 9, 552–569. [Google Scholar] [CrossRef]
- Fu, Y.-X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Provan, J.; Bennett, K.D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef]
- Kapli, P.; Botoni, D.; Ilgaz, Ç.; Kumlutaş, Y.; Avcı, A.; Rastegar-Pouyani, N.; Fathinia, B.; Lymberakis, P.; Ahmadzadeh, F.; Poulakakis, N. Molecular phylogeny and historical biogeography of the Anatolian lizard Apathya (Squamata, Lacertidae). Mol. Phylogenetics Evol. 2013, 66, 992–1001. [Google Scholar] [CrossRef]
- Najibzadeh, M.; Veith, M.; Gharzi, A.; Rastegar-Pouyani, N.; Rastegar-Pouyani, E.; Kieren, S.; Pesarakloo, A. Molecular phylogenetic relationships among Anatolian-Hyrcanian brown frog taxa (Ranidae: Rana). Amphib. -Reptil. 2017, 38, 339–350. [Google Scholar] [CrossRef]
- Asadi, A.; Montgelard, C.; Nazarizadeh, M.; Moghaddasi, A.; Fatemizadeh, F.; Simonov, E.; Kami, H.G.; Kaboli, M. Evolutionary history and postglacial colonization of an Asian pit viper (Gloydius halys caucasicus) into Transcaucasia revealed by phylogenetic and phylogeographic analyses. Sci. Rep. 2019, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Pouyani, E.R.; Noureini, S.K.; Pouyani, N.R.; Joger, U.; Wink, M. Molecular phylogeny and intraspecific differentiation of the Eremias velox complex of the Iranian Plateau and Central Asia (Sauria, Lacertidae). J. Zool. Syst. Evol. Res. 2012, 50, 220–229. [Google Scholar] [CrossRef]
- Currat, M.; Ruedi, M.; Petit, R.J.; Excoffier, L. The hidden side of invasions: Massive introgression by local genes. Evolution 2008, 62, 1908–1920. [Google Scholar] [CrossRef]
- Excoffier, L.; Foll, M.; Petit, R.J. Genetic consequences of range expansions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 481–501. [Google Scholar] [CrossRef]
- Tzedakis, P.C. Seven ambiguities in the Mediterranean palaeoenvironmental narrative. Quat. Sci. Rev. 2007, 26, 2042–2066. [Google Scholar] [CrossRef]
- Leroy, S.A.; Tudryn, A.; Chalié, F.; López-Merino, L.; Gasse, F. From the Allerød to the mid-Holocene: Palynological evidence from the south basin of the Caspian Sea. Quat. Sci. Rev. 2013, 78, 77–97. [Google Scholar] [CrossRef]
- Carnaval, A.C.; Hickerson, M.J.; Haddad, C.F.B.; Rodrigues, M.T.; Moritz, C. Stability Predicts Genetic Diversity in the Brazilian Atlantic Forest Hotspot. Science 2009, 323, 785–789. [Google Scholar] [CrossRef]
- Sandel, B.; Arge, L.; Dalsgaard, B.; Davies, R.; Gaston, K.; Sutherland, W.; Svenning, J.-C. The influence of Late Quaternary climate-change velocity on species endemism. Science 2011, 334, 660–664. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Sánchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 1999, 285, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Aguillon, S.M.; Fitzpatrick, J.W.; Bowman, R.; Schoech, S.J.; Clark, A.G.; Coop, G.; Chen, N.; Barsh, G.S. Deconstructing isolation-by-distance: The genomic consequences of limited dispersal. PLoS Genet. 2017, 13, e1006911. [Google Scholar] [CrossRef]
- Rousset, F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 1997, 145, 1219–1228. [Google Scholar] [CrossRef]
- Whitmee, S.; Orme, C.D.L.; Humphries, M. Predicting dispersal distance in mammals: A trait-based approach. J. Anim. Ecol. 2013, 82, 211–221. [Google Scholar] [CrossRef]
- Manel, S.; Schwartz, M.K.; Luikart, G.; Taberlet, P. Landscape genetics: Combining landscape ecology and population genetics. Trends Ecol. Evol. 2003, 18, 189–197. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Riddell, E.A.; Iknayan, K.J.; Wolf, B.O.; Sinervo, B.; Beissinger, S.R. Cooling requirements fueled the collapse of a desert bird community from climate change. Proc. Natl. Acad. Sci. USA 2019, 116, 21609–21615. [Google Scholar] [CrossRef]
- Weiss, S.; Ferrand, N. Phylogeography of Southern European Refugia: Evolutionary Perspectives on the Origins and Conservation of European Biodiversity; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Schmitt, T.; Varga, Z. Extra-Mediterranean refugia: The rule and not the exception? Front. Zool. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Šklíba, J.; Šumbera, R.; Chitaukali, W.N.; Burda, H. Determinants of daily activity patterns in a free-living Afrotropical solitary subterranean rodent. J. Mammal. 2007, 88, 1009–1016. [Google Scholar] [CrossRef]
- Ansell, S.W.; Stenøien, H.K.; Grundmann, M.; Russell, S.J.; Koch, M.A.; Schneider, H.; Vogel, J.C. The importance of Anatolian mountains as the cradle of global diversity in Arabis alpina, a key arctic–alpine species. Ann. Bot. 2011, 108, 241–252. [Google Scholar] [CrossRef]
- Forester, B.R.; DeChaine, E.G.; Bunn, A.G.; Wintle, B. Integrating ensemble species distribution modelling and statistical phylogeography to inform projections of climate change impacts on species distributions. Divers. Distrib. 2013, 19, 1480–1495. [Google Scholar] [CrossRef]
- Ambarlı, D.; Zeydanlı, U.S.; Balkız, Ö.; Aslan, S.; Karaçetin, E.; Sözen, M.; Ilgaz, C.; Gürsoy Ergen, A.; Lise, Y.; Çağlayan, S.D.; et al. An overview of biodiversity and conservation status of steppes of the Anatolian Biogeographical Region. Biodivers. Conserv. 2016, 25, 2491–2519. [Google Scholar] [CrossRef]
- Smelansky, I.E.; Tishkov, A.A. The steppe biome in Russia: Ecosystem services, conservation status, and actual challenges, in Eurasian steppes. In Ecological Problems and Livelihoods in a Changing World; Springer: Dordrecht, The Netherlands, 2012; pp. 45–101. [Google Scholar]
- Török, P.; Ambarlı, D.; Kamp, J.; Wesche, K.; Dengler, J. Step (pe) up! Raising the profile of the Palaearctic natural grasslands. Biodivers. Conserv. 2016, 25, 2187–2195. [Google Scholar] [CrossRef]




| Population | N | Nh | Hd | π | S | θw | Tajima’s D | p-Value |
|---|---|---|---|---|---|---|---|---|
| Eastern Anatolia | 16 | 15 | 0.9917 | 0.023761 | 74 | 22.301 | −0.231 | 0.817 |
| Central Anatolia | 55 | 50 | 0.9966 | 0.014861 | 104 | 22.730 | −1.475 | 0.140 |
| Iran–Azerbaijan | 10 | 9 | 0.9778 | 0.016692 | 51 | 18.028 | −0.869 | 0.385 |
| Aegean Region | 11 | 10 | 0.9818 | 0.007535 | 29 | 9.901 | −1.500 | 0.134 |
| Black Sea Region | 6 | 6 | 1.0000 | 0.006907 | 16 | 7.007 | −0.771 | 0.441 |
| Variable | Description | Correlation (%) | AUC (%) |
|---|---|---|---|
| bio2 | Mean Diurnal Range (°C) | 16.2 | 5.2 |
| bio3 | Isothermality (%) | 9.6 | 2.4 |
| bio4 | Temperature Seasonality (standard deviation × 100) | 4.5 | 1.4 |
| bio8 | Mean Temperature of Wettest Quarter (°C) | 6.6 | 1.9 |
| bio9 | Mean Temperature of Driest Quarter (°C) | 23.5 | 8.6 |
| bio14 | Precipitation of Driest Month (mm) | 10.0 | 5.2 |
| bio15 | Precipitation Seasonality (coefficient of variation) | 9.9 | 3.9 |
| bio19 | Precipitation of Coldest Quarter (mm) | 3.4 | 1.1 |
| Time Period | SSP1-2.6 | SSP2-4.5 | SSP3-7.0 | SSP5-8.5 |
|---|---|---|---|---|
| 2021–2040 | 48.24 | 46.92 | 39.76 | 43.46 |
| 2041–2060 | 65.39 | 69.17 | 73.82 | 81.77 |
| 2061–2080 | 67.81 | 86.55 | 79.71 | 97.34 |
| 2081–2100 | 63.69 | 90.27 | 98.41 | 93.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helvacı, Z.; Çolak, E. Phylogeography of Scarturus williamsi and Climate Change Impacts: Genetic Diversity and Projected Habitat Loss in Anatolia. Biology 2025, 14, 1184. https://doi.org/10.3390/biology14091184
Helvacı Z, Çolak E. Phylogeography of Scarturus williamsi and Climate Change Impacts: Genetic Diversity and Projected Habitat Loss in Anatolia. Biology. 2025; 14(9):1184. https://doi.org/10.3390/biology14091184
Chicago/Turabian StyleHelvacı, Zeycan, and Ercüment Çolak. 2025. "Phylogeography of Scarturus williamsi and Climate Change Impacts: Genetic Diversity and Projected Habitat Loss in Anatolia" Biology 14, no. 9: 1184. https://doi.org/10.3390/biology14091184
APA StyleHelvacı, Z., & Çolak, E. (2025). Phylogeography of Scarturus williamsi and Climate Change Impacts: Genetic Diversity and Projected Habitat Loss in Anatolia. Biology, 14(9), 1184. https://doi.org/10.3390/biology14091184






