Regulation of Light Absorption and Energy Dissipation in Sweet Sorghum Under Climate-Relevant CO2 and Temperature Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Light Response Measurement
2.3. Chlorophyll Quantification
2.4. Model Fitting and Computational Analysis
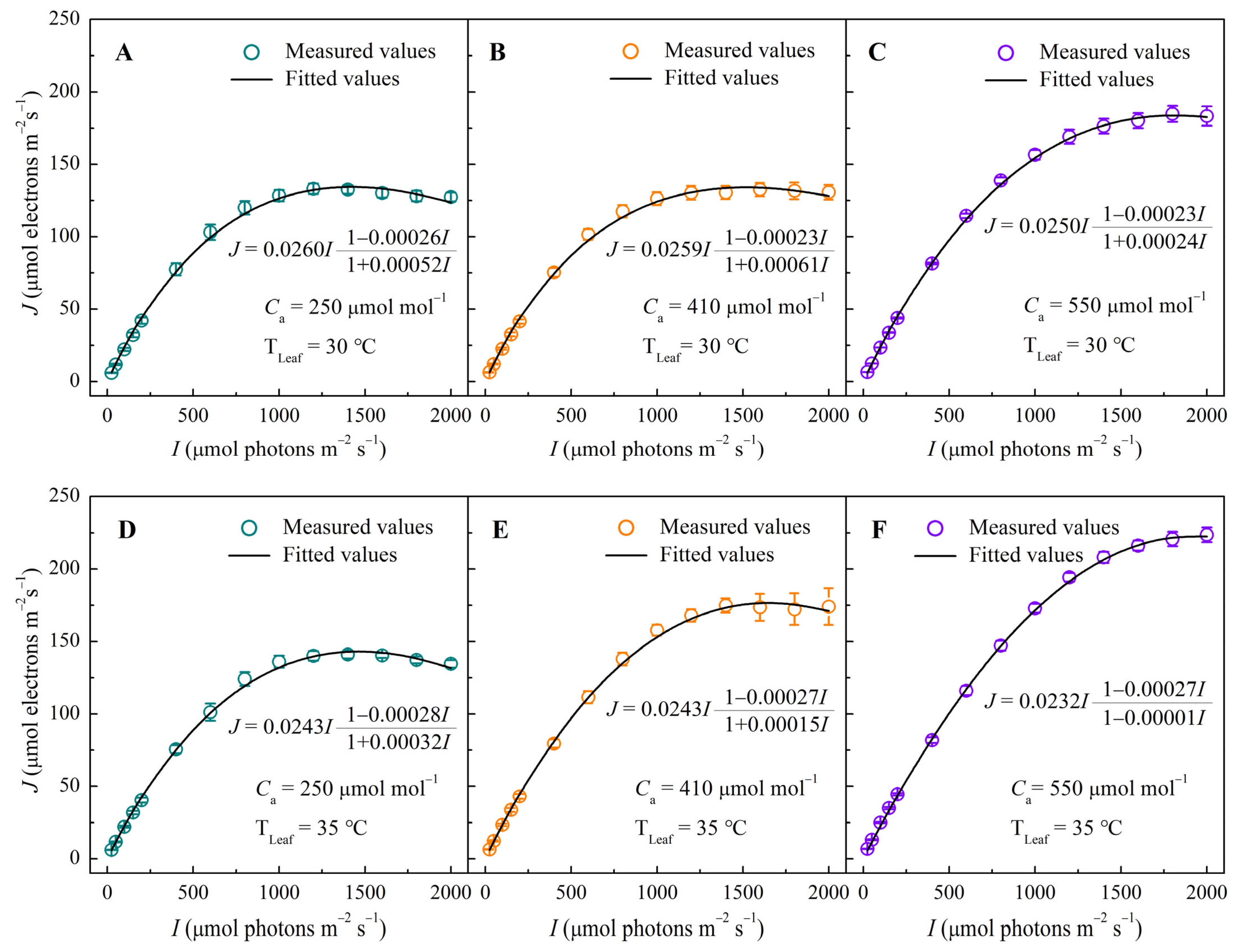
2.4.1. J–I Mechanistic Model
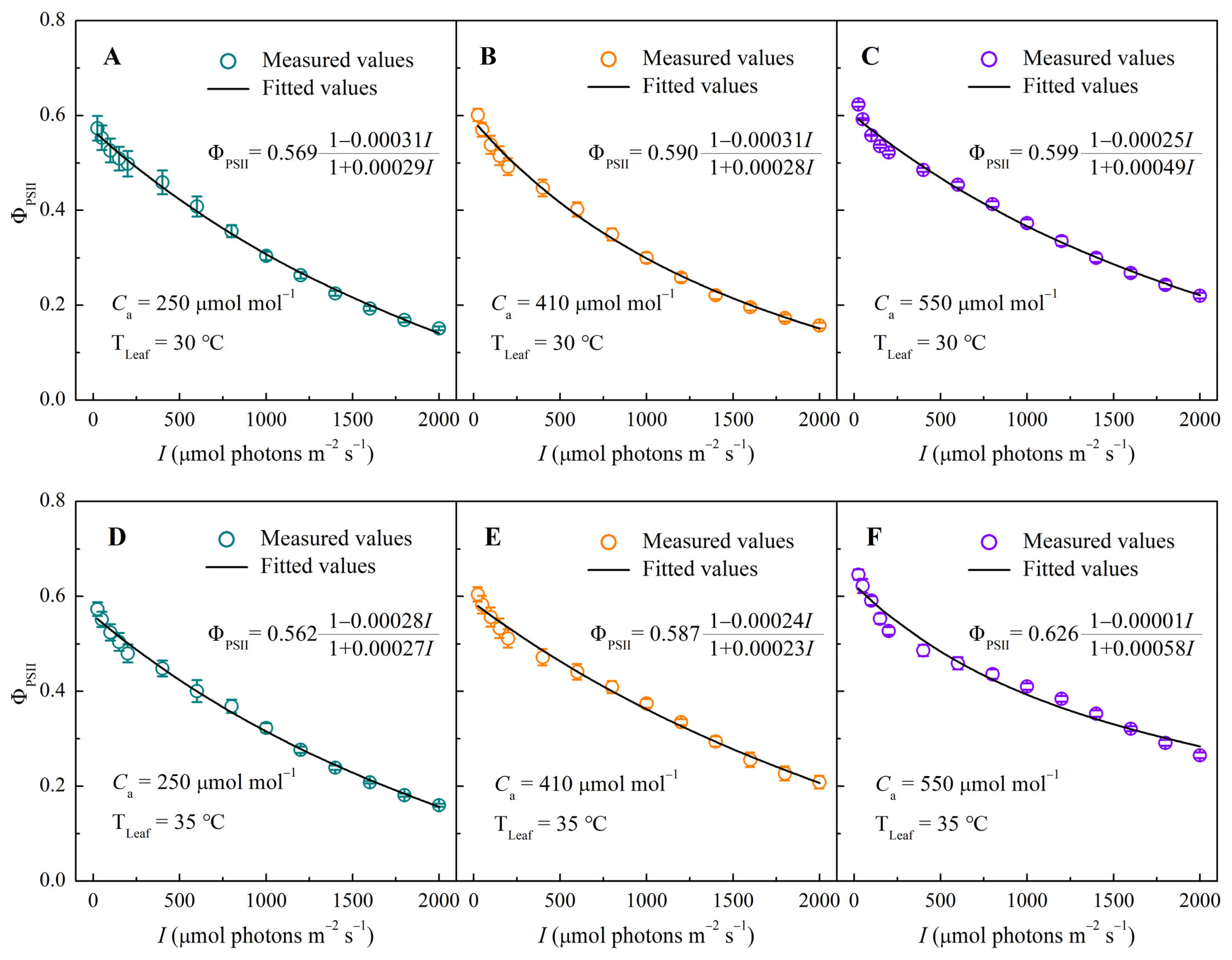
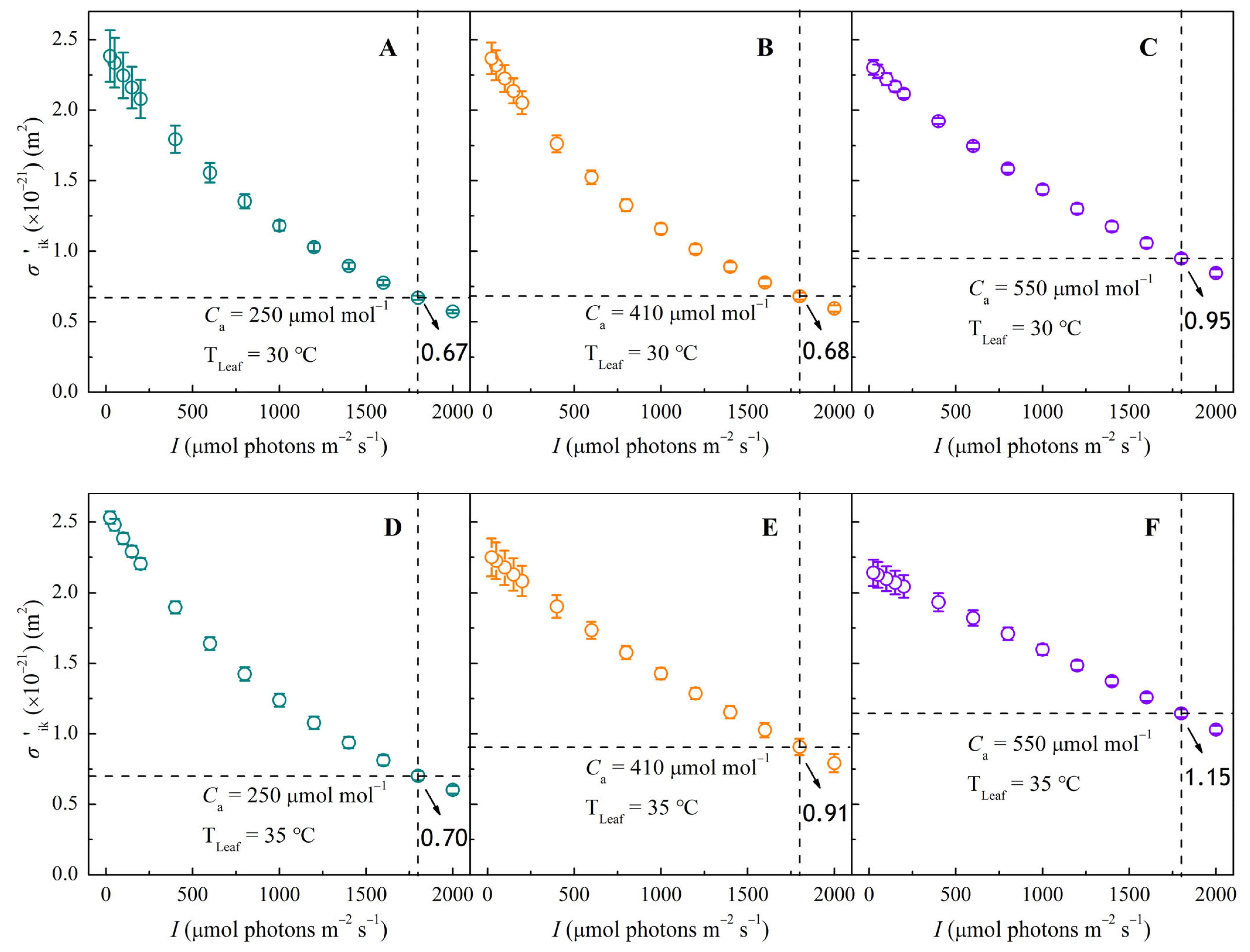
2.4.2. Mechanistic Models for ΦPSII, σ′ik, and Nk
2.5. Statistical Analysis
3. Results
3.1. J–I Response Curves Under Different CO2 and Temperature Conditions
3.2. ΦPSII–I Response Curves Under Different CO2 and Temperature Conditions
3.3. σ′ik–I Response Curves Under Different CO2 and Temperature Conditions
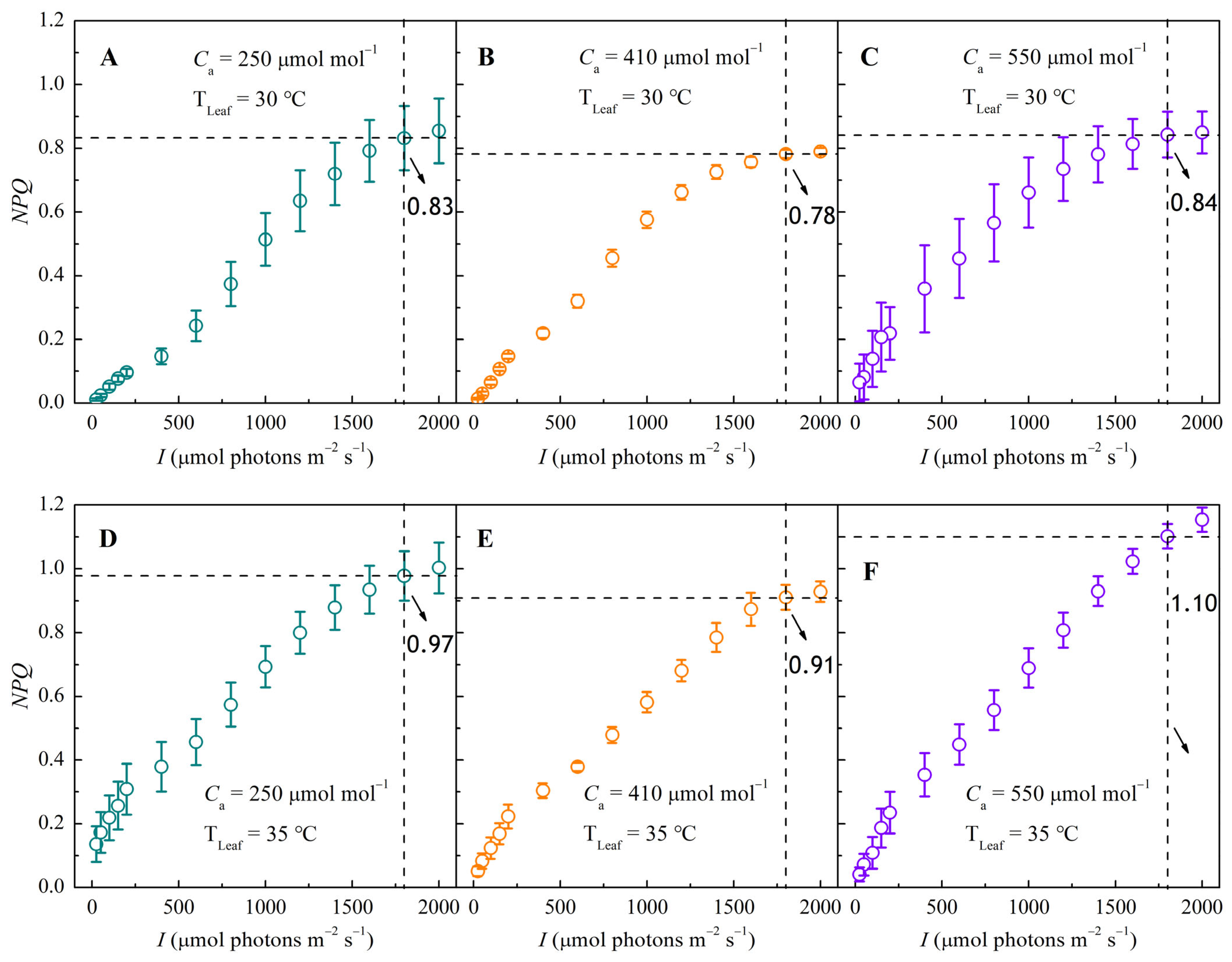
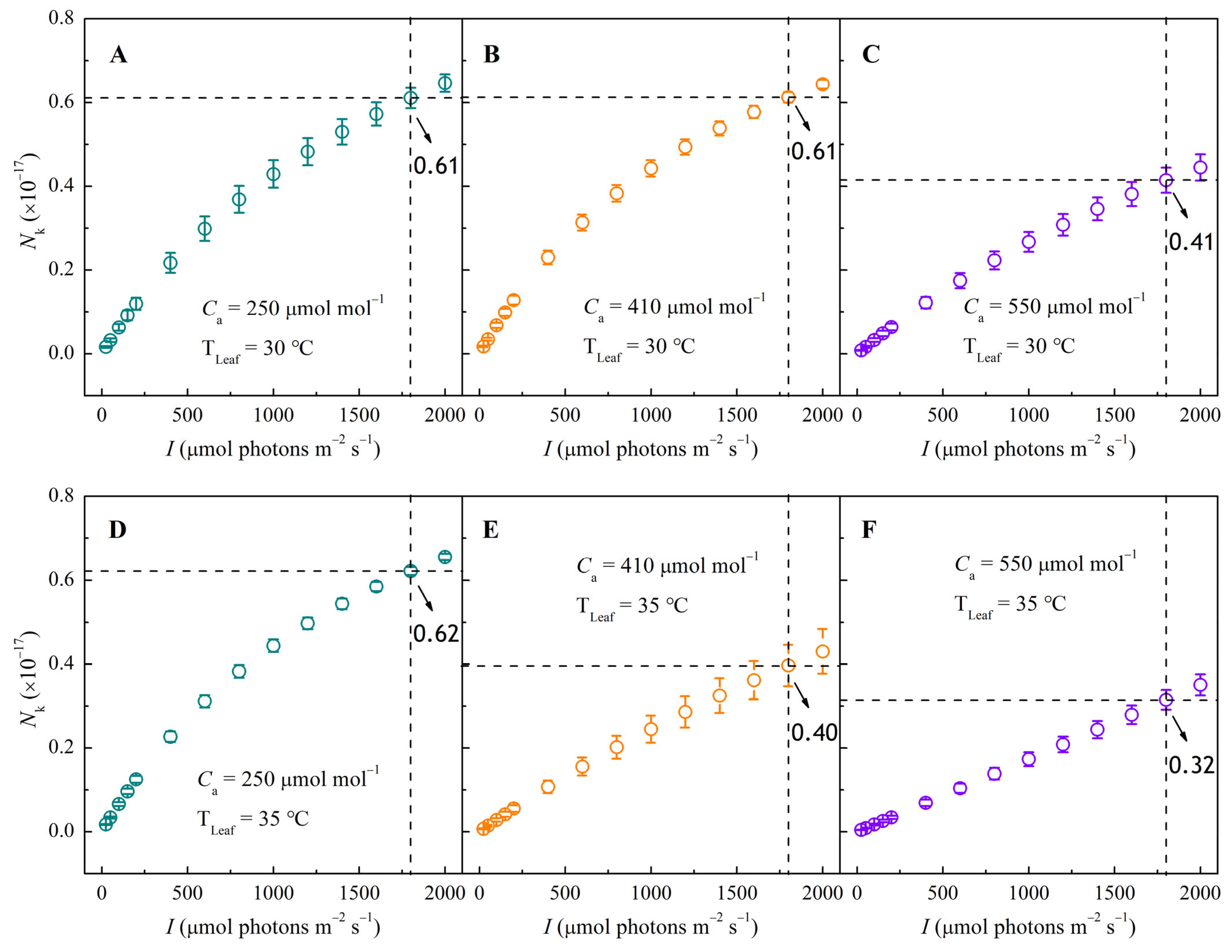
3.4. NPQ–I and Nk–I Response Curves Under Different CO2 and Temperature Conditions
4. Discussion
4.1. Regulation of Electron Transport and Light Utilization
4.2. Regulation of Excitation Energy Allocation and Photoprotection via Antenna Pigment Dynamics
4.3. Implications for Climate Resilience in C4 Crops
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on improving photosynthesis to increase crop yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef]
- Leister, D. Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef]
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 2016, 7, 12699. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. BBA-Bioenergetics 2015, 1847, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R. Effect of light intensity on photosynthesis. In Photosynthesis, Productivity and Environmental Stress; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 65–73. [Google Scholar]
- Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Elevated [CO2] negatively impacts C4 photosynthesis under heat and water stress without penalizing biomass. J. Exp. Bot. 2023, 74, 2875–2890. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, Y.; Tian, Z.; Liu, H.; Wu, H.; Li, X.; Zhang, Y.; Hu, C.; Dong, W.; Gu, L. Influence of fertilization on the dynamics of energy use in wheat. Plant Phenomics 2025, 7, 100063. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.; Tian, Y.; Zha, T.; Jia, X.; Bourque, C.P.A.; Wu, Y.; Bai, Y.; Ma, J.; Zhang, M. Light energy partitioning and photoprotection in an exotic species (Salix Psammophila) grown in a semi-arid area of northwestern China. Forests 2018, 9, 341. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Qu, M.; Ort, D.R.; Zhu, X.-G. The impact of modifying photosystem antenna size on canopy photosynthetic efficiency—Development of a new canopy photosynthesis model scaling from metabolism to canopy level processes. Plant Cell Environ. 2017, 40, 2946–2957. [Google Scholar] [CrossRef]
- Navakoudis, E.; Stergiannakos, T.; Daskalakis, V. A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein. Photosynth. Res. 2023, 156, 163–177. [Google Scholar] [CrossRef]
- Yang, X.-L.; Ma, X.-F.; Ye, Z.-P.; Yang, L.-S.; Shi, J.-B.; Wang, X.; Zhou, B.-B.; Wang, F.-B.; Deng, Z.-F. Simulating short-term light responses of photosynthesis and water use efficiency in sweet sorghum under varying temperature and CO2 conditions. Front. Plant Sci. 2024, 15, 1291630. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Eshghizadeh, H.R. Response of sorghum genotypes to water deficit stress under different CO2 and nitrogen levels. Plant Physiol. Bioch. 2021, 158, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Yu, J.; Shang, F. 2.58-Biorefinery Engineering. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 815–828. [Google Scholar]
- FAO. World Food and Agriculture–Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Cai, C.; Li, G.; Yang, H.; Yang, J.; Liu, H.; Struik, P.C.; Luo, W.; Yin, X.; Di, L.; Guo, X.; et al. Do all leaf photosynthesis parameters of rice acclimate to elevated CO2, elevated temperature, and their combination, in FACE environments? Glob. Change Biol. 2018, 24, 1685–1707. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Ahammed, G.J.; Li, X.; Shi, K. Elevated CO2 improves photosynthesis under high temperature by atenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves. Front. Plant Sci. 2018, 9, 1739. [Google Scholar] [CrossRef]
- Moynul Haque, M.; Hamid, A.; Khanam, M.; Biswas, D.K.; Karim, M.A.; Khaliq, Q.A.; Hossain, M.A.; Uprety, D.C. The effect of elevated CO2 concentration on leaf chlorophyll and nitrogen contents in rice during post-flowering phases. Biol. Plantarum 2006, 50, 69–73. [Google Scholar] [CrossRef]
- Ye, Z.P.; Stirbet, A.; An, T.; Robakowski, P.; Kang, H.J.; Yang, X.L.; Wang, F.B. Investigation on absorption cross-section of photosynthetic pigment molecules based on a mechanistic model of the photosynthetic electron flow-light response in C3, C4 species and cyanobacteria grown under various conditions. Front. Plant Sci. 2023, 14, 1234462. [Google Scholar] [CrossRef]
- Ye, Z.P.; Suggett, J.D.; Robakowski, P.; Kang, H.J. A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytol. 2013, 199, 110–120. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yang, X.L.; An, T.; Ye, Z.W.; Kang, H.J.; Robakowski, P.; Ye, Z.P.; Wang, F.B.; Zhou, S.X. Modeling light response of effective quantum efficiency of photosystem II for C3 and C4 crops. Front. Plant Sci. 2025, 16, 1478346. [Google Scholar] [CrossRef]
- Ye, Z.P. Nonlinear optical absorption of photosynthetic pigment molecules in leaves. Photosynth. Res. 2012, 112, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Ehleringer, J.J.O. Leaf absorptances of Mohave and Sonoran desert plants. Oecologia 1981, 49, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.R.G.; Wang, Y.; Evers, J.B.; Kromdijk, J. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J. Exp. Bot. 2021, 72, 5942–5960. [Google Scholar] [CrossRef]
- Feng, X.; Liu, R.; Li, C.; Zhang, H.; Slot, M. Contrasting responses of two C4 desert shrubs to drought but consistent decoupling of photosynthesis and stomatal conductance at high temperature. Environ. Exp. Bot. 2023, 209, 105295. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Sane, P.V.; Hurry, V.; Oquist, G.; Huner, N.P. Photosystem II reaction centre quenching: Mechanisms and physiological role. Photosynth. Res. 2008, 98, 565–574. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Johnson, M.P.; Goral, T.K.; Duffy, C.D.; Brain, A.P.; Mullineaux, C.W.; Ruban, A.V. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 2011, 23, 1468–1479. [Google Scholar] [CrossRef]
- Yan, K.; Chen, P.; Shao, H.; Shao, C.; Zhao, S.; Brestic, M. Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 2013, 8, e62100. [Google Scholar] [CrossRef]
- Baly, E.C.C. The kinetics of photosynthesis. Proc. R. Soc. Lond. 1935, 149, 596. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Wong, S.C. An empirical model of stomatal conductance. Aust. J. Plant Physiol. 1984, 11, 191–210. [Google Scholar] [CrossRef]
- Thornley, J.H.M. Mathematical Models in Plant Physiology; Academic Press: London, UK, 1976. [Google Scholar]
- Silsbe, G.M.; Kromkamp, J.C. Modeling the irradiance dependency of the quantum efficiency of photosynthesis. Limnol. Oceanogr.-Meth. 2012, 10, 645–652. [Google Scholar] [CrossRef]
- Smyth, T.J.; Pemberton, K.L.; Aiken, J.; Geider, R.J. A methodology to determine primary production and phytoplankton photosynthetic parameters from Fast Repetition Rate Fluorometry. J. Plankton Res. 2004, 26, 1337–1350. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Duffy, C.D.P.; Ruban, A.V. Dissipative pathways in the photosystem-II antenna in plants. J. Photoch. Photobio. B 2015, 152, 215–226. [Google Scholar] [CrossRef]
- Leiger, K.; Linnanto, J.M.; Freiberg, A. Establishment of the Qy absorption spectrum of chlorophyll a extending to near-infrared. Molecules 2020, 25, 3796. [Google Scholar] [CrossRef]
- Szabó, I.; Bergantino, E.; Giacometti, G.M. Light and oxygenic photosynthesis: Energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep. 2005, 6, 629–634. [Google Scholar] [CrossRef]
- Vasil’ev, S.; Orth, P.; Zouni, A.; Owens, T.G.; Bruce, D. Excited-state dynamics in photosystem II: Insights from the x-ray crystal structure. Proc. Natl. Acad. Sci. USA 2001, 98, 8602–8607. [Google Scholar] [CrossRef]
| Photosynthetic Parameters | TLeaf = 30 °C | TLeaf = 35 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca = 250 μmol mol−1 | Ca = 410 μmol mol−1 | Ca = 550 μmol mol−1 | Ca = 250 μmol mol−1 | Ca = 410 μmol mol−1 | Ca = 550 μmol mol−1 | |||||||
| Fitted | Measured | Fitted | Measured | Fitted | Measured | Fitted | Measured | Fitted | Measured | Fitted | Measured | |
| α | 0.262 ± 0.020 ab | ― | 0.261 ± 0.013 ab | ― | 0.252 ± 0.005 ab | ― | 0.278 ± 0.004 a | ― | 0.246 ± 0.016 ab | ― | 0.232 ± 0.009 b | ― |
| Jmax (μmol electron m−2 s−1) | 135.46 ± 3.53 c | 133.63 ± 3.30 c | 134.67 ± 5.05 c | 133.84 ± 5.52 c | 184.27 ± 5.93 b | 184.82 ± 5.49 b | 141.48 ± 6.46 c | 142.20 ± 1.92 c | 179.55 ± 8.72 b | 179.88 ± 8.45 b | 223.16 ± 4.90 a | 223.57 ± 5.06 a |
| Ie-sat (μmol photons m−2 s−1) | 1412.1 ± 43.4 d | 1280.0 ± 48.9 d | 1515.0 ± 33.3 cd | 1600.0 ± 63.2 c | 1795.7 ± 16.5 b | 1800.0 ± 0.0 b | 1411.4 ± 17.6 d | 1400.0 ± 63.2 d | 1665.4 ± 154.8 bc | 1666.7 ± 176.4 bc | 1896.5 ± 28.9 ab | 2000.00 ± 0.00 a |
| σik (×10−21 m2) | 2.43 ± 0.19 ab | ― | 2.42 ± 0.12 ab | ― | 2.33 ± 0.06 ab | ― | 2.58 ± 0.05 a | ― | 2.28 ± 0.14 b | ― | 2.16 ± 0.09 b | ― |
| τmin (ms) | 7.83 ± 0.71 a | ― | 8.65 ± 0.61 a | ― | 5.14 ± 0.52 b | ― | 7.88 ± 0.62 a | ― | 4.68 ± 0.44 b | ― | 2.93 ± 0.23 c | ― |
| N0 (×1016 m2) | 17.09 ± 0.06 a | ― | 17.10 ± 0.07 a | ― | 13.99 ± 0.09 b | ― | 17.09 ± 0.06 a | ― | 13.29 ± 0.12 b | ― | 13.51 ± 0.96 b | ― |
| ΦPSIImax | 0.569 ± 0.029 b | 0.573 ± 0.026 b | 0.590 ± 0.018 a | 0.601 ± 0.013 a | 0.599 ± 0.003 b | 0.623 ± 0.005 ab | 0.562 ± 0.018 b | 0.573 ± 0.015 b | 0.587 ± 0.018 b | 0.604 ± 0.015 ab | 0.626 ± 0.009 a | 0.646 ± 0.011 a |
| R2 (J–I curves) | 0.9970 ± 0.0006 | ― | 0.9979 ± 0.0005 | ― | 0.9994 ± 0.0001 | ― | 0.9972 ± 0.0007 | ― | 0.9975 ± 0.0018 | ― | 0.9996 ± 0.0001 | ― |
| R2 (ΦPSII–I curves) | 0.9945 ± 0.0014 | ― | 0.9932 ± 0.0023 | ― | 0.9889 ± 0.0034 | ― | 0.9895 ± 0.0039 | ― | 0.9900 ± 0.0031 | ― | 0.9770 ± 0.0009 | ― |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-J.; Liu, L.-H.; Ye, Z.-P.; Zhang, C.-W.; Yang, X.-L. Regulation of Light Absorption and Energy Dissipation in Sweet Sorghum Under Climate-Relevant CO2 and Temperature Conditions. Biology 2025, 14, 1185. https://doi.org/10.3390/biology14091185
Li J-J, Liu L-H, Ye Z-P, Zhang C-W, Yang X-L. Regulation of Light Absorption and Energy Dissipation in Sweet Sorghum Under Climate-Relevant CO2 and Temperature Conditions. Biology. 2025; 14(9):1185. https://doi.org/10.3390/biology14091185
Chicago/Turabian StyleLi, Jin-Jing, Li-Hua Liu, Zi-Piao Ye, Chao-Wei Zhang, and Xiao-Long Yang. 2025. "Regulation of Light Absorption and Energy Dissipation in Sweet Sorghum Under Climate-Relevant CO2 and Temperature Conditions" Biology 14, no. 9: 1185. https://doi.org/10.3390/biology14091185
APA StyleLi, J.-J., Liu, L.-H., Ye, Z.-P., Zhang, C.-W., & Yang, X.-L. (2025). Regulation of Light Absorption and Energy Dissipation in Sweet Sorghum Under Climate-Relevant CO2 and Temperature Conditions. Biology, 14(9), 1185. https://doi.org/10.3390/biology14091185






