Antioxidant Defense Strategies Against Diaporthe eres Infection in Hongyang Kiwifruit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Pathogen
2.2. Pathogen Inoculation and Sampling
2.3. Enzyme Activity Assays
2.3.1. SOD Activity Assay
2.3.2. POD Activity Assay
2.4. Measurement of Malondialdehyde (MDA) Content
2.5. Transcriptome Sequencing and Analysis
2.6. Bioinformatic Analysis
2.7. Statistical Analysis
3. Results
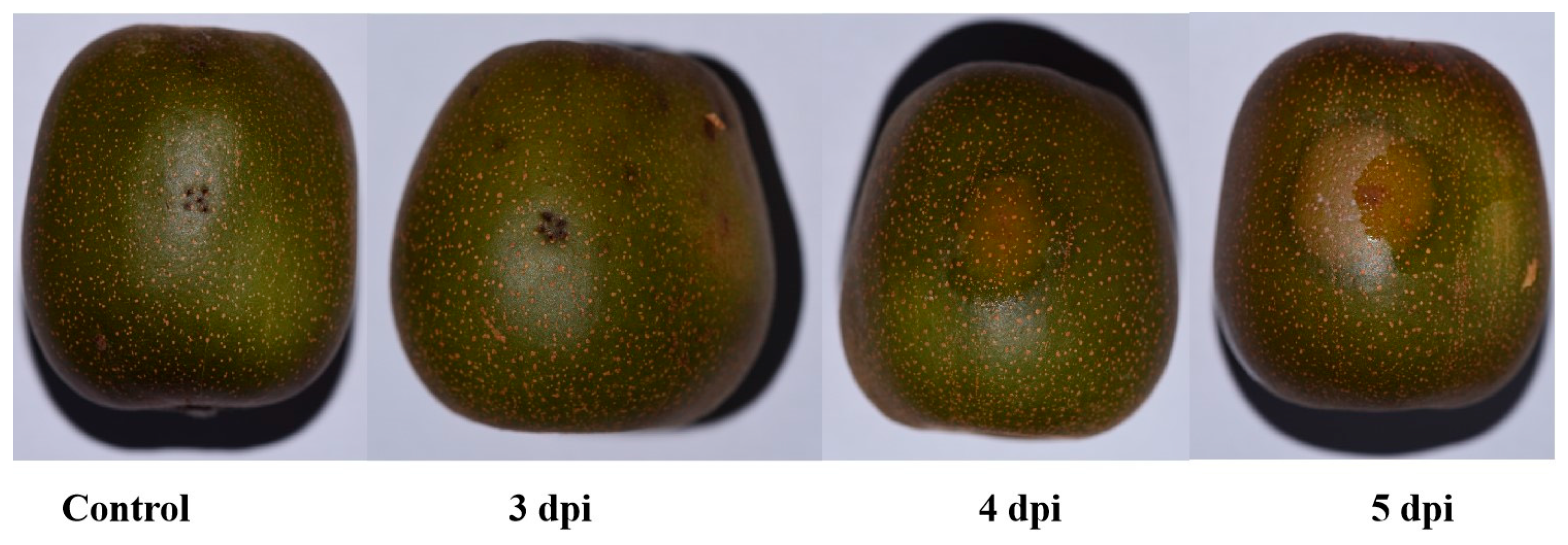
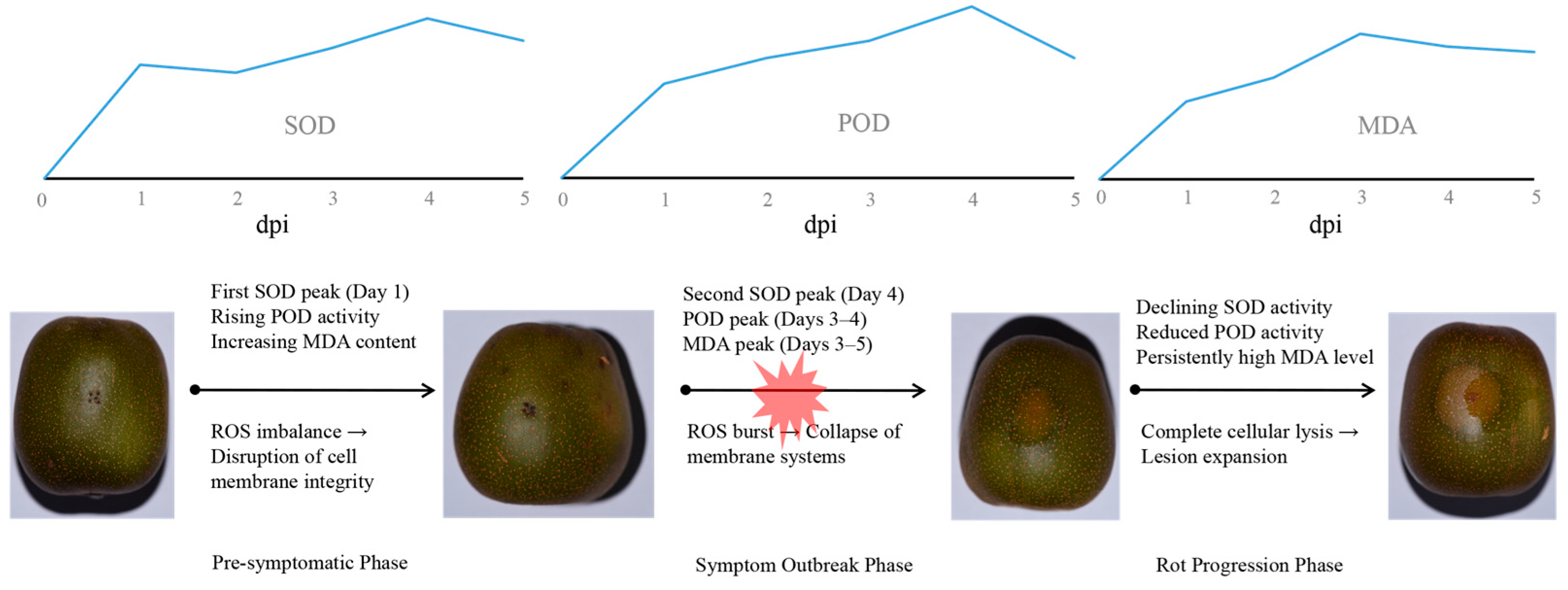
3.1. Disease Progression and Incubation Period Dynamics
3.2. Bimodal SOD Activation Patterns in Diaporthe-Infected Hongyang Kiwifruit
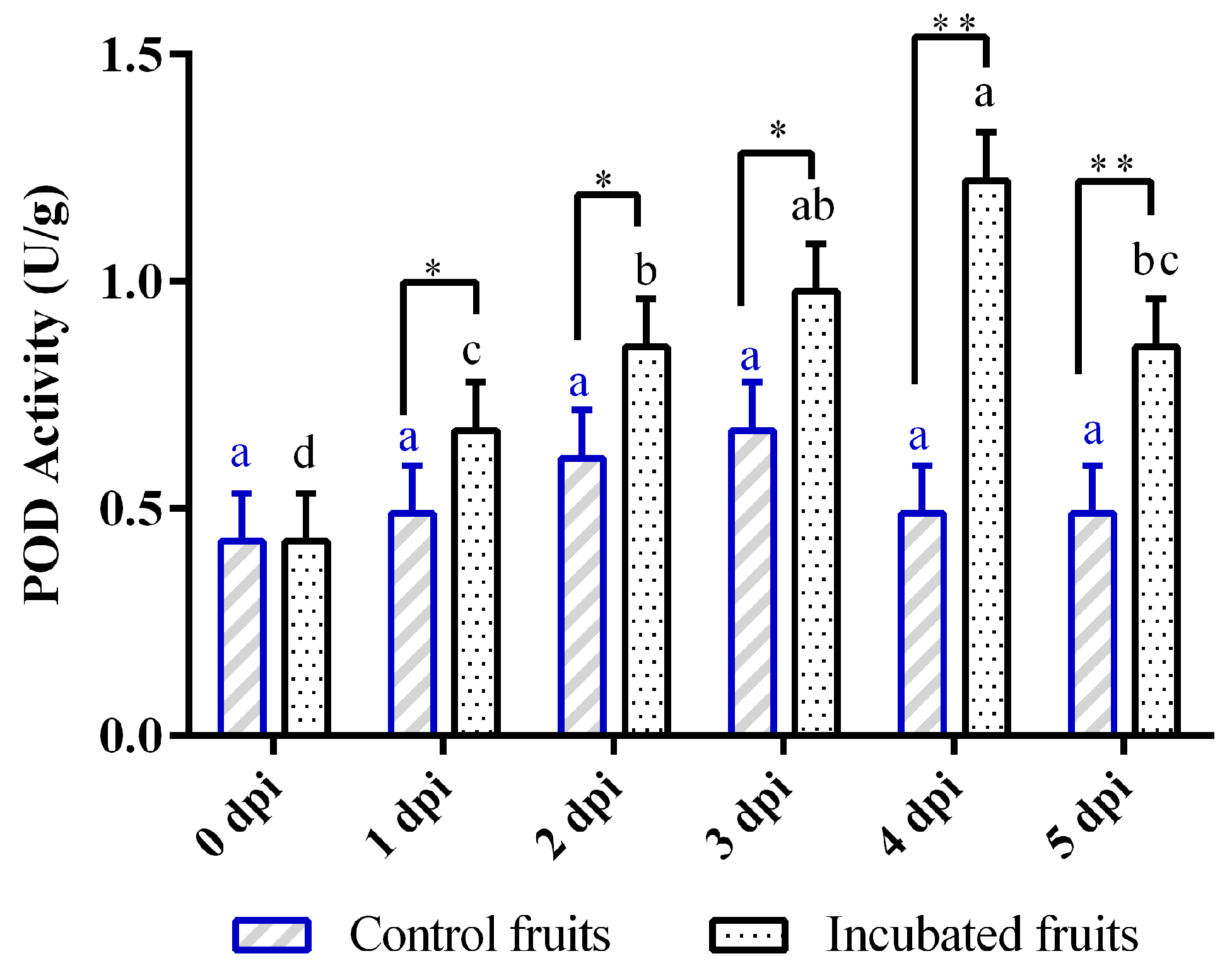
3.3. Temporal Changes in POD Activity During Diaporthe-Infected Hongyang Kiwifruit
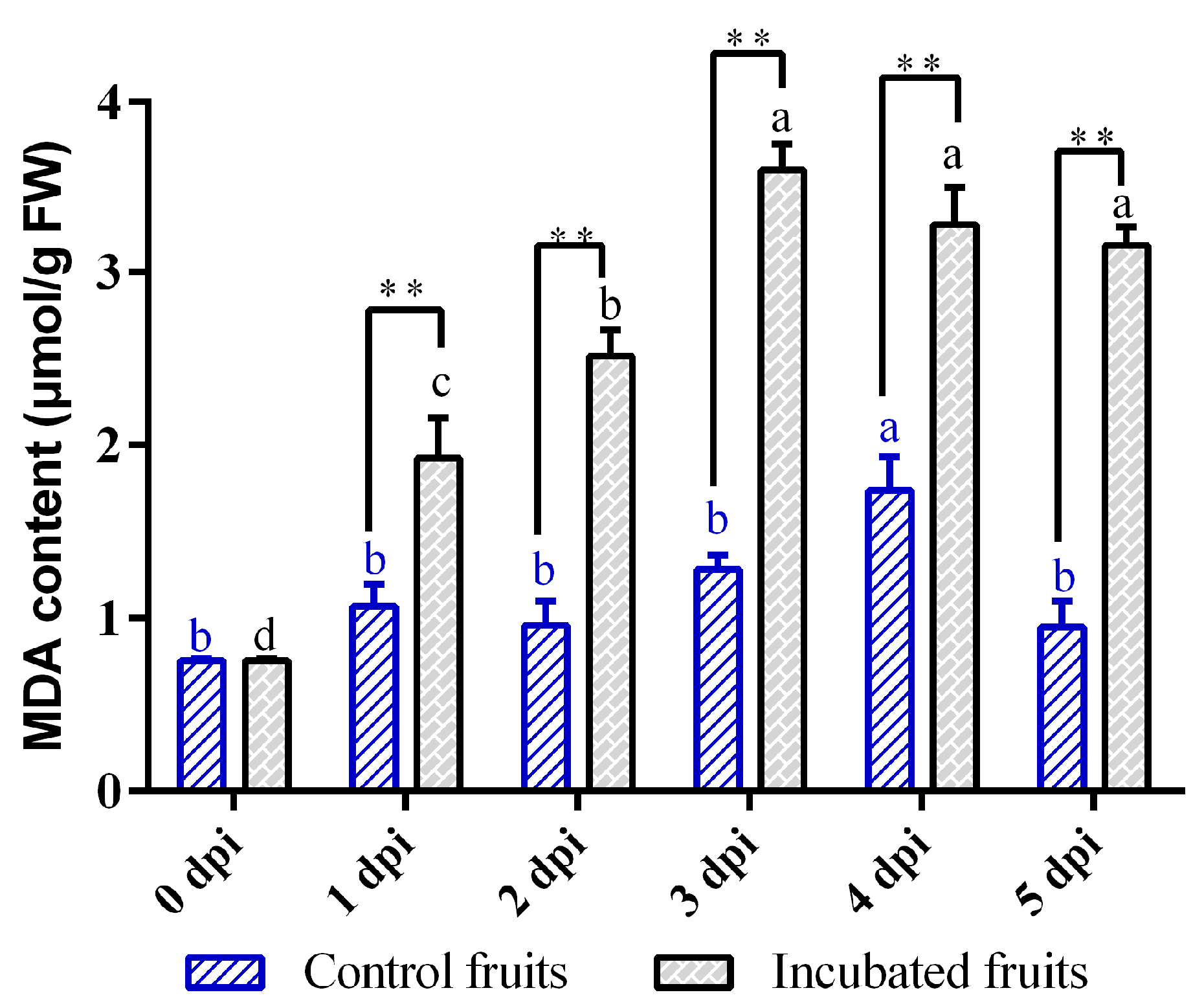
3.4. Dynamics of Malondialdehyde (MDA) Accumulation
3.5. Transcriptome Analysis of the SOD and POD Genes
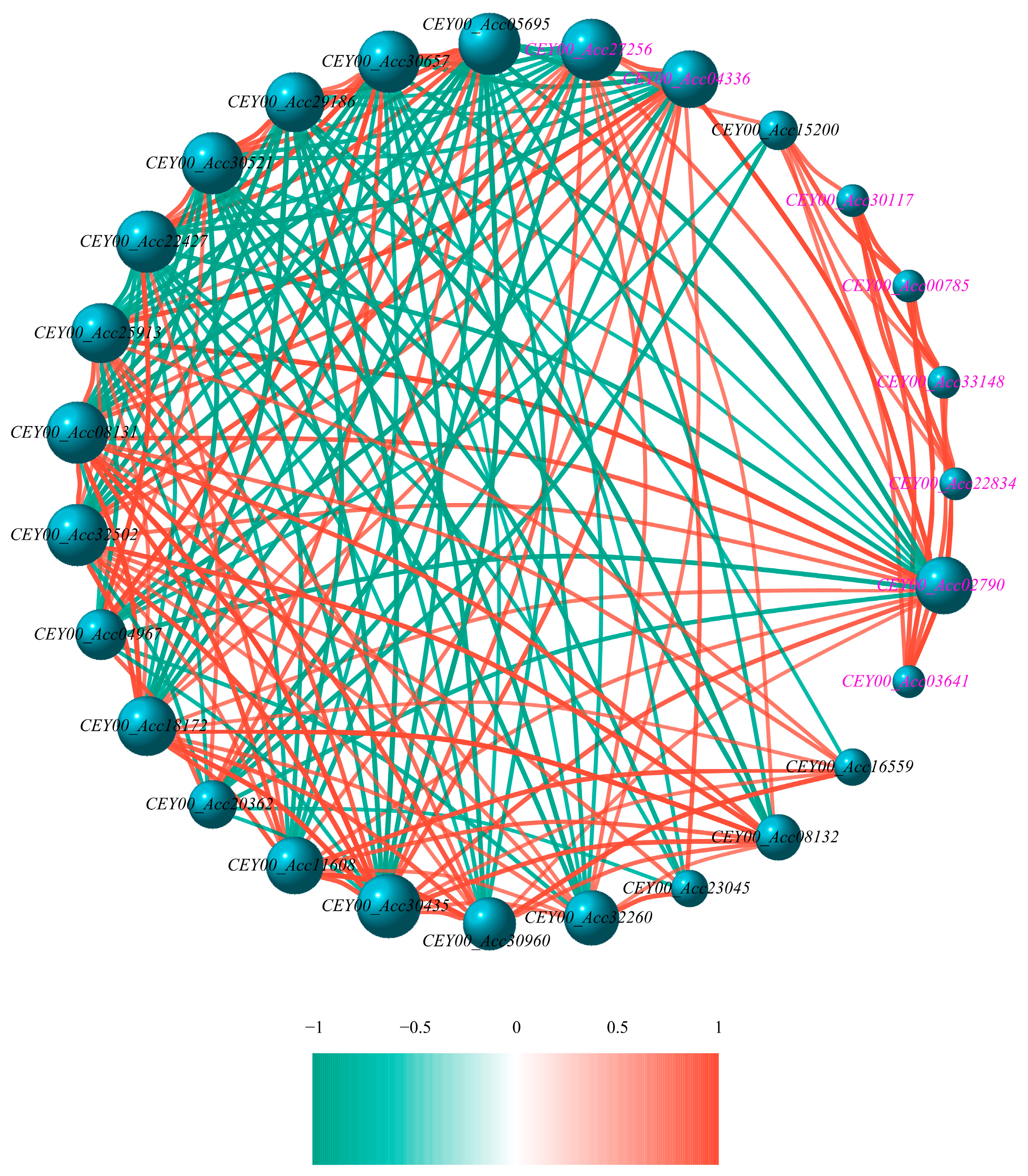
3.6. Bioinformatic Analysis of CEY00_Acc02790 Regulatory Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferguson, A.R.; Huang, H. Genetic resources of kiwifruit: Domestication and breeding. Hortic. Rev. 2007, 33, 1–121. [Google Scholar]
- Nishiyama, I. Fruits of the Actinidia genus. Adv. Food Nutr. Res. 2007, 52, 293–324. [Google Scholar]
- Hongwen, H. The Genus Actinidia a World Monograph; Science Press: Beijing, China, 2016. [Google Scholar]
- Liu, Y.; Qi, Y.; Chen, X.; He, H.; Liu, Z.; Zhang, Z.; Ren, Y.; Ren, X. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Res. Int. 2019, 116, 291–301. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S.; Drummond, L. The nutritional composition of Zespri® SunGold Kiwifruit and Zespri® Sweet Green Kiwifruit. Food Chem. 2018, 238, 195–202. [Google Scholar] [CrossRef]
- Ling, L.; Chen, L.; Tu, D.; Zhang, S. Isolation and identification of pathogenic fungi causing fruit rot of Hongyang kiwifruit during cold storage. Anhui Agric. Sci. Bull. 2023, 29, 114–118. [Google Scholar]
- Zhou, Y.; Gong, G.; Cui, Y.; Zhang, D.; Chang, X.; Hu, R.; Liu, N.; Sun, X. Identification of Botryosphaeriaceae species causing kiwifruit rot in Sichuan province, China. Plant Dis. 2015, 99, 699–708. [Google Scholar] [CrossRef]
- Díaz, G.A.; Latorre, B.A.; Lolas, M.; Ferrada, E.; Naranjo, P.; Zoffoli, J.P. Identification and characterization of Diaporthe ambigua, D. australafricana, D. novem, and D. rudis causing a postharvest fruit rot in kiwifruit. Plant Dis. 2017, 101, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Díaz, G.A.; Latorre, B.A.; Jara, S.; Ferrada, E.; Naranjo, P.; Rodríguez, J.; Zoffoli, J.P. First report of Diaporthe novem causing postharvest rot of kiwifruit during controlled atmosphere storage in Chile. Plant Dis. 2014, 98, 1274. [Google Scholar] [CrossRef]
- Lei, J.; Wu, W.; Liu, Y.; Long, X.; Li, W.; Wang, R. Identification of pathogen isolation and pathogenicity difference of ‘Hongyang’ kiwifruit ripe rot in liupanshui city, Guizhou province. North. Hortic. 2019, 04, 31–38. (In Chinese) [Google Scholar]
- Zhang, S.; Liu, W.; Ling, L. Inhibitory effects of four traditional Chinese medicine extracts on postharvest fungal Phomopsis sp.in ‘Hongyang’ kiwifruit. North. Hortic. 2023, 24, 95–101. [Google Scholar]
- Chen, L.; Zhang, S.; Liu, W.; Han, X.; Ling, L. Inhibitory effects of Syzygium aromaticum ethanol extract on three pathogenic fungi causing soft rot of ‘Hongyang’ kiwifruit. North. Hortic. 2024, 22, 15–23. [Google Scholar]
- Singh, V.P.; Jaiswal, S.; Wang, Y.; Feng, S.; Tripathi, D.K.; Singh, S.; Gupta, R.; Xue, D.; Xu, S.; Chen, Z.H.; et al. Evolution of reactive oxygen species cellular targets for plant development. Trends Plant Sci. 2024, 29, 865–877. [Google Scholar] [CrossRef]
- Vuorinen, K.; Zamora, O.; Vaahtera, L.; Overmyer, K.; Brosché, M. Dissecting contrasts in cell death, hormone, and defense signaling in response to Botrytis cinerea and reactive oxygen species. Mol. Plant-Microbe Interact. 2021, 34, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Nordzieke, D.E.; Medraño-Fernandez, I. The plasma membrane: A platform for intra- and intercellular redox signaling. Antioxidants 2018, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Jovanovi, S.V.; Kukavica, B.; Vidovi, M.; Morina, F.; Menckhoff, L. Class III Peroxidases: Functions, localization and redox regulation of isoenzymes. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 269–300. [Google Scholar]
- Freitas, C.D.; Costa, J.H.; Germano, T.A.; Rocha, R.D.O.; Ramos, M.V.; Bezerra, L.P. Class III plant peroxidases: From classification to physiological functions. Int. J. Biol. Macromol. 2024, 263, 130306. [Google Scholar] [CrossRef]
- Patel, M.K.; Fanyuk, M.; Feyngenberg, O.; Maurer, D.; Sela, N.; Ovadia, R.; Oren-Shamir, M.; Alkan, N. Phenylalanine induces mango fruit resistance against chilling injuries during storage at suboptimal temperature. Food Chem. 2023, 405, 134909. [Google Scholar] [CrossRef] [PubMed]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef]
- Ling, L.Z.; Chen, L.L.; Liu, Z.Z.; Luo, L.Y.; Tai, S.H.; Zhang, S.D. Genome sequencing and CAZymes repertoire analysis of Diaporthe eres P3-1W causing postharvest fruit rot of ‘Hongyang’ kiwifruit in China. PeerJ 2024, 12, e17715. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, L.; Li, X.; Tan, X.; Zhu, Y.; Li, X.; Wang, D.; Ren, X.; Kong, Q. Early identification and monitoring of soft rot contamination in postharvest kiwifruit based on volatile fingerprints using headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2025, 474, 143195. [Google Scholar] [CrossRef] [PubMed]
- Dandan, F.; Lei, D.; Qiya, S.; Dejiang, L.; Caihong, Z.; Li, L. Green fluorescent protein gene markers and infection of Diaporthe actinidiae. Mycosystema 2022, 41, 450–458. [Google Scholar]
- Alvarez, M.E.; Pennell, R.I.; Meijer, P.J.; Ishikawa, A.; Dixon, R.A.; Lamb, C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef]
- Park, J.; Son, H. Antioxidant systems of plant pathogenic fungi: Functions in oxidative stress response and their regulatory mechanisms. Plant Pathol. J. 2024, 40, 235–250. [Google Scholar] [CrossRef]
- Jha, S.; Singh, R.; Jha, G.; Singh, P.; Aggarwal, D.; Shukla, M.; Dikshit, A. Plant Peroxidases: Biomarkers of environmental stresses and signaling in plants. In Antioxidant Defense in Plants: Molecular Basis of Regulation; Aftab, T., Hakeem, K.R., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 135–156. [Google Scholar]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- Liao, H.; Fang, Y.; Yin, J.; He, M.; Wei, Y.; Zhang, J.; Yong, S.; Cha, J.; Song, L.; Zhu, X.; et al. Rice transcription factor bHLH25 confers resistance to multiple diseases by sensing H2O2. Cell Res. 2025, 35, 205–219. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Sui, N.; Zhang, F. Class III peroxidase: An essential enzyme for enhancing plant physiological and developmental process by maintaining the ROS level: A review. Int. J. Biol. Macromol. 2024, 283, 137331. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef]
- Delledonne, M.; Zeier, J.; Marocco, A.; Lamb, C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. USA 2001, 98, 13454–13459. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, L.; Yang, T.; Long, X.; Pan, S.; Zhang, S. Antioxidant Defense Strategies Against Diaporthe eres Infection in Hongyang Kiwifruit. Biology 2025, 14, 1169. https://doi.org/10.3390/biology14091169
Ling L, Yang T, Long X, Pan S, Zhang S. Antioxidant Defense Strategies Against Diaporthe eres Infection in Hongyang Kiwifruit. Biology. 2025; 14(9):1169. https://doi.org/10.3390/biology14091169
Chicago/Turabian StyleLing, Lizhen, Tao Yang, Xiaoqing Long, Shengyu Pan, and Shudong Zhang. 2025. "Antioxidant Defense Strategies Against Diaporthe eres Infection in Hongyang Kiwifruit" Biology 14, no. 9: 1169. https://doi.org/10.3390/biology14091169
APA StyleLing, L., Yang, T., Long, X., Pan, S., & Zhang, S. (2025). Antioxidant Defense Strategies Against Diaporthe eres Infection in Hongyang Kiwifruit. Biology, 14(9), 1169. https://doi.org/10.3390/biology14091169






