7-Methoxyflavanone Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR4/NF-κB p65 and ROS/Txnip/NLRP3 Signaling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Cell Viability
2.3. Measurement of NO
2.4. Animal Experiment
2.5. Hematoxylin and Eosin Staining
2.6. Giemsa Staining
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Lung Tissues Wet/Dry (W/D) Ratio
2.9. Measurement of MDA and SOD
2.10. Immunofluorescence
2.11. Immunoblotting
2.12. qPCR Analysis
2.13. Statistical Analysis
3. Results
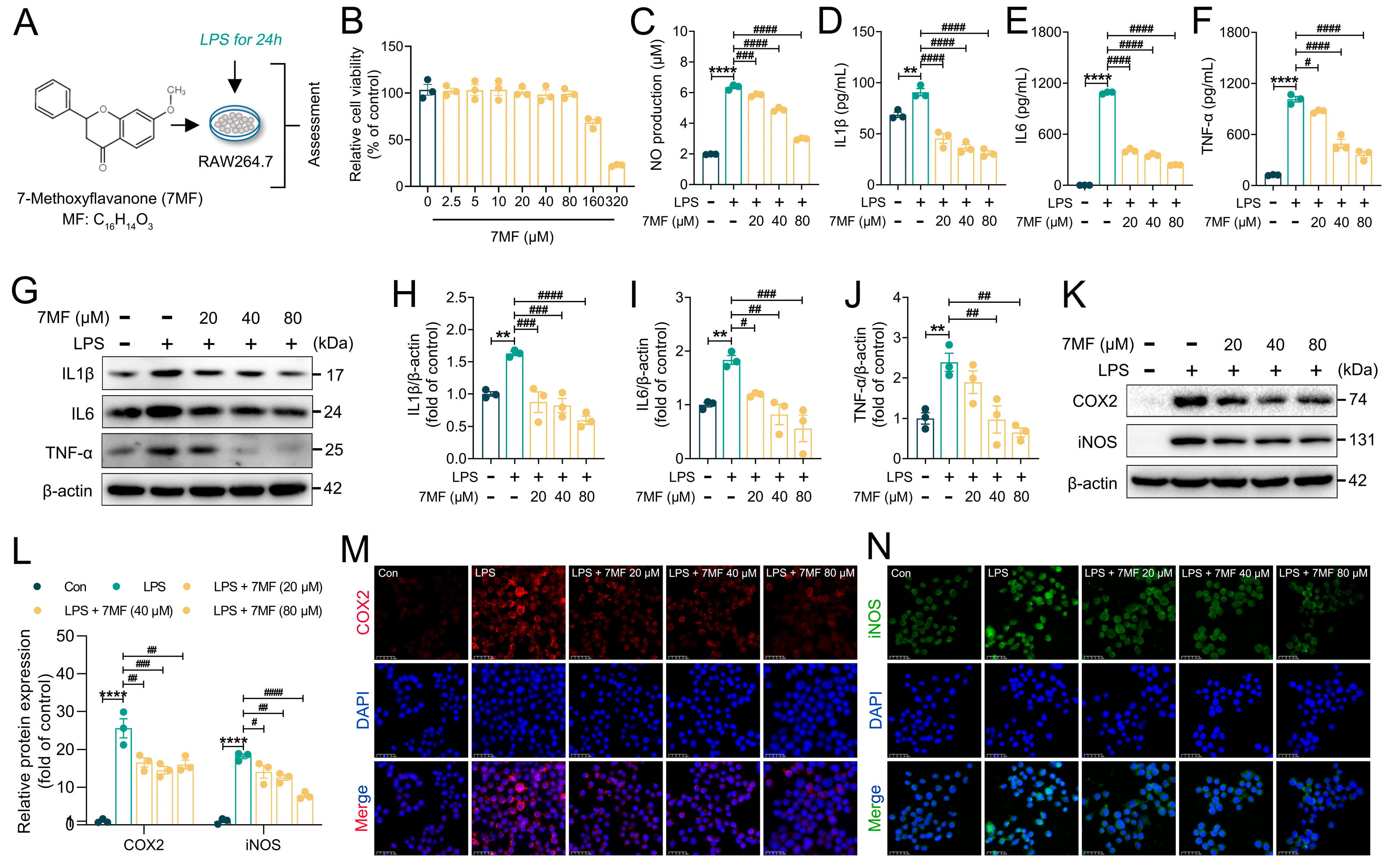
3.1. 7MF Inhibited LPS-Induced Inflammatory Response in RAW264.7 Cells
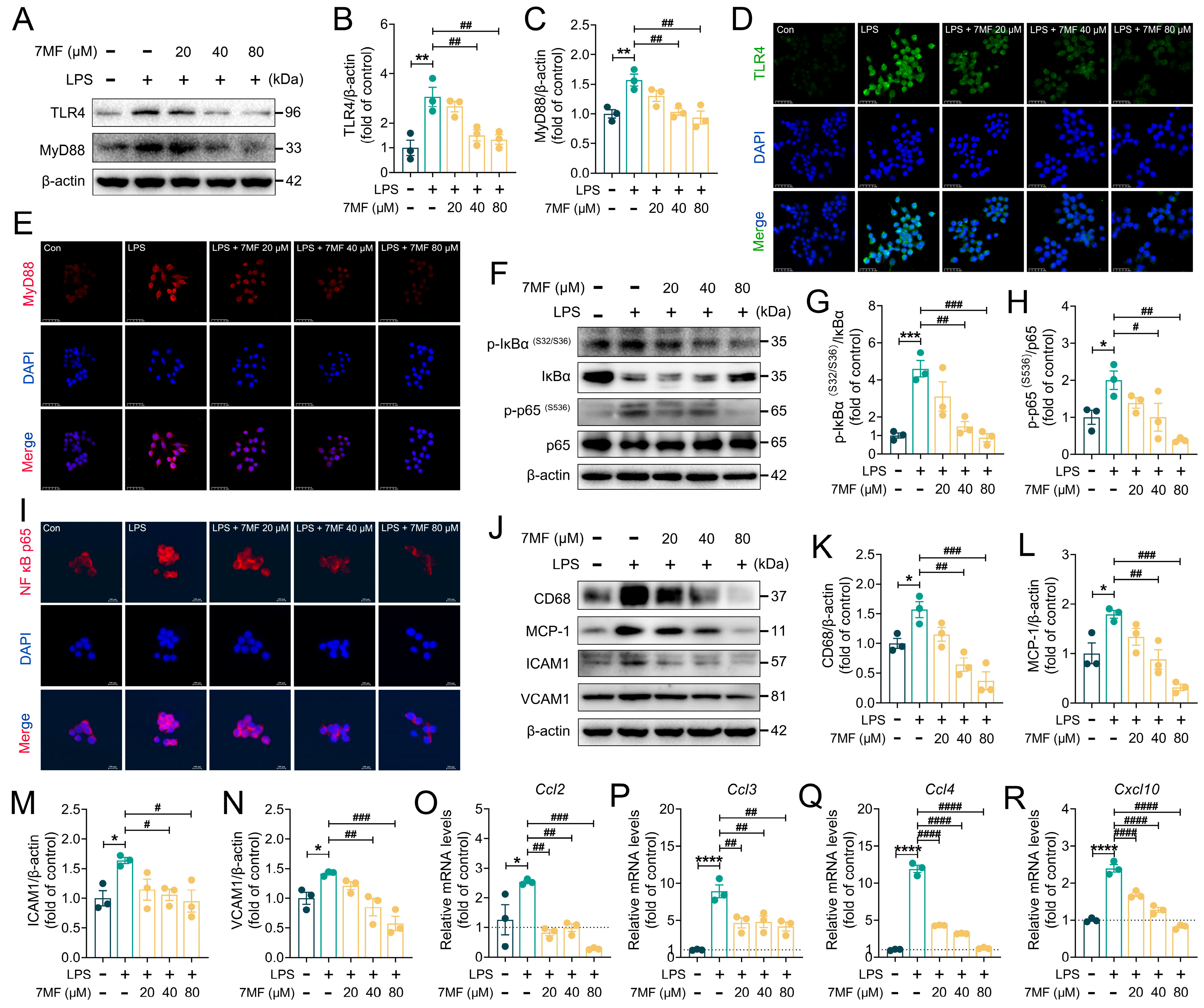
3.2. 7MF Suppressed TLR4/NF-κB p65 Signaling-Mediated Inflammatory Injury in RAW264.7 Cells
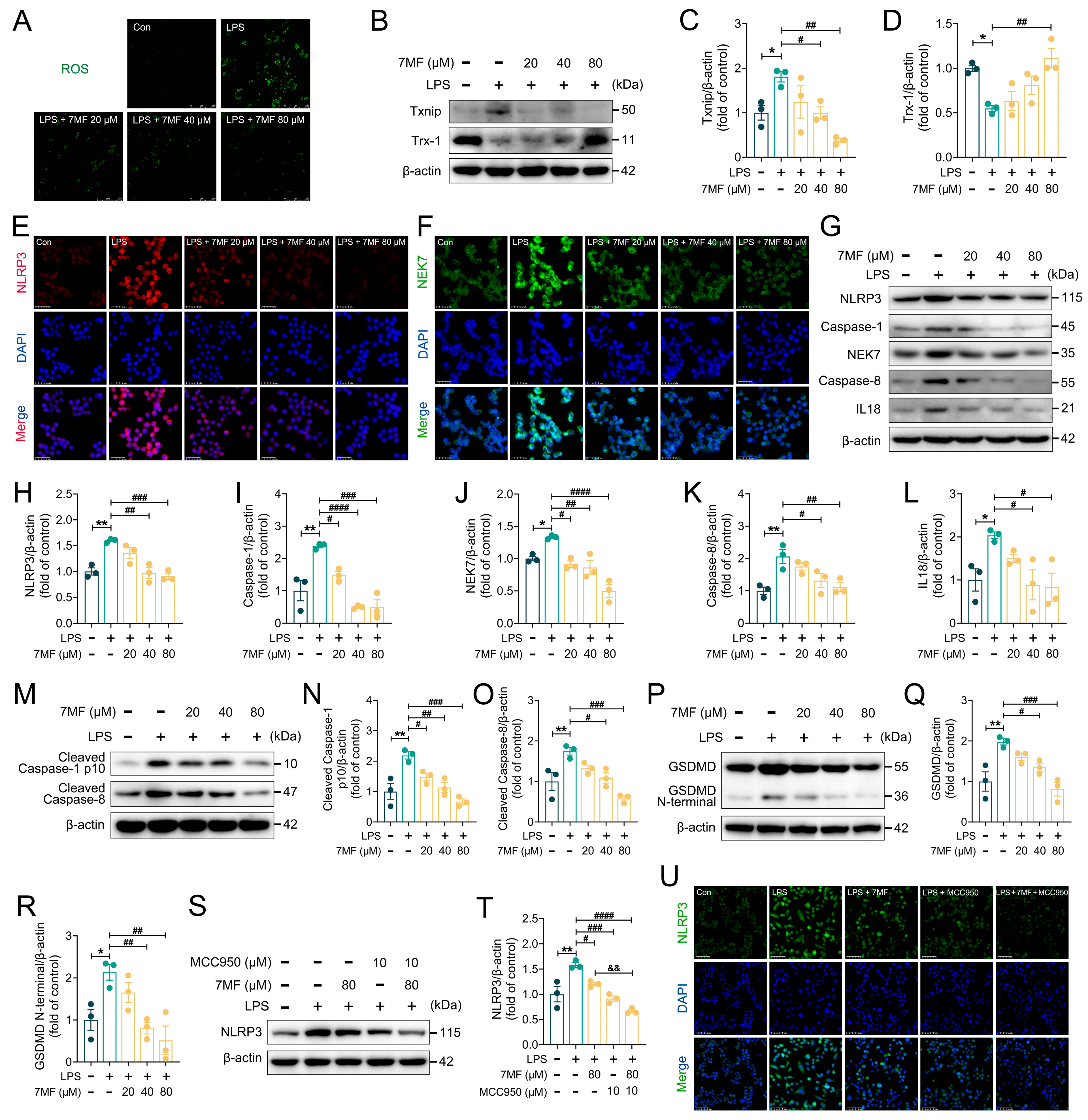
3.3. 7MF Inhibited ROS/Txnip/NLRP3 Signaling-Mediated Pyroptosis in RAW264.7 Cells
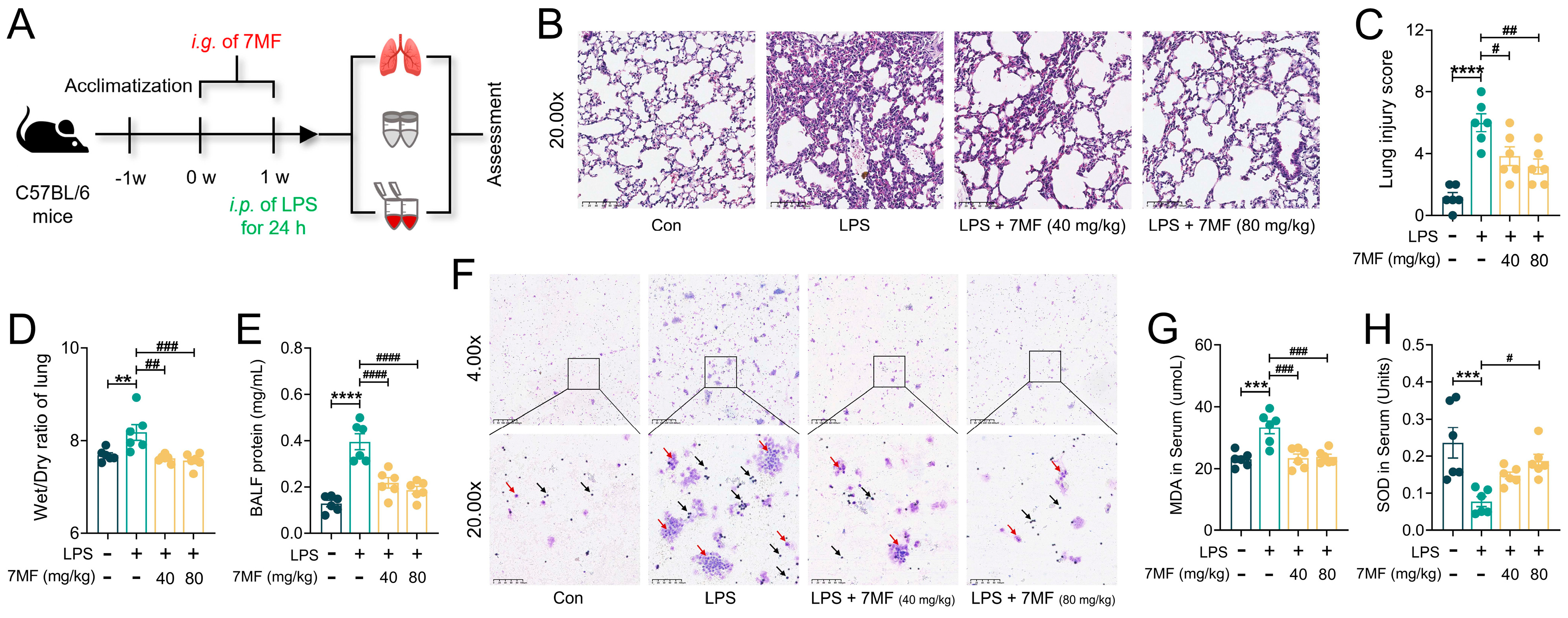
3.4. 7MF Reduced Infiltration and Exudation of Pro-Inflammatory Cells in ALI Mice
3.5. 7MF Alleviated ALI in Mice by Inhibiting Inflammatory Response
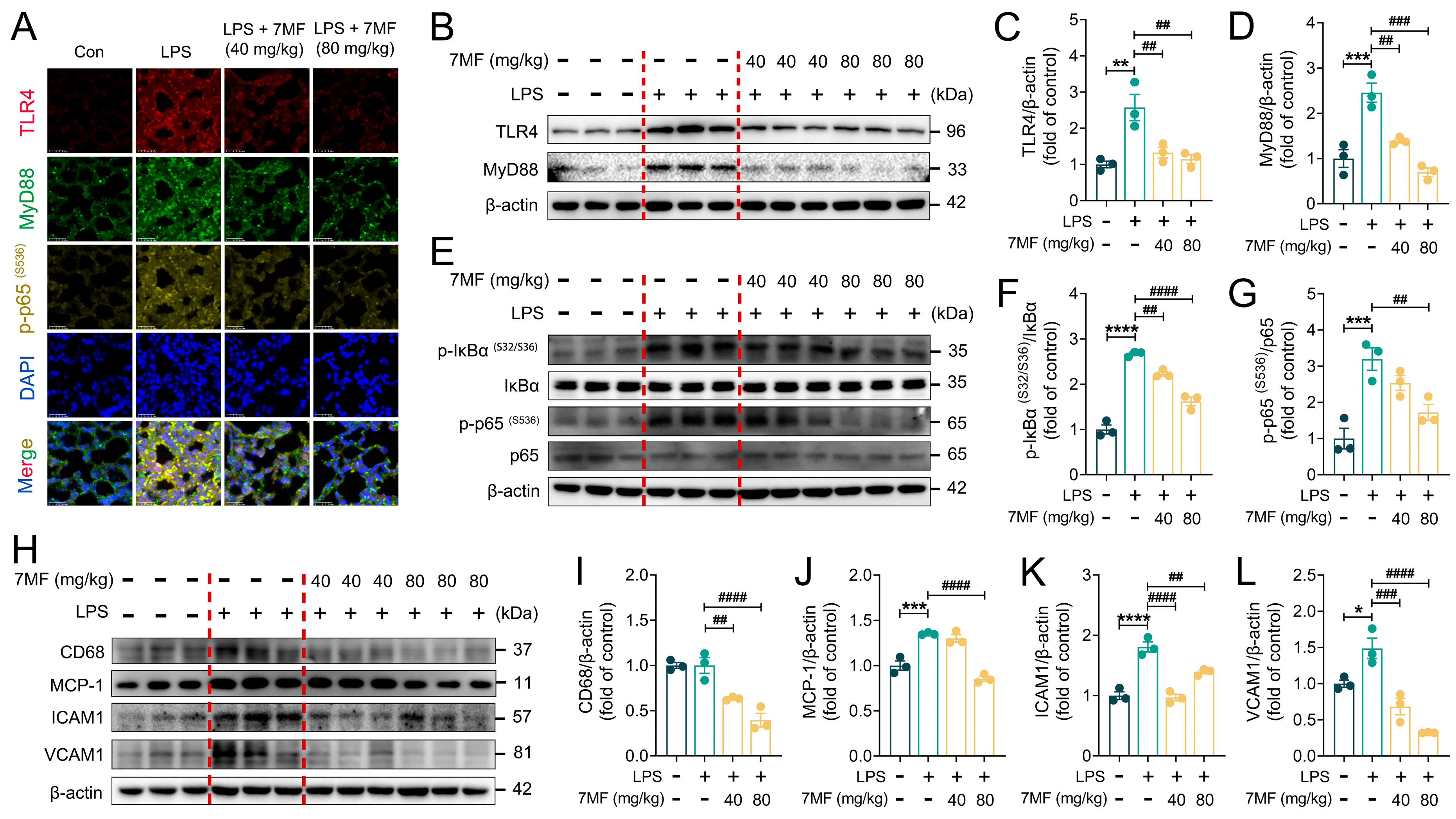
3.6. 7MF Inhibited TLR4/NF-κB p65 Signaling-Mediated Inflammatory Injury in ALI Mice
3.7. 7MF Suppressed Txnip/NLRP3 Signaling-Mediated Pyroptosis in ALI Mice
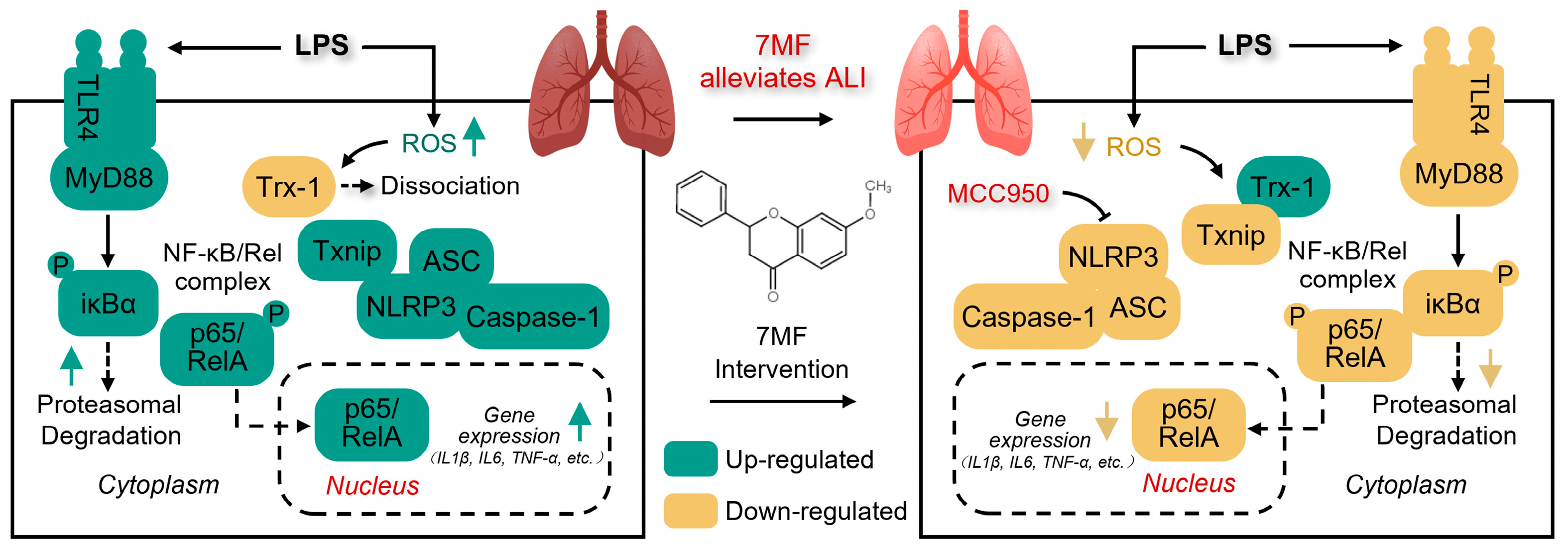
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | Acute lung injury |
| BALF | Bronchoalveolar lavage fluid |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ELISA | Enzyme-linked immunosorbent assay |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| HRP | Horseradish peroxidase |
| ICAM1 | Intercellular cell adhesion molecule-1 |
| LPS | Lipopolysaccharide |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitrogen oxide |
| PAGE | Polyacrylamide gel electrophoresis |
| PMSF | Phenylmethylsulfonyl fluoride |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulfate |
| SEM | Standard error of mean |
| SOD | Superoxide dismutase |
| TCM | Traditional Chinese medicine |
| TLR4 | Toll-like receptor 4 |
| Trx-1 | Thioredoxin-1 |
| Txnip | Thioredoxin-interacting protein |
| 7MF | Methoxyflavanone |
References
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Budinger, G.R. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2006, 354, 416–417. [Google Scholar] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Wang, R.; Shen, Y.F.; Zhao, J.L.; Zou, Y.S.; Wang, G.Z. Biomarkers, signaling pathways, and programmed cell death in acute lung injury and its treatment with Traditional Chinese Medicine: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 10157–10170. [Google Scholar]
- Spadaro, S.; Park, M.; Turrini, C.; Tunstall, T.; Thwaites, R.; Mauri, T.; Ragazzi, R.; Ruggeri, P.; Hansel, T.T.; Caramori, G.; et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. 2019, 16, 1. [Google Scholar] [CrossRef]
- Millar, M.W.; Fazal, F.; Rahman, A. Therapeutic Targeting of NF-κB in Acute Lung Injury: A Double-Edged Sword. Cells 2022, 11, 3317. [Google Scholar] [CrossRef]
- Lv, H.M.; Liu, Q.M.; Wen, Z.M.; Feng, H.H.; Deng, X.M.; Ci, X.X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Xu, Z.B.; Li, J.Y.; Zhou, K.L.; Wang, K.Y.; Hu, H.Y.; Hu, Y.J.; Gao, Y.; Luo, Z.H.; Huang, J.W. Exocarpium Citri Grandis ameliorates LPS-induced acute lung injury by suppressing inflammation, NLRP3 inflammasome, and ferroptosis. J. Ethnopharmacol. 2024, 329, 118162. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Choi, E.H.; Park, S.J. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp. Mol. Med. 2023, 55, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Grailer, J.J.; Canning, B.A.; Kalbitz, M.; Haggadone, M.D.; Dhond, R.M.; Andjelkovic, A.V.; Zetoune, F.S.; Ward, P.A. Critical role for the NLRP3 inflammasome during acute lung injury. J. Immunol. 2014, 192, 5974–5983. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhou, C.C.; Yu, L.Y.; Wang, L.; Deng, J.L.; Tao, Y.L.; Zhang, F.; Chen, W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2020, 163, 105224. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, W.L.; Zhang, H.R.; Liang, N.; Chen, D.N.; Li, J.W.; Ding, W.; He, Z.Z.; Yuan, Y.L.; Chu, C.; et al. Plant-derived natural compounds for the treatment of acute lung injury: A systematic review of their anti-inflammatory effects in animal models. Int. Immunopharmacol. 2025, 146, 113807. [Google Scholar] [CrossRef]
- Qu, Z.; Chen, Y.; Luo, Z.H.; Shen, X.L.; Hu, Y.J. 7-methoxyflavanone alleviates neuroinflammation in lipopolysaccharide-stimulated microglial cells by inhibiting TLR4/MyD88/MAPK signalling and activating the Nrf2/NQO-1 pathway. J. Pharm. Pharmacol. 2020, 72, 385–395. [Google Scholar] [CrossRef]
- Chen, C. COX-2’s new role in inflammation. Nat. Chem. Biol. 2000, 6, 401–402. [Google Scholar] [CrossRef]
- Kröncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Xu, Z.B.; Wang, K.Y.; Hu, H.Y.; Hu, Y.J.; Huang, J.W.; Luo, Z.H. Sinensetin attenuates LPS-induced acute pulmonary inflammation in mice and RAW264.7 cells by modulating NF-κB p65-mediated immune resistance and STAT3-mediated tissue resilience. Int. Immunopharmacol. 2025, 148, 114101. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Smith, K.M.; Mrozek, J.D.; Simonton, S.C.; Bing, D.R.; Meyers, P.A.; Connett, J.E.; Mammel, M.C. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: Gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit. Care Med. 1997, 25, 1888–1897. [Google Scholar] [CrossRef]
- Deng, J.C.; Standiford, T.J. Growth factors and cytokines in acute lung injury. Compr. Physiol. 2011, 1, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.B.; Hu, H.Y.; Wang, K.Y.; Zhou, Z.Y.; He, X.Q.; Huang, X.A.; Hu, Y.J.; Huang, J.W.; Luo, Z.H. Sinensetin, a polymethoxyflavone from citrus fruits, ameliorates LPS-induced acute lung injury by suppressing Txnip/NLRP3/Caspase-1/GSDMD signaling-mediated inflammatory responses and pyroptosis. Food Funct. 2024, 15, 7592–7604. [Google Scholar] [CrossRef]
- Luster, A.D. Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998, 338, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379-399. [Google Scholar] [CrossRef]
- de Martin, R.; Vanhove, B.; Cheng, Q.; Hofer, E.; Csizmadia, V.; Winkler, H.; Bach, F.H. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J. 1993, 12, 2773–2779. [Google Scholar] [CrossRef]
- Fan, W.N.; Tang, Y.Y.; Liu, Y.M.; Ran, Y.; Pan, G.R.; Song, X.; Mai, L.; Jiang, X.; Chen, D.; Song, F.Z.; et al. ROS-responsive nanoparticles for bioimaging and treating acute lung injury by releasing dexamethasone and improving alveolar macrophage homeostasis. J. Nanobiotechnol. 2024, 22, 729. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.E.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, C.; Russo, H.M.; Sanadi, C.E.; Martin, B.N.; Li, X.X.; Kaiser, W.J.; Mocarski, E.S.; Dubyak, G.R. Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling. J. Biol. Chem. 2015, 290, 20167–20184. [Google Scholar] [CrossRef]
- He, Y.; Zeng, M.Y.; Yang, D.H.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhao, Y.; Wang, K.; Shi, X.Y.; Wang, Y.; Huang, H.W.; Zhuang, Y.H.; Cai, T.; Wang, F.C.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.B.; Ruan, J.B.; Pan, Y.D.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Sborgi, L.; Rühl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Müller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- Wang, Y.; Sharma, P.; Jefferson, M.; Zhang, W.; Bone, B.; Kipar, A.; Bitto, D.; Coombes, J.L.; Pearson, T.; Man, A.; et al. Non-canonical autophagy functions of ATG16L1 in epithelial cells limit lethal infection by influenza A virus. EMBO J. 2021, 40, e105543. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, L.; Ramírez-Suástegui, C.; Strobl, D.C.; Gillett, T.E.; Zappia, L.; Madissoon, E.; Markov, N.S.; Zaragosi, L.E.; Ji, Y.; Ansari, M.; et al. An integrated cell atlas of the lung in health and disease. Nat. Med. 2023, 29, 1563–1577. [Google Scholar] [CrossRef]
- Shenoy, A.T.; Lyon De Ana, C.; Arafa, E.I.; Salwig, I.; Barker, K.A.; Korkmaz, F.T.; Ramanujan, A.; Etesami, N.S.; Soucy, A.M.; Martin, I.M.C.; et al. Antigen presentation by lung epithelial cells directs CD4 T cell function and regulates barrier immunity. Nat. Commun. 2021, 12, 5834. [Google Scholar] [CrossRef]
- Cong, B.; Dong, X.; Yang, Z.; Yu, P.; Chai, Y.; Liu, J.; Zhang, M.; Zang, Y.; Kang, J.; Feng, Y.; et al. Single-cell spatiotemporal analysis reveals alveolar dendritic cell-T cell immunity hubs defending against pulmonary infection. Cell Discov. 2024, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lyu, C.; Lai, D.; Shu, Q. Advances on physiology and pathology of subpopulations of macrophages in the lung tissue. Zhejiang Da Xue Xue Bao Yi Xue Ban 2024, 53, 650–658. [Google Scholar] [CrossRef]
- Song, J.Q.; Shen, L.J.; Wang, H.J.; Liu, Q.B.; Ye, L.B.; Liu, K.; Shi, L.; Cai, B.; Lin, H.S.; Pang, T. Discovery of Balasubramide Derivative with Tissue-Specific Anti-Inflammatory Activity Against Acute Lung Injury by Targeting VDAC1. Adv. Sci. 2024, 11, e2410550. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Dong, K.; Dai, J.; Zhang, A.; Chen, P.; Chen, Z.; Guo, M.; Sun, C.; Yang, J.; Huang, N.; et al. Discovery of c-Kit as a New Therapeutic Target in LPS-Induced Acute Lung Injury through Novel Phenylamide Derivative D9. J. Med. Chem. 2025, 68, 7499–7517. [Google Scholar] [CrossRef]
- Li, B.; Xia, C.; He, W.; Liu, J.; Duan, R.; Ji, Z.; Pan, X.; Zhou, Y.; Yu, G.; Wang, L. The Thyroid Hormone Analog GC-1 Mitigates Acute Lung Injury by Inhibiting M1 Macrophage Polarization. Adv. Sci. 2024, 11, e2401931. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, M.; Han, H. Mycoplasma pneumoniaeInteraction between alveolar macrophages and epithelial cells during infection. Front. Cell Infect. Microbiol. 2023, 13, 1052020. [Google Scholar] [CrossRef]
- Gao, C.L.; Song, J.Q.; Yang, Z.N.; Wang, H.; Wu, X.Y.; Shao, C.; Dai, H.X.; Chen, K.; Guo, Y.W.; Pang, T.; et al. Chemoproteomics of Marine Natural Product Naamidine J Unveils CSE1L as a Therapeutic Target in Acute Lung Injury. J. Am. Chem. Soc. 2024, 146, 28384–28397. [Google Scholar] [CrossRef]
- Gong, T.; Fu, Y.; Wang, Q.; Loughran, P.A.; Li, Y.; Billiar, T.R.; Wen, Z.; Liu, Y.; Fan, J. Decoding the multiple functions of ZBP1 in the mechanism of sepsis-induced acute lung injury. Commun. Biol. 2024, 7, 1361. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Hu, H.; Xu, Z.; Chen, Y.; Qiu, Y.; Hu, Y.; Huang, J.; Luo, Z. 7-Methoxyflavanone Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR4/NF-κB p65 and ROS/Txnip/NLRP3 Signaling. Biology 2025, 14, 1170. https://doi.org/10.3390/biology14091170
Wang K, Hu H, Xu Z, Chen Y, Qiu Y, Hu Y, Huang J, Luo Z. 7-Methoxyflavanone Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR4/NF-κB p65 and ROS/Txnip/NLRP3 Signaling. Biology. 2025; 14(9):1170. https://doi.org/10.3390/biology14091170
Chicago/Turabian StyleWang, Kongyan, Huiyu Hu, Zaibin Xu, Yan Chen, Yi Qiu, Yingjie Hu, Jiawen Huang, and Zhuohui Luo. 2025. "7-Methoxyflavanone Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR4/NF-κB p65 and ROS/Txnip/NLRP3 Signaling" Biology 14, no. 9: 1170. https://doi.org/10.3390/biology14091170
APA StyleWang, K., Hu, H., Xu, Z., Chen, Y., Qiu, Y., Hu, Y., Huang, J., & Luo, Z. (2025). 7-Methoxyflavanone Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR4/NF-κB p65 and ROS/Txnip/NLRP3 Signaling. Biology, 14(9), 1170. https://doi.org/10.3390/biology14091170





