From Lab to Clinic and Farm: Leveraging Drosophila Feeding Studies to Combat Eating Disorders and Pest Challenges
Simple Summary
Abstract
1. Introduction
2. Feeding Research in Drosophila
2.1. The Feeding Apparatus
2.2. Drosophila Feeding Assays
3. Drosophila Feeding Research and Applications in Human Health
3.1. Human Eating Behaviour Dysregulation
3.1.1. Fly Feeding Research and Energy Homeostasis/Metabolic Disorders
3.1.2. Fly Feeding Research and Psychological Eating Disorders
3.1.3. Limitation of Drosophila as a Model for Human Eating Dysregulation
3.2. Infectious Diseases and Medical Pests
3.2.1. Elucidating the Feeding Behaviour of Hematophagous Insects
3.2.2. Development of Insect Repellent and Control Agents
4. Drosophila Feeding Research Applications for Agriculture and Food Production
4.1. Economic Loss Caused by Agricultural Pests
4.2. Drosophila as a Model for Pest Behaviour and Feeding Regulation
4.3. Drosophila as a Model for Beneficial Insects and Pollinator Protection
4.4. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wirtz, R.A.; Andre, R.G.; Das, Y.T. Insect Models of Biomedical Research. In Non-Mammalian Animal Models for Biomedical Research; CRC Press Inc.: Boca Raton, FL, USA, 1989. [Google Scholar]
- Mahishi, D.; Huetteroth, W. The Prandial Process in Flies. Curr. Opin. Insect Sci. 2019, 36, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Benton, R. Drosophila Olfaction: Past, Present and Future. Proc. R. Soc. B 2022, 289, 2054. [Google Scholar] [CrossRef] [PubMed]
- Thoma, V.; Kobayashi, K.; Tanimoto, H. The Role of the Gustatory System in the Coordination of Feeding. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, O.; Bohra, A.A.; Liu, X.; Reichert, H.; Vijayraghavan, K.; Pielage, J. Motor Control of Drosophila Feeding Behavior. eLife 2017, 6, e19892. [Google Scholar] [CrossRef] [PubMed]
- Itskov, P.M.; Ribeiro, C. The Dilemmas of the Gourmet Fly: The Molecular and Neuronal Mechanisms of Feeding and Nutrient Decision Making in Drosophila. Front. Neurosci. 2013, 7, 24088. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Senapati, B.; Tsao, C.H. Neural Basis of Hunger-Driven Behaviour in Drosophila. Open Biol. 2019, 9, 180259. [Google Scholar] [CrossRef] [PubMed]
- Pool, A.H.; Scott, K. Feeding Regulation in Drosophila. Curr. Opin. Neurobiol. 2014, 29, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Wang, L.-M. Recent Advances in the Neural Regulation of Feeding Behavior in Adult Drosophila. J. Zhejiang Univ. Sci. B 2019, 20, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Edgecomb, R.S.; Harth, C.E.; Schneiderman, A.M. Regulation of Feeding Behavior in Adult Drosophila Melanogaster Varies with Feeding Regime and Nutritional State. J. Exp. Biol. 1994, 197, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.B.; Kapahi, P.; Benzer, S. Compensatory Ingestion upon Dietary Restriction in Drosophila Melanogaster. Nat. Methods 2005, 2, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Itskov, P.M.; Moreira, J.M.; Vinnik, E.; Lopes, G.; Safarik, S.; Dickinson, M.H.; Ribeiro, C. Automated Monitoring and Quantitative Analysis of Feeding Behaviour in Drosophila. Nat. Commun. 2014, 5, 4560. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhan, Y.; Zhu, Y. Combining Quantitative Food-Intake Assays and Forcibly Activating Neurons to Study Appetite in Drosophila. J. Vis. Exp. 2018, 2018, e56900. [Google Scholar] [CrossRef] [PubMed]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; De La Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE Assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef] [PubMed]
- Sellier, M.J.; Reeb, P.; Marion-Poll, F. Consumption of Bitter Alkaloids in Drosophila Melanogaster in Multiple-Choice Test Conditions. Chem. Senses 2011, 36, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Yang, Z.; Lin, Z.; Park, J.Y.; Suh, G.S.B.; Wang, L. A Quantitative Feeding Assay in Adult Drosophila Reveals Rapid Modulation of Food Ingestion by Its Nutritional Value. Mol. Brain 2015, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Segu, A.; Radhakrishnan, D.; Kannan, N.N. Modified Capillary Feeder Assay Using Micro-Tips to Measure Real-Time Feeding in Drosophila. STAR Protoc. 2023, 4, 102393. [Google Scholar] [CrossRef] [PubMed]
- Yapici, N.; Cohn, R.; Schusterreiter, C.; Ruta, V.; Vosshall, L.B. A Taste Circuit That Regulates Ingestion by Integrating Food and Hunger Signals. Cell 2016, 165, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Dhakal, S.; Shrestha, B.; Nath, D.K.; Kim, Y.; Ganguly, A.; Montell, C.; Lee, Y. A Single Pair of Pharyngeal Neurons Functions as a Commander to Reject High Salt in Drosophila Melanogaster. eLife 2023, 12, e93464. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.D.; Hatfield, J.S.; Baker, B.B.; Mackay, T.F.C.; Anholt, R.R.H. A High Throughput Microplate Feeder Assay for Quantification of Consumption in Drosophila. J. Vis. Exp. 2021, 172. [Google Scholar] [CrossRef] [PubMed]
- Thakare, M.R.; Choudhary, P.; Pydah, B.; Sunke, S.; Yadav, R.S.P.; Agrawal, P.; Das, G. DIETS: A Simple and Sensitive Assay to Measure and Control the Intake of Complex Solid Foods, like High-Fat Diets, in Drosophila. eLife 2024, 13, RP97706. [Google Scholar] [CrossRef]
- Mair, W.; Piper, M.D.W.; Partridge, L. Calories Do Not Explain Extension of Life Span by Dietary Restriction in Drosophila. PLoS Biol. 2005, 3, e223. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Piper, M.D.W.; Wertheim, B.; Partridge, L. Quantification of Food Intake in Drosophila. PLoS ONE 2009, 4, e6063. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, G.; Park, S.J.; Gao, Y.; Ja, W.W.; Yang, M. Excreta Quantification (EX-Q) for Longitudinal Measurements of Food Intake in Drosophila. iScience 2020, 23, 100776. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.-M.; Itskov, P.M.; Goldschmidt, D.; Baltazar, C.; Steck, K.; Tastekin, I.; Walker, S.J.; Ribeiro, C. OptoPAD, a Closed-Loop Optogenetics System to Study the Circuit Basis of Feeding Behaviors. eLife 2019, 8, e43924. [Google Scholar] [CrossRef] [PubMed]
- Musso, P.Y.; Junca, P.; Jelen, M.; Feldman-Kiss, D.; Zhang, H.; Chan, R.C.W.; Gordon, M.D. Closed-Loop Optogenetic Activation of Peripheral or Central Neurons Modulates Feeding in Freely Moving Drosophila. eLife 2019, 8, e45636. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.; Harvanek, Z.M.; Pletcher, S.D. FLIC: High-Throughput, Continuous Analysis of Feeding Behaviors in Drosophila. PLoS ONE 2014, 9, e101107. [Google Scholar] [CrossRef] [PubMed]
- May, C.E.; Vaziri, A.; Lin, Y.Q.; Grushko, O.; Khabiri, M.; Wang, Q.P.; Holme, K.J.; Pletcher, S.D.; Freddolino, P.L.; Neely, G.G.; et al. High Dietary Sugar Reshapes Sweet Taste to Promote Feeding Behavior in Drosophila Melanogaster. Cell Rep. 2019, 27, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. Metabolic and Hedonic Drives in the Neural Control of Appetite: Who Is the Boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Zheng, H.; Berthoud, H.R. An Expanded View of Energy Homeostasis: Neural Integration of Metabolic, Cognitive, and Emotional Drives to Eat. Physiol. Behav. 2009, 97, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Elmquist, J.K.; Coppari, R.; Balthasar, N.; Ichinose, M.; Lowell, B.B. Identifying Hypothalamic Pathways Controlling Food Intake, Body Weight, and Glucose Homeostasis. J. Comp. Neurol. 2005, 493, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.; White, M.; Potenza, M. Binge Eating Disorder and Food Addiction. Curr. Drug Abuse Rev. 2011, 4, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.J.; Morris, M.J. The Role of Reward Circuitry and Food Addiction in the Obesity Epidemic: An Update. Biol. Psychol. 2018, 131, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Ho, C.Y.; Richard, J.M.; Difeliceantonio, A.G. The Tempted Brain Eats: Pleasure and Desire Circuits in Obesity and Eating Disorders. Brain Res. 2010, 1350, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W. Altered Brain Reward Circuits in Eating Disorders: Chicken or Egg? Curr. Psychiatry Rep. 2013, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Hardaway, J.A.; Crowley, N.A.; Bulik, C.M.; Kash, T.L. Integrated Circuits and Molecular Components for Stress and Feeding: Implications for Eating Disorders. Genes Brain Behav. 2015, 14, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of Food Intake in Health and Disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425541. [Google Scholar]
- Casper, R.C. Depression and Eating Disorders. Depress. Anxiety 1998, 8, 96–104. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Bulik, C.M.; Thornton, L.; Barbarich, N.; Masters, K. Comorbidity of Anxiety Disorders with Anorexia and Bulimia Nervosa. Am. J. Psychiatry 2004, 161, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, E.; Perrimon, N. Modeling Metabolic Homeostasis and Nutrient Sensing in Drosophila: Implications for Aging and Metabolic Diseases. DMM Dis. Models Mech. 2014, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Vatashchuk, M.V.; Bayliak, M.M.; Hurza, V.V.; Storey, K.B.; Lushchak, V.I. Metabolic Syndrome: Lessons from Rodent and Drosophila Models. BioMed Res. Int. 2022, 2022, 5850507. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Wagner, A.E. Drosophila Melanogaster as a Model Organism for Obesity and Type-2 Diabetes Mellitus by Applying High-Sugar and High-Fat Diets. Biomolecules 2022, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.V.; Tekin, I.; Reis, T. Drosophila as a Genetic Model System to Study Organismal Energy Metabolism. Biomolecules 2025, 15, 652. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.P.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Hathiramani, S.S.; Cagan, R.L.; Baranski, T.J. A High-Sugar Diet Produces Obesity and Insulin Resistance in Wild-Type Drosophila. DMM Dis. Models Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-Fat-Diet-Induced Obesity and Heart Dysfunction Are Regulated by the TOR Pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, K.J.; Kierdorf, K.; Pouchelon, C.A.; Vivancos, V.; Dionne, M.S.; Geissmann, F. Macrophage-Derived Upd3 Cytokine Causes Impaired Glucose Homeostasis and Reduced Lifespan in Drosophila Fed a Lipid-Rich Diet. Immunity 2015, 42, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Kang, M.; Lee, K.S.; Yu, K. High Fat Diet-Induced TGF-β/Gbb Signaling Provokes Insulin Resistance through the Tribbles Expression. Sci. Rep. 2016, 6, 30265. [Google Scholar] [CrossRef] [PubMed]
- Tiefenböck, S.K.; Baltzer, C.; Egli, N.A.; Frei, C. The Drosophila PGC-1 Homologue Spargel Coordinates Mitochondrial Activity to Insulin Signalling. EMBO J. 2010, 29, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Diop, S.B.; Bisharat-Kernizan, J.; Birse, R.T.; Oldham, S.; Ocorr, K.; Bodmer, R. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 2015, 10, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Hull-Thompson, J.; Muffat, J.; Sanchez, D.; Walker, D.W.; Benzer, S.; Ganfornina, M.D.; Jasper, H. Control of Metabolic Homeostasis by Stress Signaling Is Mediated by the Lipocalin NLaz. PLoS Genet. 2009, 5, e1000460. [Google Scholar] [CrossRef] [PubMed]
- Pasco, M.Y.; Léopold, P. High Sugar-Induced Insulin Resistance in Drosophila Relies on the Lipocalin Neural Lazarillo. PLoS ONE 2012, 7, e36583. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Musselman, L.P.; Pendse, J.; Baranski, T.J.; Bodmer, R.; Ocorr, K.; Cagan, R. A Drosophila Model of High Sugar Diet-Induced Cardiomyopathy. PLoS Genet. 2013, 9, e1003175. [Google Scholar] [CrossRef] [PubMed]
- van Dam, E.; van Leeuwen, L.A.G.; dos Santos, E.; James, J.; Best, L.; Lennicke, C.; Vincent, A.J.; Marinos, G.; Foley, A.; Buricova, M.; et al. Sugar-Induced Obesity and Insulin Resistance Are Uncoupled from Shortened Survival in Drosophila. Cell Metab. 2020, 31, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Pospisilik, J.A.; Schramek, D.; Schnidar, H.; Cronin, S.J.F.; Nehme, N.T.; Zhang, X.; Knauf, C.; Cani, P.D.; Aumayr, K.; Todoric, J.; et al. Drosophila Genome-Wide Obesity Screen Reveals Hedgehog as a Determinant of Brown versus White Adipose Cell Fate. Cell 2010, 140, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, J.; Meyer, C.; Neschen, S.; Augustin, R.; Schmolz, K.; Kluge, R.; Al-Hasani, H.; Jürgens, H.; Eulenberg, K.; Wehr, R.; et al. Ablation of the Cholesterol Transporter Adenosine Triphosphate-Binding Cassette Transporter G1 Reduces Adipose Cell Size and Protects against Diet-Induced Obesity. Endocrinology 2007, 148, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.; Tung, Y.C.L.; Liu, C.H.; O’Kane, C.J.; O’Rahilly, S.; Yeo, G.S.H. A Multicomponent Screen for Feeding Behaviour and Nutritional Status in Drosophila to Interrogate Mammalian Appetite-Related Genes. Mol. Metab. 2021, 43, 101127. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Dhakal, S.; Lee, Y. Cucurbitacin b Suppresses Hyperglycemia Associated with a High Sugar Diet and Promotes Sleep in Drosophila Melanogaster. Mol. Cells 2021, 44, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, W.; Wu, Q.; Zhang, Y.; Tong, H. Fucoidan Alleviates High Sucrose-Induced Metabolic Disorders and Enhances Intestinal Homeostasis through Modulation of Notch Signaling. J. Adv. Res. 2025, 71, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ortiz, C.; Gracia-Rodriguez, C.; Belcher, S.; Flores-Iga, G.; Das, A.; Nimmakayala, P.; Balagurusamy, N.; Reddy, U.K. Drosophila Melanogaster as a Translational Model System to Explore the Impact of Phytochemicals on Human Health. Int. J. Mol. Sci. 2023, 24, 13365. [Google Scholar] [CrossRef] [PubMed]
- Men, T.T.; Van Thanh, D.N.; Yamaguchi, M.; Suzuki, T.; Hattori, G.; Arii, M.; Huy, N.T.; Kamei, K. A Drosophila Model for Screening Antiobesity Agents. BioMed Res. Int. 2016, 2016, 6293163. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, A.; Sahoo, K.; Sahoo, B.; Choudhury, A.; Sufi, N.; Kumar, R. Childhood Obesity: Causes and Consequences. J. Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy Progress on Obesity Prevention: Emerging Examples, Entrenched Barriers, and New Thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating Disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Guarda, A.S. Treatment of Anorexia Nervosa: Insights and Obstacles. Physiol. Behav. 2008, 94, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Treasure, J. Psychopharmacological Advances in Eating Disorders. Expert Rev. Clin. Pharmacol. 2018, 11, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Yellman, C.; Tao, H.; He, B.; Hirsh, J. Conserved and Sexually Dimorphic Behavioral Responses to Biogenic Amines in Decapitated Drosophila. Proc. Natl. Acad. Sci. USA 1997, 94, 4131–4136. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.S.; Schneider, D.S. The Role of Anorexia in Resistance and Tolerance to Infections in Drosophila. PLoS Biol. 2009, 7, e1000150. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.R.; Rupassara, S.I.; Markelz, R.J.C.; Leakey, A.D.B.; Muir, W.M.; Pittendrigh, B.R. Methamphetamine Causes Anorexia in Drosophila Melanogaster, Exhausting Metabolic Reserves and Contributing to Mortality. J. Toxicol. Sci. 2012, 37, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Ryuda, M.; Tsuzuki, S.; Matsumoto, H.; Oda, Y.; Tanimura, T.; Hayakawa, Y. Identification of a Novel Gene, Anorexia, Regulating Feeding Activity via Insulin Signaling in Drosophila Melanogaster. J. Biol. Chem. 2011, 286, 38417–38426. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.P.; Liu, L.; Zhu, Y. Taotie Neurons Regulate Appetite in Drosophila. Nat. Commun. 2016, 7, 13633. [Google Scholar] [CrossRef] [PubMed]
- Moulin, T.C.; Ferro, F.; Berkins, S.; Hoyer, A.; Williams, M.J.; Schiöth, H.B. Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila Melanogaster Adults. Brain Sci. 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Pool, A.H.; Kvello, P.; Mann, K.; Cheung, S.K.; Gordon, M.D.; Wang, L.; Scott, K. Four GABAergic Interneurons Impose Feeding Restraint in Drosophila. Neuron 2014, 83, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Alberti, L.; Gilardini, A.; Girola, A.; Moro, M.; Cavagnini, F.; Invitti, L. Adiponectin Receptors Gene Expression in Lymphocytes of Obese and Anorexic Patients. Diabetes Obes. Metab. 2007, 9, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Karlik, A.; Koran, E.; Maceluch, J.; Niedziela, M. Molecular Analysis of the Corticotropin-Releasing Hormone Receptor Type 2 Gene Fragment in Anorexia Nervosa. Arch. Psychiatry Psychother. 2009, 11, 75–80. [Google Scholar]
- Pinheiro, A.P.; Bulik, C.M.; Thornton, L.M.; Sullivan, P.F.; Root, T.L.; Bloss, C.S.; Berrettini, W.H.; Schork, N.J.; Kaye, W.H.; Bergen, A.W.; et al. Association Study of 182 Candidate Genes in Anorexia Nervosa. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.D.; Dahanukar, A. DH44 Neurons: Gut-Brain Amino Acid Sensors. Cell Res. 2018, 28, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Dus, M.; Lai, J.S.Y.; Gunapala, K.M.; Min, S.; Tayler, T.D.; Hergarden, A.C.; Geraud, E.; Joseph, C.M.; Suh, G.S.B. Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron 2015, 87, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Hadano, S.; Ueda, M.T.; Nakagawa, S.; Komaki, G.; Ando, T. Rare CRHR2 and GRM8 Variants Identified as Candidate Factors Associated with Eating Disorders in Japanese Patients by Whole Exome Sequencing. Heliyon 2024, 10, e28643. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Woo, J.M.; Heo, S.Y.; Kim, J.H.; Lim, S.J.; Yu, B.H. An Association Study of the A218C Polymorphism of the Tryptiophan Hydroxylase I Gene with Eating Disorders in a Korean Population: A Pilot Study. Psychiatry Investig. 2009, 6, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Strumila, R.; Lengvenyte, A.; Guillaume, S.; Courtet, P. Effects of Polymorphisms of COMT, TPH1 and SERT Gene Mutations on Perceived Childhood Trauma in Suicide Attempters with Eating Disorders. Neurosci. Appl. 2022, 1, 100751. [Google Scholar] [CrossRef]
- Neckameyer, W.S.; Coleman, C.M.; Eadie, S.; Goodwin, S.F. Compartmentalization of Neuronal and Peripheral Serotonin Synthesis in Drosophila Melanogaster. Genes Brain Behav. 2007, 6, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Banu, A.; Gowda, S.B.M.; Salim, S.; Mohammad, F. Serotonergic Control of Feeding Microstructure in Drosophila. Front. Behav. Neurosci. 2023, 16, 1105579. [Google Scholar] [CrossRef] [PubMed]
- Schaper, S.J.; Wölk, E.; Hofmann, T.; Friedrich, T.; Römer, M.; de Punder, K.; Rose, M.; Stengel, A. NUCB2/Nesfatin-1 in the Acute Stress Response of Obese Women with High and Low Anxiety. Psychoneuroendocrinology 2023, 155, 106325. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, K.; Asakawa, A.; Amitani, H.; Nakahara, T.; Ushikai, M.; Haruta, I.; Koyama, K.I.; Amitani, M.; Harada, T.; Yasuhara, D.; et al. Plasma Nesfatin-1 Concentrations in Restricting-Type Anorexia Nervosa. Peptides 2011, 32, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Vitolo, M.R.; Campagnolo, P.D.B.; Mattevi, V.S.; Genro, J.P.; Almeida, S. DRD4 and SLC6A3 Gene Polymorphisms Are Associated with Food Intake and Nutritional Status in Children in Early Stages of Development. J. Nutr. Biochem. 2015, 26, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Frieling, H.; Rómer, K.D.; Scholz, S.; Mittelbach, F.; Wilhelm, J.; De Zwaan, M.; Jacoby, G.E.; Kornhuber, J.; Hillemacher, T.; Bleich, S. Epigenetic Dysregulation of Dopaminergic Genes in Eating Disorders. Int. J. Eat. Disord. 2010, 43, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hersrud, S.L.; Stoltenberg, S.F. Epistatic Interaction between COMT and DAT1 Genes on Eating Behavior: A Pilot Study. Eat. Behav. 2009, 10, 131–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ballinger, A.; McLoughlin, L.; Medbak, S.; Clark, M. Cholecystokinin Is a Satiety Hormone in Humans at Physiological Post-Prandial Plasma Concentrations. Clin. Sci. 1995, 89, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.H. Cholecystokinin and Satiety: Current Perspectives. Nutrition 2000, 16, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Fedina, T.Y.; Cummins, E.T.; Promislow, D.E.L.; Pletcher, S.D. The Neuropeptide Drosulfakinin Enhances Choosiness and Protects Males from the Aging Effects of Social Perception. Proc. Natl. Acad. Sci. USA 2023, 120, e2308305120. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, J.A.E.; Carlsson, M.A.; Nässel, D.R. Insulin-Producing Cells in the Drosophila Brain Also Express Satiety-Inducing Cholecystokinin-like Peptide, Drosulfakinin. Front. Endocrinol. 2012, 3, 109. [Google Scholar] [CrossRef] [PubMed]
- Strike, M.K.; Norris, S.; Kearney, S.; Norris, M.L. More than Just Milk: A Review of Prolactin’s Impact on the Treatment of Anorexia Nervosa. Eur. Eat. Disord. Rev. 2012, 20, e85–e90. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, P.; Monteleone, P.; D’Istria, M.; Fuschino, A.; Serino, I.; Maj, M. Aggressive Behavioral Characteristics and Endogenous Hormones in Women with Bulimia Nervosa. Neuropsychobiology 2000, 42, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Ha, J.; Shin, D.W.; Woo, H.Y.; Kim, K.H. Associations between the Serotonin-1A Receptor C(-1019)G Polymorphism and Disordered Eating Symptoms in Female Adolescents. J. Neural Transm. 2010, 117, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Bailer, U.F.; Frank, G.K.; Henry, S.E.; Price, J.C.; Meltzer, C.C.; Mathis, C.A.; Wagner, A.; Thornton, L.; Hoge, J.; Ziolko, S.K.; et al. Exaggerated 5-HT1A but Normal 5-HT2A Receptor Activity in Individuals Ill with Anorexia Nervosa. Biol. Psychiatry 2007, 61, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Bailer, U.F.; Frank, G.K.; Henry, S.E.; Price, J.C.; Meltzer, C.C.; Weissfeld, L.; Mathis, C.A.; Drevets, W.C.; Wagner, A.; Hoge, J.; et al. Altered Brain Serotonin 5-HT1A Receptor Binding after Recovery from Anorexia Nervosa Measured by Positron Emission Tomography and [Carbonyl11C]WAY-100635. Arch. Gen. Psychiatry 2005, 62, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Wierenga, C.E.; Bailer, U.F.; Simmons, A.N.; Bischoff-Grethe, A. Nothing Tastes as Good as Skinny Feels: The Neurobiology of Anorexia Nervosa. Trends Neurosci. 2013, 36, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Keski-Rahkonen, A.; Löppönen, M.; Muhonen, M.; Kajander, J.; Allonen, T.; Någren, K.; Hietala, J.; Rissanen, A. Brain Serotonin 1A Receptor Binding in Bulimia Nervosa. Biol. Psychiatry 2004, 55, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Bailer, U.F.; Bloss, C.S.; Frank, G.K.; Price, J.C.; Meltzer, C.C.; Mathis, C.A.; Geyer, M.A.; Wagner, A.; Becker, C.R.; Schork, N.J.; et al. 5-HT1A Receptor Binding Is Increased after Recovery from Bulimia Nervosa Compared to Control Women and Is Associated with Behavioral Inhibition in Both Groups. Int. J. Eat. Disord. 2011, 44, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Muñoz, S.; Camarena-Medellin, B. Role of Serotonin Transporter Gene in Eating Disorders. Rev. Colomb. Psiquiatr. 2014, 43, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Yokokura, M.; Terada, T.; Bunai, T.; Nakaizumi, K.; Kato, Y.; Yoshikawa, E.; Futatsubashi, M.; Suzuki, K.; Yamasue, H.; Ouchi, Y. Alterations in Serotonin Transporter and Body Image-Related Cognition in Anorexia Nervosa. Neuroimage Clin. 2019, 23, 101928. [Google Scholar] [CrossRef] [PubMed]
- Majuri, J.; Joutsa, J.; Johansson, J.; Voon, V.; Parkkola, R.; Alho, H.; Arponen, E.; Kaasinen, V. Serotonin Transporter Density in Binge Eating Disorder and Pathological Gambling: A PET Study with [11C]MADAM. Eur. Neuropsychopharmacol. 2017, 27, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Orecchini, E.; Porreca, A.; Mondanelli, G.; Orabona, C.; Dalla Ragione, L.; Di Nicola, M.; Stuppia, L.; Vitacolonna, E.; Beccari, T.; et al. SLC6A4 DNA Methylation Levels and Serum Kynurenine/Tryptophan Ratio in Eating Disorders: A Possible Link with Psychopathological Traits? Nutrients 2023, 15, 406. [Google Scholar] [CrossRef] [PubMed]
- Knapp, E.M.; Kaiser, A.; Arnold, R.C.; Sampson, M.M.; Ruppert, M.; Xu, L.; Anderson, M.I.; Bonanno, S.L.; Scholz, H.; Donlea, J.M.; et al. Mutation of the Drosophila Melanogaster Serotonin Transporter DSERT Impacts Sleep, Courtship, and Feeding Behaviors. PLoS Genet. 2022, 18, e1010289. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.; Rose, M.; Mönnikes, H.; Reutter, W.; Keller, W.; Klapp, B.F. Eating Disorders: A Role for Dipeptidyl Peptidase IV in Nutritional Control. Nutrition 2001, 17, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Fliege, H.; Rose, M.; Klapp, B.F.; Hildebrandt, M. DPP IV, Immune Parameters, and Psychometrical Variables in Patients with Eating Disorders. Adv. Exp. Med. Biol. 2003, 524, 313–320. [Google Scholar] [PubMed]
- Hildebrandt, M.; Rose, M.; Mayr, C.; Arck, P.; Schüler, C.; Reutter, W.; Salama, A.; Klapp, B.F. Dipeptidyl Peptidase IV (DPP IV, CD26) in Patients with Mental Eating Disorders. Adv. Exp. Med. Biol. 2000, 477, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Santini, F.; La Porta, D.; Cimino, S. Association of Serotonin Receptor Gene Polymorphisms with Anorexia Nervosa: A Systematic Review and Meta-Analysis. Eat. Weight Disord. 2024, 29, 31. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, L.; Accoto, A.; Couyoumdjian, A.; Conversi, D. A Systematic Review of Genetic Polymorphisms Associated with Binge Eating Disorder. Nutrients 2021, 13, 848. [Google Scholar] [CrossRef] [PubMed]
- Bergen, A.W.; Yeager, M.; Welch, R.A.; Haque, K.; Ganjei, J.K.; Van Den Bree, M.B.M.; Mazzanti, C.; Nardi, I.; Fichter, M.M.; Halmi, K.A.; et al. Association of Multiple DRD2 Polymorphisms with Anorexia Nervosa. Neuropsychopharmacology 2005, 30, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yu, S.; Wang, Q.; Kang, Q.; Zhang, Y.; Zhang, R.; Jiang, W.; Qian, Y.; Zhang, H.; Zhang, M.; et al. Dopamine Receptor D2 and Catechol-O-Methyltransferase Gene Polymorphisms Associated with Anorexia Nervosa in Chinese Han Population. DRD2 and COMT Gene Polymorphisms Were Associated with AN. Neurosci. Lett. 2016, 616, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.A.; Levitan, R.D.; Reid, C.; Carter, J.C.; Kaplan, A.S.; Patte, K.A.; King, N.; Curtis, C.; Kennedy, J.L. Dopamine for Wanting and Opioids for Liking: A Comparison of Obese Adults with and without Binge Eating. Obesity 2009, 17, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Brunani, A.; Borgomainerio, E.; Tonello, C.; Dioni, L.; Briscini, L.; Redaelli, G.; Molinari, E.; Cavagnini, F.; Carruba, M.O. D2 Dopamine Receptor (DRD2) Gene Taq1A Polymorphism and the Eating-Related Psychological Traits in Eating Disorders (Anorexia Nervosa and Bulimia) and Obesity. Eat. Weight Disord. 2007, 12, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Steiger, H.; Thaler, L.; Gauvin, L.; Joober, R.; Labbe, A.; Israel, M.; Kucer, A. Epistatic Interactions Involving DRD2, DRD4, and COMT Polymorphisms and Risk of Substance Abuse in Women with Binge-Purge Eating Disturbances. J. Psychiatr. Res. 2016, 77, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Groleau, P.; Joober, R.; Israel, M.; Zeramdini, N.; DeGuzman, R.; Steiger, H. Methylation of the Dopamine D2 Receptor (DRD2) Gene Promoter in Women with a Bulimia-Spectrum Disorder: Associations with Borderline Personality Disorder and Exposure to Childhood Abuse. J. Psychiatr. Res. 2014, 48, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Dey, M.; Scott, C.; Duong, V.K.; Dahanukar, A.A. Dietary Macronutrient Imbalances Lead to Compensatory Changes in Peripheral Taste via Independent Signaling Pathways. J. Neurosci. 2021, 41, 10222–10246. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Hernández, J.M.; Olivares, G.H.; Olguín, P.; Glavic, A. Low-Nutrient Diet in Drosophila Larvae Stage Causes Enhancement in Dopamine Modulation in Adult Brain Due Epigenetic Imprinting. Open Biol. 2023, 13, 230049. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.D.; De Ridder, D. Fatty Acid Amide Hydrolase: An Integrative Clinical Perspective. Cannabis Cannabinoid Res. 2023, 8, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Bifulco, M.; Di Filippo, C.; Gazzerro, P.; Canestrelli, B.; Monteleone, F.; Proto, M.C.; Di Genio, M.; Grimaldi, C.; Maj, M. Association of CNR1 and FAAH Endocannabinoid Gene Polymorphisms with Anorexia Nervosa and Bulimia Nervosa: Evidence for Synergistic Effects. Genes Brain Behav. 2009, 8, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Carsten, L.D.; Watts, T.; Markow, T.A. Gene Expression Patterns Accompanying a Dietary Shift in Drosophila Melanogaster. Mol. Ecol. 2005, 14, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Eickelberg, V.; Rimbach, G.; Seidler, Y.; Hasler, M.; Staats, S.; Lüersen, K. Fat Quality Impacts the Effect of a High-Fat Diet on the Fatty Acid Profile, Life History Traits and Gene Expression in Drosophila Melanogaster. Cells 2022, 11, 4043. [Google Scholar] [CrossRef] [PubMed]
- Wiers, R.W.; Rinck, M.; Dictus, M.; Van Den Wildenberg, E. Relatively Strong Automatic Appetitive Action-Tendencies in Male Carriers of the OPRM1 G-Allele. Genes Brain Behav. 2009, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.; Al-Sulaiti, A.; Nasser, E.A.A.; Najjar, H.; Kamareddine, L. Drosophila as a Model Organism in Host–Pathogen Interaction Studies. Front. Cell. Infect. Microbiol. 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Michael Harnish, J.; Link, N.; Yamamoto, S. Drosophila as a Model for Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 2724. [Google Scholar] [CrossRef] [PubMed]
- Panayidou, S.; Ioannidou, E.; Apidianakis, Y. Human Pathogenic Bacteria, Fungi, and Viruses in Drosophila. Virulence 2014, 5, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.J.; O’Neill, L.A.J. Of Flies and Men—The Discovery of TLRs. Cells 2022, 11, 3127. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.L. Overview of Drosophila Immunity: A Historical Perspective. Dev. Comp. Immunol. 2014, 42, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila Melanogaster-from Microbial Recognition to Whole-Organism Physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- van de Leemput, J.; Han, Z. Drosophila, a Powerful Model to Study Virus-Host Interactions and Pathogenicity in the Fight against SARS-CoV-2. Cell Biosci. 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. Using Drosophila as a Model Insect. Nat. Rev. Genet. 2000, 1, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.; Ontaro, D.; Wall, R.L. The Encyclopaedia of Medical and Veterinary Entomology; CABI: Wallingford, UK, 2013. [Google Scholar]
- World Health Organization. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Schneider, D.; Shahabuddin, M. Malaria Parasite Development in a Drosophila Model. Science 2000, 288, 2376–2379. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.M.; Jaramillo-Gutierrez, G.; Kumar, S.; Barillas-Mury, C.; Schneider, D.S. Use of a Drosophila Model to Identify Genes Regulating Plasmodium Growth in the Mosquito. Genetics 2008, 180, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Tonk, M.; Pierrot, C.; Cabezas-Cruz, A.; Rahnamaeian, M.; Khalife, J.; Vilcinskas, A. The Drosophila Melanogaster Antimicrobial Peptides Mtk-1 and Mtk-2 Are Active against the Malarial Parasite Plasmodium Falciparum. Parasitol. Res. 2019, 118, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Hjerrild, K.A.; Jin, J.; Wright, K.E.; Brown, R.E.; Marshall, J.M.; Labbé, G.M.; Silk, S.E.; Cherry, C.J.; Clemmensen, S.B.; Jørgensen, T.; et al. Production of Full-Length Soluble Plasmodium Falciparum RH5 Protein Vaccine Using a Drosophila Melanogaster Schneider 2 Stable Cell Line System. Sci. Rep. 2016, 6, 30357. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tarrant, R.D.; Bolam, E.J.; Angell-Manning, P.; Soegaard, M.; Pattinson, D.J.; Dulal, P.; Silk, S.E.; Marshall, J.M.; Dabbs, R.A.; et al. Production, Quality Control, Stability, and Potency of CGMP-Produced Plasmodium Falciparum RH5.1 Protein Vaccine Expressed in Drosophila S2 Cells. npj Vaccines 2018, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Dutra, H.L.C.; Caragata, E.P.; Moreira, L.A. The Re-Emerging Arboviral Threat: Hidden Enemies: The Emergence of Obscure Arboviral Diseases, and the Potential Use of Wolbachia in Their Control. BioEssays 2017, 39, 1600175. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.H.; Dittmar, M.; Gordesky-Gold, B.; Hofmann, J.; Cherry, S. Drosophila Melanogaster as a Model for Arbovirus Infection of Adult Salivary Glands. Virology 2020, 543, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, A.L.; Arnold, P.A.; Johnson, K.N. Wolbachia-Mediated Antiviral Protection in Drosophila Larvae and Adults Following Oral Infection. Appl. Environ. Microbiol. 2015, 81, 8215–8223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asselin, A.; Johnson, K. The Infectivity of Virus Particles from Wolbachia-Infected Drosophila. BMC Microbiol. 2025, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Takken, W. Feeding Strategies of Anthropophilic Mosquitoes Result in Increased Risk of Pathogen Transmission. Trends Parasitol. 2012, 28, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Cozens, D.W.; Ferdous, Z.; Armstrong, P.M.; Brackneyi, D.E. Increased Blood Meal Size and Feeding Frequency Compromise Aedes Aegypti Midgut Integrity and Enhance Dengue Virus Dissemination. PLoS Negl. Trop. Dis. 2023, 17, e0011703. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.N.; Darriet, F.; Chabrerie, O.; Lenoir, J.; Courchamp, F.; Claeys, C.; Robert, V.; Jourdain, F.; Ulmer, R.; Diagne, C.; et al. Invasive Hematophagous Arthropods and Associated Diseases in a Changing World. Parasites Vectors 2023, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Bursali, F.; Touray, M. The Complexities of Blood-Feeding Patterns in Mosquitoes and Sandflies and the Burden of Disease: A Minireview. Vet. Med. Sci. 2024, 10, e1580. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, A.C.; Blass, C.; Meister, S.; Schmidt, S.; MacCallum, R.M.; Soares, M.B.; Collins, F.H.; Benes, V.; Zdobnov, E.; Kafatos, F.C.; et al. Life Cycle Transcriptome of the Malaria Mosquito Anopheles Gambiae and Comparison with the Fruitfly Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 2007, 104, 11304–11309. [Google Scholar] [CrossRef] [PubMed]
- Behura, S.K.; Haugen, M.; Flannery, E.; Sarro, J.; Tessier, C.R.; Severson, D.W.; Duman-Scheel, M. Comparative Genomic Analysis of Drosophila Melanogaster and Vector Mosquito Developmental Genes. PLoS ONE 2011, 6, e21504. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.J.; Rund, S.S.C.; Reece, S.E. Time-of-Day of Blood-Feeding: Effects on Mosquito Life History and Malaria Transmission. Parasites Vectors 2019, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Hickner, P.V.; Mori, A.; Rund, S.S.C.; Severson, D.W. Quantitative Trait Locus Determining the Time of Blood Feeding in Culex Pipiens (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, L.F.; Hernández, B.J.; Guzmán, P.A.; Alfonso-Parra, C.; Avila, F.W. The Effects of Female Age on Blood-Feeding, Insemination, Sperm Storage, and Fertility in the Dengue Vector Mosquito Aedes Aegypti (Diptera: Culicidae). J. Insect Physiol. 2023, 150, 104570. [Google Scholar] [CrossRef] [PubMed]
- Valzania, L.; Mattee, M.T.; Strand, M.R.; Brown, M.R. Blood Feeding Activates the Vitellogenic Stage of Oogenesis in the Mosquito Aedes Aegypti through Inhibition of Glycogen Synthase Kinase 3 by the Insulin and TOR Pathways. Dev. Biol. 2019, 454, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Jaime, V.; Broderick, N.A.; Maya-Maldonado, K. Metal Ions in Insect Reproduction: A Crosstalk between Reproductive Physiology and Immunity. Curr. Opin. Insect Sci. 2022, 52, 100924. [Google Scholar] [CrossRef] [PubMed]
- Seay, D.J.; Thummel, C.S. The Circadian Clock, Light, and Cryptochrome Regulate Feeding and Metabolism in Drosophila. J. Biol. Rhythm. 2011, 26, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.F.; Wang, G.; Su, C.Y.; Zwiebel, L.J.; Carlson, J.R. Odorant Reception in the Malaria Mosquito Anopheles Gambiae. Nature 2010, 464, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Jové, V.; Gong, Z.; Hol, F.J.H.; Zhao, Z.; Sorrells, T.R.; Carroll, T.S.; Prakash, M.; McBride, C.S.; Vosshall, L.B. Sensory Discrimination of Blood and Floral Nectar by Aedes Aegypti Mosquitoes. Neuron 2020, 108, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo-Colmenares, K.; Cardo, M.V.; Vezzani, D. Blood Feeding Habits of Mosquitoes: Hardly a Bite in South America. Parasitol. Res. 2022, 121, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Henriques-Santos, B.M.; Xiong, C.; Pietrantonio, P.V. Automated Analysis of Feeding Behaviors of Females of the Mosquito Aedes Aegypti Using a Modified FlyPAD System. Sci. Rep. 2023, 13, 20188. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.; Lazzari, C.; Barrozo, R. Beyond Blood: The Flexibility of Triatomine Bug Food Search and Recognition. Curr. Opin. Insect Sci. 2025, 68, 101301. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Malele, I.; Abd-Alla, A.M.; Njiokou, F. Blood Feeding Tsetse Flies as Hosts and Vectors of Mammals-Pre-Adapted African Trypanosoma: Current and Expected Research Directions 06 Biological Sciences 0604 Genetics 06 Biological Sciences 0605 Microbiology. BMC Microbiol. 2018, 18, 162. [Google Scholar]
- Brackney, D.E.; LaReau, J.C.; Smith, R.C. Frequency Matters: How Successive Feeding Episodes by Blood-Feeding Insect Vectors Influences Disease Transmission. PLoS Pathog. 2021, 17, e1009590. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.F.B.; Vimal, D.; Owusu-Ansah, E. Using Fruit Flies to Delve into Mosquito Insecticide Resistance. Front. Malar. 2023, 1, 1073761. [Google Scholar] [CrossRef]
- Reeder, N.L.; Ganz, P.J.; Carlson, J.R.; Saunders, C.W. Isolation of a Deet-Insensitive Mutant of Drosophila Melanogaster (Diptera: Drosophilidae). J. Econ. Entomol. 2001, 94, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Ditzen, M.; Pellegrino, M.; Vosshall, L.B. Insect Odorant Receptors Are Molecular Targets of the Insect Repellent DEET. Science 2008, 319, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.H.; Montell, C. Avoiding DEET through Insect Gustatory Receptors. Neuron 2010, 67, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Clere, N.; Apaire-Marchais, V.; Faure, S.; Lapied, B. Unusual Modes of Action of the Repellent DEET in Insects Highlight Some Human Side Effects. Eur. J. Pharmacol. 2018, 825, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Pelletier, J.; Flounders, E.; Chitolina, R.F.; Leal, W.S. Generic Insect Repellent Detector from the Fruit Fly Drosophila Melanogaster. PLoS ONE 2011, 6, e17705. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, S.H.; Ronderos, D.S.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Smith, D.P.; Montell, C. Drosophila TRPA1 Channel Is Required to Avoid the Naturally Occurring Insect Repellent Citronellal. Curr. Biol. 2010, 20, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Du, E.J.; Ahn, T.J.; Choi, M.S.; Kwon, I.; Kim, H.W.; Kwon, J.Y.; Kang, K.J. The Mosquito Repellent Citronellal Directly Potentiates Drosophila TRPA1, Facilitating Feeding Suppression. Mol. Cells 2015, 38, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Knols, B.; Takken, W. Olfaction in Vector-Host Interactions; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011. [Google Scholar]
- Reisenman, C.E.; Scott, K. Food-Derived Volatiles Enhance Consumption in Drosophila Melanogaster. J. Exp. Biol. 2019, 222, jeb202762. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.F.; Carlson, J.R. Insect Olfaction from Model Systems to Disease Control. Proc. Natl. Acad. Sci. USA 2011, 108, 12987–12995. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Lee, Y.; Jeong, Y.T.; Kim, Y.; Lee, M.G.; Montell, C.; Moon, S.J. The Full Repertoire of Drosophila Gustatory Receptors for Detecting an Aversive Compound. Nat. Commun. 2015, 6, 8867. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.L.; Siriyasatien, P.; Kohl, A. Toxorhynchites Species: A Review of Current Knowledge. Insects 2020, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Sukupayo, P.R.; Poudel, R.C.; Ghimire, T.R. Nature’s Solution to Aedes Vectors: Toxorhynchites as a Biocontrol Agent. J. Trop. Med. 2024, 2024, 3529261. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Phloem-Sap Feeding by Animals: Problems and Solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, P.G.; Beanland, L. Insect Vectors of Phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Entling, W.; Hoffmann, C. Single and Combined Effects of Drosophila Suzukii and Drosophila Melanogaster on Sour Rot Development in Viticulture. J. Appl. Entomol. 2020, 144, 153–160. [Google Scholar] [CrossRef]
- Mertz, R.W.; Hesler, S.; Pfannenstiel, L.J.; Norris, R.H.; Loeb, G.; Scott, J.G. Insecticide Resistance in Drosophila Melanogaster in Vineyards and Evaluation of Alternative Insecticides. Pest Manag. Sci. 2022, 78, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.S.; Fairlamb, G.M.; Bell, P.; Nachman, R.J.; Audsley, N.; Isaac, R.E. Peptidergic Control in a Fruit Crop Pest: The Spotted-Wing Drosophila, Drosophila Suzukii. PLoS ONE 2017, 12, e0188021. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Quevedo, K.; Lankheet, M.J.; Pen, I.; Trienens, M.; Helsen, H.H.M.; Wertheim, B. Studying Foraging Behavior to Improve Bait Sprays Application to Control Drosophila Suzukii. BMC Ecol. Evol. 2024, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Amrein, H. Diverse Roles for the Drosophila Fructose Sensor Gr43a. Fly 2014, 8, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, H.J.; Anderson, A. A Sugar Gustatory Receptor Identified from the Foregut of Cotton Bollworm Helicoverpa Armigera. J. Chem. Ecol. 2012, 38, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yan, Q.; Yang, Y.L.; Hou, W.; Miao, C.L.; Peng, Y.C.; Dong, S.L. A Gustatory Receptor GR8 Tunes Specifically to D-Fructose in the Common Cutworm Spodoptera Litura. Insects 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-S.; Wang, P.-C.; Ning, C.; Yang, K.; Li, G.-C.; Cao, L.-L.; Huang, L.-Q.; Wang, C.-Z. The Larva and Adult of Helicoverpa Armigera Use Differential Gustatory Receptors to Sense Sucrose. eLife 2024, 12, e91711. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular Response of Drosophila Melanogaster Tyramine Receptor Cascade to Plant Essential Oils. Insect Biochem. Mol. Biol. 2005, 35, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and Physiological Responses of Drosophila Melanogaster and D. Suzukii to Volatiles from Plant Essential Oils. Pest Manag. Sci. 2021, 77, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Bošković, D.; Vuković, S.; Lazić, S.; Baser, N.; Čulum, D.; Tekić, D.; Žunić, A.; Šušnjar, A.; Šunjka, D. Insecticidal Activity of Selected Essential Oils against Drosophila Suzukii (Diptera: Drosophilidae). Plants 2023, 12, 3727. [Google Scholar] [CrossRef] [PubMed]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or Lures? Biological and Behavioral Effects of Plant Essential Oils on Tephritidae Fruit Flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef] [PubMed]
- Marella, S.; Fischler, W.; Kong, P.; Asgarian, S.; Rueckert, E.; Scott, K. Imaging Taste Responses in the Fly Brain Reveals a Functional Map of Taste Category and Behavior. Neuron 2006, 49, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Fellner, A.; Bresgen, N.; Fefer, M.; Liu, J.; Plaetzer, K. Fly into the Light: Eliminating Drosophila Melanogaster with Chlorophyllin-Based Photodynamic Inactivation. Photochem. Photobiol. Sci. 2024, 23, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Buchon, N. Drosophila Melanogaster as a Powerful Tool for Studying Insect Toxicology. Pestic. Biochem. Physiol. 2019, 161, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Batterham, P. Harnessing Model Organisms to Study Insecticide Resistance. Curr. Opin. Insect Sci. 2018, 27, 61–67. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, L.A.L.; Conacher, A.J. The Role of Termites and Ants in Soil Modification: A Review. Aust. J. Soil Res. 1990, 28, 55–93. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Yocgo, R.E.E.; Hitimana, I.; Hakizimana, M.; Birachi, E.A. Insect Pollinators Can Unlock an Annual Monetary Value of More than US $100 Million from Crop Production in Rwanda. Sci. Rep. 2023, 13, 20108. [Google Scholar] [CrossRef] [PubMed]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread Losses of Pollinating Insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- FAO. Protecting Pollinators from Pesticides—Urgent Need for Action; FAO: Rome, Italy, 2022. [Google Scholar]
- Tasman, K.; Rands, S.A.; Hodge, J.J.L. The Power of Drosophila Melanogaster for Modeling Neonicotinoid Effects on Pollinators and Identifying Novel Mechanisms. Front. Physiol. 2021, 12, 659440. [Google Scholar] [CrossRef] [PubMed]
- Pratomo, A.R.; Salim, E.; Hori, A.; Kuraishi, T. Drosophila as an Animal Model for Testing Plant-Based Immunomodulators. Int. J. Mol. Sci. 2022, 23, 14801. [Google Scholar] [CrossRef] [PubMed]
- Panchal, K.; Tiwari, A.K. Drosophila Melanogaster “a Potential Model Organism” for Identification of Pharmacological Properties of Plants/Plant-Derived Components. Biomed. Pharmacother. 2017, 89, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Tatarko, A.R.; Leonard, A.S.; Mathew, D. A Neonicotinoid Pesticide Alters Drosophila Olfactory Processing. Sci. Rep. 2023, 13, 10606. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Franz, H.R.; Deimel, S.H.; Widmann, A. Exploring Neonicotinoid Effects on Drosophila: Insights into Olfactory Memory, Neurotransmission, and Synaptic Connectivity. Front. Physiol. 2024, 15, 1363943. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.J.; Smagghe, G. RNAi Technology for Insect Management and Protection of Beneficial Insects from Diseases: Lessons, Challenges and Risk Assessments. Neotrop. Entomol. 2015, 44, 197–213. [Google Scholar] [CrossRef] [PubMed]
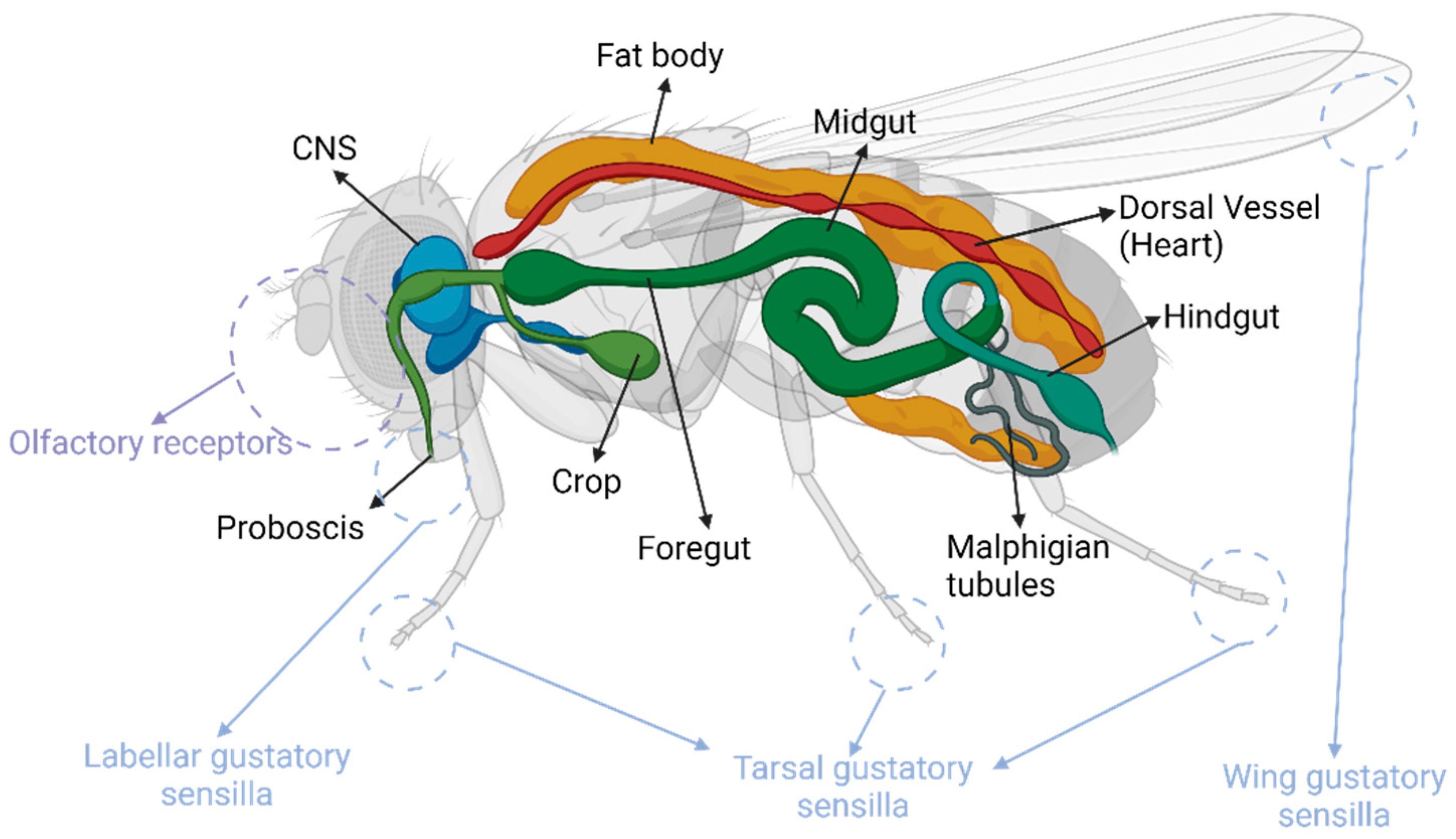


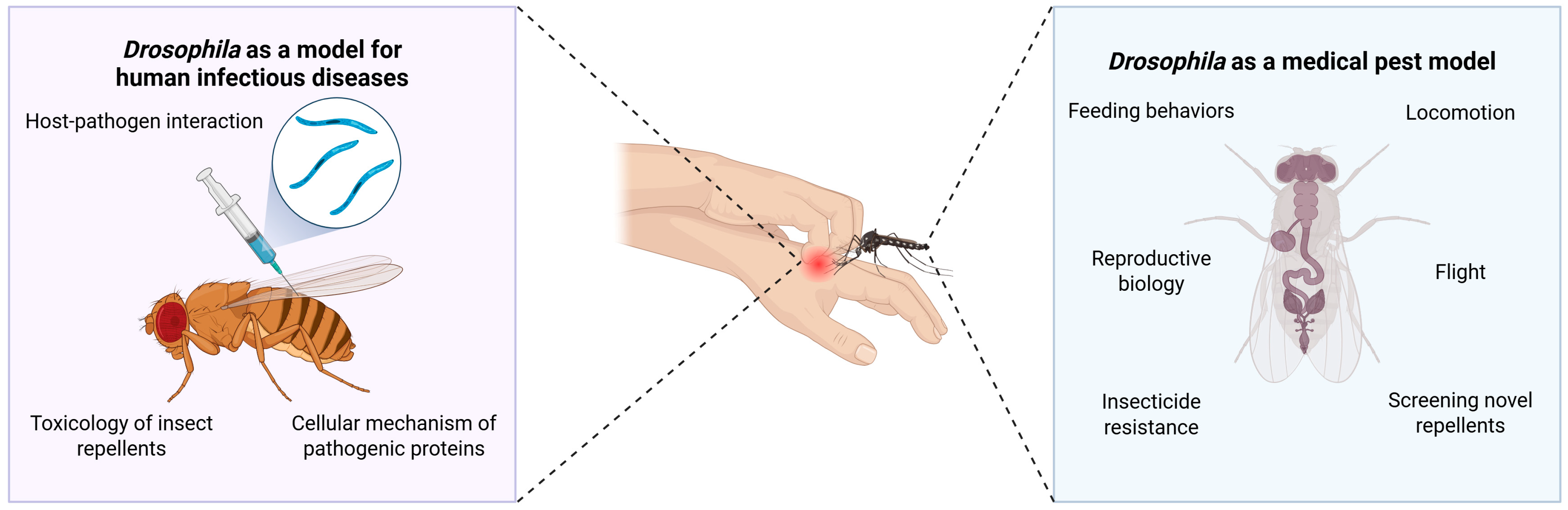
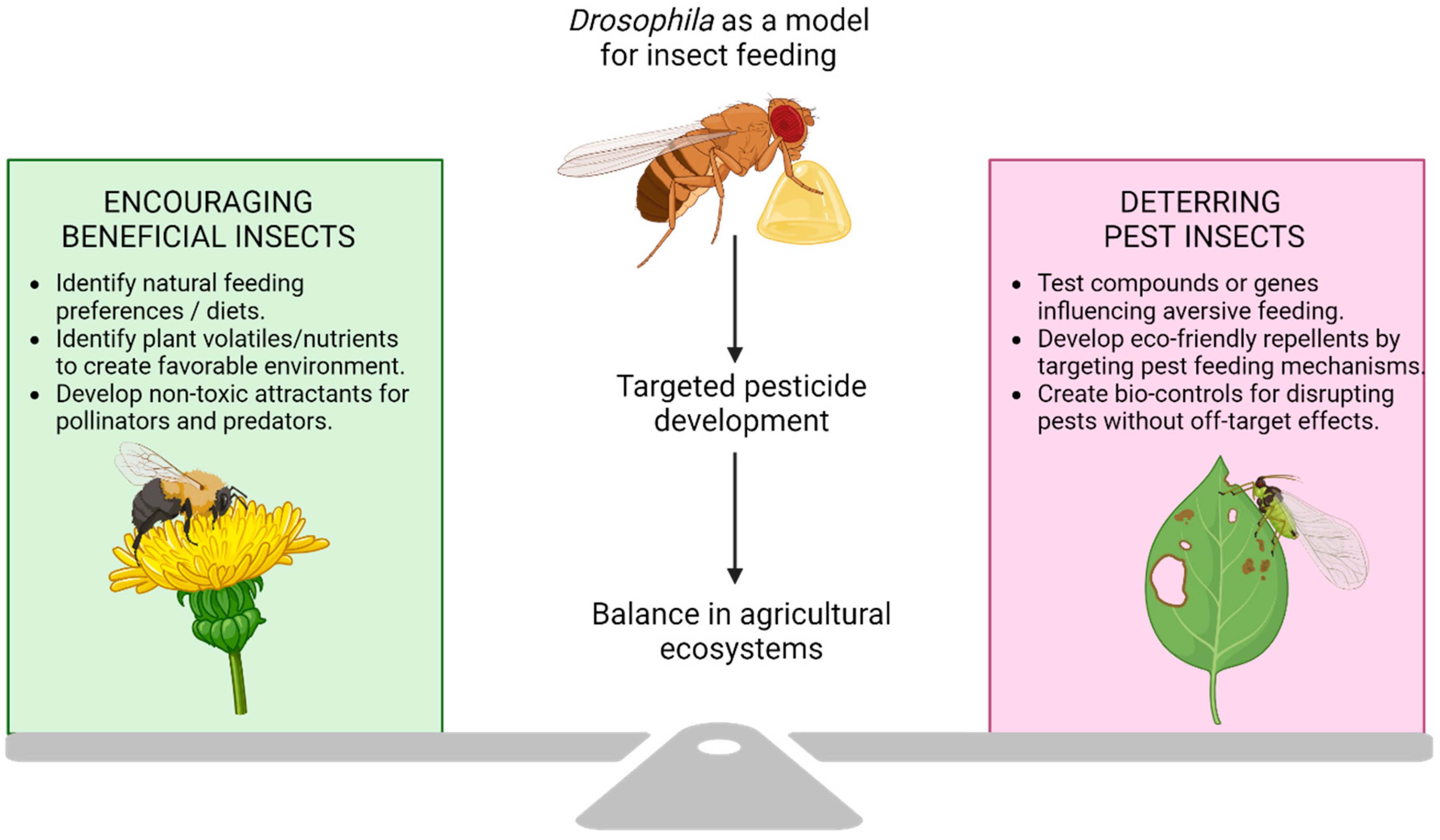
| Human Gene | Effect on Feeding and Related Behaviour in Humans | Drosophila Ortholog | Orthology Scores | Effect on Feeding and Related Behaviour in Drosophila |
|---|---|---|---|---|
| ADIPOR1 | Altered mRNA levels in AN patients [75] | AdipoR | 14/14 | - |
| CRHR1 | Mediates response to stress [76], SNP associated with AN [77] | Dh44-R1, Dh44-R2 | 13/14 | Nutrient sensors [78,79] |
| CRHR2 | Regulates appetite [76], SNV associated with eating disorders [80] | Dh44-R1 | 13/14 | Nutrient sensors [78,79] |
| TPH1 | Polymorphisms linked with genetic susceptibility to BN [81] and may increase perception of adversity in individuals with ED [82] | Trhn | 13/14 | Trh-null mutant flies show reduced feeding ability in both larval and adult stages [83]; Trh-attp mutants showed altered feeding microstructure [84] |
| NUCB2 | Anorexigenic peptide hormone [85], decreased levels in AN [86] | NUCB1 | 12/14 | - |
| SLC6A3 | Influences food intake and food reward [87], genetic and epigenetic dysregulation in AN [88] and BN [88,89] | DAT | 12/14 | - |
| KCNN3 | SNP associated with AN [77], key contributor to AN predisposition | SK | 12/14 | - |
| CCK | Satiety hormone [90,91], altered protein levels in AN and B | CCKLR-17D1, CCKLR-17D3 | 11/14 | The neuropeptide drosulfakinin (DSK) regulates feeding through CCKLRs [92,93] |
| PRL | Women with AN demonstrate abnormal PRL regulation [94], plasma PRL levels decreased in BN patients [95] | PRL-1 | 11/14 | - |
| HTR1A | Polymorphism associated with ED symptoms in adolescents [96], altered receptor activity in patients ill with and recovering from AN [97,98] and BN [99,100,101] | 5-HT1A | 11/14 | Mutants show changes in microstructure of feeding behaviour [84] |
| SLC6A4 | Much-studied polymorphisms associated with ED [102]. Alterations in transporter activity associated with body image distortions in AN [103] and altered activity in different brain areas in BED [104], DNA methylation levels altered in AN compared to HC and BED [105] | SerT | 12/14 | Food intake is significantly reduced in starved dSERT mutants [106] |
| DPP4 | Modulates nutrition control [107], higher activity in AN [108] and BN [109] | CG11034 | 10/14 | - |
| HTR2A | Well-studied polymorphism significantly associated with AN [110], altered receptor activity in AN and BN [99] | 5-HT2B | 9/14 | Mutants show changes in microstructure of feeding behaviour [84] |
| DRD2 | Influences eating behaviours [111], SNPs associated with AN [112,113], epigenetic changes in gene in AN [88], SNP associated with BN [114,115,116], epigenetic changes in gene in BN [117] | Dop2R | 8/14 | Signalling modulates feeding preference for sugar and amino acid [118], response to nutrition restriction [119] |
| HTR2C | - | 5-HT2B | 8/14 | Mutants show changes in microstructure of feeding behaviour [84] |
| TTR | - | CG30016 | 6/14 | - |
| FAAH | Regulates appetite [120], SNPs associated with AN and BN [121] | CG5112, CG7900, CG7910 | 5/14 | Altered CG5112 expression in response to a dietary shift [122], upregulated CG7910 expression upon high-fat diet consumption [123] |
| OPRM1 | Rewarding effect of craving [124], SNP associated with BN [114] | AstC-R2 | 5/14 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banu, A.; Salim, S.; Mohammad, F. From Lab to Clinic and Farm: Leveraging Drosophila Feeding Studies to Combat Eating Disorders and Pest Challenges. Biology 2025, 14, 1168. https://doi.org/10.3390/biology14091168
Banu A, Salim S, Mohammad F. From Lab to Clinic and Farm: Leveraging Drosophila Feeding Studies to Combat Eating Disorders and Pest Challenges. Biology. 2025; 14(9):1168. https://doi.org/10.3390/biology14091168
Chicago/Turabian StyleBanu, Ayesha, Safa Salim, and Farhan Mohammad. 2025. "From Lab to Clinic and Farm: Leveraging Drosophila Feeding Studies to Combat Eating Disorders and Pest Challenges" Biology 14, no. 9: 1168. https://doi.org/10.3390/biology14091168
APA StyleBanu, A., Salim, S., & Mohammad, F. (2025). From Lab to Clinic and Farm: Leveraging Drosophila Feeding Studies to Combat Eating Disorders and Pest Challenges. Biology, 14(9), 1168. https://doi.org/10.3390/biology14091168







