Simple Summary
A novel pesticide based on sprayed double-stranded RNA (dsRNA) has recently been developed to control insect pests, and its application is expected to expand for controlling various other pests. This study demonstrates its application to control a soil nematode, Acrobeloides nanus, by a drenching method. In this study, a bioassay used a soil sample containing the nematodes, which was treated with a formulated dsRNA with chitosan. Among the three target genes, vATPase subunit B was the most potent for killing the nematodes when its expression was specifically suppressed by the gene-specific dsRNA treatment. In this treatment, the chitosan formulation significantly enhanced dsRNA stability under soil conditions. These findings suggest a promising approach using chitosan-formulated dsRNA to control various soil-dwelling nematodes that damage crops.
Abstract
Acrobeloides nanus is a cosmopolitan, parthenogenetic soil nematode that is widely distributed across various terrestrial environments, including forests, sand dunes, and agricultural lands. In Korea, this nematode was first isolated from soil collected from a potato farm. It has been used as a biological indicator for monitoring contamination caused by divalent metals such as copper and zinc. In this study, A. nanus was isolated from the soil collected from a cucumber farm, and its identity was confirmed using both morphological and molecular markers. Spray-induced gene silencing using double-stranded RNA (dsRNA) represents a promising new strategy for pest control. Here, we tested a spraying dsRNA that would specifically suppress the target genes in A. nanus. Three genes (Pat-10, Unc-87, and vATPase-B) were targeted, and their expression levels were assessed following treatment with their corresponding dsRNAs. The dsRNAs were sprayed onto the nematode diet. As the concentration of dsRNA increased, the expression levels of the target genes were significantly reduced, leading to notable nematode mortality. However, nematicidal activity varied among the three different dsRNAs. To practically assess these dsRNAs under field conditions, the dsRNAs were applied to the soil containing the nematodes by a drenching application. Significant mortality was observed in treatments with dsRNAs targeting vATPase-B or Pat-10, but not with dsRNA targeting Unc-87. To enhance nematicidal activity in soil, the dsRNAs were formulated with chitosan. This formulation significantly improved the stability of dsRNAs under soil conditions and increased their control efficacy against A. nanus. This study suggests that the drenching technique offers an effective strategy to the control of soil-dwelling nematode pests affecting agricultural crops.
1. Introduction
RNA interference (RNAi) is a cellular process to control gene expression at a posttranscriptional level [1]. Its gene-specific control is accomplished by a complementary short-interference single-stranded RNA produced by a specific RNase called Argonaute (Ago) in an RNA-induced silencing complex (RISC) [2]. Even though it is an endogenous process that regulates development, it is useful to defend against viral infections in plants and animals [3]. Based on this background, the exogenous application of double-stranded RNA (dsRNA) has been devised, and its successful manipulation of specific target genes in various fields including pest control in agriculture has been demonstrated [4]. Recently, the development of a spray-induced gene silencing (SIGS) technique has allowed us to practically apply dsRNAs to control various insect and plant pests [5].
Nematodes are a protostome invertebrate phylum and are classified into 12 different clades [6]. De Ley and Blaxter [7] grouped the clades into two classes, i.e., Enoplea (clades 1 and 2) and Chromadorea (clades 3 to 12), based on molecular and morphological criteria. Thus, a model nematode, Caenorhabditis elegans, is classified into clade 9A in Chromadorea. RNAi has been described as systemic in C. elegans, and it can also be transmitted to the next generation (parental RNAi) upon dsRNA injection or feeding [8]. This suggests that RNAi can be a novel tactic to control nematode pests in agriculture.
Root-knot nematodes cause significant crop losses by forming root galls to prevent nitrogen fixation, which may lead to total failure in yield [9]. Pioneering works to apply RNAi to control the nematode pests were performed using transgenic plants expressing dsRNA. For instance, a previous observation [10] demonstrated that the transgenic strains of Arabidopsis thaliana expressed against the dsRNA specific to the 16D10 gene, which is responsible for nematode–host integration, resulted in the reduced infectivity of four species of Meloidogyne (M. incognita, M. javanica, M. arenaria, and M. hapla). A similar host-induced gene silencing (HIGS) event occurred in another crop, Vitis sp. (grape), and reduced the infection of M. incognita [11]. The use of RNAi to control parasitic root nematodes was also effective for other targets in Meloidogyne such as FMRFamide-like peptide (FLP) genes responsible for modulating nerve and muscle activities [12], as well as for other genera such as Pratylenchus and Heterodera [13,14].
To avoid any concerns regarding genetically modified organisms, SIGS would be an ideal strategy to control the nematode pests. SIGS can effectively deliver dsRNA to target pests using nanoformulations such as chitosan or BioClay. However, few studies have evaluated SIGS, probably due to a lack of suitable dsRNA formulations against soil nematodes. For example, SIGS-based nematode control was devised through dsRNA formulations using a kaolinite nanoclay, in which Globodera pallida, a white potato cyst nematode and a major economic pest that causes substantial potato yield losses, is the target nematode [15]. dsRNAs specific to three FLP genes were formulated using the kaolinite nanoclay. The drenching of potato with the dsRNA–kaolinite formulations induced the deformation and premature death of J2 juveniles, compared with untreated J2s that entered J3 or J4 stages.
This study aimed to test a SIGS-based strategy for controlling soil nematodes. To this end, we used a soil nematode, Acrobeloides nanus, because of its ease of collection and relatively rapid reproduction in laboratory conditions [16,17]. After screening the optimal target genes using the unformulated dsRNAs, this study demonstrated the efficacy of the chitosan-based formulation on the nematicidal activity in the soil conditions.
2. Materials and Methods
2.1. Nematode Collection and Rearing
Nematode specimens were isolated from soil samples collected from a cucumber (Cucumis sativus L.) farm in Gunwi, Korea (36°11′32″ N, 128°33′51″ E) using a combination of sieving and the Baermann funnel method [18]. The isolated nematodes were then transferred to 1% agar plate containing a bacterial lawn of Pseudomonas fluorescens ANU101. The plates were incubated under darkness at 25 °C to facilitate nematode growth.
2.2. Nematode Identification Using Morphological Characteristics
Adult females were fixed in warm water (~60 °C) in 4% formaldehyde solution. Following fixation, they underwent dehydration using Seinhorst’s method [19] and were subsequently mounted in pure glycerin on slides [20]. A glass rod was used to prevent the samples from flattening while the coverslip was overlaid. Morphological characteristics were examined using a stereoscopic microscope (Stemi SV11, Zeiss, Jena, Germany) and a light microscope (Eclipse 80i, Nikon, Tokyo, Japan) at 50× magnification.
2.3. Chemicals Common in Different Treatments
Diethyl pyrocarbonate (DEPC)-treated water was prepared by adding DEPC to the deionized distilled water to 0.1% and autoclaved. Metafectene PRO was obtained from Biontex (München, Germany) to create liposomes following its addition to dsRNA. Proteinase K (20 µg/mL) was obtained from Biofact (Daejeon, Republic of Korea). Dithiothreitol (DTT), Tween-20, yeast tRNA, and N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) were purchased from Sigma-Aldrich Korea (Seoul, Republic of Korea). Methanol was obtained from Samchun Chemical (Seoul, Republic of Korea). Formamide, sodium citrate, sodium chloride, heparin, acetic anhydride, triethanolamine, dimethyl sulfoxide (DMSO), formaldehyde, and sodium dodecyl sulfate were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Phosphate-buffered saline (PBS) was prepared with 0.1 M phosphate and 0.7% NaCl and its pH was adjusted to 7.4.
2.4. Nematode Identification Using Molecular Marker
For sequencing internal transcribed spacer (ITS) to identify the nematode isolate, genomic DNA (gDNA) was extracted from 500–600 nematodes which were collected from the laboratory population. Briefly, nematodes were crushed with a pestle in 50 μL of DNA extraction solution (LGC Bioresearch Technologies, Hoddesdon, UK) and heated at 100 °C for 10 min. After cooling on ice for 2 min, the suspension was centrifuged at 14,000× g for 5 min. The resulting supernatant (1–2 ng DNA/μL) was used as the gDNA sample.
Primers to amplify the ITS region were 5′-TCCGTAGGTGAACCTGCGG-3′ and 5′-TCCTCCGCTTATTGATATGC-3′ [21]. Each 25 μL PCR reaction consisted of 3 μL of gDNA, 2 mM MgCl2, 0.2 mM dNTP, 4 pmol of each primer, and 1 unit of Taq DNA polymerase (GeneAll, Seoul, Republic of Korea). PCR was performed on a My Cycler Personal Thermal Cycler (Bio-Rad, Hercules, CA, USA) using the following cycling conditions: initial heating at 94 °C for 3 min, 35 cycles of denaturation at 94 °C for 1 min, annealing at 48 °C for 1 min, and polymerization at 72 °C for 10 min, followed by keeping at 4 °C. PCR products were cloned into PCR2.1 cloning vector (Invitrogen, Carlsbad, CA, USA) and transformed into Escherichia coli TOP 10 chemically competent cells. Plasmids were obtained after cloning and used for bidirectional sequence analyses using M13F and M13R universal primers. Sequencing was performed by Macrogen (Seoul, Republic of Korea).
2.5. Prediction of Three Target Genes from the Nematode Genome
To screen the optimal target genes for developing dsRNA pesticide against A. nanus, Profilin/Actin-binding protein-10 (Pat-10), vacuolar type ATPase subunit B (vATPase-B) of C. elegans, and Uncoordinated-87 (Unc-87) of Pratylenchus goodeyi were used to obtain the orthologous sequences from A. nanus genome using BlastN (https://www.ncbi.nlm.nih.gov/, accessed on 1 July 2025). To obtain the open reading frame (ORF) of the collected sequences, the ORF finder tool from NCBI was used (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 1 July 2025). Prediction of the protein domain structure was performed using EMBL-EBI (www.ebi.ac.uk, accessed on 1 July 2025) and Pfam (http://pfam.xfam.org, accessed on 1 July 2025). Phylogenetic analysis was performed using the Neighbor-Joining method and the Poisson correction model using MEGA6.06 software (www.megasoftware.net, accessed on 1 July 2025). Bootstrapping values were obtained with 1000 replications to test the supports on each node in the resulting phylogenetic tree.
2.6. RNA Extraction, cDNA Preparation, and RT-PCR
Total RNA was extracted from approximately ~500–600 nematodes using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The extracted RNA was resuspended in nuclease-free water and its concentration was measured with a spectrophotometer (NanoDrop, Thermo Scientific, Wilmington, DE, USA). For cDNA synthesis, 65 ng of RNA per reaction was used with RT-Premix (Intron Biotechnology, Seongnam, Republic of Korea) containing an oligo(dT) primer, following the manufacturer’s protocol.
For RT-PCR, the synthesized cDNAs were used for PCR amplification with Taq DNA polymerase and gene-specific primers under the following conditions: an initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 1 min, different annealing temperatures (Table S1) for 1 min, and extension at 72 °C for 1 min. The PCR reaction mixture (25 µL) consisted of a DNA template, dNTPs (2.5 mM each), 10 pmol of each primer, and Taq polymerase (2.5 units/µL).
Quantitative PCR (qPCR) was performed using a real-time PCR instrument (Step One Plus Real-Time PCR System, Applied Biosystems, Singapore) and Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA, USA), following the guidelines of Bustin et al. [22]. The qPCR reaction mixture (20 µL) included 10 µL of Power SYBR Green PCR Mix, 2 µL of cDNA template (65 ng/µL), and 1 µL each of forward and reverse primers (Table S1). Elongation factor 1 alpha (EF1α) was used as the reference gene with its specific primers listed in Table S1. Melting curve analysis was performed to verify the presence of a single specific PCR product. Quantitative analysis was conducted using the comparative CT (2−∆∆CT) method [23] with three independent replicates for each experiment.
2.7. dsRNA Preparation
Template DNA was amplified by PCR using gene-specific primers (Table S1) with the T7 promoter sequence attached to their 5′ ends. The manufacturer’s instructions subjected the PCR products to in vitro transcription using the MEGAscript RNAi Kit (Ambion, Austin, TX, USA). The synthesized dsRNAs were mixed with Metafectene PRO at a 1:1 volume ratio and incubated at room temperature for 30 min to create a dsRNA–liposome complex. RNAi efficiency was evaluated via RT-qPCR 72 h after treatment.
2.8. Bioassay to Test Gene Silencing by dsRNA Application in an Agar Plate Assay
For the bioassay, 1% agar solution was prepared by dissolving 1 g of agar in 100 mL of distilled water and sterilizing it in an autoclave. The agar solution (3 mL) was dispensed to each well of a 24-well plate. dsRNA solutions were prepared at final concentrations of 50, 100, and 500 µg/µL by dilution with distilled water. A 20 µL volume of each dsRNA solution, including the control dsRNA (dsCON), was added to the designated well. Nematodes were suspended in 100 µL of PBS on a slide glass, and 40 individuals were transferred into each well using a 10 µL pipette. The plate was sealed with a lid and sealing tape and wrapped in aluminum foil to prevent light exposure. After three days at 25 °C, live nematodes were counted under a stereomicroscope by ‘S’ body shape and movement. Each treatment was replicated three times.
2.9. Bioassay to Test Gene Silencing by dsRNA Application in Soil
Instead of agar, this assay used soil (1.13 g/well). The sterilized soil was mixed with dsRNA suspension (100 µL) at 50, 100, and 500 µg/g. Control soil was mixed with PBS. A total of 100 nematodes were added to each well in PBS. The plates were then incubated in darkness at room temperature for seven days. Then the live nematodes in each well were counted using the Baermann funnel method. Each treatment was replicated three times.
2.10. Whole-Mount In Situ Hybridization of vATPase-B
To investigate the mRNA expression of vATPase-B in the juvenile stage of A. nanus, a whole-mount in situ hybridization was performed according to the method of Wang et al. [24]. After dsRNA feeding, nematodes were incubated in 1 mL of a lysis buffer (10 mM DTT, 0.1% Tween-20 in DEPC-treated water, pH 9.0) for 15 min at room temperature and then washed three times with ice-cold PBS. Samples were digested with proteinase K (20 µg/mL; Sigma-Aldrich Korea) for 10–15 min at room temperature and post-fixed in 3.7% formaldehyde (Sigma-Aldrich Korea) for 10 min, followed by additional PBS washes. Fixation was carried out in pre-chilled Dent’s fixative (methanol: DMSO, 8:2, v/v) for 20 min on ice. Samples were rehydrated in 50% methanol in PBS for 10 min and then post-fixed again in 3.7% formaldehyde in PBS for 10 min at room temperature. An additional fixation step was performed in 3.7% formaldehyde prepared in HEPES-buffered PBS (pH 7.4) for 60 min at room temperature. To improve probe penetration, nematodes were further digested with proteinase K (20 µg/mL) for 30 min at room temperature. To reduce non-specific probe binding, samples were treated with 0.1% triethanolamine (Sigma-Aldrich Korea) for 2 min, followed by incubation in 0.05% acetic anhydride in triethanolamine for 10 min. Hybridization was performed in hybridization buffer containing 50% formamide, 5× SSC, 0.1% Tween-20, 100 µg/mL heparin, and 100 µg/mL tRNA at 65 °C for 18 h with gentle rotation. A total of 1 µL of FAM-labeled antisense or sense probe (10 pmol) was used for each reaction. Post-hybridization washes were conducted using 2× SSC with 0.1% Tween-20 at room temperature, followed by a stringent wash in 0.2× SSC at 65 °C for 30 min and a final rinse in PBS. Imaging was carried out using an inverted fluorescence microscope (Eclipse 80i, Nikon, Tokyo, Japan) at 100× magnification using appropriate filters for FAM fluorescence detection.
2.11. Preparation of Chitosan-Formulated dsRNA
Chitosan formulation followed the method described in our previous study [25]. Chitosan (3.8–20 kDa, ≥75% deacetylated; Sigma-Aldrich Korea), derived from crab shells, was dissolved in 0.1 M sodium acetate buffer (prepared by mixing 0.1 M sodium acetate and 0.1 M acetic acid in deionized water, pH 4.5) and maintained at room temperature. For nanoparticle formation, 50 µL of dsRNA (0.5 µg/µL) was mixed with 100 µL of 100 mM sodium sulfate solution (Na2SO4 dissolved in deionized water). This solution was added to 100 µL of the chitosan solution (1 µg/µL) and incubated at 55 °C for 1 min, followed by mixing for 30 s. The resulting suspension was centrifuged at 14,000× g for 10 min at room temperature. The pellet (~300 nm in diameter) was washed three times with deionized water and resuspended in Milli-Q ultrapure water (Merck KGaA, Darmstadt, Germany). Nanoparticles were sonicated for 5 min at 25 °C using an ultrasonic liquid processor (Powersonic 405, Hwashin, Shanghai, China) prior to use in subsequent experiments. The final chitosan formulation contained dsRNA at a concentration of 500 µg/mL.
2.12. Statistical Analysis
All analyses were performed using one-way ANOVA with the PROC GLM procedure in the SAS program version 9.45 [26]. Mortality data were subjected to arcsine transformation before ANOVA. Mean comparisons were conducted using the least significant difference (LSD) test. The study included three independent biological replicates, and results were expressed as the mean ± standard error, generated with GraphPad v8.1 and Sigma Plot v10.
3. Results
3.1. Identification of a Nematode Isolate Collected from an Agricultural Land
Female adult nematode isolate was identified with nine morphological characteristics at the mouth, pharynx, esophagus, anus, and tail (Figure 1). The morphometric characteristics of the isolate matched to those of Acrobeloides nanus (Table 1). To confirm the morphological identification, the ITS sequence of the isolate was sequenced (Figure S1). Blast analysis to the GenBank supported the morphological identification with the highest match score to A. nanus (Table 2). A phylogenetic analysis indicated that the isolate is classified into one of the family Cephalobidae, in which it is clustered with A. nanus (Figure 2).
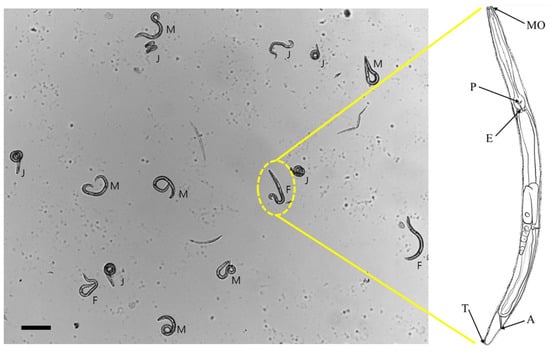
Figure 1.
Nematode identification using morphological characteristics. Anatomical illustration of a nematode, highlighting key structures: mouth (‘MO’), esophagus (‘E’), pharynx (‘P’), tail (‘T’), and anus (‘A’). Juveniles (‘J’) are distinct from reproductive adults, which have ovary for females (‘F’) and testis for males (‘M’). Scale bar represents 100 μm.

Table 1.
A morphometric comparison with characteristics of other nematode species (Acrobeloides nanus, Pratylenchus goodeyi, and Meloidogyne incognita). Homology percentages indicate morphological similarities between the isolate and the reference species.

Table 2.
Molecular identification and phylogenetic analysis of a nematode isolate Gunwi using its ITS sequence (GenBank accession number: PV981766). BLAST analysis of the ITS region of the nematode isolates against the GenBank database. The table lists the five top-matching species, accession numbers, match scores, query cover, E-value, and sequence identity.

Figure 2.
Phylogenetic analysis of the nematode isolate (red-boxed) based on ITS sequence. The phylogenetic tree was constructed using the Neighbor-Joining method in MEGA6.0, with bootstrap values calculated from 1000 replicates to support branch confidence. Sequences were retrieved from GenBank, with accession numbers provided in the corresponding dataset (Table S2).
3.2. Prediction of Three Target Genes of RNAi from A. nanus Genome
Two structural genes, Pat-10 and Unc-87, are essential for nematode survival and movement and their RNAi led to significant mortalities in C. elegans and plant parasitic nematode [27]. In addition, vATPase-B is a gene encoding a catalytic subunit (Figure 3) of the enzyme, which actively transports proton to the lumen of the midgut for the digested nutrients to be absorbed and is crucial for survival for several insects [25,28]. These three genes were retrieved from A. nanus genome and confirmed by Blast search to the GenBank (Table 3).
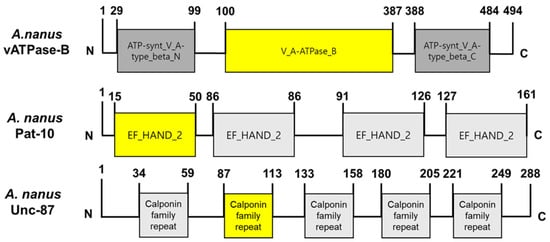
Figure 3.
Three candidate genes of A. nanus for dsRNA construction. Domain analysis was conducted using InterPro (https://www.ebi.ac.uk/interpro/, accessed on 1 July 2025). Yellow colors indicate the signature domains of the specific genes.

Table 3.
Bioinformatic analysis of three distinct genes of A. nanus obtained from the GenBank database. The BLAST tables list the five top-matching genes, species, accession numbers, match scores, query coverage, E-value, and sequence identity.
Their expressions were confirmed from the growing nematodes of A. nanus by RT-qPCR in two different developmental stages (Figure 4). Expression levels of the three genes were not different at the juvenile stage but they were at the adult stage, at which Pat-10 was highly expressed more than other two genes.

Figure 4.
Expression profile of three genes at juvenile (‘J’) and adult stages of A. nanus. An-EFα1, an elongation factor, was employed to normalize the expression levels. Three replications were conducted for each measurement. The standard deviation bars with different letters indicate significant differences among means in each developmental stage at a Type I error = 0.05 (LSD test).
3.3. Nematicidal Effects of Three dsRNAs Against A. nanus Using an Agar Plate Assay
On the growing medium, the nematodes of A. nanus were exposed to dsRNAs (Supplementary Figure S2) specific to three target genes (Figure 5). All three dsRNAs gave significant mortalities (F = 30.87; df = 2, 24; p < 0.0001) to the nematodes in a dose-dependent manner (F = 359.67; df = 3, 24; p < 0.0001) (Figure 5a). However, there was a significant difference (F = 8.56; df = 6, 24; p < 0.0001) among three dsRNA treatments, in which dsRNA specific to vATPase-B showed the highest nematicidal effect with the lowest LC50 value (Table S3). To confirm the mortality caused by the reduction in the target genes, the target genes were monitored in their amounts in the treated nematodes (Figure 5b). All three dsRNA treatments led to over 50% reduction in the expression levels of their target genes at 24–48 h after the dsRNA treatments.
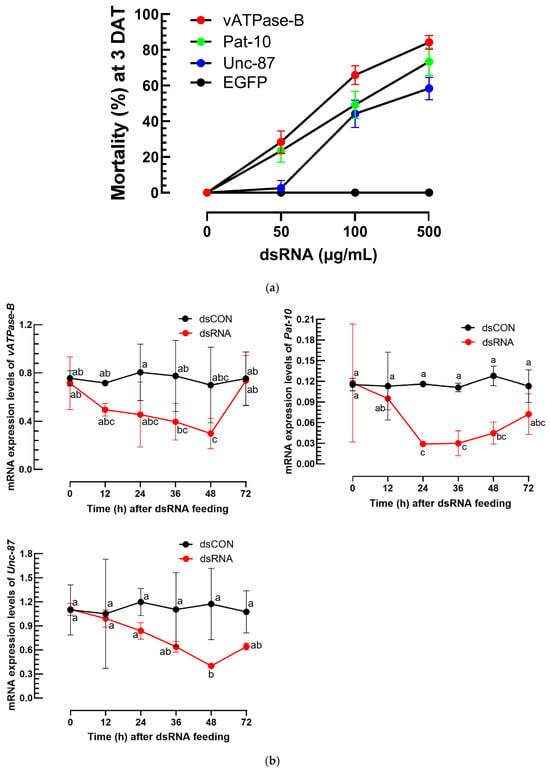
Figure 5.
Efficacy of dsRNA application against A. nanus using an agar plate assay. (a) Nematode susceptibility to three different dsRNAs at different doses. EGFP was used as a control for a non-target gene. Mortality (%) was assessed 3 days after treatment (‘DAT’). Each plate contained 40 nematodes (a mixture of larvae and adults) and was replicated three times. Median lethal concentrations were estimated and shown in Table S3. (b) Quantification of target gene expression levels after the RNAi treatments, where expression levels were normalized using an elongation factor, EF1α, for A. nanus. Control (‘dsCON’) utilized dsRNA specific to a green fluorescence protein gene, EGFP, serving as a non-target gene. Data represent mean ± standard deviation from three biological replicates. Different letters at the dots indicate significant difference among means at Type I error = 0.05 (LSD test).
To visualize the mRNA reduction caused by the dsRNA treatment, a fluorescence probe specific to vATPase-B mRNA was applied to the test nematodes (Figure 6). This FISH analysis showed that this gene was highly expressed in the gut in control (Figure 6a). The mRNA signal was markedly reduced in the nematode treated with dsRNA specific to vATPase-B. Based on the fluorescence signal intensity, the dsRNA treatment reduced the mRNA levels by more than 12-fold (Figure 6b).
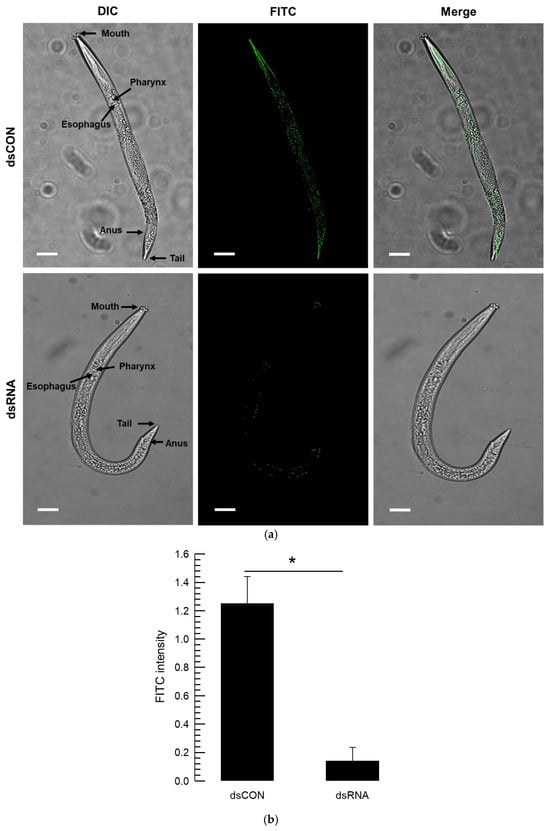
Figure 6.
Comparative analysis of RNAi efficiency of a dsRNA treatment specific to vATPase-B in A. nanus using FISH. (a) Whole-mount in situ hybridization analysis in the nematode using antisense probes. Sense probes were employed to confirm specificity and did not show any signal. A fluorescent microscope (DM2500; Leica, Wetzlar, Germany) was used to examine the samples in fluorescence (‘FITC’ against vATPase-B). The overall nematode morphology was examined in differential interference contrast (‘DIC’) mode at 100× magnification, with a scale bar indicating 0.1 mm. (b) Fluorescence intensity was quantified by calculating the FITC signal. Each treatment was replicated three times, with each replication including three larvae. The asterisk above the standard deviation bars indicates the significant difference between means at Type I error = 0.05 (LSD test).
3.4. Nematicidal Effects of Three dsRNAs Against A. nanus in Soil
To be practical for the dsRNA control against the soil nematode, the infective juveniles were applied to soil along with dsRNA (Figure 7). After 7 days, the nematodes in the treated soil were collected and assessed in the control efficacies of the three different dsRNA treatments. Unlike in the microplate assay, the dsRNAs specific to Unc-87 or Pat-10 gave low control efficacies below 40% and did not exhibit significant dose-dependent mortality against A. nanus (Figure 7a). In contrast, the dsRNA treatment specific to vATPase-B gave significant dose-dependent mortalities to the nematodes, in which dsRNA specific to vATPase-B achieved approximately 57% control efficacy at 500 µg/g (Figure 7b).
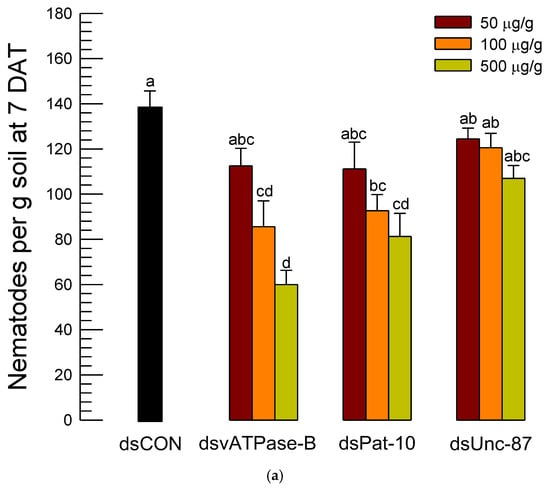
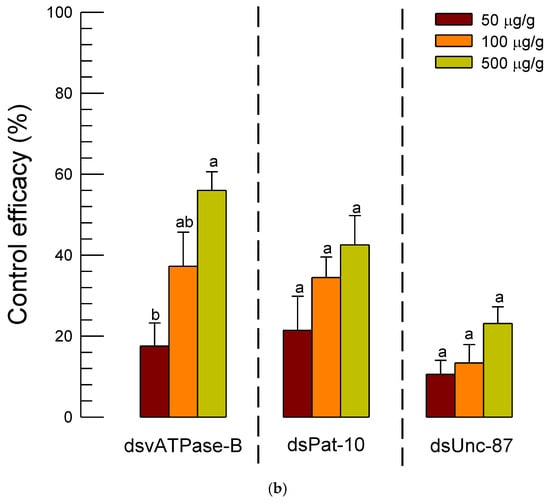
Figure 7.
Efficacy of dsRNA application against A. nanus in soil. (a) Number of live nematodes at seven days after treatment (‘DAT’) in each dsRNA treatment: dsRNA specific to vATPase-B (‘dsvATPase-B’), dsRNA specific to Pat-10 (‘dsPat-10’), and dsRNA specific to Unc-87 (‘dsUnc-87’). Control (‘dsCON’) represented dsRNA treatment specific to a non-target gene, EGFP. Bars with different letters mean significant difference among means at Type I error = 0.05 (LSD test). (b) Control efficacies of the dsRNA treatments.
3.5. Chitosan-Formulated dsRNA to Protect dsRNA Stability in Soil
To increase the control efficacy of the dsRNAs in the soil by enhancing dsRNA stability, chitosan was used to formulate the dsRNAs (Figure 8). After the formulated dsRNA was first applied to soil, the test nematodes were periodically released to the treated soil. In unformulated dsRNA treatments, the control efficacies significantly decreased with the time in the soil before the nematode treatment (Figure 8a). Furthermore, they did not show any nematicidal activities in any treatments when the nematodes were exposed to dsRNA already sprayed 7 days previously in the soil. Interestingly, the formulated dsRNA did not exhibit a decline in nematicidal activity against A. nanus. The formulated dsRNA specific to vATPase-B significantly enhanced the nematicidal activity, achieving approximately 80% mortality against A. nanus (Figure 8b).

Figure 8.
Influence of chitosan formulation of dsRNA on the control efficacy against A. nanus. (a) Comparison of dsRNA stability in soil conditions between formulated or unformulated dsRNAs. After incubation period of dsRNA in soil, the test nematodes (100 nematodes (a mixture of larvae and adults) per replicate) were applied to the pre-incubated soil–dsRNA mixture. Three dsRNA treatments (dsvATPase-B, dsPat-10, and dsUnc-87) were tested at 500 µg/g, while the control (dsCON) received dsRNA specific to EGFP. Each treatment was replicated three times. Different letters above the standard deviation bars indicate the significant difference between means at Type I error = 0.05 (LSD test). (b) Relatively longer control efficacy of chitosan-formulated dsRNA.
4. Discussion
This study tested the hypothesis that nematodes can be controlled via environmental application of dsRNA. A model nematode for this study used A. nanus, which were identified by morphological and molecular characteristics. Even though A. nanus is not a pest but rather a decomposer in the ecosystem, it is an ideal nematode species for developing a novel control agent using RNAi due to its well-known gene expression system [29,30]. In particular, its habitat in soil allowed us to test dsRNA application in soil using a chitosan formulation. This would be necessary to develop dsRNA to kill nematodes, which cause serious economic damage to crops because several nematodes such as root-knot nematodes, root lesion nematodes, or cyst nematodes live in soil environments. The isolate used in this study was collected from an agricultural farm cultivating cucumber. In contrast, the first report of this species in Korea was isolated in the soil collected from a potato farm [31]. This explains its habitats in a wide range of terrestrial areas, including forests, sand dunes, and agricultural farms [32].
Three different genes of A. nanus were silenced by treating specific dsRNAs to the living habitats. In a Petri dish, the dsRNA sprayed onto the growth medium significantly reduced the target gene expression and led to significant mortalities. Several nematode genes have been determined as effective targets of RNAi for pest control. Dong et al. [33] tested two kinds of genes of FLPs (FMRFamide-like peptides: modulating locomotory, feeding, and reproductive functions of nematodes) and mitogen-activated protein (MAP) kinase (regulating transcription factors and protein kinases) to control Meloidogyne incognita. In this study, test nematodes soaked in the dsRNA suspension exhibited a significant reduction in the target genes and suffered from poor infectivity of host plants by reducing root-knot numbers along with significant fecundity reduction in producing egg masses. Another two genes, Unc-87 and Pat-10, of C. elegans are essential components of the body wall muscle and are thus required for nematode movement. Joseph et al. [34] used these genes as RNAi targets to control a banana root nematode, Pratylenchus coffeae. The nematode soaking treatment of these dsRNAs resulted in significant reductions in the mRNA levels in a sequence-specific manner and led to behavioral abnormalities, with the nematodes exhibiting a straight and rigid form in Pat-10 RNAi treatment, while they exhibited coiled behavior in Unc-87 RNAi treatment, in contrast to the regular sinusoidal movement of the control nematodes. Another structural gene, Unc-15, was an effective RNAi target for the control of a stem nematode, Ditylenchus destructor, which is one of the most serious diseases limiting the productivity and quality of sweet potato [35]. Two cuticle collagen genes, Mi-col-1 and Lemmi-5, of M. incognita are involved in the synthesis and maintenance of the nematode cuticle and highly expressed in adult females. Transgenic tomatoes expressing these dsRNAs reduced the number of the adult females, which critically impaired the nematode fecundity [36]. In addition, two transcriptional factors, DAF-16 and SKN-1, and two nematode effectors, Mi-msp10 and Mi-msp23, are RNAi targets to control M. incognita [37,38]. Thorat et al. [39] tested nematode-responsive promoter of A. thaliana in tomato in order to express the dsRNA specifically upon the nematode damage and showed pAt1g74770 as a persistently inducible promoter during the nematode infection.
Among three candidate genes, vATPase-B was highly effective for controlling the nematode, A. nanus, in microplate and soil assays. However, the other two target genes were less potent due to their poor lethality to the nematode. In general, at least ~40% of genes are essential for maintaining life in most organisms [40]. Thus, any interruption of the expression of the essential genes leads to fatal lethality. However, the lethal effects after RNAi vary among these essential genes, probably due to the presence of paralogs or overexpression of alternative gene(s) [41]. For vATPase, its proton pumping function is crucial for nutrient absorption in the midgut in insects and nematodes [42,43]. Our current FISH assay showed that vATPase-B was highly expressed in the nematode gut of A. nanus. The mortality induced by the reduction in vATPase-B mRNA level has been reported in several insects and C. elegans [44,45].
This study developed a technique to apply dsRNA for controlling nematodes in soil. For this application, this study applied a chitosan formulation of dsRNA to enhance its chemical stability in the soil environment. In general, the half-life of dsRNA in soil is approximately 24 h due to various environmental factors including microbial degradation or absorption to soil particles [46]. The stabilized chitosan formulation significantly enhanced the nematicidal activity of the dsRNA. Chitosan formulation activated clathrin-dependent endocytosis pathway to enhance uptake efficiency of dsRNA by inducing the gene expression of the key clathrin heavy chain, which led to a significant increase in RNAi efficiency [47]. Chitosan formulation is also helpful to minimize its cellular degradation in the target cells by evacuating the dsRNA–chitosan from lysosomal endosomes [48]. A similar dsRNA formulation using a kaolinite nanoclay was devised in controlling a soil nematode, G. pallida, infesting potato crop [15]. Its drenching application led to significant impairment of the nematode juveniles, suggesting a potential to control the nematodes using dsRNA. Although little is known about effective dsRNA delivery methods to control nematodes in soil, this study suggests the chitosan formulation in addition to kaolinite nanoclay as a promising method. Even though our bioassay showed the nematicidal effect on the nematodes in soil, the chitosan-formulated dsRNA should be assessed for control efficacy against nematodes within plant roots because a number of the plant-parasitic nematodes reside in the plant tissues. In addition, because the amount of dsRNA required may be high to expect a successful control efficacy, this nematode control must be economically practical by screening highly efficient target genes to increase the RNAi efficiency and lead to high mortality. Because RNAi is sequence-specific, dsRNA-based nematode control must be target-specific. The optimal dsRNA should minimize off-target risks by selecting highly specific regions within effective target genes.
5. Conclusions
This study suggests a novel control tactic of soil-dwelling nematodes by drenching with a formulated dsRNA. It also provides a model nematode, A. nanus, to screen candidate genes to select highly nematicidal dsRNAs. The resulting dsRNA may be applicable for developing a highly efficient control method against soil nematode pests damaging crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14091161/s1, Figure S1: Molecular identification and phylogenetic analysis of a nematode isolate using ITS sequence data. Nucleotide sequence of the internal transcribed spacer (ITS) region of a nematode isolate. The sequence was analyzed and color-coded to indicate different putative rDNA regions: partial 18S rDNA (yellow), ITS-1 (green), 5.8S rDNA (cyan), ITS-2 (magenta), and partial 28S rDNA (blue). These regions were identified based on sequence comparison with ITS sequences of Heterorhabditis spp. [49]; Figure S2: DNA sequences of A. nanus to produce the dsRNAs used in this study. Open reading frames of Pat-10 (A), Unc-87 (B), and vATPase-B (C) genes. The yellow-colored sequences represent the regions encoding their dsRNAs; Table S1: Primer sequences used in this study; Table S2: GenBank accession numbers if ITS genes used to construct a phylogeny tree in Figure 2b; Table S3: Median lethal doses of three different dsRNAs in a microplate assay in Figure 5a.
Author Contributions
Conceptualization, Y.K.; methodology, T.S. and F.K.; software, T.S. and F.K.; validation, T.S. and F.K.; formal analysis, T.S. and F.K.; investigation, T.S. and F.K.; resources, Y.K.; data curation, T.S. and F.K.; writing—original draft preparation, T.S., F.K. and Y.K.; writing—review and editing, Y.K.; visualization, T.S. and F.K.; supervision, Y.K.; project administration, Y.K.; funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant (No. 2022R1A2B5B03001792) from the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea. It was also supported by the research grant from a Glocal Project of Gyeongkuk National University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tomari, Y. RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochim. Biophys. Acta 2016, 1859, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Silver, K.; Cooper, A.M.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021, 77, 2645–2658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef]
- De Ley, P.; Blaxter, M.L. Systematic position and phylogeny. In The Biology of Nematodes; Lee, D.L., Ed.; Taylor and Francis: London, UK, 2002; pp. 1–30. [Google Scholar]
- Timmons, L.; Fire, A. Specific interference by ingested dsRNA. Nature 1998, 395, 854. [Google Scholar] [CrossRef]
- Hewezi, T.; Baum, T.J. Manipulation of plant cells by cyst and root-knot nematode effectors. Mol. Plant Microbe Interact. 2013, 26, 9–16. [Google Scholar] [CrossRef]
- Huang, G.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA 2006, 103, 14302–14306. [Google Scholar] [CrossRef]
- Yang, Y.; Jittayasothorn, Y.; Chronis, D.; Wang, X.; Cousins, P.; Zhong, G.Y. Molecular characteristics and efficacy of 16D10 siRNAs in inhibiting root-knot nematode infection in transgenic grape hairy roots. PLoS ONE 2013, 8, e69463. [Google Scholar] [CrossRef]
- Papolu, P.K.; Gantasala, N.P.; Kamaraju, D.; Banakar, P.; Sreevathsa, R.; Rao, U. Utility of host delivered RNAi of two FMRF amide like peptides, flp-14 and flp-18, for the management of root knot nematode, Meloidogyne incognita. PLoS ONE 2013, 8, e80603. [Google Scholar] [CrossRef]
- Youssef, R.M.; Kim, K.H.; Haroon, S.A.; Matthews, B.F. Post-transcriptional gene silencing of the gene encoding aldolase from soybean cyst nematode by transformed soybean roots. Exp. Parasitol. 2013, 134, 266–274. [Google Scholar] [CrossRef]
- Walawage, S.L.; Britton, M.T.; Leslie, C.; Uratsu, S.L.; Li, Y.; Dandekar, A.M. Stacking resistance to crown gall and nematodes in walnut rootstocks. BMC Genom. 2012, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, A.; Dipta, B.; Siddappa, S.; Singh, B.; Sharma, N.; Naga, K.C.; Mhatre, P.H.; Sharma, S.; Venkatasalam, E.P.; Singh, B. Kaolinite nanoclay-shielded dsRNA drenching for management of Globodera pallida: An environmentally friendly pest management approach. Protoplasma 2024, 261, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.F.; Ryder, M.H. Feeding of the nematode Acrobeloides nanus on bacteria. J. Nematol. 1993, 25, 493–499. [Google Scholar] [PubMed]
- Alvarez, O.A.; Jager, T.; Redondo, E.M.; Kammenga, J.E. Physiological modes of action of toxic chemicals in the nematode Acrobeloides nanus. Environ. Toxicol. Chem. 2006, 25, 3230–3237. [Google Scholar] [CrossRef]
- Baermann, G. A simple method for detecting Ankylostomum (nematodes) larvae in soil samples. Med. J. Dutch East Indie 1917, 57, 131–137. [Google Scholar]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- Shirayama, Y.; Kaku, T.; Higgins, R.P. Double-sided microscopic observation of meiofauna using an HS-slide. Benthos Res. 1993, 44, 41–44. [Google Scholar] [CrossRef]
- Swibawa, I.G.; Fitriana, Y.U.; Suharjo, R.; Susilo, F.X.; Rani, E.K.A. Morpho-molecular identification and pathogenicity test on fungal parasites of guava root-knot nematode eggs in Lampung, Indonesia. Biodiversitas 2020, 21, 1108–1115. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, W.M.; Shipley, L.G.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schimittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, C.; Zhuge, J.; Tang, S.; Zhou, X.; Zhou, L.; Guo, K. Comparative effects of two in situ hybridization methods for the pinewood nematode (Bursaphelenchus xylophilus). Front. Microbiol. 2023, 14, 234895. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Jin, G.; Kim, Y. Spraying dsRNA with chitosan formulation improves control of the western flower thrips, Frankliniella occidentalis, in a greenhouse. Insect Mol. Biol. 2024, 34, 552–569. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STAT User’s Guide; Release 6.03; SAS Institute Inc.: Cary, NC, USA, 1989. [Google Scholar]
- Nsengimana, J.; Bauters, L.; Haegeman, A.; Gheysen, G. Silencing of Mg-pat-10 and Mg-unc-87 in the plant parasitic nematode Meloidogyne graminicola using siRNAs. Agriculture 2013, 3, 567–578. [Google Scholar] [CrossRef]
- Khan, F.; Esmaeily, M.; Jin, G.; Sevin, S.; Jung, C.; Ham, E.; Kim, Y. A sprayable long hairpin dsRNA formulated with layered double hydroxide against the western flower thrips, Frankliniella occidentalis: Control efficacy in a greenhouse and influence on beneficial insects. Pestic. Biochem. Physiol. 2025, 209, 106331. [Google Scholar] [CrossRef]
- Hao, W.; Li, Q.; Zhang, J.; Jiang, Y.; Liang, W. Utility of nematode Acrobeloides nanus for assessing subacute toxicity of heavy metals. Environ. Monit. Assess. 2010, 164, 273–278. [Google Scholar] [CrossRef]
- Schiffer, P.H.; Polsky, A.L.; Cole, A.G.; Camps, J.I.R.; Kroiher, M.; Silver, D.H.; Grishkevich, V.; Anavy, L.; Koutsovoulos, G.; Hashimshony, T.; et al. The gene regulatory program of Acrobeloides nanus reveals conservation of phylum-specific expression. Proc. Natl. Acad. Sci. USA 2018, 115, 4459–4464. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Bae, Y.J.; Park, J. First record of Acrobeloides nanus (Cephalobidae: Rhabditida: Nematoda) from Korea. Anim. Syst. Evol. Divers. 2016, 32, 258–265. [Google Scholar] [CrossRef]
- Pervez, R. Acrobeloides ishraqi sp. n. and Acrobeloides mushtaqi sp. n. (Nematoda: Rhabditida) from chickpea rhizosphere, Uttar Pradesh, India. Arch. Phytopathol. Plant Prot. 2011, 44, 1438–1446. [Google Scholar] [CrossRef]
- Dong, L.; Xu, J.; Chen, S.; Li, X.; Zuo, Y. Mi-flp-18 and Mi-mpk-1 genes are potential targets for Meloidogyne incognita control. J. Parasitol. 2016, 102, 208–213. [Google Scholar] [CrossRef]
- Joseph, S.; Gheysen, G.; Subramaniam, K. RNA interference in Pratylenchus coffeae: Knock down of Pc-pat-10 and Pc-unc-87 impedes migration. Mol. Biochem. Parasitol. 2012, 186, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wei, Z.; Zhang, M.; Ma, P.; Liu, G.; Zheng, J.; Guo, X.; Zhang, P. Resistance to Ditylenchus destructor infection in sweet potato by the expression of small interfering RNAs targeting unc-15, a movement-related gene. Phytopathology 2015, 105, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Gill, S.S.; Gawade, B.H.; Jain, P.K.; Subramaniam, K.; Sirohi, A. Host delivered RNAi of two cuticle collagen genes, Mi-col-1 and Lemmi-5 hampers structure and fecundity in Meloidogyne incognita. Front. Plant Sci. 2018, 8, 2266. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.F.; Lourenço-Tessutti, I.T.; Mendes, R.A.G.; Pinto, C.E.M.; Bournaud, C.; Gillet, F.X.; Togawa, R.C.; de Macedo, L.L.P.; de Almeida Engler, J.; Grossi-de-Sa, M.F. MiDaf16-like and MiSkn1-like gene families are reliable targets to develop biotechnological tools for the control and management of Meloidogyne incognita. Sci. Rep. 2020, 10, 6991. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, I.; Changwal, C.; Sirohi, A.; Jain, P.K. Host-delivered RNAi-mediated silencing of the root-knot nematode (Meloidogyne incognita) effector genes, Mi-msp10 and Mi-msp23, confers resistance in Arabidopsis and impairs reproductive ability of the root-knot nematode. Planta 2022, 256, 74. [Google Scholar] [CrossRef]
- Thorat, Y.E.; Dutta, T.K.; Jain, P.K.; Subramaniam, K.; Sirohi, A. A nematode-inducible promoter can effectively drive RNAi construct to confer Meloidogyne incognita resistance in tomato. Plant Cell Rep. 2023, 43, 3. [Google Scholar] [CrossRef]
- Schmitt-Engel, C.; Schultheis, D.; Schwirz, J.; Ströhlein, N.; Troelenberg, N.; Majumdar, U.; Dao, V.A.; Grossmann, D.; Richter, T.; Tech, M.; et al. The iBeetle large-scale RNAi screen reveals gene functions for insect development and physiology. Nat. Commun. 2015, 8, 7822. [Google Scholar] [CrossRef]
- Ulrich, J.; Dao, V.A.; Majumdar, U.; Schmitt-Engel, C.; Schwirz, J.; Schultheis, D.; Ströhlein, N.; Troelenberg, N.; Grossmann, D.; Richter, T.; et al. Large scale RNAi screen in Tribolium reveals novel target genes for pest control and the proteasome as prime target. BMC Genom. 2015, 16, 674. [Google Scholar] [CrossRef]
- Oka, T.; Toyomura, T.; Honjo, K.; Wada, Y.; Futai, M. Four subunit a isoforms of Caenorhabditis elegans vacuolar H+-ATPase. Cell-specific expression during development. J. Biol. Chem. 2001, 276, 33079–33085. [Google Scholar] [CrossRef]
- Barroso, I.G.; Santos, C.S.; Bertotti, M.; Ferreira, C.; Terra, W.R. Molecular mechanisms associated with acidification and alkalization along the larval midgut of Musca domestica. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 237, 110535. [Google Scholar] [CrossRef]
- Choi, K.Y.; Ji, Y.J.; Dhakal, B.K.; Yu, J.R.; Cho, C.; Song, W.K.; Ahnn, J. Vacuolar-type H+-ATPase E subunit is required for embryogenesis and yolk transfer in Caenorhabditis elegans. Gene 2003, 311, 13–23. [Google Scholar] [CrossRef]
- Khan, F.; Kim, M.; Kim, Y. Greenhouse test of spraying dsRNA to control the western flower thrips, Frankliniella occidentalis, infesting hot peppers. BMC Biotechnol. 2023, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Dubelman, S.; Fischer, J.; Zapata, F.; Huizinga, K.; Jiang, C.; Uffman, J.; Levine, S.; Carson, D. Environmental fate of double-stranded RNA in agricultural soils. PLoS ONE 2014, 9, e93155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wan, F.; Jian, Y.; Guo, F.; Zhang, M.; Shi, S.; Yang, L.; Li, S.; Liu, Y.; Ding, W. Chitosan/dsRNA polyplex nanoparticles advance environmental RNA interference efficiency through activating clathrin-dependent endocytosis. Int. J. Biol. Macromol. 2023, 253, 127021. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, D.; Mogilicherla, K.; Palli, S.R. Chitosan nanoparticles help double-stranded RNA escape from endosomes and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21677. [Google Scholar] [CrossRef]
- Adams, B.J.; Burnell, A.M.; Powers, T.O. A phylogenetic analysis of Heterorhabditis (Nemata: Rhabditidae) based on internal transcribed spacer 1 DNA sequence data. J. Nematol. 1998, 30, 22–39. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).