Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture
Simple Summary
Abstract
1. Introduction
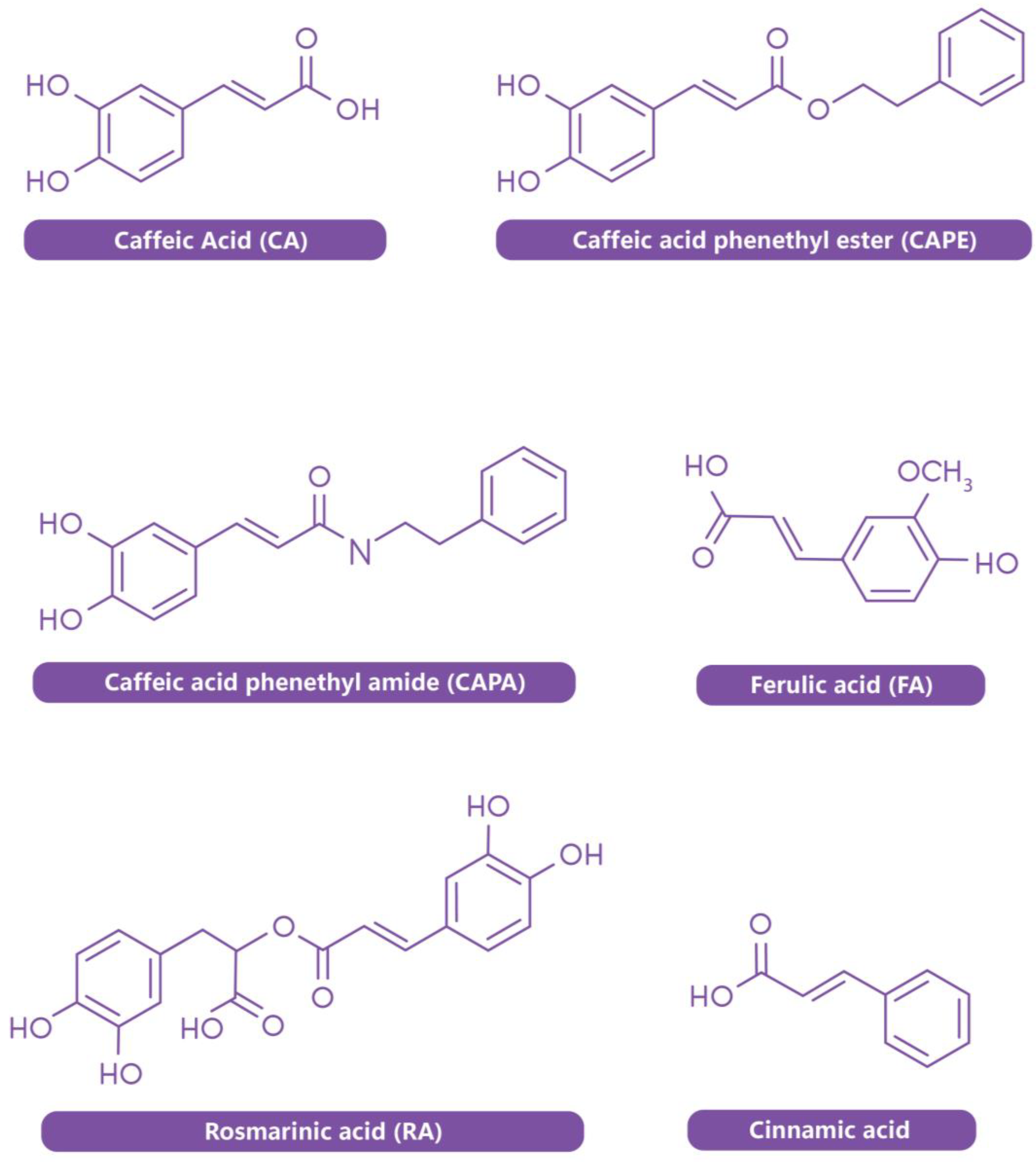
2. Sources of CA and Its Derivatives
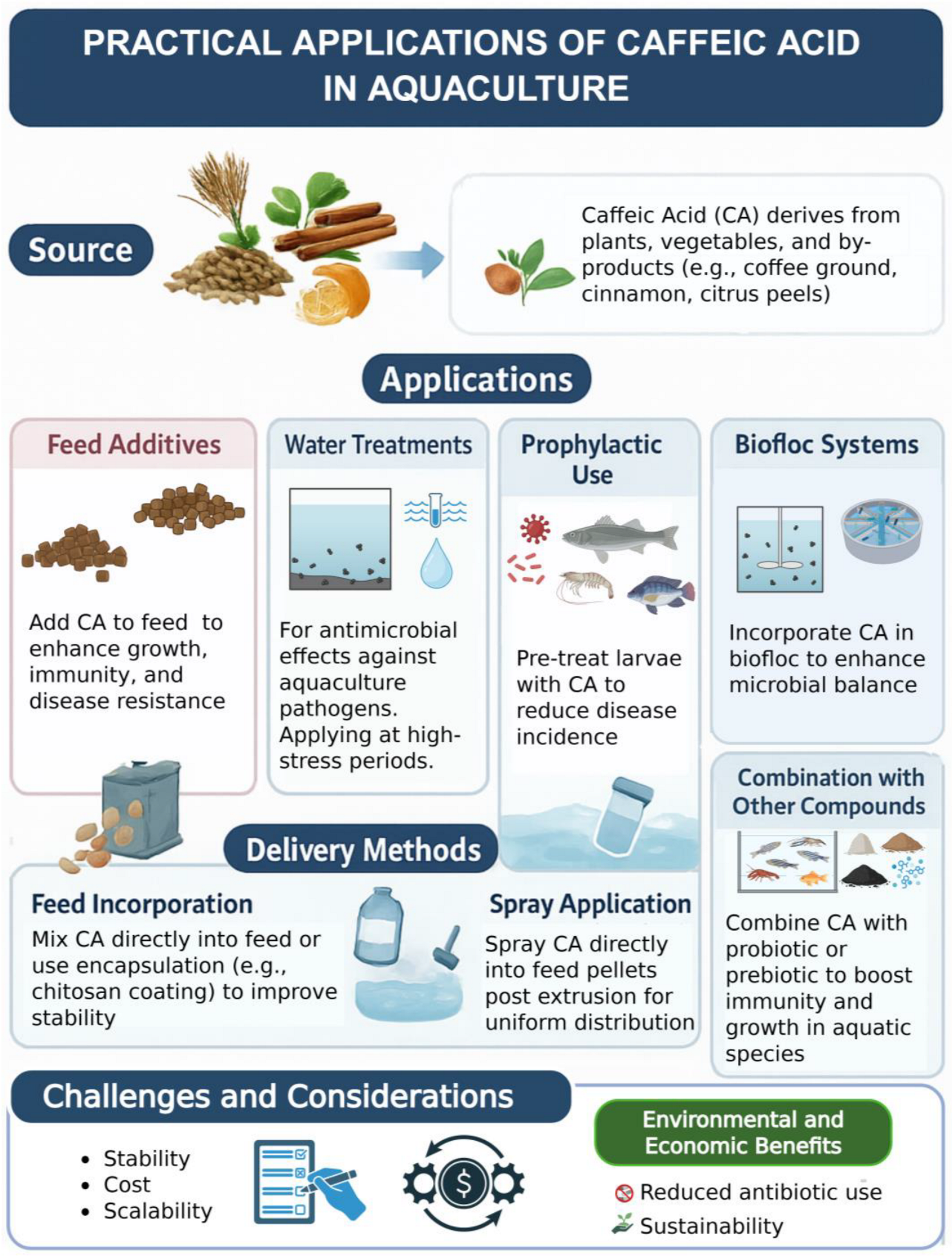
3. Applications of CA and Its Derivatives in Aquaculture
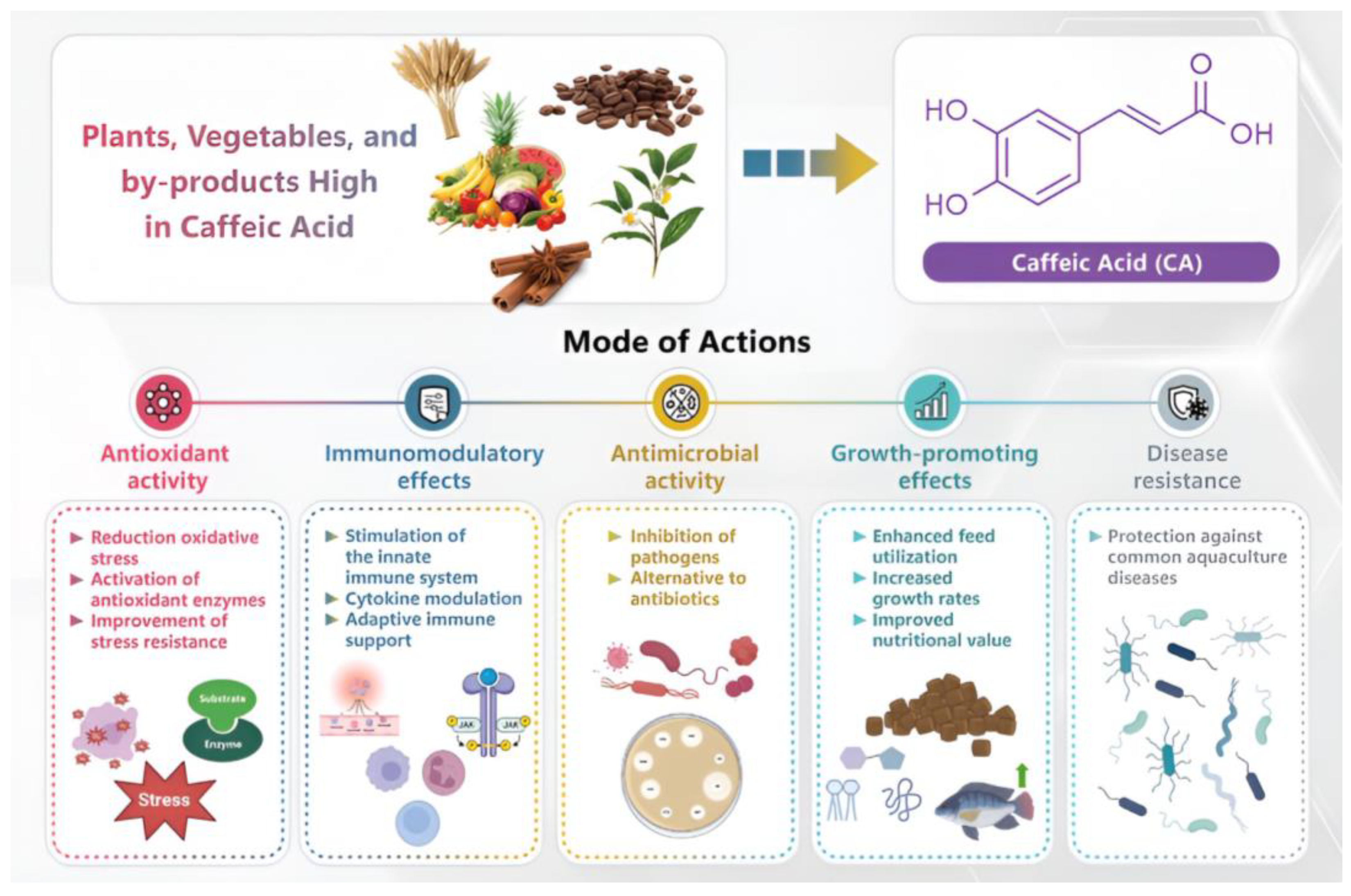
3.1. Applications of Caffeic Acid
3.2. Applications of CA Derivatives
3.3. Challenges and Gaps in Applying CA and Its Derivatives in Aquaculture
4. Mechanisms of CA and Its Derivatives in Aquaculture
4.1. Antioxidant Properties
4.2. Pro-Inflammatory and Anti-Inflammatory Properties
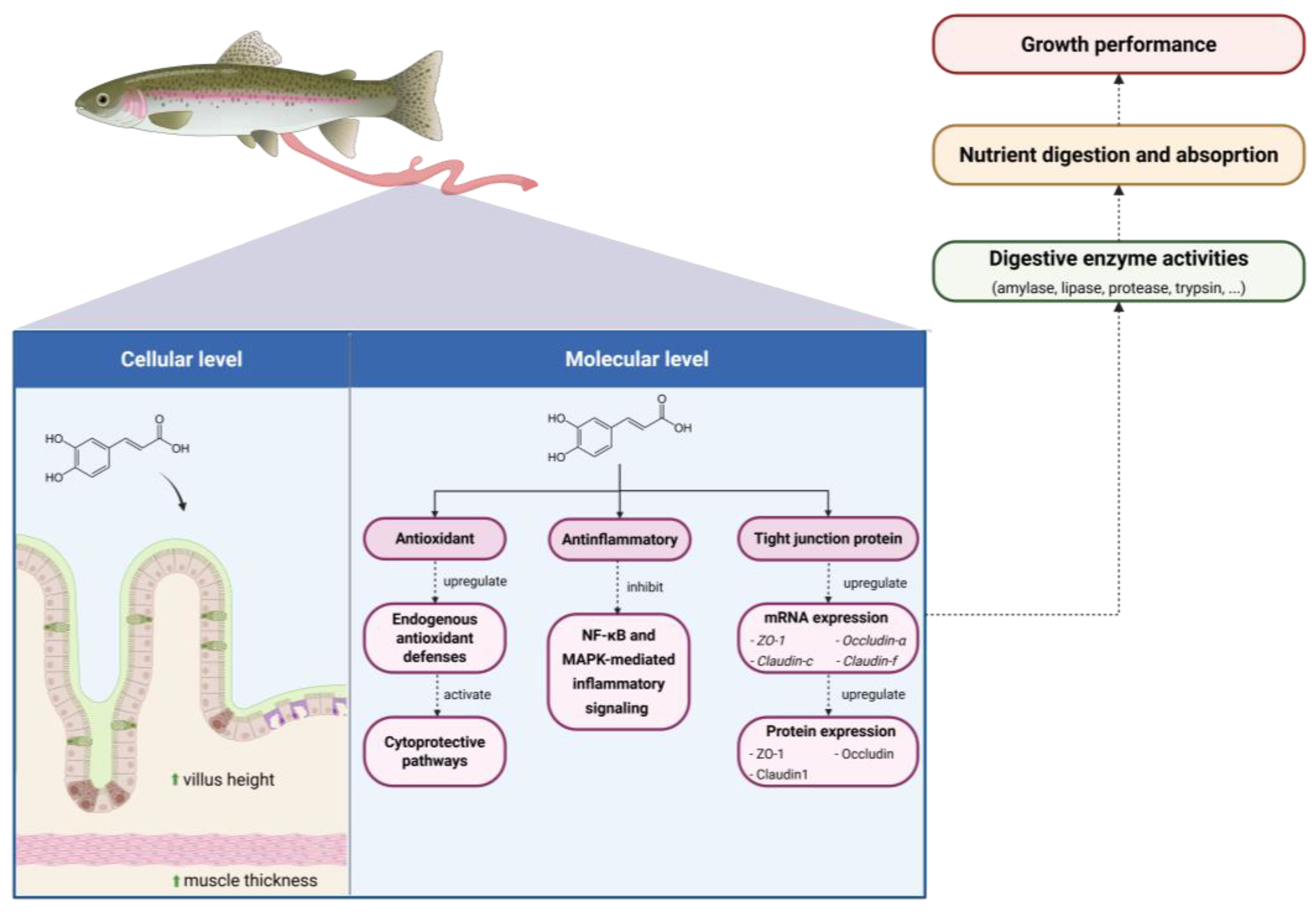
4.3. Digestive System
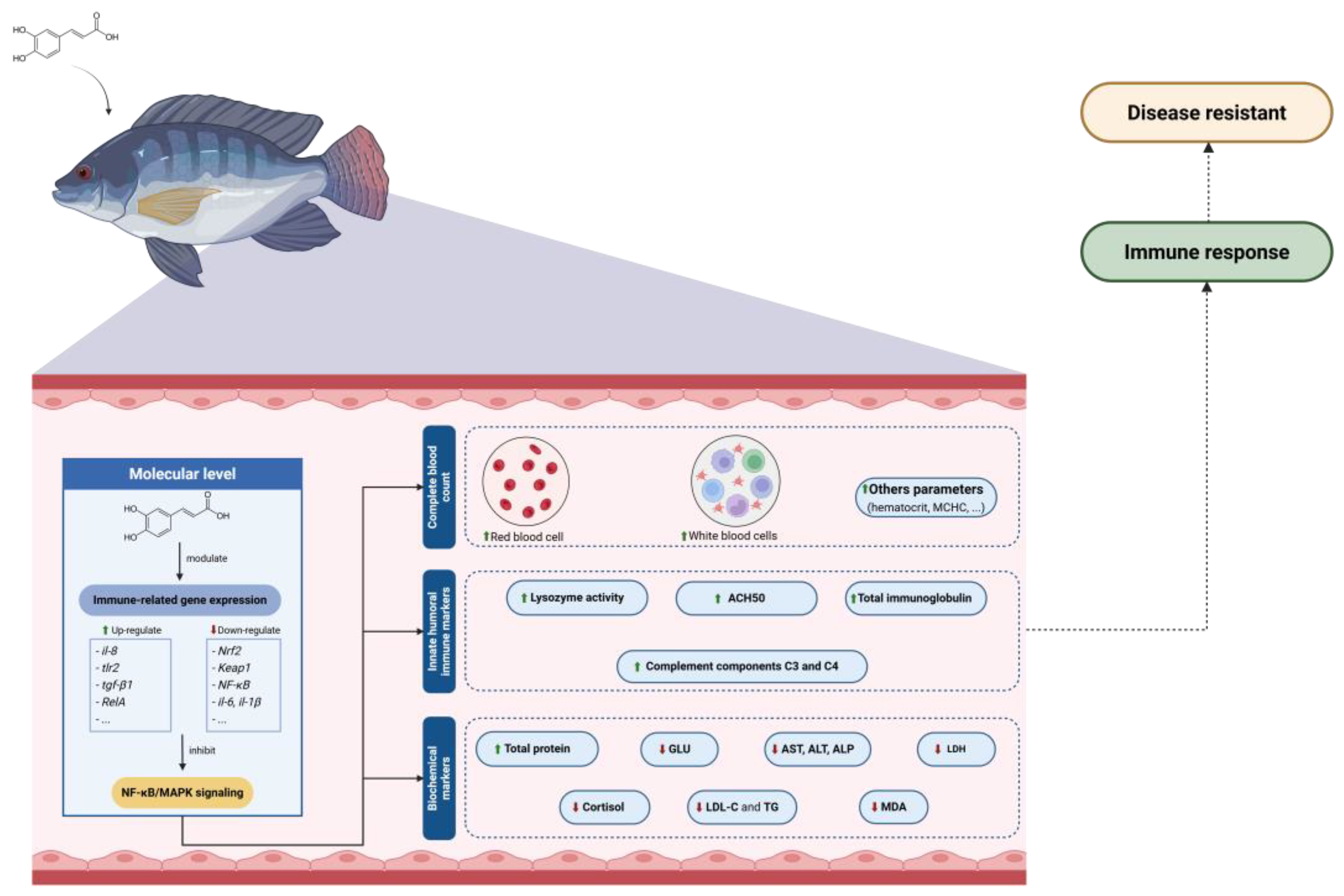
4.4. Immunological Properties
4.5. Using as an Alternative to Antibiotics
5. Challenges and Limitations
6. Future Perspective and Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACH50 | Alternative Complement Hemolytic 50 Activity |
| ALB | Albumin |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| AVM | Abamectin |
| BUN | Blood Urea Nitrogen |
| CA | Caffeic Acid |
| CAPA | Caffeic Acid Phenethyl Amide |
| CAPE | Caffeic Acid Phenethyl Ester |
| CAT | Catalase |
| CF | Condition Factor |
| CGA | Chlorogenic Acid |
| CS-g-CA | Chitosan-grafted Caffeic Acid |
| DAO DAMPs | Diamine Oxidase Damage-associated molecular patterns |
| ET-1 | Endothelin-1 |
| FA | Ferulic Acid |
| FCR | Feed Conversion Ratio |
| FER | Feed Efficiency Ratio |
| FBW | Final Body Weight |
| FI | Feed Intake |
| FW | Final Weight |
| GLO | Globulin |
| GLU | Glucose |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione |
| GSH-Px | Glutathione Peroxidase |
| GST | Glutathione S-Transferase |
| HDL | High-Density Lipoprotein |
| HFD | High-Fat Diet |
| HO-1 | Heme Oxygenase-1 |
| Ig | Immunoglobulin |
| IgM | Immunoglobulin M |
| IL | Interleukin |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LD | Lactate Dehydrogenase |
| LDH | Lactate Dehydrogenase |
| LDLC | Low-Density Lipoprotein Cholesterol |
| LPS | Lipopolysaccharide |
| LYM | Lymphocyte |
| LYZ | Lysozyme |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| MTs | Metallothioneins |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NFI-3 NLRs | Nuclear Factor Interleukin-3 NOD-like receptors |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| Nrf2 | Nuclear Factor Erythroid 2-related Factor 2 |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PER | Protein Efficiency Ratio |
| PP | Pediococcus pentosaceus |
| PRRs | Pattern Recognition Receptors |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| ROS | Reactive Oxygen Species |
| RA | Rosmarinic Acid |
| RLRs | RIG-I-Like Receptors |
| SGR | Specific Growth Rate |
| SOD | Superoxide Dismutase |
| SNP | Sodium Nitroprusside |
| TAS | Total Antioxidant Status |
| TC | Total Cholesterol |
| TGs | Triglycerides |
| TGF-β | Transforming Growth Factor Beta |
| TLR | Toll-Like Receptor |
| TP | Total Protein |
| WG | Weight Gain |
| WGR | Weight Gain Rate |
| WSSV | White Spot Syndrome Virus |
| ZO-1 | Zonula Occludens-1 |
References
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. Comments on FAOs state of world fisheries and aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- Action, S. World fisheries and aquaculture. Food Agric. Organ. 2020, 2020, 1–244. [Google Scholar]
- Arshad, S.; Arshad, S.; Afzal, S.; Tasleem, F. Environmental Impact and Sustainable Practices in Aquaculture: A Comprehensive Review. Haya Saudi J. Life Sci. 2024, 9, 447–454. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Dinh-Hung, N.; Srisapoome, P.; Na-Nakorn, U.; Chatchaiphan, S. Genomics-driven prophylactic measures to increase streptococcosis resistance in tilapia. J. Fish Dis. 2023, 46, 597–610. [Google Scholar] [CrossRef]
- Manage, P.M. Heavy use of antibiotics in aquaculture: Emerging human and animal health problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Lubis, A.R.; Sumon, M.A.A.; Dinh-Hung, N.; Dhar, A.K.; Delamare-Deboutteville, J.; Kim, D.-H.; Shinn, A.P.; Kanjanasopa, D.; Permpoonpattana, P.; Doan, H.V.; et al. Review of quorum-quenching probiotics: A promising non-antibiotic-based strategy for sustainable aquaculture. J. Fish Dis. 2024, 47, e13941. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Dong, H.T.; Senapin, S.; Shinn, A.P.; Linh, N.V.; Dien, L.T.; Soontara, C.; Hirono, I.; Chatchaiphan, S.; Rodkhum, C. Using ozone nanobubbles to mitigate the risk of mycobacteriosis in Siamese fighting fish (Betta splendens). Aquaculture 2024, 581, 740390. [Google Scholar] [CrossRef]
- Linh, N.V.; Lubis, A.R.; Dinh-Hung, N.; Wannavijit, S.; Montha, N.; Fontana, C.M.; Lengkidworraphiphat, P.; Srinual, O.; Jung, W.-K.; Paolucci, M.; et al. Effects of Shrimp Shell-Derived Chitosan on Growth, Immunity, Intestinal Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System. Mar. Drugs 2024, 22, 150. [Google Scholar] [CrossRef]
- Linh, N.V.; Wannavijit, S.; Sumon, M.A.A.; Tayyamath, K.; Dinh-Hung, N.; Brown, C.L.; Nititanarapee, T.; Permpoonpattana, P.; Tapingkae, W.; Srinual, O.; et al. Immunomodulatory and growth-promoting effects of supplementing red yeast (Sporidiobolus pararoseus) in fish meal-based diets for koi carp (Cyprinus carpio var. koi) cultured in a biofloc system. Aquac. Int. 2024, 33, 17. [Google Scholar] [CrossRef]
- Linh, N.V.; Wannavijit, S.; Tayyamath, K.; Dinh-Hung, N.; Nititanarapee, T.; Sumon, M.A.A.; Srinual, O.; Permpoonpattana, P.; Doan, H.V.; Brown, C.L. Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio var. koi). Fishes 2024, 9, 53. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Birková, A.; Hubková, B.; Bolerázska, B.; Mareková, M.; Čižmárová, B. Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Joneidi, S.; Alizadeh, S.R.; Ebrahimzadeh, M.A. Chlorogenic Acid Derivatives: Structural Modifications, Drug Design, and Biological Activities: A Review. Mini Rev. Med. Chem. 2024, 24, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Mi, H.; Ren, M.; Huang, D.; Aboseif, A.M.; Liang, H.; Zhang, L. Chlorogenic acid plays an important role in improving the growth and antioxidant status and weakening the inflammatory response of largemouth bass (Micropterus salmoides). Animals 2024, 14, 2871. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghipour, I.; Nasimi Shad, A.; Askari, V.R.; Maharati, A.; Baradaran Rahimi, V. How caffeic acid and its derivatives combat diabetes and its complications: A systematic review. J. Funct. Foods 2023, 110, 105862. [Google Scholar] [CrossRef]
- Medina, I.; Gallardo, J.; González, M.J.; Lois, S.; Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Mohammadzadeh, S.; Kalhor, N.; Salehi, F.; Eslami, M.; Zaretabar, A.; Moghadam, M.S.; Hoseinifar, S.H.; Van Doan, H. Effects of caffeic acid on the growth performance, growth genes, digestive enzyme activity, and serum immune parameters of beluga (Huso huso). J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 715–723. [Google Scholar] [CrossRef]
- Alavinejad, S.S.; Soltani, M.; Mirzargar, S.S.; Shohreh, P.; Taherimirghaed, A. Influence of caffeic acid and Bacillus coagulans supplementation on growth, digestive enzymes, immune response, and antioxidant gene expression in common carp (Cyprinus carpio) and its resistance to Aeromonas hydrophila infection. Aquac. Rep. 2025, 40, 102515. [Google Scholar] [CrossRef]
- Yilmaz, S. Effects of dietary caffeic acid supplement on antioxidant, immunological and liver gene expression responses, and resistance of Nile tilapia, Oreochromis niloticus to Aeromonas veronii. Fish Shellfish Immunol. 2019, 86, 384–392. [Google Scholar] [CrossRef]
- Li, Q.; Fu, M.; Zhu, S.; Liu, J.; Li, Y.; Xue, Z.; Zhou, Z.; Yu, L. Effects of dietary hydroxy-cinnamic acid derivatives on growth, muscle, and intestinal parameters of Tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2025, 51, 4. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, E.; Babin, P.J.; Gutierrez, J.; Capilla, E.; Navarro, I. Caffeic acid and hydroxytyrosol have anti-obesogenic properties in zebrafish and rainbow trout models. PLoS ONE 2017, 12, e0178833. [Google Scholar] [CrossRef]
- Guan, Y.; Lan, W.-q.; Sun, Y.-q.; Liu, L.; Zhou, D.-p.; Xie, J. Effect of ultrasonic treatment combined with caffeic acid on the quality of sea bass (Lateolabrax japonicas) during refrigerated storage. Food Sci. 2022, 43, 207–214. [Google Scholar]
- Chung, M.J.; Walker, P.A.; Hogstrand, C. Dietary phenolic antioxidants, caffeic acid and Trolox, protect rainbow trout gill cells from nitric oxide-induced apoptosis. Aquat. Toxicol. 2006, 80, 321–328. [Google Scholar] [CrossRef]
- Li, H.; Ji, S.; Song, L.; Wei, M.; Tian, Z.; Ji, H.; Sun, J. Caffeic acid phenethyl ester promotes adipocyte hyperplasia and improves the growth performance, lipid metabolism, and inflammation in grass carp, Ctenopharyngodon idellus fed with high-fat diet. Aquaculture 2025, 599, 742201. [Google Scholar] [CrossRef]
- Ji, S.; Song, L.; Tian, Z.; Wei, M.; Ji, H.; Sun, J. Caffeic acid phenethyl ester improves high-carbohydrate diet utilization by promoting adipocyte hyperplasia in grass carp (Ctenopharyngodon idellus). Anim. Nutr. 2025, 22, 154–164. [Google Scholar] [CrossRef]
- Park, M.K.; Im, G.J.; Chang, J.; Chae, S.W.; Yoo, J.; Han, W.-g.; Hwang, G.H.; Jung, J.Y.; Choi, J.; Jung, H.H. Protective effects of caffeic acid phenethyl ester (CAPE) against neomycin-induced hair cell damage in zebrafish. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1311–1315. [Google Scholar] [CrossRef]
- Cravioveanu, M.G.; Gheorghe, S.; Lucaciu, I.; Stoica, L.; Constantin, C. Assessment of aquatic toxicity of the caffeic acid complexed with Cr (III) and Pb (II) in the flotation process. Rev. Chim 2014, 65, 339–343. [Google Scholar]
- Lan, W.; Zhang, B.; Liu, S.; Sun, Y.; Xie, J. Carbodiimide-mediated grafting of caffeic acid on chitosan to improve its physicochemical and biological properties: Used for Pompano (Trachinotus ovatus) preservation. Int. J. Food Sci. Technol. 2023, 58, 4683–4696. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, J.; Wei, X.; Sun, Y.; Liu, S.; Sun, X. Chitosan-grafted-caffeic acid combined with ultrasound inhibits the oxidation and degradation of myofibrillar proteins in pompano (Trachinotus ovatus) during ice storage. Food Funct. 2023, 14, 4595–4606. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, S.; Li, D.; Liu, S.; Li, L.; Wu, L. Dietary chlorogenic acid supplementation protects against lipopolysaccharide-induced oxidative stress, inflammation and apoptosis in intestine of amur ide (Leuciscus waleckii). Aquat. Toxicol. 2025, 279, 107223. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, C.; Yuan, Q.; Lv, W.; Qiu, J.; Li, M.; Zhang, Q.; Zhou, W. Effects of Dietary Chlorogenic Acid on the Growth, Lipid Metabolism, Antioxidant Capacity, and Non-Specific Immunity of Asian Swamp Eel (Monopterus albus). Fishes 2024, 9, 496. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Lin, J.; He, X.; Rong, H.; Lin, F.; Wen, X. Dietary Chlorogenic Acid Improved Muscle Quality, Antioxidant Capacity, and Pro-Inflammatory Responses of Blackspotted Croaker Protonibea diacanthus. Aquac. Nutr. 2024, 2024, 7867796. [Google Scholar] [CrossRef]
- Bakhtiari, F.; Ahmadifar, E.; Moghadam, M.S.; Mohammadzadeh, S.; Mahboub, H.H. Dual effects of dietary Lactobacillus helveticus and chlorogenic acid on growth performance, digestibility, immune-antioxidant capacity and resistance against heat stress of juvenile common carp. Aquac. Int. 2024, 32, 7911–7927. [Google Scholar] [CrossRef]
- Jin, X.; Su, M.; Liang, Y.; Li, Y. Effects of chlorogenic acid on growth, metabolism, antioxidation, immunity, and intestinal flora of crucian carp (Carassius auratus). Front. Microbiol. 2023, 13, 1084500. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Xu, H.; Chen, J.; Xu, X.; Leng, X. Effects of dietary chlorogenic acid on growth, flesh quality and serum biochemical indices of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2017, 23, 1254–1263. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Li, X.; Leng, X. Individual and combined effects of dietary chlorogenic acid and quercetin supplementation on the growth, lipid metabolism and flesh quality of grass carp, Ctenopharyngodon idellus. Anim. Feed Sci. Technol. 2024, 318, 116129. [Google Scholar] [CrossRef]
- Xu, G.; Xing, W.; Yu, H.; Jiang, N.; Ma, Z.; Luo, L.; Li, T. Evaluation of chlorogenic acid supplementation in koi (Cyprinus carpio) diet: Growth performance, body color, antioxidant activity, serum biochemical parameters, and immune response. Aquac. Nutr. 2022, 2022, 2717003. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; Fang, H.; Tian, L.; Liu, Y.; Niu, J. Chlorogenic acid improves health in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets: Involvement of lipid metabolism, antioxidant ability, inflammatory response, and intestinal integrity. Aquaculture 2021, 545, 737169. [Google Scholar] [CrossRef]
- Liu, X.-r.; Ma, X.; Zhang, N.; Li, M.; Li, K.; Jiao, S.-q.; Wang, G.-q.; Kong, Y.-d. Effects of chlorogenic acid in feed on the growth performance, digestive enzyme activity, immune function, and antioxidant capacity of loach (Misgurnus anguillicaudatus). J. Fish. China 2023, 47, 99–112. [Google Scholar]
- Zhai, Q.; Chang, Z.; Li, J.; Li, J. Effects of combined florfenicol and chlorogenic acid to treat acute hepatopancreatic necrosis disease in Litopenaeus vannamei caused by Vibrio parahaemolyticus. Aquaculture 2022, 547, 737462. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, J.; Duan, Y.-F.; Niu, J.; Wang, J.; Huang, Z.; Lin, H.-Z. Effects of dietary chlorogenic acid on growth performance, antioxidant capacity of white shrimp Litopenaeus vannamei under normal condition and combined stress of low-salinity and nitrite. Fish Shellfish Immunol. 2015, 43, 337–345. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Nedaei, S.; Hoseinifar, S.H.; Van Doan, H. Effect of different levels of chlorogenic acid on growth performance, immunological responses, antioxidant defense, and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles. Aquac. Nutr. 2023, 2023, 3679002. [Google Scholar] [CrossRef]
- Chen, C.; Wang, T.; Shen, J.-L.; Liang, C.-S.; Ling, F.; Li, P.-F.; Wang, G.-X. Evaluation of the antiviral activity of chlorogenic acid against white spot syndrome virus. Aquaculture 2024, 579, 740242. [Google Scholar] [CrossRef]
- Ma, J.; Kong, L.; Zhou, S.; Lin, H.; Lin, Y.; Qin, H.; Long, Z.; Liu, L.; Huang, Z.; Li, Z. Effect of supplementation of chlorogenic acid to high-fat diet on growth, lipid metabolism, intestinal and hepatic histology, and gut microbiota of spotted sea bass (Lateolabrax maculatus). Metabolites 2023, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, J.; Yan, Z.; Gao, M.; Lin, K.; Zhan, Y.; Li, Y.; Liang, J.; Han, S. Effects of chlorogenic acid on growth performance, immunity, antioxidant capacity, intestinal microbiota, and liver transcriptome in Mauremys mutica. Aquac. Rep. 2025, 42, 102851. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergun, S.; Şanver Çelik, E.; Yigit, M.; Bayizit, C. Dietary trans-cinnamic acid application for rainbow trout (Oncorhynchus mykiss): II. Effect on antioxidant status, digestive enzyme, blood biochemistry and liver antioxidant gene expression responses. Aquac. Nutr. 2019, 25, 1207–1217. [Google Scholar] [CrossRef]
- Yılmaz, S.; Ergun, S.; Yigit, M.; Çelik, E.Ş. Effect of combination of dietary Bacillus subtilis and trans-cinnamic acid on innate immune responses and resistance of rainbow trout, Oncorhynchus mykiss to Yersinia ruckeri. Aquac. Res. 2020, 51, 441–454. [Google Scholar] [CrossRef]
- Lin, Y.; Miao, L.; Li, X.; Qian, L.; Mu, Q.; Liu, B.; Ge, X.; Leng, X. Effects of ferulic acid on the growth performance, physiological and biochemical functions, and hepato-intestinal health of blunt snout bream, Megalobrama amblycephala. Aquac. Rep. 2023, 33, 101879. [Google Scholar] [CrossRef]
- Wang, G.; Guo, J.; Ma, Y.; Xin, Y.; Ji, X.; Sun, Y.; Zhang, J.; Dong, J. Ferulic acid alleviates carp brain damage and growth inhibition caused by avermectin by modulating the Nrf2/Keap1 and NF-κB signaling pathways. Pestic. Biochem. Physiol. 2023, 196, 105590. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, M.; Pan, E.; Li, Y.; Yan, W.; Li, Y.; Ji, G.; Dong, J. Protective effect of feed additive ferulic acid on respiratory depression and oxidation imbalance of carp induced by pesticide difenoconazole via ROS/NF-κB/NLRP3 axis. Fish Shellfish Immunol. 2024, 151, 109659. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, S.; Pan, E.; Ji, X.; Zhou, M.; Zhang, S.; Xu, B.; Feng, H.; Yin, J.; Dong, Z. Ferulic acid alleviates long-term avermectin-induced damage to the spleen of carp and restores its inflammatory response and oxidative balance. J. Environ. Sci. 2025, 151, 616–626. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Moghadam, M.S.; Dawood, M.A.; Hoseinifar, S.H. Lactobacillus fermentum and/or ferulic acid improved the immune responses, antioxidative defence and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2019, 94, 916–923. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Wu, S.; Ai, C.; Yang, Q.; Jia, J.; Xu, X.; Wu, M.; Jiang, J. Effects of ferulic acid on respiratory metabolism, oxidative lesions, and apoptotic parameters in gills and red blood cells of carp (Cyprinus carpio var. Jian) response to copper. Antioxidants 2024, 13, 314. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Y.; Shi, H.; Miao, L.; Liu, B.; Ge, X. Dietary ferulic acid supplementation improved cottonseed meal-based diet utilization by enhancing intestinal physical barrier function and liver antioxidant capacity in grass carp (Ctenopharyngodon Idellus). Front. Physiol. 2022, 13, 922037. [Google Scholar] [CrossRef]
- Yu, L.; Wen, H.; Jiang, M.; Wu, F.; Tian, J.; Lu, X.; Xiao, J.; Liu, W. Effects of ferulic acid on intestinal enzyme activities, morphology, microbiome composition of genetically improved farmed tilapia (Oreochromis niloticus) fed oxidized fish oil. Aquaculture 2020, 528, 735543. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.-S.; El-Sharawy, M.E.; Ghozlan, A.M.; Abdel-Latif, H.M.; Van Doan, H.; Ali, M.A. The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 2020, 525, 735320. [Google Scholar] [CrossRef]
- Lu, M.; Jing, F.; Liu, R.; Chen, Z.; Tong, R.; Li, Y.; Pan, L. The effects and mechanisms of dietary ferulic acid (FA) and dihydromyricetin (DMY) on growth and physiological responses of the shrimp (Litopenaeus vannamei). Aquaculture 2024, 589, 740967. [Google Scholar] [CrossRef]
- Habibnia, M.; Bahram, S.; Bahrekazemi, M.; Javadian, S.R.; Hedayatifard, M.; Abdel-Tawwab, M. Effects of dietary Pediocuccus pentosaceus and/or ferulic acid on performance, digestive enzymes, blood biochemistry, and growth-related genes expression in rainbow trout (Oncorhynchus mykiss). Aquac. Fish. 2024, 10, 628–635. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Liu, B.; Zhou, Q.; Sun, C.; Zheng, X.; Han, Y. Dietary effect of ferulic acid on growth performance, physiological response, non-specific immunity and disease resistance of oriental river prawn (Macrobrachium nipponense). Aquac. Rep. 2022, 24, 101162. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Zhou, Q.; Zheng, X.; Jiang, S.; Wang, A.; Han, Y.; Xu, G.; Liu, B. Ferulic Acid Relieves the Oxidative Stress Induced by Oxidized Fish Oil in Oriental River Prawn (Macrobrachium nipponense) with an Emphasis on Lipid Metabolism and Gut Microbiota. Antioxidants 2024, 13, 1463. [Google Scholar] [CrossRef]
- Chi, C.; Lin, Y.; Miao, L.; Liu, B.; Ge, X. Effects of dietary supplementation of a mixture of ferulic acid and probiotics on the fillet quality of Megalobrama amblycephala fed with oxidized oil. Aquaculture 2022, 549, 737786. [Google Scholar] [CrossRef]
- Xu, W.; Huang, W.; Yao, C.; Liu, Y.; Yin, Z.; Mai, K.; Ai, Q. Effects of supplemental ferulic acid (FA) on survival, growth performance, digestive enzyme activities, antioxidant capacity and lipid metabolism of large yellow croaker (Larimichthys crocea) larvae. Fish Physiol. Biochem. 2022, 48, 1635–1648. [Google Scholar] [CrossRef]
- Shahraki, S.; Ahmadifar, E.; Moghadam, M.S.; Sheikhzadeh, N.; Mohammadzadeh, S.; Hoseinifar, S.H.; Van Doan, H. Protective efficacy of dietary rosmarinic acid and Bacillus subtilis on growth, health parameters and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio). Anim. Feed Sci. Technol. 2025, 323, 116276. [Google Scholar] [CrossRef]
- Shohreh, P.; Mohammadzadeh, S.; Mahboub, H.H.; Ahmadifar, E.; Elsheshtawy, H.M.; Kalhor, N.; Moghadam, M.S.; Abdel-Tawwab, M. Growth performance, hematological profile, and related genes expression in goldfish (Carassius auratus) fed on rosmarinic acid-enriched diets and subjected to ambient ammonia. Aquaculture 2024, 587, 740861. [Google Scholar] [CrossRef]
- Hajirezaee, S.; Khanjani, M.H. Rosmarinic acid alone or in combination with Lactobacillus rhamnosus ameliorated resistance to ammonia stress in the rainbow trout, Oncorhynchus mykiss: Growth, immunity, antioxidant defense and liver functions. Ann. Anim. Sci. 2023, 23, 819–831. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirao, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Teixeira Filho, J.; Wagner, R.; Godoy, H.T. Chlorogenic and caffeic acids in 64 fruits consumed in Brazil. Food Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef]
- Machado, M.; Machado, S.; Ferreira, H.; Oliveira, M.B.P.; Alves, R.C. Chlorogenic acids profile of Coffee arabica by-products (Cascara and silverskin): A comparison with green and roasted beans. Biol. Life Sci. Forum 2022, 18, 57. [Google Scholar] [CrossRef]
- Machado, M.; Espírito Santo, L.; Machado, S.; Lobo, J.C.; Costa, A.S.; Oliveira, M.B.P.; Ferreira, H.; Alves, R.C. Bioactive potential and chemical composition of coffee by-products: From pulp to silverskin. Foods 2023, 12, 2354. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Sun, Y.; Li, S.; Wang, H. Purification of caffeic acid, chlorogenic acid and luteolin from Caulis lonicerae by high-speed counter-current chromatography. Sep. Purif. Technol. 2008, 63, 721–724. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. by-products: Phenolic composition and effect on Caco-2 cells viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Prakash, D.; Upadhyay, G.; Pushpangadan, P.; Gupta, C. Antioxidant and free radical scavenging activities of some fruits. J. Complement. Integr. Med. 2011, 8, 1–16. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Mandura Jarić, A.; Natucci Pasquino, M.; Cazalens, E.; Šeremet, D.; Pudić, R.; Vojvodić Cebin, A.; Komes, D. The assesement of bioactive potential and sensory acceptability of coffee and its byproducts-cascara and silverskin. Hrvat. časopis Za Prehrambenu Tehnol. Biotehnol. I Nutr. 2021, 16, 35–40. [Google Scholar] [CrossRef]
- Chaowuttikul, C.; Palanuvej, C.; Ruangrungsi, N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020, 56, e17547. [Google Scholar] [CrossRef]
- Pyne, S.; Paria, K. Optimization of extraction process parameters of caffeic acid from microalgae by supercritical carbon dioxide green technology. BMC Chem. 2022, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Drăghici-Popa, A.M.; Boscornea, A.C.; Brezoiu, A.M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of Extraction Process Factors on the Composition and Antioxidant Activity of Blackthorn (Prunus spinosa L.) Fruit Extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Pazos, M.; Andersen, M.L.; Skibsted, L.H.; Medina, I. Caffeic acid as antioxidant in fish muscle: Mechanism of synergism with endogenous ascorbic acid and α-tocopherol. J. Agric. Food Chem. 2009, 57, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Tapan, F.; Ergun, S.; Yilmaz, S. The determination of the antibacterial effects of caffeic acid on fish pathogens: Caffeic acid on fish pathogens. Aquat. Anim. Rep. 2023, 1, 1–8. [Google Scholar]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Galmarini, M.V.; Maury, C.; Mehinagic, E.; Sanchez, V.; Baeza, R.I.; Mignot, S.; Zamora, M.C.; Chirife, J. Stability of individual phenolic compounds and antioxidant activity during storage of a red wine powder. Food Bioprocess Technol. 2013, 6, 3585–3595. [Google Scholar] [CrossRef]
- Villanueva, M.P.; Gioia, C.; Sisti, L.; Martí, L.; Llorens-Chiralt, R.; Verstichel, S.; Celli, A. Valorization of ferulic acid from agro-industrial by-products for application in agriculture. Polymers 2022, 14, 2874. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. The influence of pH on the thermal stability of 5-O-caffeoylquinic acids in aqueous solutions. Eur. Food Res. Technol. 2011, 233, 223–232. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Gurram, S.; Jha, D.K.; Shah, D.S.; Kshirsagar, M.M.; Amin, P.D. Insights on the critical parameters affecting the probiotic viability during stabilization process and formulation development. AAPS PharmSciTech 2021, 22, 156. [Google Scholar] [CrossRef]
- Santivarangkna, C. Storage Stability of Probiotic Powder. In Advances in Probiotic Technology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hu, X.; Ma, W.; Zhang, D.; Tian, Z.; Yang, Y.; Huang, Y.; Hong, Y. Application of natural antioxidants as feed additives in aquaculture: A review. Biology 2025, 14, 87. [Google Scholar] [CrossRef]
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 125–160. [Google Scholar]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio, M.; Araújo, D.; da Costa, J.G.M. Chemical properties of caffeic and ferulic acids in biological system: Implications in cancer therapy. A review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu (II) ions: Mechanism and structure–activity relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef]
- Cai, H.; Huang, X.; Xu, S.; Shen, H.; Zhang, P.; Huang, Y.; Jiang, J.; Sun, Y.; Jiang, B.; Wu, X. Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur. J. Med. Chem. 2016, 108, 89–103. [Google Scholar] [CrossRef]
- Galati, G.; O’brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Banerjee, S. Inhibition of mackerel (Scomber scombrus) muscle lipoxygenase by green tea polyphenols. Food Res. Int. 2006, 39, 486–491. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Shukry, M.; Abd-Elaziz, R.A. Clinico-pathological findings and expression of inflammatory cytokines, apoptosis, and oxidative stress-related genes draw mechanistic insights in Nile tilapia reared under ammonia-N exposure and Aeromonas hydrophila challenge. Fish Shellfish Immunol. 2022, 127, 1–12. [Google Scholar] [CrossRef]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.; Dayal, S. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Dong, H.T.; Senapin, S.; Linh, N.V.; Shinn, A.P.; Pirarat, N.; Hirono, I.; Chatchaiphan, S.; Rodkhum, C. Infection and histopathological consequences in Siamese fighting fish (Betta splendens) due to exposure to a pathogenic Mycobacterium chelonae via different routes. Aquaculture 2024, 579, 740191. [Google Scholar] [CrossRef]
- Neerukonda, S.N.; Katneni, U. Avian pattern recognition receptor sensing and signaling. Vet. Sci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Jørgensen, J.B. The innate immune response in fish. In Fish Vaccination; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 85–103. [Google Scholar]
- Chu, Q.; Sun, Y.; Cui, J.; Xu, T. MicroRNA-3570 modulates the NF-κB pathway in teleost fish by targeting MyD88. J. Immunol. 2017, 198, 3274–3282. [Google Scholar] [CrossRef]
- Kondo, T.; Kawai, T.; Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012, 33, 449–458. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Lin, M.; Guo, X.; Xu, X.; Chang, C.; Le, T.N.; Cai, H.; Zhao, M. Caffeic Acid Phenethyl Ester Alleviates Alcohol-Induced Inflammation Associated with Pancreatic Secretion and Gut Microbiota in Zebrafish. Biomolecules 2025, 15, 918. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.; Nunan, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Labbe, R.; Shetty, K. Inhibition of Staphylococcus aureus by phenolic phytochemicals of selected clonal herbs species of Lamiaceae family and likely mode of action through proline oxidation. Food Biotechnol. 2007, 21, 71–89. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Kyselka, J.; Rabiej, D.; Dragoun, M.; Kreps, F.; Burčová, Z.; Němečková, I.; Smolová, J.; Bjelková, M.; Szydłowska-Czerniak, A.; Schmidt, Š. Antioxidant and antimicrobial activity of linseed lignans and phenolic acids. Eur. Food Res. Technol. 2017, 243, 1633–1644. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Servet, C.; Ghelis, T.; Richard, L.; Zilberstein, A.; Savoure, A. Proline dehydrogenase: A key enzyme in controlling cellular homeostasis. Front Biosci 2012, 17, 607. [Google Scholar] [CrossRef]
- Dos Santos, J.F.; Tintino, S.R.; de Freitas, T.S.; Campina, F.F.; Menezes, I.R.d.A.; Siqueira-Júnior, J.P.; Coutinho, H.D.; Cunha, F.A. In vitro e in silico evaluation of the inhibition of Staphylococcus aureus efflux pumps by caffeic and gallic acid. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 22–28. [Google Scholar] [CrossRef]
- Sivakumar, S.; Girija, A.S.; Priyadharsini, J.V. Evaluation of the inhibitory effect of caffeic acid and gallic acid on tetR and tetM efflux pumps mediating tetracycline resistance in Streptococcus sp., using computational approach. J. King Saud Univ.-Sci. 2020, 32, 904–909. [Google Scholar] [CrossRef]
- Pinho, E.; Ferreira, I.C.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simões, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Kwiatek, K.; Janiuk, J.; Witeska, M.; Pękala-Safińska, A. Antibacterial activity of commercial phytochemicals against Aeromonas species isolated from fish. Pathogens 2019, 8, 142. [Google Scholar] [CrossRef]
- Almajano, M.; Carbo, R.; Delgado, M.; Gordon, M. Effect of pH on the antimicrobial activity and oxidative stability of oil-in-water emulsions containing caffeic acid. J. Food Sci. 2007, 72, C258–C263. [Google Scholar] [CrossRef] [PubMed]
- Meneses, C.C.F.; de Sousa, P.R.M.; Lima, K.C.N.; Souza, L.M.M.d.A.; Feio, W.P.; Remédios, C.M.R.; Jouin, J.; Thomas, P.; Masson, O.; Alves, C.N. Caffeic Acid-Zinc Basic Salt/Chitosan Nanohybrid Possesses Controlled Release Properties and Exhibits In Vivo Anti-Inflammatory Activities. Molecules 2023, 28, 4973. [Google Scholar] [CrossRef]
- Aziz, N.; Farag, S.; Mousa, L.; Abo-Zaid, M. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 1998, 93, 43–54. [Google Scholar] [PubMed]
- Rungsimakan, S.; Rowan, M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochem. 2014, 108, 177–188. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Yu, Z.-J.; Cui, Z.-Q.; Peng, L.-Y.; Li, H.-R.; Zhang, C.-L.; Shen, H.-Q.; Yi, P.-F.; Fu, B.-D. In vitro antiviral efficacy of caffeic acid against canine distemper virus. Microb. Pathog. 2017, 110, 240–244. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial activity of the phenolic compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Al-Mughrabi, K.I.; Shahrour, W.; Anfoka, G.H.; Aburaj, T.A. Antifungal activity of olive cake extracts. Phytopathol. Mediterr. 2001, 40, 1000–1005. [Google Scholar]
- BOWLES, B.L.; Miller, A.J. Caffeic acid activity against Clostridium botulinum spores. J. Food Sci. 1994, 59, 905–908. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.M.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Gauthier, L.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, B.M.; Lim, C.S. Bactericidal antibiotic-phytochemical combinations against methicillin resistant Staphylococcus aureus. Braz. J. Microbiol. 2012, 43, 938–945. [Google Scholar] [CrossRef]
- Perumal, S.; Mahmud, R.; Ismail, S. Mechanism of action of isolated caffeic acid and epicatechin 3-gallate from Euphorbia hirta against Pseudomonas aeruginosa. Pharmacogn. Mag. 2017, 13, S311. [Google Scholar] [CrossRef]
- Ogawa, M.; Shirasago, Y.; Ando, S.; Shimojima, M.; Saijo, M.; Fukasawa, M. Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J. Infect. Chemother. 2018, 24, 597–601. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, K.; Zhang, Z.-M.; Fang, R.-Q.; Zhu, H.-L. Synthesis, structure and structure–activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45, 2638–2643. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Al-Dhabi, N.A.; Balakrishna, K.; Kalia, N.P.; Rajput, V.S.; Khan, I.A.; Ignacimuthu, S. Antimicrobial and antimycobacterial activities of methyl caffeate isolated from Solanum torvum Swartz. fruit. Indian J. Microbiol. 2012, 52, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; HRádkoVá, I.; FIlIp, V.; ŠMIdRkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef]
- Sardi, J.d.C.O.; Gullo, F.P.; Freires, I.A.; de Souza Pitangui, N.; Segalla, M.P.; Fusco-Almeida, A.M.; Rosalen, P.L.; Regasini, L.O.; Mendes-Giannini, M.J.S. Synthesis, antifungal activity of caffeic acid derivative esters, and their synergism with fluconazole and nystatin against Candida spp. Diagn. Microbiol. Infect. Dis. 2016, 86, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.; Benfeito, S.; Soares, P.; e Silva, D.M.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-tuning of the hydrophobicity of caffeic acid: Studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Shen, H.; Yamashita, A.; Nakakoshi, M.; Yokoe, H.; Sudo, M.; Kasai, H.; Tanaka, T.; Fujimoto, Y.; Ikeda, M.; Kato, N. Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus. PLoS ONE 2013, 8, e82299. [Google Scholar] [CrossRef]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; Di Santo, R. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorganic Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef]
- Araujo, M.O.; Freire Pessoa, H.L.; Lira, A.B.; Castillo, Y.P.; de Sousa, D.P. Synthesis, antibacterial evaluation, and QSAR of caffeic acid derivatives. J. Chem. 2019, 2019, 3408315. [Google Scholar] [CrossRef]
- Collins, W.; Lowen, N.; Blake, D.J. Caffeic acid esters are effective bactericidal compounds against Paenibacillus larvae by altering intracellular oxidant and antioxidant levels. Biomolecules 2019, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-L.; Zheng, J.; Yu, F.; Cai, Y.-X.; Zhan, X.-L.; Wang, H.-F.; Chen, Q.-X. Anti-tyrosinase kinetics and antibacterial process of caffeic acid N-nonyl ester in Chinese Olive (Canarium album) postharvest. Int. J. Biol. Macromol. 2016, 91, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Casanova, Y.; Bravo-Chaucanés, C.P.; Martínez, A.X.H.; Costa, G.M.; Contreras-Herrera, J.L.; Medina, R.F.; Rivera-Monroy, Z.J.; García-Castañeda, J.E.; Parra-Giraldo, C.M. Combining the peptide RWQWRWQWR and an ethanolic extract of Bidens pilosa enhances the activity against sensitive and resistant Candida albicans and C. auris strains. J. Fungi 2023, 9, 817. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial effect of caffeic acid phenethyl ester on cariogenic bacteria and Streptococcus mutans biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kakino, Y.; Iwai, K.; Mochida, K.; Fujita, T. In vitro antibacterial, antimutagenic and anti-influenza virus activity of caffeic acid phenethyl esters. Biocontrol Sci. 2005, 10, 155–161. [Google Scholar] [CrossRef]
- Meyuhas, S.; Assali, M.; Huleihil, M.; Huleihel, M. Antimicrobial activities of caffeic acid phenethyl ester. J. Mol. Biochem. 2015, 4, 21–31. [Google Scholar]
- Park, M.-Y.; Kang, D.-H. Antibacterial activity of caffeic acid combined with UV-a light against Escherichia coli O157: H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e0063121. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and antibiofilm activity against Streptococcus mutans of individual and mixtures of the main polyphenolic compounds found in Chilean propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef]
- Kataria, R.; Khatkar, A. In-silico design, synthesis, ADMET studies and biological evaluation of novel derivatives of Chlorogenic acid against Urease protein and H. Pylori bacterium. BMC Chem. 2019, 13, 1–17. [Google Scholar] [CrossRef]
- Pinho, E.; Machado, S.; Soares, G. Smart hydrogel for the pH-selective drug delivery of antimicrobial compounds. Macromol. Symp. 2019, 385, 1800182. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Dang, S.; Gupta, S.; Bansal, R.; Ali, J.; Gabrani, R. Nano-encapsulation of a natural polyphenol, green tea catechins: Way to preserve its antioxidative potential. In Free Radicals in Human Health and Disease; Springer: New Delhi, India, 2015; pp. 397–415. [Google Scholar]
- Kasote, D.M.; Jayaprakasha, G.; Patil, B.S. Encapsulation of polyphenols: An effective way to enhance their bioavailability for gut health. Adv. Plant Phenolics Chem. Hum. Health 2018, 13, 239–259. [Google Scholar]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Verardo, V.; Robert, P.; Segura-Carretero, A.; Martínez-Férez, A. Nanoencapsulation strategies applied to maximize target delivery of intact polyphenols. In Encapsulations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 559–595. [Google Scholar]
- Bora, A.F.M.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Richard, N.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Metabolomics and fish nutrition: A review in the context of sustainable feed development. Rev. Aquac. 2020, 12, 261–282. [Google Scholar] [CrossRef]
- Lulijwa, R.; Alfaro, A.C.; Young, T. Metabolomics in salmonid aquaculture research: Applications and future perspectives. Rev. Aquac. 2022, 14, 547–577. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic acid on metabolic syndrome: A review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ge, J.; He, X.; Sheng, Y.; Zheng, S.; Zhang, C.; Xu, W.; Huang, K. Caffeic acid reduces body weight by regulating gut microbiota in diet-induced-obese mice. J. Funct. Foods 2020, 74, 104061. [Google Scholar] [CrossRef]
| Compound | Species | Supplementation | Observed Effects | References |
|---|---|---|---|---|
| Caffeic acid | Atlantic horse mackerel (Trauchurus trauchurus) | 10 to 200 ppm (w/w, 0.001–0.020%) |
| [19] |
| Beluga sturgeon (Huso huso) | 5–10 g/kg CA over 56 days |
| [20] | |
| Common carp (Cyprinus carpio) | 5 g/kg CA + Bacillus coagulans (2 × 107 CFU/g feed) |
| [21] | |
| Nile tilapia (Oreochromis niloticus) | 5 g/kg CA in diet |
| [22] | |
| 0.52 mmol/kg CA |
| [23] | ||
| Rainbow trout (Oncorhynchus mykiss) adipocyte cell | Incubated with vehicle + 50 μM CA |
| [24] | |
| Sea bass (Lateolabrax japonicas) | Dipping fresh sea bass fillets in 2.0 g/L CA, ultrasonic + 2.0 g/L CA |
| [25] | |
| Zebrafish larval (Danio rerio) | Exposure in 50 μM CA |
| [24] | |
| Rainbow trout (Oncorhynchus mykiss) | CA treatment in gill cells exposed to oxidative stress |
| [26] | |
| Caffeic acid phenethyl ester | Grass carp (Ctenopharyngodon idellus) | 0, 200, 500, and 800 mg/kg in high-fat diet |
| [27] |
| 0, 200, 500, and 800 mg/kg in high-carbohydrate diet |
| [28] | ||
| Five-day post-fertilization zebrafish (Danio rerio) larvae | Exposed to 125 μM neomycin and CAPE (50, 100, 250, 500, or 1000 μM) in 1 h |
| [29] | |
| Caffeic acid complexed with Cr(III) and Pb(II) | Common carp (Cyprinus carpio), water flea (Daphnia magna), green algae (Selenastrum capricornutum) | Exposure |
| [30] |
| Carbodiimide-mediated grafting of caffeic acid on chitosan | Pompano (Trachinotus ovatus) | Immersed fresh fillet with 1% CS and CS-g-CA solution |
| [31] |
| Chitosan-grafted caffeic acid | Pompano (Trachinotus ovatus) | Fresh fish slices were treated with ultrasonic, CS-g-CA, and ultrasonic + CS-g-CA |
| [32] |
| Chlorogenic acid | Amur ide (Leuciscus waleckii) | 0.04% |
| [33] |
| Asian swamp eel (Monopterus albus) | 250, 500, 750 mg/kg |
| [34] | |
| Blackspotted croaker (Protonibea diacanthus) | 0, 100, 200, 400, 800, 1600 mg/kg |
| [35] | |
| Common carp (Cyprinus carpio) | 107 CFU/g Lactobacillus helveticus, 550 mg/kg CGA, and a combination of both elements |
| [36] | |
| Crucian carp (Carassius auratus) | 200 mg/kg |
| [37] | |
| 100, 200, 400, 800 mg/kg |
| [38] | ||
| Grass carp (Ctenopharyngodon idellus) | CGA (400 mg/kg), quercetin (QC, 400 mg/kg), and their combinations |
| [39] | |
| Koi carp (Cyprinus carpio) | 200, 400, 600, 800 mg/kg |
| [40] | |
| Largemouth bass (Micropterus salmoides) | 60, 120, 180, and 240 mg/kg |
| [17] | |
| 300 and 600 mg/kg in high-fat diet |
| [41] | ||
| Loach (Misgurnus anguillicaudatus) | 200, 400, 600 and 800 mg/kg |
| [42] | |
| Pacific white shrimp (Litopenaeus vannamei) | CGA (200 mg/kg), low-dose drug combination (100 mg/kg CGA + 7.5 mg/kg FFC), moderate-dose drug combination (200 mg/kg CGA + 15 mg/kg FFC), and a high-dose drug combination (400 mg/kg CGA + 30 mg/kg FFC) |
| [43] | |
| 100, 200 and 400 mg/kg |
| [44] | ||
| Rainbow trout (Oncorhynchus mykiss) | 200, 400, 800 mg/kg |
| [45] | |
| Red swamp crayfish (Procambarus clarkii) | Injected 50 mg/kg |
| [46] | |
| Spotted sea bass (Lateolabrax maculatus) | 100, 200, 300, and 400 mg/kg CGA in high-fat diet |
| [47] | |
| Yellow pond turtles (Mauremys mutica) | 100, 200, 400, 800 mg/kg |
| [48] | |
| Cinnamic acid | Rainbow trout (Oncorhynchus mykiss) | 0.25, 0.50, 0.75, 1.50 |
| [49] |
| Mix of Bacillus subtilis and trans-cinnamic acid (25 mg/kg-25trcBS, 50 mg/kg-50trcBS, 75 mg/kg-75 trcBS, 150 mg/kg-150 trcBS) |
| [50] | ||
| Ferulic acid | Blunt snout bream (Megalobrama amblycephala) | 100, 200 mg/kg |
| [51] |
| Carp (Cyprinus carpio) | 200 mg/kg |
| [52] | |
| 400 mg/kg |
| [53] | ||
| 400 mg/kg |
| [54] | ||
| Lactobacillus fermentum (108 CFU/g) and/or ferulic acid (100 mg/kg) |
| [55] | ||
| Carp (Cyprinus carpio var. Jian) | 0.10, 0.20, 0.30, 0.40 g/kg |
| [56] | |
| Grass carp (Ctenopharyngodon Idellus) | 50, 100, 200 mg/kg |
| [57] | |
| Nile tilapia (Oreochromis niloticus) | Highly oxidized fish oil + FA (0 or 400 mg/kg) |
| [58] | |
| 20, 40, 80, 160 mg/kg |
| [59] | ||
| Pacific white shrimp (Litopenaeus vannamei) | Ferulic acid (0, 50, 100 mg/kg) or/and FA-dihydromyricetin (0, 100, 200 mg/kg) |
| [60] | |
| Rainbow trout (Oncorhynchus mykiss) | 108 CFU Pediocuccus pentosaceus/g, 100 mg/kg of FA, and a combination of PP and FA |
| [61] | |
| River prawn (Macrobrachium nipponense) | 20, 40, 80, 160, 320 mg/kg |
| [62] | |
| 3% oxidized fish oil + 160 and 320 mg/kg of FA |
| [63] | ||
| Wuchang bream (Megalobrama amblycephala) | Oxidized soybean oil + 0.06% FA + probiotics |
| [64] | |
| Yellow croaker (Larimichthys crocea) larvae | 20, 40, 80 mg/kg |
| [65] | |
| Rosmarinic acid | Common carp (Cyprinus carpio) | 5 × 109 CFU Bacillus subtilis/g feed, 600 mg rosmarinic acid/kg feed, combination of these additives |
| [66] |
| Goldfish (Carassius auratus) | 400, 600, 800 mg/kg |
| [67] | |
| Rainbow trout (Oncorhynchus mykiss) | 1.5 or 3 × 108 Lactobacillus rhamnosus, 1 or 3 g RS/kg, and combination of both elements |
| [68] |
| Source/By-Product | CA Concentration (mg/g) | References |
|---|---|---|
| Fruits | ||
| Wild cherry | 10.00–12.00 | [74] |
| Coconut (Cocos nucifera) fruit | 0.0485–2.231 | [75] |
| Earleaf acacia (Acacia auriculiformis) | 0.0485–2.231 | [75] |
| Emblica officinalis fruits | 0.0485–2.231 | [75] |
| Mulberry | 0.200–0.570 | [69] |
| Quince | 0.200–0.570 | [69] |
| Sweet granadilla (Passiflora ligularis) | 0.200–0.570 | [69] |
| Rowanberry | 0.59–0.96 | [72] |
| Chokeberry | 0.59–0.96 | [72] |
| Sweet rowanberry | 0.59–0.96 | [72] |
| Saskatoon berry | 0.59–0.96 | [72] |
| Blueberry | 0.59–0.96 | [72] |
| Blueberries | 0.20–1.00 | [76] |
| Apple (Valkea kuulas) | 0.28 | [72] |
| Dark plum (Syzygium cumini) | 0.28 | [72] |
| Plums | 0.005–0.02 | [76] |
| Kiwis | 0.005–0.02 | [76] |
| Coffee and related products | ||
| Green coffee beans (dw) | 49.57 | [70,71] |
| Defective coffee beans (dw) | 38.00 | [70,71] |
| Green coffee beverage (dw) | 33.70 | [77] |
| Coffee canephora seeds | 12.33 | [78] |
| Coffee pulp (dw) | 2.00 | [71] |
| Cascara (dw) | 1.10 | [70,71] |
| Coffee husk (dw) | 0.839 | [70,71] |
| Teas and Other Sources | ||
| Green/black teas | 0.30–0.36 | [72] |
| Compound | Pathogens | Effective Concentrations | References |
|---|---|---|---|
| Caffeic acid | Aspergillus brasiliensis |
| [113] |
| Aspergillus flavus |
| [124] | |
| Aspergillus parasiticus |
| [124] | |
| Bacillus cereus |
| [124,125] | |
| Candida albicans |
| [113] | |
| Canine distemper virus |
| [126] | |
| Citrobacter freundii |
| [110,127] | |
| Cladosporium sp. |
| [128] | |
| Clostridium botulinum |
| [129] | |
| Colletotrichum sp. |
| [128] | |
| Enterobacter aerogenes |
| [110,127] | |
| Enterobacter cloacae |
| [110,127] | |
| Enterococcus faecalis |
| [125] | |
| Escherichia coli |
| [113,114,120,127,130] | |
| Fusarium graminearum |
| [131] | |
| Fusarium oxysporum |
| [128] | |
| Hepatitis C virus |
| [126] | |
| Herpes Simplex Virus type 1 |
| [132] | |
| Influenza A virus |
| [133] | |
| Klebsiella oxytoca |
| [127,130] | |
| Klebsiella pneumoniae |
| [113,124] | |
| Methicillin-resistant Staphylococcus aureus |
| [130,134] | |
| Methicillin-susceptible Staphylococcus aureus |
| [130] | |
| Mucor sp. |
| [128] | |
| Proteus hauseri |
| [127,130] | |
| Proteus mirabilis |
| [127,130] | |
| Pseudomonas aeruginosa |
| [113,114,135] | |
| Pythium sp. |
| [128] | |
| Rhizoctonia solani |
| [128] | |
| Rhizopus sp. |
| [128] | |
| Salmonella enterica |
| [127,130] | |
| Serratia marcescens |
| [130] | |
| Staphylococcus aureus |
| [111,112,113,114,117,119,120,125] | |
| Staphylococcus epidermidis |
| [119,130] | |
| Thrombocytopenia syndrome virus |
| [136] | |
| Verticillium sp. |
| [128] | |
| Caffeic acid-amides | Bacillus subtilis |
| [137] |
| Caffeic acid-alkyl esters | Aspergillus flavus |
| [138] |
| Bacillus cereus |
| [139] | |
| Candida albicans |
| [138,140] | |
| Escherichia coli |
| [139,141] | |
| Fusarium culmorum |
| [139] | |
| Hepatitis C virus |
| [142] | |
| Proteus vulgaris |
| [138] | |
| Saccharomyces cerevisiae |
| [139] | |
| Staphylococcus aureus |
| [141] | |
| Trichophyton mentagrophytes |
| [138] | |
| Caffeic acid-ester derivatives | Alternaria alternata |
| [143] |
| Candida albicans |
| [144] | |
| Colletotrichum truncatum |
| [143] | |
| Escherichia coli |
| [145] | |
| Fusarium equiseti |
| [143] | |
| Fusarium graminearum |
| [143] | |
| Paenibacillus larvae |
| [146] | |
| Phomopsis longicolla |
| [143] | |
| Septoria bataticola |
| [143] | |
| Staphylococcus aureus |
| [145] | |
| Caffeic acid-N-nonyl ester | Agrobacterium tumefaciens |
| [147] |
| Bacillus subtilis |
| ||
| Escherichia coli |
| ||
| Klebsiella rhinoscleromatis |
| ||
| Pseudomonas aeruginosa |
| ||
| Salmonella sp. |
| ||
| Staphylococcus aureus |
| ||
| Caffeic acid nanoparticles | Ralstonia solanacearum |
| [148] |
| Caffeic acid-phenethyl ester | Actinomyces viscosus |
| [149] |
| Aspergillus niger |
| [150] | |
| Bacillus subtilis |
| [150] | |
| Bacillus megaterium |
| [151] | |
| Candida albicans |
| [150] | |
| Influenza virus type A and B |
| [150] | |
| Klebsiella spp. |
| [151] | |
| Lactobacillus acidophilus |
| [149] | |
| Pseudomonas aeruginosa |
| [150] | |
| Staphylococcus aureus |
| [150] | |
| Streptococcus olysgalactiae |
| [151] | |
| Streptococcus mitis |
| [151] | |
| Streptococcus mutans |
| [149] | |
| Streptococcus sobrinus |
| [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh-Hung, N.; Khang, L.T.P.; Wisetkaeo, S.; Tran, N.T.; Po-Tsang, L.; Brown, C.L.; Sangsawad, P.; Dwinanti, S.H.; Permpoonpattana, P.; Linh, N.V. Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture. Biology 2025, 14, 1160. https://doi.org/10.3390/biology14091160
Dinh-Hung N, Khang LTP, Wisetkaeo S, Tran NT, Po-Tsang L, Brown CL, Sangsawad P, Dwinanti SH, Permpoonpattana P, Linh NV. Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture. Biology. 2025; 14(9):1160. https://doi.org/10.3390/biology14091160
Chicago/Turabian StyleDinh-Hung, Nguyen, Luu Tang Phuc Khang, Suwanna Wisetkaeo, Ngoc Tuan Tran, Lee Po-Tsang, Christopher L. Brown, Papungkorn Sangsawad, Sefti Heza Dwinanti, Patima Permpoonpattana, and Nguyen Vu Linh. 2025. "Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture" Biology 14, no. 9: 1160. https://doi.org/10.3390/biology14091160
APA StyleDinh-Hung, N., Khang, L. T. P., Wisetkaeo, S., Tran, N. T., Po-Tsang, L., Brown, C. L., Sangsawad, P., Dwinanti, S. H., Permpoonpattana, P., & Linh, N. V. (2025). Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture. Biology, 14(9), 1160. https://doi.org/10.3390/biology14091160







