Morphological and Behavioral Abnormalities Induced by Hydrogen Peroxide in Drosophila melanogaster
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Obtaining Third-Instar Larvae of Drosophila Melanogaster
2.3. Larval–Adult Viability and LC50 of H2O2
2.4. Morphological Evaluation
2.5. Behavioral Tests
2.6. Statistical Analysis
3. Results
3.1. Larval–Adult Viability
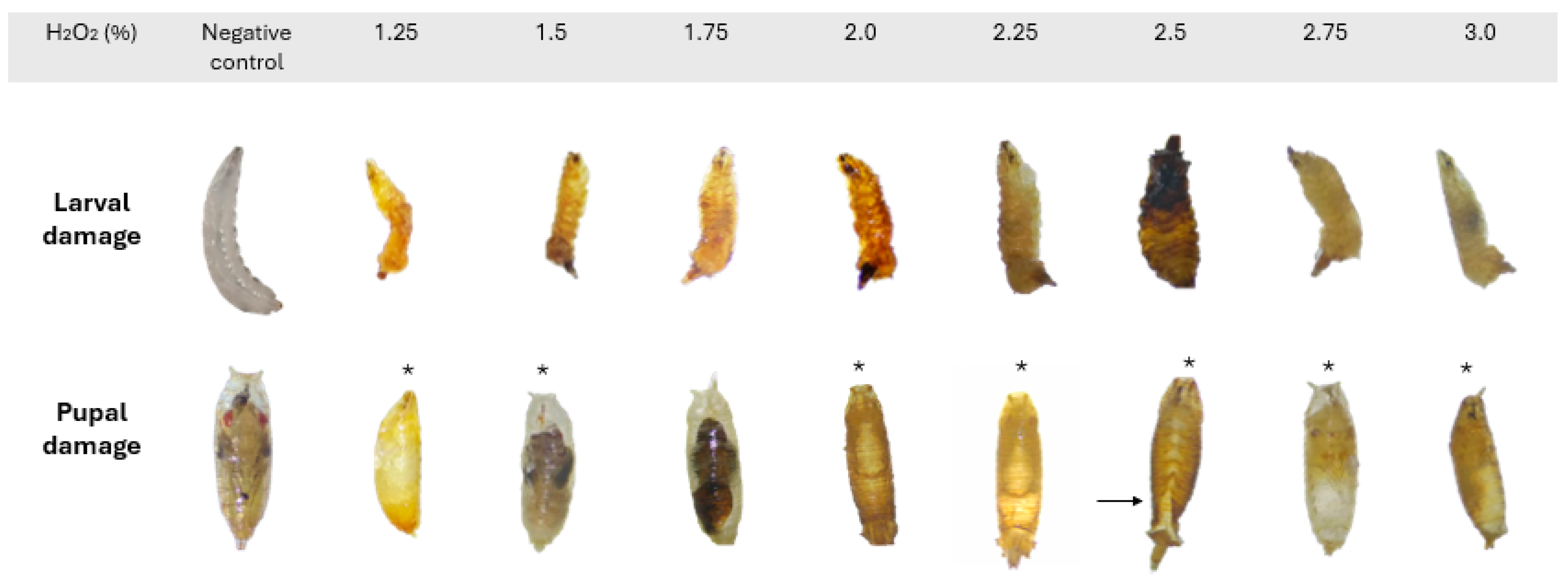
3.2. Morphological Evaluation
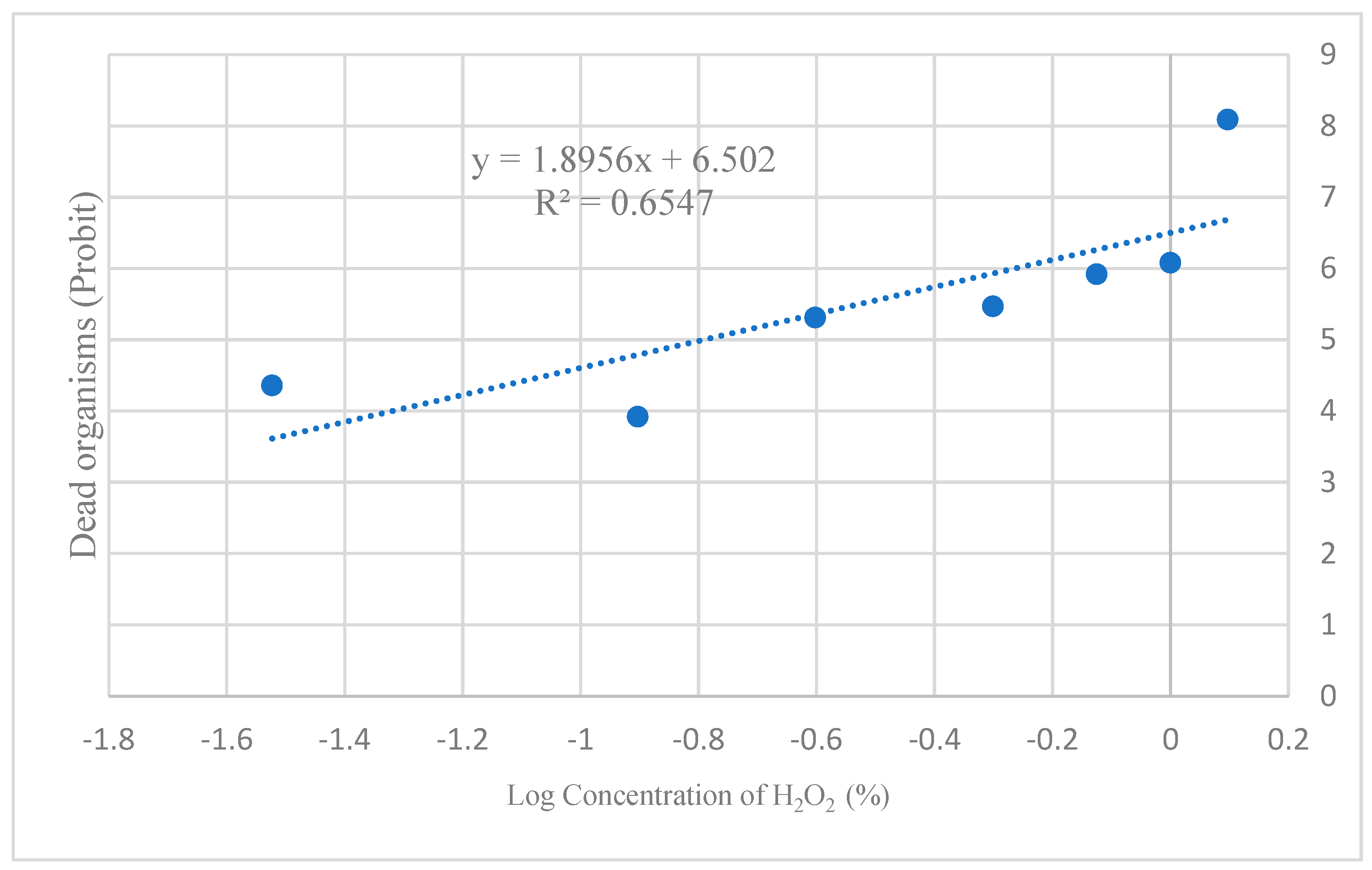
3.3. LC50 of H2O2
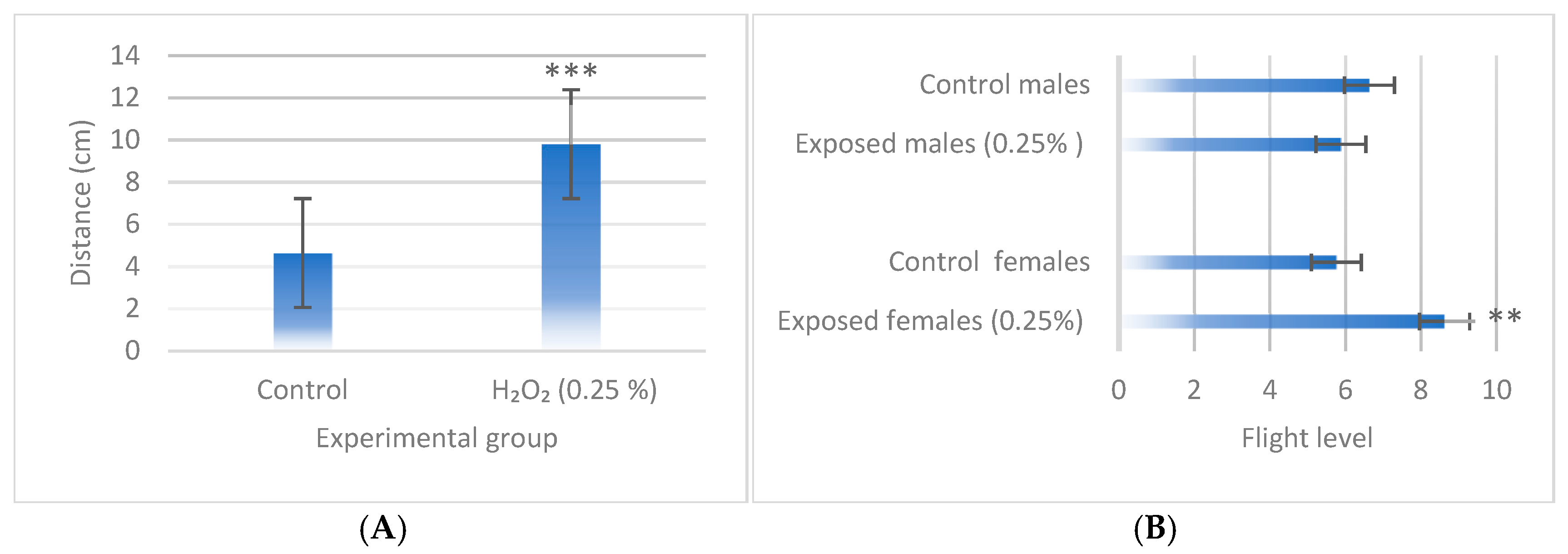
3.4. Behavioral Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNA | Desoxyribonucleic acid |
| EC50 | Effective concentration 50% |
| FDA | Food and Drug Administration |
| LC50 | Lethal Concentration 50% |
| ROS | Reactive oxygen species |
| SE | Standard error |
| TI | Teratogenic index |
References
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Liu, P.; Xu, S.; Chen, D.; Liu, J.N.; Xie, W. Microplastics exposure causes oxidative stress and microbiota dysbiosis in planarian Dugesia japonica. Environ. Sci. Pollut. Res. Int. 2022, 29, 28973–28983. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Xiang, C.; Li, X.; Chen, H.; Shi, X.; Li, X.; Huang, C.; Yu, Y.; Qi, J.; Li, A.J.; et al. Photoaged microplastics induce neurotoxicity via oxidative stress and abnormal neurotransmission in zebrafish larvae (Danio rerio). Sci. Total Environ. 2023, 881, 163480. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhao, Y.; Zhao, J.; Yu, T.; Yao, Y.; Zhao, R.; Yu, R.; Liu, J.; Su, J. Reproductive toxicity of microplastics in female mice and their offspring from induction of oxidative stress. Environ. Pollut. 2023, 327, 121482. [Google Scholar] [CrossRef]
- de Sousa, A.K.A.; Pires, K.S.N.; Cavalcante, I.H.; Cavalcante, I.C.L.; Santos, J.D.; Queiroz, M.I.C.; Leite, A.C.R.; Crispim, A.C.; da Rocha Junior, E.R.; Aquino, T.M.; et al. Polystyrene microplastics exposition on human placental explants induces time-dependent cytotoxicity, oxidative stress and metabolic alterations. Front. Endocrinol. Lausanne 2024, 15, 1481014. [Google Scholar] [CrossRef]
- Ranjan, H.; Senthil Kumar, S.; Priscilla, S.; Swaminathan, S.; Umezawa, M.; Sheik Mohideen, S. Polyethylene microplastics affect behavioural, oxidative stress, and molecular responses in the Drosophila model. Environ. Sci. Process. Impacts 2024, 26, 2203–2214. [Google Scholar] [CrossRef]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Johnmark, N.; Kinyi, H.W. Amaranth leaf extract protects against hydrogen peroxide induced oxidative stress in Drosophila melanogaster. BMC Res. Notes 2021, 14, 188. [Google Scholar] [CrossRef]
- Urban, M.V.; Rath, T.; Radtke, C. Hydrogen peroxide (H2O2): A review of its use in surgery. Wasserstoffperoxid (H2O2)—Eine Übersicht zur Verwendung in der Chirurgie. Wien. Med. Wochenschr. 2019, 169, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Zhengfen, W.; Nam-Trung, N.; Yongsheng, G.; Li, Q. Laser induced graphene for biosensors. In Sustainable Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2020; Volume 25, p. e00205. [Google Scholar] [CrossRef]
- Ulloa, M.; Macías, F.; Martínez de la Escalera, G.; Arnold, E. Role of hydrogen peroxide (H2O2) as a redox signaling molecule and in the diabetes mellitus-related oxidative stress. TIP Rev. Espec. Cienc. Químico-Biológicas 2023, 26, e597. [Google Scholar] [CrossRef]
- Davies, A.; Pottage, T.; Bennett, A.; Walker, J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect. 2011, 77, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.K.; Rajavelu, I.; Pereira, M.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Inside the genome: Understanding genetic influences on oxidative stress. Front. Genet. 2024, 15, 1397352. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Jang, W.; Kim, C. Dual Oxidase, a Hydrogen-Peroxide-Producing Enzyme, Regulates Neuronal Oxidative Damage and Animal Lifespan in Drosophila melanogaster. Cells 2022, 11, 2059. [Google Scholar] [CrossRef]
- Wells, P.G.; McCallum, G.P.; Chen, C.S.; Henderson, J.T.; Lee, C.J.; Perstin, J.; Preston, T.J.; Wiley, M.J.; Wong, A.W. Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 2009, 108, 4–18. [Google Scholar] [CrossRef]
- Hansen, J.M.; Harris, C. Redox control of teratogenesis. Reprod. Toxicol. 2013, 35, 165–179. [Google Scholar] [CrossRef]
- Hansen, J.M.; Jacob, B.R.; Piorczynski, T.B. Oxidative stress during development: Chemical-Induced teratogenesis. Curr. Opin. Toxicol. 2018, 7, 110–115. [Google Scholar] [CrossRef]
- Wells, P.G.; Winn, L.M. The Role of Biotransformation in Developmental Toxicity. In Comprehensive Toxicology, 2nd ed.; Charlene, A., Queen, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 95–115. [Google Scholar]
- Tyl, R.W. Toxicity Testing, Developmental. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 656–668. ISBN 9780123864550. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, J.W.; Lin, T.; Lee, J.E.; Jin, D.I. Teratogenic potential of antiepileptic drugs in the zebrafish model. Biomed Res. Int. 2013, 2013, 726478. [Google Scholar] [CrossRef]
- Sachdeva, P.; Patel, B.G.; Patel, B.K. Drug use in pregnancy; a point to ponder! Indian J. Pharm. Sci. 2009, 71, 1–7. [Google Scholar] [CrossRef]
- Grover, D.; Ford, D.; Brown, C.; Hoe, N.; Erdem, A.; Tavaré, S.; Tower, J. Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS ONE 2009, 4, e7580. [Google Scholar] [CrossRef]
- Mishra, M.; Barik, B.K. Behavioral Teratogenesis in Drosophila melanogaster. Methods Mol. Biol. 2018, 1797, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- droso4LatAm. Available online: https://droso4latam.wordpress.com/recursos/ (accessed on 23 June 2025).
- Prokop, A.; Patel, S. Biology Lessons for Schools Using the Fruit Fly Drosophila. 2018. Figshare. Dataset. Available online: https://figshare.com/articles/dataset/Biology_lessons_for_schools_using_the_fruit_fly_Drosophila/1352064/31 (accessed on 25 May 2025).
- IBM SPSS Statistics for Windows, version 25.0; IBM Corp: Armonk, NY, USA, 2007.
- Jarque, S.; Rubio-Brotons, M.; Ibarra, J.; Ordoñez, V.; Dyballa, S.; Miñana, R.; Terriente, J. Morphometric analysis of developing zebrafish embryos allows predicting teratogenicity modes of action in higher vertebrates. Reprod. Toxicol. 2020, 96, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Vismara, C.; Bacchetta, R.; DiMuzio, A.; Mantecca, P.; Tarca, S.; Vailati, G.; Colombo, R. H2O2 induces abnormal tail flexure in Xenopus embryos: Similarities with Paraquat teratogenic effects. Birth Defects Res. B Dev. Reprod. Toxicol. 2006, 77, 238–243. [Google Scholar] [CrossRef]
- Jordan, K.W.; Morgan, T.J.; Mackay, T.F. Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genetics 2006, 174, 271–284. [Google Scholar] [CrossRef]
- Oram, T.B.; Card, G.M. Context-dependent control of behavior in Drosophila. Curr. Opin. Neurobiol. 2022, 73, 102523. [Google Scholar] [CrossRef]
- Long, T.A.; Rice, W.R. Adult locomotory activity mediates intralocus sexual conflict in a laboratory-adapted population of Drosophila melanogaster. Proc. Biol. Sci. 2007, 274, 3105–3112. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci. Rep. 2017, 7, 15617. [Google Scholar] [CrossRef]
- Lessel, C.E.; Parkes, T.L.; Dickinson, J.; Merritt, T.J.S. Sex and Genetic Background Influence Superoxide Dismutase (cSOD)-Related Phenotypic Variation in Drosophila melanogaster. G3 Genes 2017, 7, 2651–2664. [Google Scholar] [CrossRef]
- Adedara, A.O.; Otenaike, T.A.; Olabiyi, A.A.; Adedara, I.A.; Abolaji, A.O. Neurotoxic and behavioral deficit in Drosophila melanogaster co-exposed to rotenone and iron. Metab. Brain Dis. 2023, 38, 349–360. [Google Scholar] [CrossRef]
- Jones, M.A.; Grotewiel, M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011, 46, 320–325. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Calderón, M.L.; Gallegos-Moreno, A.; Miranda-Camacho, A.Y.; Sánchez-Jiménez, C.L.; Díaz-Barriga-Arceo, S.; Aguirre-Joya, J.A.; Torres-León, C.; Aguillón-Gutiérrez, D.R. Morphological and Behavioral Abnormalities Induced by Hydrogen Peroxide in Drosophila melanogaster. Biology 2025, 14, 1122. https://doi.org/10.3390/biology14091122
Hernández-Calderón ML, Gallegos-Moreno A, Miranda-Camacho AY, Sánchez-Jiménez CL, Díaz-Barriga-Arceo S, Aguirre-Joya JA, Torres-León C, Aguillón-Gutiérrez DR. Morphological and Behavioral Abnormalities Induced by Hydrogen Peroxide in Drosophila melanogaster. Biology. 2025; 14(9):1122. https://doi.org/10.3390/biology14091122
Chicago/Turabian StyleHernández-Calderón, María Llasbeth, Alondra Gallegos-Moreno, Aneet Yamely Miranda-Camacho, Claudia Linette Sánchez-Jiménez, Sandra Díaz-Barriga-Arceo, Jorge Alejandro Aguirre-Joya, Cristian Torres-León, and David Ramiro Aguillón-Gutiérrez. 2025. "Morphological and Behavioral Abnormalities Induced by Hydrogen Peroxide in Drosophila melanogaster" Biology 14, no. 9: 1122. https://doi.org/10.3390/biology14091122
APA StyleHernández-Calderón, M. L., Gallegos-Moreno, A., Miranda-Camacho, A. Y., Sánchez-Jiménez, C. L., Díaz-Barriga-Arceo, S., Aguirre-Joya, J. A., Torres-León, C., & Aguillón-Gutiérrez, D. R. (2025). Morphological and Behavioral Abnormalities Induced by Hydrogen Peroxide in Drosophila melanogaster. Biology, 14(9), 1122. https://doi.org/10.3390/biology14091122







