Bone-Derived Factors: Regulating Brain and Treating Alzheimer’s Disease
Simple Summary
Abstract
1. Introduction
2. Anatomy of Bone-Brain Communication
2.1. Mechanisms of Blood–Brain Barrier Penetration
2.2. Mechanism of Circumventricular Organs Penetration
3. Bone Cell Network Signaling Modulates Neurological Pathologies
3.1. BMSCs Regulate Brain Function
3.1.1. BMSCs-Derived Trophic Factors Regulate Brain Function
BMSCs-Derived BDNF Regulates Brain Function
BMSCs-Derived NGF Regulates Brain Function
BMSCs-Derived VEGF Regulates Brain Function
3.1.2. BMSCs-Derived Exosomes Regulate Brain Function
BMSCs-EXOs Regulate Brain Function by Regulating Neurons, Microvessels, and Intracerebral Factors
BMSCs-EXOs Regulate Brain Function by Regulating Inflammatory and Oxidative Stress
BMSCs-EXOs Regulate Brain Function by Secreting microRNA
3.1.3. The Effects and Mechanisms of BMSCs on AD
3.2. Osteoblasts Regulate Brain Function
3.2.1. OBs-Derived OCN Regulates Brain Function
3.2.2. OBs-Derived LCN2 Regulates Brain Function
3.2.3. The Effects and Mechanisms of Osteoblasts on AD
3.3. Osteocytes Regulate Brain Function
3.3.1. Osteocytes-Derived RANKL Regulates Brain Function
3.3.2. Osteocytes-Derived FGF23 Regulates Brain Function
3.3.3. Osteocytes-Derived EVs Regulate Brain Function
3.3.4. The Effects and Mechanisms of Osteocytes on AD
3.4. BMMs Regulate Brain Function
3.4.1. Transplanted BMMs Regulate Brain Function
3.4.2. Preosteoclasts-Derived PDGF-BB Regulates Brain Function
3.4.3. The Effects and Mechanisms of Osteoclasts on AD
4. Osteoporosis and AD: A Common Pathophysiology
4.1. The Pathogenesis Mechanisms of AD
4.2. The Treatment Mechanisms of AD
4.3. Low Bone Density Exacerbates AD
4.4. AD Exacerbates Bone Loss
5. Dual Modulation of Bone and Brain Function by Drugs
5.1. Effects of Osteoporosis Medications on Cognitive Functioning
5.2. Neuropharmacological Modulation of Bone Density
6. Clinical Applications—Whole Body Vibration Therapy to Regulate Bone Brain Crosstalk
7. Future Directions and Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A cluster of differentiation | CD |
| Alzheimer’s disease | AD |
| Blood–brain barrier | BBB |
| BMSCs-derived exosomes | BMSCs-EXOs |
| Bone marrow-derived mononuclear cells | BMMs |
| Bone marrow mesenchymal stem cells | BMSCs |
| Bone morphogenetic proteins | BMPs |
| Bone-derived cytokines | BDCs |
| Brain-derived neurotrophic factor | BDNF |
| Carboxylated osteocalcin | cOCN |
| Central nervous system | CNS |
| Cerebrospinal fluid | CSF |
| chemokine receptor | Cx3cr1 |
| chronic kidney disease | CKD |
| C-X-C chemokine receptor type 4 | CXCR4 |
| Cyclooxygenase 2 | COX2 |
| Early growth response protein 1 | Egr1 |
| Exosomes | EXOs |
| Experimental allergic encephalomyelitis | EAE |
| Extracellular signal-regulated kinases 1 and 2 | ERK1/2 |
| Extracellular vesicles | EVs |
| Fibroblast growth factor2 | FGF2 |
| Fibrosarcoma 1 | Raf1 |
| High-mobility group box 1 protein | HMGB1 |
| Hypoxic–ischemic brain damage | HIBD |
| Inducible nitric oxide synthase | iNOS |
| Interferon-γ | IFN-γ |
| Interleukin-1 receptor-associated kinase1 | IRAK1 |
| Interleukin-6 | IL-6 |
| Intracerebral hemorrhage | ICH |
| Ischemia/reperfusion | I/R |
| Ischemic stroke | IS |
| Klotho | KL |
| Knockout | KO |
| Lipocalin-2 | LCN2 |
| Malondialdehyde | MDA |
| Matrix metalloproteinase | MMP |
| Melanocortin 4 receptor | MC4R |
| Membrane-bound RANKL | mRANKL |
| Mesenchymal stem cell | MSC |
| Multiple sclerosis | MS |
| Nerve growth factor | NGF |
| Neuropeptide Y | NPY |
| Nuclear factor kappa B | NF-κB |
| Nuclear factor of activated T cells 5 | NFAT5 |
| Nuclear factor-κB ligand | RANKL |
| Osteoblasts | Obs |
| Osteocalcin | OCN |
| Osteoclasts | Ocs |
| Oxygen-glucose deprivation/reperfusion | OGD/R |
| Parkinson’s disease | PD |
| PDGF-BB/PDGF receptor | PDGFR β |
| Permanent middle cerebral artery occlusion | pMCAO |
| Phosphatase gene | PTEN |
| Platelet-derived growth factor BB | PDGF-BB |
| Postsynaptic density protein 95 | PSD95 |
| Soluble RANKL | sRANKL |
| Sphingosine-1-phosphate | S1P |
| Streptozotocin | STZ |
| Stromal cell-derived factor 1 | SDF-1 |
| Subarachnoid hemorrhage | SAH |
| Superoxide dismutase | SOD |
| Tartrate-resistant acid phosphatase positive | TRAP+ |
| The common carotid arteries | 2VO |
| Toll-like receptor-4 | TLR4 |
| Transient middle cerebral artery occlusion | TMCAO |
| Traumatic brain injuries | TBI |
| Tumor necrosis factor-α | TNF-α |
| Uncarboxylated osteocalcin | ucOCN |
| Vascular endothelial growth factor | VEGF |
| Vitamin D | VD |
| γ-aminobutyric acid | GABA |
References
- Pang, Y.; Jia, D.; Ye, F.; Liu, F.; Li, J.; Zhu, S.; Wang, B.; Yao, M.; Du, L.; Yang, C.; et al. Bone Marrow-Derived Myeloid Cells Drive Neuroinflammation in Alzheimer’s Disease: Insights from the FAD(4T) Mouse Model. J. Orthop. Transl. 2025, 53, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, N.; Honea, R.A.; Brooks, W.M.; Burns, J.M. Reduced Limbic and Hypothalamic Volumes Correlate with Bone Density in Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, 313–322. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood-Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the Transferrin Receptor for Brain Drug Delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-Based Blood-Brain-Barrier (BBB) Crossing Strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome Engineering in Cell Therapy and Drug Delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Counil, H.; Silva, R.O.; Rabanel, J.M.; Zaouter, C.; Haddad, M.; Ben Khedher, M.R.; Brambilla, D.; Fülöp, T.; Patten, S.A.; Ramassamy, C. Brain Penetration of Peripheral Extracellular Vesicles from Alzheimer’s Patients and Induction of Microglia Activation. J. Extracell. Biol. 2025, 4, e70027. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.A.; De Felice, F.G. The Crosstalk between Brain and Periphery: Implications for Brain Health and Disease. Neuropharmacology 2021, 197, 108728. [Google Scholar] [CrossRef] [PubMed]
- Balland, E.; Dam, J.; Langlet, F.; Caron, E.; Steculorum, S.; Messina, A.; Rasika, S.; Falluel-Morel, A.; Anouar, Y.; Dehouck, B.; et al. Hypothalamic Tanycytes Are an ERK-Gated Conduit for Leptin into the Brain. Cell Metab. 2014, 19, 293–301. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of Human Bone Marrow-Derived Mesenchymal Stem Cells Promotes Behavioral Recovery and Endogenous Neurogenesis after Cerebral Ischemia in Rats. Brain Res. 2011, 1367, 103–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic Administration of Cell-Free Exosomes Generated by Human Bone Marrow Derived Mesenchymal Stem Cells Cultured under 2D and 3D Conditions Improves Functional Recovery in Rats after Traumatic Brain Injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wu, Y.; Kong, F.Y.; Ma, S.; Fu, L.Y.; Geng, J. BMSCs Differentiated into Neurons, Astrocytes and Oligodendrocytes Alleviated the Inflammation and Demyelination of EAE Mice Models. PLoS ONE 2021, 16, e0243014. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Balani, D.H.; Kronenberg, H.M. Stem and Progenitor Cells in Skeletal Development. Curr. Top. Dev. Biol. 2019, 133, 1–24. [Google Scholar] [CrossRef]
- Seyfried, D.; Ding, J.; Han, Y.; Li, Y.; Chen, J.; Chopp, M. Effects of Intravenous Administration of Human Bone Marrow Stromal Cells after Intracerebral Hemorrhage in Rats. J. Neurosurg. 2006, 104, 313–318. [Google Scholar] [CrossRef]
- Jo, H.; Jung, M.; Seo, D.J.; Park, D.J. The Effect of Rat Bone Marrow Derived Mesenchymal Stem Cells Transplantation for Restoration of Olfactory Disorder. Biochem. Biophys. Res. Commun. 2015, 467, 395–399. [Google Scholar] [CrossRef]
- Zacharek, A.; Chen, J.; Cui, X.; Li, A.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/Tie2 and VEGF/Flk1 Induced by MSC Treatment Amplifies Angiogenesis and Vascular Stabilization after Stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef]
- El-Akabawy, G.; Aabed, K.; Rashed, L.A.; Amin, S.N.; AlSaati, I.; Al-Fayez, M. Preventive Effects of Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in a D-Galactose-Induced Brain Aging in Rats. Folia Morphol. 2022, 81, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizue, Y.; Fujimiya, M. Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetes-Induced Cognitive Impairment by Exosome Transfer into Damaged Neurons and Astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef]
- Song, M.; Mohamad, O.; Gu, X.; Wei, L.; Yu, S.P. Restoration of Intracortical and Thalamocortical Circuits after Transplantation of Bone Marrow Mesenchymal Stem Cells into the Ischemic Brain of Mice. Cell Transplant. 2013, 22, 2001–2015. [Google Scholar] [CrossRef]
- Chang, Y.C.; Shyu, W.C.; Lin, S.Z.; Li, H. Regenerative Therapy for Stroke. Cell Transplant. 2007, 16, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Shichinohe, H.; Kuroda, S.; Yano, S.; Hida, K.; Iwasaki, Y. Role of SDF-1/CXCR4 System in Survival and Migration of Bone Marrow Stromal Cells after Transplantation into Mice Cerebral Infarct. Brain Res. 2007, 1183, 138–147. [Google Scholar] [CrossRef]
- Shen, L.H.; Li, Y.; Chen, J.; Cui, Y.; Zhang, C.; Kapke, A.; Lu, M.; Savant-Bhonsale, S.; Chopp, M. One-Year Follow-up after Bone Marrow Stromal Cell Treatment in Middle-Aged Female Rats with Stroke. Stroke 2007, 38, 2150–2156. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chen, X.G.; Wang, L.; Gautam, S.C.; Xu, Y.X.; Katakowski, M.; Zhang, L.J.; Lu, M.; Janakiraman, N.; et al. Human Marrow Stromal Cell Therapy for Stroke in Rat: Neurotrophins and Functional Recovery. Neurology 2002, 59, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Katakowski, M.; Chen, X.; Wang, L.; Lu, D.; Lu, M.; Gautam, S.C.; Chopp, M. Intravenous Bone Marrow Stromal Cell Therapy Reduces Apoptosis and Promotes Endogenous Cell Proliferation after Stroke in Female Rat. J. Neurosci. Res. 2003, 73, 778–786. [Google Scholar] [CrossRef]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A Dynamic Duo in Age-Related Neuronal Plasticity and Neurodegenerative Disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef]
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S. Intravenous Brain-Derived Neurotrophic Factor Reduces Infarct Size and Counterregulates Bax and Bcl-2 Expression after Temporary Focal Cerebral Ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar] [CrossRef]
- Liu, X.L.; Zu, Q.Q.; Wang, B.; Lu, S.S.; Xu, X.Q.; Liu, S.; Shi, H.B. Differentiation of Genetically Modified Canine Bone Mesenchymal Stem Cells Labeled with Superparamagnetic Iron Oxide into Neural-Like Cells. Mol. Med. Rep. 2018, 17, 7902–7910. [Google Scholar] [CrossRef]
- Shichinohe, H.; Ishihara, T.; Takahashi, K.; Tanaka, Y.; Miyamoto, M.; Yamauchi, T.; Saito, H.; Takemoto, H.; Houkin, K.; Kuroda, S. Bone Marrow Stromal Cells Rescue Ischemic Brain by Trophic Effects and Phenotypic Change toward Neural Cells. Neurorehabilit. Neural Repair 2015, 29, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Makar, T.K.; Bever, C.T.; Singh, I.S.; Royal, W.; Sahu, S.N.; Sura, T.P.; Sultana, S.; Sura, K.T.; Patel, N.; Dhib-Jalbut, S.; et al. Brain-Derived Neurotrophic Factor Gene Delivery in an Animal Model of Multiple Sclerosis Using Bone Marrow Stem Cells as a Vehicle. J. Neuroimmunol. 2009, 210, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cheng, Y.; Gao, S.; Chen, J. Effects of Nerve Growth Factor and Noggin-Modified Bone Marrow Stromal Cells on Stroke in Rats. J. Neurosci. Res. 2011, 89, 222–230. [Google Scholar] [CrossRef]
- Riccio, A.; Ahn, S.; Davenport, C.M.; Blendy, J.A.; Ginty, D.D. Mediation by a CREB Family Transcription Factor of NGF-Dependent Survival of Sympathetic Neurons. Science 1999, 286, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.W.; Ginty, D.D. Long-Distance Retrograde Neurotrophic Factor Signalling in Neurons. Nat. Rev. Neurosci. 2013, 14, 177–187. [Google Scholar] [CrossRef]
- Levi-Montalcini, R.; Aloe, L.; Mugnaini, E.; Oesch, F.; Thoenen, H. Nerve Growth Factor Induces Volume Increase and Enhances Tyrosine Hydroxylase Synthesis in Chemically Axotomized Sympathetic Ganglia of Newborn Rats. Proc. Natl. Acad. Sci. USA 1975, 72, 595–599. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kuroda, S.; Kobayashi, H.; Shichinohe, H.; Yano, S.; Hida, K.; Shinpo, K.; Kikuchi, S.; Iwasaki, Y. The Effects of Neuronal Induction on Gene Expression Profile in Bone Marrow Stromal Cells (BMSC)—A Preliminary Study Using Microarray Analysis. Brain Res. 2006, 1087, 15–27. [Google Scholar] [CrossRef]
- Fang, C.N.; Tan, H.Q.; Song, A.B.; Jiang, N.; Liu, Q.R.; Song, T. NGF/TrkA Promotes the Vitality, Migration and Adhesion of Bone Marrow Stromal Cells in Hypoxia by Regulating the Nrf2 Pathway. Metab. Brain Dis. 2022, 37, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, S.S.; Kim, J.Y.; Tchah, H. Nerve Growth Factor Attenuates Apoptosis and Inflammation in the Diabetic Cornea. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6767–6775. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guan, H.; Zhang, Y.; Li, S.; Li, K.; Hu, S.; Zuo, E.; Zhang, C.; Zhang, X.; Gong, G.; et al. Bone Marrow Mesenchymal Stem Cells Promote Remyelination in Spinal Cord by Driving Oligodendrocyte Progenitor Cell Differentiation via TNFα/RelB-Hes1 Pathway: A Rat Model Study of 2,5-Hexanedione-Induced Neurotoxicity. Stem Cell Res. Ther. 2021, 12, 436. [Google Scholar] [CrossRef]
- Kermani, P.; Rafii, D.; Jin, D.K.; Whitlock, P.; Schaffer, W.; Chiang, A.; Vincent, L.; Friedrich, M.; Shido, K.; Hackett, N.R.; et al. Neurotrophins Promote Revascularization by Local Recruitment of TrkB+ Endothelial Cells and Systemic Mobilization of Hematopoietic Progenitors. J. Clin. Investig. 2005, 115, 653–663. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, X.; Xu, A.; Xu, F.; Liang, Q. Bone Marrow Stromal Cell Transplantation through Tail Vein Injection Promotes Angiogenesis and Vascular Endothelial Growth Factor Expression in Cerebral Infarct Area in Rats. Cytotherapy 2015, 17, 1200–1212. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Q.; Wang, P.; Yao, L.; Leong, K.; Tan, Z.; Huang, Z. Enhanced Neuroprotective Efficacy of Bone Marrow Mesenchymal Stem Cells Co-Overexpressing BDNF and VEGF in a Rat Model of Cardiac Arrest-Induced Global Cerebral Ischemia. Cell Death Dis. 2017, 8, e2774. [Google Scholar] [CrossRef]
- Zong, X.; Wu, S.; Li, F.; Lv, L.; Han, D.; Zhao, N.; Yan, X.; Hu, S.; Xu, T. Transplantation of VEGF-Mediated Bone Marrow Mesenchymal Stem Cells Promotes Functional Improvement in a Rat Acute Cerebral Infarction Model. Brain Res. 2017, 1676, 9–18. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, F.; Li, Q.Y.; Gong, A.; Lan, Q. Protective Effect of Ad-VEGF-Bone Mesenchymal Stem Cells on Cerebral Infarction. Turk. Neurosurg. 2016, 26, 8–15. [Google Scholar] [CrossRef]
- Guo, S.; Zhen, Y.; Wang, A. Transplantation of Bone Mesenchymal Stem Cells Promotes Angiogenesis and Improves Neurological Function after Traumatic Brain Injury in Mouse. Neuropsychiatr. Dis. Treat. 2017, 13, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, L.; Su, Y.; Su, L.; Lan, X.; Wu, D.; Han, S.; Li, J.; Kvederis, L.; Corey, S.; et al. Hypoxia Conditioning Enhances Neuroprotective Effects of Aged Human Bone Marrow Mesenchymal Stem Cell-Derived Conditioned Medium against Cerebral Ischemia in Vitro. Brain Res. 2019, 1725, 146432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, Q.; Shen, L.; Chen, J.; Gan, D.; Sun, Y.; Ding, L.; Wang, G. Hypoxic Preconditioning Enhances the Differentiation of Bone Marrow Stromal Cells into Mature Oligodendrocytes via the mTOR/HIF-1α/VEGF Pathway in Traumatic Brain Injury. Brain Behav. 2020, 10, e01675. [Google Scholar] [CrossRef]
- Sluijter, J.P.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and Exosomes for Intracardiac Communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; et al. Hyaluronan-Based Hydrogel Integrating Exosomes for Traumatic Brain Injury Repair by Promoting Angiogenesis and Neurogenesis. Carbohydr. Polym. 2023, 306, 120578. [Google Scholar] [CrossRef]
- Li, X.; Bi, T.; Yang, S. Exosomal Microrna-150-5p from Bone Marrow Mesenchymal Stromal Cells Mitigates Cerebral Ischemia/Reperfusion Injury via Targeting Toll-Like Receptor 5. Bioengineered 2022, 13, 3030–3043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Chopp, M.; Pang, H.; Zhang, Z.G.; Mahmood, A.; Xiong, Y. MiR-17-92 Cluster-Enriched Exosomes Derived from Human Bone Marrow Mesenchymal Stromal Cells Improve Tissue and Functional Recovery in Rats after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1535–1550. [Google Scholar] [CrossRef]
- Mot, Y.Y.; Moses, E.J.; Mohd Yusoff, N.; Ling, K.H.; Yong, Y.K.; Tan, J.J. Mesenchymal Stromal Cells-Derived Exosome and the Roles in the Treatment of Traumatic Brain Injury. Cell. Mol. Neurobiol. 2023, 43, 469–489. [Google Scholar] [CrossRef]
- Wang, H.; Huber, C.C.; Li, X.P. Mesenchymal and Neural Stem Cell-Derived Exosomes in Treating Alzheimer’s Disease. Bioengineering 2023, 10, 253. [Google Scholar] [CrossRef]
- Yin, T.; Liu, Y.; Ji, W.; Zhuang, J.; Chen, X.; Gong, B.; Chu, J.; Liang, W.; Gao, J.; Yin, Y. Engineered Mesenchymal Stem Cell-Derived Extracellular Vesicles: A State-of-the-Art Multifunctional Weapon against Alzheimer’s Disease. Theranostics 2023, 13, 1264–1285. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Minjarez, B.; Gutiérrez-Mercado, Y.K.; Márquez-Aguirre, A.L.; Canales-Aguirre, A.A. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, S.; Zangbar, H.S.; Farajdokht, F.; Rahbarghazi, R.; Ghiasi, F.; Mohaddes, G. Mesenchymal Stem Cell-Derived Exosomes Improve Neurogenesis and Cognitive Function of Mice with Methamphetamine Addiction: A Novel Treatment Approach. CNS Neurosci. Ther. 2024, 30, e14719. [Google Scholar] [CrossRef]
- Oveili, E.; Vafaei, S.; Bazavar, H.; Eslami, Y.; Mamaghanizadeh, E.; Yasamineh, S.; Gholizadeh, O. The Potential Use of Mesenchymal Stem Cells-Derived Exosomes as Micrornas Delivery Systems in Different Diseases. Cell Commun. Signal. 2023, 21, 20. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in AD Mice by Inhibiting Hyperphosphorylation of the Tau Protein through the AKT/GSK-3β Pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Hu, L.T.; Wang, B.Y.; Fan, Y.H.; He, Z.Y.; Zheng, W.X. Exosomal miR-23b from Bone Marrow Mesenchymal Stem Cells Alleviates Oxidative Stress and Pyroptosis after Intracerebral Hemorrhage. Neural Regen. Res. 2023, 18, 560–567. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, M.; Luo, J.; Zhang, X.; Dai, Z.; Zhang, B.; Chen, H.; Xue, J.; He, M.; Xu, H.; et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Attenuate Neurological Damage in Traumatic Brain Injury by Alleviating Glutamate-Mediated Excitotoxicity. Exp. Neurol. 2022, 357, 114182. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, F.; Cao, J.; Wang, C. Exosomes Derived from Microrna-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des. Dev. Ther. 2020, 14, 3143–3158. [Google Scholar] [CrossRef]
- Li, X.T.; Zhao, J.; Xu, D.S.; Zhang, Y.; Zhou, S.T. Bone Marrow Mesenchymal Stem Cell Exosomes Promote Brain Microvascular Endothelial Cell Proliferation and Migration in Rats. J. Sichuan Univ. (Med. Sci.) 2020, 51, 599–604. [Google Scholar] [CrossRef]
- Xiong, L.; Sun, L.; Zhang, Y.; Peng, J.; Yan, J.; Liu, X. Exosomes from Bone Marrow Mesenchymal Stem Cells Can Alleviate Early Brain Injury after Subarachnoid Hemorrhage through miRNA129-5p-HMGB1 Pathway. Stem Cells Dev. 2020, 29, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Cao, Y.; Guo, X.; Chu, X.; Li, T.; Xue, H.; Xin, D.; Yuan, L.; Ke, H.; Li, G.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Microglial M2 Polarization after Subarachnoid Hemorrhage in Rats and Involve the AMPK/NF-κB Signaling Pathway. Biomed. Pharmacother. 2021, 133, 111048. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Gu, J.; Xu, X.; Chen, H.; Gui, Y. Exosomes Isolated During Dopaminergic Neuron Differentiation Suppressed Neuronal Inflammation in a Rodent Model of Parkinson’s Disease. Neurosci. Lett. 2022, 771, 136414. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Xu, J.X.; Yang, L.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Exosomes Derived from Bone-Marrow Mesenchymal Stem Cells Alleviate Cognitive Decline in AD-Like Mice by Improving BDNF-Related Neuropathology. J. Neuroinflamm. 2022, 19, 35. [Google Scholar] [CrossRef]
- El-Derany, M.O.; Noureldein, M.H. Bone Marrow Mesenchymal Stem Cells and Their Derived Exosomes Resolve Doxorubicin-Induced Chemobrain: Critical Role of Their Mirna Cargo. Stem Cell Res. Ther. 2021, 12, 322. [Google Scholar] [CrossRef]
- Liu, C.; Yang, T.H.; Li, H.D.; Li, G.Z.; Liang, J.; Wang, P. Exosomes from Bone Marrow Mesenchymal Stem Cells Are a Potential Treatment for Ischemic Stroke. Neural Regen. Res. 2023, 18, 2246–2251. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.D.; Shen, D.F.; Zheng, P.D.; Tu, M.D.; You, W.D.; Zhu, Y.R.; Wang, H.; Feng, J.F.; Yang, X.F. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Inhibit Neuroinflammation after Traumatic Brain Injury. Neural Regen. Res. 2022, 17, 2717–2724. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Carrying Long Noncoding RNA ZFAS1 Alleviate Oxidative Stress and Inflammation in Ischemic Stroke by Inhibiting Microrna-15a-5p. Metab. Brain Dis. 2022, 37, 2545–2557. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, L.; Wang, X.; Zhang, Y. Correlation of Oxidative Stress-Related Biomarkers with Postmenopausal Osteoporosis: A Systematic Review and Meta-Analysis. Arch. Osteoporos. 2021, 16, 4. [Google Scholar] [CrossRef]
- Shu, J.; Jiang, L.; Wang, M.; Wang, R.; Wang, X.; Gao, C.; Xia, Z. Human Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Protect against Nerve Injury via Regulating Immune Microenvironment in Neonatal Hypoxic-Ischemic Brain Damage Model. Immunobiology 2022, 227, 152178. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, D.; Gao, F.; Lv, H.; Zhang, G.; Sun, X.; Liu, L.; Mo, D.; Ma, N.; Song, L.; et al. Correction to: Exosomes Derived from Microrna-138-5poverexpressing Bone Marrow-Derived Mesenchymal Stem Cells Confer Neuroprotection to Astrocytes Following Ischemic Stroke via Inhibition of LCN2. J. Biol. Eng. 2022, 16, 8. [Google Scholar] [CrossRef]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone Marrow-Derived Mesenchymal Stem Cells Improve Cognitive Impairment in an Alzheimer’s Disease Model by Increasing the Expression of Microrna-146a in Hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef]
- Xiao, Y.; Geng, F.; Wang, G.; Li, X.; Zhu, J.; Zhu, W. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Prevent Oligodendrocyte Apoptosis through Exosomal Mir-134 by Targeting Caspase-8. J. Cell. Biochem. 2019, 120, 2109–2118. [Google Scholar] [CrossRef]
- Min, W.; Wu, Y.; Fang, Y.; Hong, B.; Dai, D.; Zhou, Y.; Liu, J.; Li, Q. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal microRNA-124-3p Attenuates Hypoxic-Ischemic Brain Damage through Depressing Tumor Necrosis Factor Receptor Associated Factor 6 in Newborn Rats. Bioengineered 2022, 13, 3194–3206. [Google Scholar] [CrossRef]
- Wang, J.; Bonacquisti, E.E.; Brown, A.D.; Nguyen, J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells 2020, 9, 660. [Google Scholar] [CrossRef]
- Xiong, W.P.; Yao, W.Q.; Wang, B.; Liu, K. BMSCs-Exosomes Containing GDF-15 Alleviated SH-SY5Y Cell Injury Model of Alzheimer’s Disease via AKT/GSK-3β/β-Catenin. Brain Res. Bull. 2021, 177, 92–102. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, Y.; Wu, X.; Xu, X.; Zhu, C.; Huang, W. Breviscapine Combined with BMSCs Reduces Aβ Deposition in Rat with Alzheimer’s Disease by Regulating Circular RNA ciRS-7. Curr. Mol. Med. 2023, 23, 76–86. [Google Scholar] [CrossRef]
- Yu, J.; Yan, Y.; Gu, Q.; Kumar, G.; Yu, H.; Zhao, Y.; Liu, C.; Gao, Y.; Chai, Z.; Chumber, J.; et al. Fasudil in Combination with Bone Marrow Stromal Cells (BMSCs) Attenuates Alzheimer’s Disease-Related Changes through the Regulation of the Peripheral Immune System. Front. Aging Neurosci. 2018, 10, 216. [Google Scholar] [CrossRef]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2017, 25, 218. [Google Scholar] [CrossRef]
- Jin, F.; Liu, M.; Zhang, D.; Wang, X. Translational Perspective on Bone-Derived Cytokines in Inter-Organ Communications. Innovation 2023, 4, 100365. [Google Scholar] [CrossRef]
- Lombardi, G.; Perego, S.; Luzi, L.; Banfi, G. A Four-Season Molecule: Osteocalcin. Updates in Its Physiological Roles. Endocrine 2015, 48, 394–404. [Google Scholar] [CrossRef]
- Oury, F.; Khrimian, L.; Denny, C.A.; Gardin, A.; Chamouni, A.; Goeden, N.; Huang, Y.Y.; Lee, H.; Srinivas, P.; Gao, X.B.; et al. Maternal and Offspring Pools of Osteocalcin Influence Brain Development and Functions. Cell 2013, 155, 228–241. [Google Scholar] [CrossRef]
- Shan, C.; Ghosh, A.; Guo, X.Z.; Wang, S.M.; Hou, Y.F.; Li, S.T.; Liu, J.M. Roles for Osteocalcin in Brain Signalling: Implications in Cognition- and Motor-Related Disorders. Mol. Brain 2019, 12, 23. [Google Scholar] [CrossRef]
- Kosmidis, S.; Polyzos, A.; Harvey, L.; Youssef, M.; Denny, C.A.; Dranovsky, A.; Kandel, E.R. RbAp48 Protein Is a Critical Component of GPR158/OCN Signaling and Ameliorates Age-Related Memory Loss. Cell Rep. 2018, 25, 959–973.e6. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased Bone Formation in Osteocalcin-Deficient Mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the Brain: From Embryonic Development to Age-Related Decline in Cognition. Nat. Rev. Endocrinol. 2018, 14, 174–182. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 Mediates Osteocalcin’s Regulation of Cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef]
- Bu, X.L.; Liu, Z.T.; Xin, J.Y.; Huang, M.; Bai, Y.D.; Zhou, J.; Bao, Y.Y.; Li, J.H.; Liu, Z.H.; Zeng, G.H.; et al. Associations of Plasma and CSF Osteocalcin Levels with CSF ATN Biomarkers and Cognitive Functions in Alzheimer’s Disease. MedComm 2025, 6, e70255. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of Neutrophil Gelatinase-Associated Lipocalin as a Novel Matrix Protein of Specific Granules in Human Neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The Neutrophil Lipocalin NGAL Is a Bacteriostatic Agent that Interferes with Siderophore-Mediated Iron Acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Dá Mesquita, S.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Palha, J.A.; Marques, F. From the Periphery to the Brain: Lipocalin-2, a Friend or Foe? Prog. Neurobiol. 2015, 131, 120–136. [Google Scholar] [CrossRef]
- Mosialou, I.; Shikhel, S.; Liu, J.M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. MC4R-Dependent Suppression of Appetite by Bone-Derived Lipocalin 2. Nature 2017, 543, 385–390. [Google Scholar] [CrossRef]
- Petropoulou, P.I.; Mosialou, I.; Shikhel, S.; Hao, L.; Panitsas, K.; Bisikirska, B.; Luo, N.; Bahna, F.; Kim, J.; Carberry, P.; et al. Lipocalin-2 Is an Anorexigenic Signal in Primates. eLife 2020, 9, e58949. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, P.W.; Lee, H.W.; Jeong, J.Y.; Lee, M.G.; Kim, J.H.; Lee, W.H.; Yu, R.; Oh, W.J.; Suk, K. Astrocyte-Derived Lipocalin-2 Mediates Hippocampal Damage and Cognitive Deficits in Experimental Models of Vascular Dementia. Glia 2017, 65, 1471–1490. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Weng, Y.C.; Chiang, I.C.; Huang, Y.T.; Liao, Y.C.; Chen, Y.C.; Kao, C.Y.; Liu, Y.L.; Lee, T.H.; Chou, W.H. Neutralization of Lipocalin-2 Diminishes Stroke-Reperfusion Injury. Int. J. Mol. Sci. 2020, 21, 6253. [Google Scholar] [CrossRef]
- Shin, H.J.; Jeong, E.A.; Lee, J.Y.; An, H.S.; Jang, H.M.; Ahn, Y.J.; Lee, J.; Kim, K.E.; Roh, G.S. Lipocalin-2 Deficiency Reduces Oxidative Stress and Neuroinflammation and Results in Attenuation of Kainic Acid-Induced Hippocampal Cell Death. Antioxidants 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Norman, J.E.; Srinivasan, V.J.; Rutledge, J.C. Metabolic, Inflammatory, and Microvascular Determinants of White Matter Disease and Cognitive Decline. Am. J. Neurodegener. Dis. 2016, 5, 171–177. [Google Scholar]
- Hametner, S.; Wimmer, I.; Haider, L.; Pfeifenbring, S.; Brück, W.; Lassmann, H. Iron and Neurodegeneration in the Multiple Sclerosis Brain. Ann. Neurol. 2013, 74, 848–861. [Google Scholar] [CrossRef]
- Zhang, X.; Haaf, M.; Todorich, B.; Grosstephan, E.; Schieremberg, H.; Surguladze, N.; Connor, J.R. Cytokine Toxicity to Oligodendrocyte Precursors Is Mediated by Iron. Glia 2005, 52, 199–208. [Google Scholar] [CrossRef]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple Sclerosis Deep Grey Matter: The Relation between Demyelination, Neurodegeneration, Inflammation and Iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef]
- Egashira, Y.; Hua, Y.; Keep, R.F.; Xi, G. Acute White Matter Injury after Experimental Subarachnoid Hemorrhage: Potential Role of Lipocalin 2. Stroke 2014, 45, 2141–2143. [Google Scholar] [CrossRef]
- Al Nimer, F.; Elliott, C.; Bergman, J.; Khademi, M.; Dring, A.M.; Aeinehband, S.; Bergenheim, T.; Romme Christensen, J.; Sellebjerg, F.; Svenningsson, A.; et al. Lipocalin-2 Is Increased in Progressive Multiple Sclerosis and Inhibits Remyelination. Neuroimmunol. Neuroinflamm. 2016, 3, e191. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Santos, T.; Sampaio-Marques, B.; Novais, A.; Mesquita, S.D.; Ludovico, P.; Bernardino, L.; Correia-Neves, M.; Sousa, N.; Palha, J.A.; et al. Lipocalin-2 Regulates Adult Neurogenesis and Contextual Discriminative Behaviours. Mol. Psychiatry 2018, 23, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Lee, H.; Choi, T.Y.; Joo, Y.; Kim, S.J.; Kim, H.; Kim, J.Y.; Jahng, J.W.; Lee, S.; Choi, S.Y.; et al. Negr1 Controls Adult Hippocampal Neurogenesis and Affective Behaviors. Mol. Psychiatry 2019, 24, 1189–1205. [Google Scholar] [CrossRef]
- Xing, C.; Wang, X.; Cheng, C.; Montaner, J.; Mandeville, E.; Leung, W.; van Leyen, K.; Lok, J.; Wang, X.; Lo, E.H. Neuronal Production of Lipocalin-2 as a Help-Me Signal for Glial Activation. Stroke 2014, 45, 2085–2092. [Google Scholar] [CrossRef]
- Berard, J.L.; Zarruk, J.G.; Arbour, N.; Prat, A.; Yong, V.W.; Jacques, F.H.; Akira, S.; David, S. Lipocalin 2 Is a Novel Immune Mediator of Experimental Autoimmune Encephalomyelitis Pathogenesis and Is Modulated in Multiple Sclerosis. Glia 2012, 60, 1145–1159. [Google Scholar] [CrossRef]
- Lee, S.; Lee, W.H.; Lee, M.S.; Mori, K.; Suk, K. Regulation by lipocalin-2 of Neuronal Cell Death, Migration, and Morphology. J. Neurosci. Res. 2012, 90, 540–550. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.Y.; Lee, W.H.; Kim, H.; Park, H.C.; Mori, K.; Suk, K. Lipocalin-2 Is an Autocrine Mediator of Reactive Astrocytosis. J. Neurosci. 2009, 29, 234–249. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and Endocrine Actions of Bone-the Functions of Secretory Proteins from Osteoblasts, Osteocytes, and Osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Mike, E.V.; Makinde, H.M.; Gulinello, M.; Vanarsa, K.; Herlitz, L.; Gadhvi, G.; Winter, D.R.; Mohan, C.; Hanly, J.G.; Mok, C.C.; et al. Lipocalin-2 Is a Pathogenic Determinant and Biomarker of Neuropsychiatric Lupus. J. Autoimmun. 2019, 96, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Dekens, D.W.; Naudé, P.J.; Engelborghs, S.; Vermeiren, Y.; Van Dam, D.; Oude Voshaar, R.C.; Eisel, U.L.; De Deyn, P.P. Neutrophil Gelatinase-Associated Lipocalin and Its Receptors in Alzheimer’s Disease (AD) Brain Regions: Differential Findings in AD with and without Depression. J. Alzheimer’s Dis. 2017, 55, 763–776. [Google Scholar] [CrossRef]
- Mucha, M.; Skrzypiec, A.E.; Schiavon, E.; Attwood, B.K.; Kucerova, E.; Pawlak, R. Lipocalin-2 Controls Neuronal Excitability and Anxiety by Regulating Dendritic Spine Formation and Maturation. Proc. Natl. Acad. Sci. USA 2011, 108, 18436–18441. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Novais, A.; Sousa, N.; Sousa, J.C.; Marques, F. Voluntary Running Rescues the Defective Hippocampal Neurogenesis and Behaviour Observed in Lipocalin 2-Null Mice. Sci. Rep. 2019, 9, 1649. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Pinto, V.; Dá Mesquita, S.; Novais, A.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Palha, J.A.; Marques, F. Lipocalin-2 Is Involved in Emotional Behaviors and Cognitive Function. Front. Cell. Neurosci. 2013, 7, 122. [Google Scholar] [CrossRef]
- Dekens, D.W.; Naudé, P.J.W.; Keijser, J.N.; Boerema, A.S.; De Deyn, P.P.; Eisel, U.L.M. Lipocalin 2 Contributes to Brain Iron Dysregulation but Does Not Affect Cognition, Plaque Load, and Glial Activation in the J20 Alzheimer Mouse Model. J. Neuroinflamm. 2018, 15, 330. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.W.; Suk, K. Increased Plasma Levels of Lipocalin 2 in Mild Cognitive Impairment. J. Neurol. Sci. 2011, 305, 28–33. [Google Scholar] [CrossRef]
- Naudé, P.J.; Eisel, U.L.; Comijs, H.C.; Groenewold, N.A.; De Deyn, P.P.; Bosker, F.J.; Luiten, P.G.; den Boer, J.A.; Oude Voshaar, R.C. Neutrophil Gelatinase-Associated Lipocalin: A Novel Inflammatory Marker Associated with Late-Life Depression. J. Psychosom. Res. 2013, 75, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Mesquita, S.D.; Sousa, J.C.; Coppola, G.; Gao, F.; Geschwind, D.H.; Columba-Cabezas, S.; Aloisi, F.; Degn, M.; Cerqueira, J.J.; et al. Lipocalin 2 Is Present in the EAE Brain and Is Modulated by Natalizumab. Front. Cell. Neurosci. 2012, 6, 33. [Google Scholar] [CrossRef]
- Dekens, D.W.; Eisel, U.L.M.; Gouweleeuw, L.; Schoemaker, R.G.; De Deyn, P.P.; Naudé, P.J.W. Lipocalin 2 as a Link between Ageing, Risk Factor Conditions and Age-Related Brain Diseases. Ageing Res. Rev. 2021, 70, 101414. [Google Scholar] [CrossRef]
- Naudé, P.J.; Nyakas, C.; Eiden, L.E.; Ait-Ali, D.; van der Heide, R.; Engelborghs, S.; Luiten, P.G.; De Deyn, P.P.; den Boer, J.A.; Eisel, U.L. Lipocalin 2: Novel Component of Proinflammatory Signaling in Alzheimer’s Disease. FASEB J. 2012, 26, 2811–2823. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Gazin, C.; Zhu, X.; Green, M.R. A Cell-Surface Receptor for Lipocalin 24p3 Selectively Mediates Apoptosis and Iron Uptake. Cell 2005, 123, 1293–1305. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Bush, A.I. Iron Neurochemistry in Alzheimer’s Disease and Parkinson’s Disease: Targets for Therapeutics. J. Neurochem. 2016, 139, 179–197. [Google Scholar] [CrossRef]
- Ni, W.; Zheng, M.; Xi, G.; Keep, R.F.; Hua, Y. Role of Lipocalin-2 in Brain Injury after Intracerebral Hemorrhage. J. Cereb. Blood Flow Metab. 2015, 35, 1454–1461. [Google Scholar] [CrossRef]
- Xu, G.; Ahn, J.; Chang, S.; Eguchi, M.; Ogier, A.; Han, S.; Park, Y.; Shim, C.; Jang, Y.; Yang, B.; et al. Lipocalin-2 Induces Cardiomyocyte Apoptosis by Increasing Intracellular Iron Accumulation. J. Biol. Chem. 2012, 287, 4808–4817. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Haschka, D.; Dichtl, S.; Sonnweber, T.; Theurl, I.; Theurl, M.; Lindner, E.; Demetz, E.; Aßhoff, M.; et al. Lipocalin-2 Ensures Host Defense against Salmonella Typhimurium by Controlling Macrophage Iron Homeostasis and Immune Response. Eur. J. Immunol. 2015, 45, 3073–3086. [Google Scholar] [CrossRef]
- Hochmeister, S.; Engel, O.; Adzemovic, M.Z.; Pekar, T.; Kendlbacher, P.; Zeitelhofer, M.; Haindl, M.; Meisel, A.; Fazekas, F.; Seifert-Held, T. Lipocalin-2 as an Infection-Related Biomarker to Predict Clinical Outcome in Ischemic Stroke. PLoS ONE 2016, 11, e0154797. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Renner, A.; Langkammer, C.; Enzinger, C.; Ropele, S.; Stojakovic, T.; Scharnagl, H.; Bachmaier, G.; Pichler, A.; Archelos, J.J.; et al. Cerebrospinal Fluid Lipocalin 2 in Patients with Clinically Isolated Syndromes and Early Multiple Sclerosis. Mult. Scler. 2016, 22, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Jeong, K.H.; Kim, J.H.; Jin, M.; Kim, J.H.; Lee, M.G.; Choi, D.K.; Won, S.Y.; McLean, C.; Jeon, M.T.; et al. Pathogenic Upregulation of Glial Lipocalin-2 in the Parkinsonian Dopaminergic System. J. Neurosci. 2016, 36, 5608–5622. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, D.; Ma, D.N.; Hou, Y.F.; Zhuang, Q.Q.; Gong, Y.L.; Sun, L.H.; Zhao, H.Y.; Tao, B.; Yang, Y.Y.; et al. Osteocalcin Ameliorates Cognitive Dysfunctions in a Mouse Model of Alzheimer’s Disease by Reducing Amyloid β Burden and Upregulating Glycolysis in Neuroglia. Cell Death Discov. 2023, 9, 46. [Google Scholar] [CrossRef]
- Wu, B.W.; Guo, J.D.; Wu, M.S.; Liu, Y.; Lu, M.; Zhou, Y.H.; Han, H.W. Osteoblast-Derived Lipocalin-2 Regulated by Mirna-96-5p/Foxo1 Advances the Progression of Alzheimer’s Disease. Epigenomics 2020, 12, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. The Osteocyte: Key Player in Regulating Bone Turnover. RMD Open 2015, 1, e000049. [Google Scholar] [CrossRef]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kasai, M.; Utsuyama, M.; Hirokawa, K. Determination of Three Isoforms of the Receptor Activator of Nuclear Factor-Kappab Ligand and Their Differential Expression in Bone and Thymus. Endocrinology 2001, 142, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Bao, B.; Wu, W. The Hidden Secrets of Soluble RANKL in Bone Biology. Cytokine 2021, 144, 155559. [Google Scholar] [CrossRef]
- Kaneko, K.; Kawai, S. Glucocorticoid and Bone. Significance of Serum Soluble RANKL Measurement in Patients under Glucocorticoid Therapy. Clin. Calcium 2014, 24, 1361–1370. [Google Scholar] [PubMed]
- Ma, T.; Miyanishi, K.; Suen, A.; Epstein, N.J.; Tomita, T.; Smith, R.L.; Goodman, S.B. Human Interleukin-1-Induced Murine Osteoclastogenesis Is Dependent on RANKL, but Independent of TNF-Alpha. Cytokine 2004, 26, 138–144. [Google Scholar] [CrossRef]
- Schlöndorff, J.; Lum, L.; Blobel, C.P. Biochemical and Pharmacological Criteria Define Two Shedding Activities for TRANCE/OPGL that Are Distinct from the Tumor Necrosis Factor Alpha Convertase. J. Biol. Chem. 2001, 276, 14665–14674. [Google Scholar] [CrossRef]
- Yasuda, H. Bone and Bone Related Biochemical Examinations. Bone and Collagen Related Metabolites. Receptor Activator of NF-Kappab Ligand (RANKL). Clin. Calcium 2006, 16, 964–970. [Google Scholar]
- Zhu, P.; Zhang, Z.; Huang, X.; Liang, S.; Khandekar, N.; Song, Z.; Lin, S. RANKL Reduces Body Weight and Food Intake via the Modulation of Hypothalamic NPY/CART Expression. Int. J. Med. Sci. 2018, 15, 969–977. [Google Scholar] [CrossRef]
- Enomoto, T.; Furuya, Y.; Tomimori, Y.; Mori, K.; Miyazaki, J.; Yasuda, H. Establishment of a New Murine Model of Hypercalcemia with Anorexia by Overexpression of Soluble Receptor Activator of NF-κB Ligand Using an Adenovirus Vector. J. Bone Miner. Metab. 2011, 29, 414–421. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Ziora, K.; Oświęcimska, J.; Swiętochowska, E.; Wołkowska-Pokrywa, K. Dehydroepiandrosterone Sulfate, Osteoprotegerin and Its Soluble Ligand sRANKL and Bone Metabolism in Girls with Anorexia Nervosa. Postep. Hig. Med. Dosw. 2012, 66, 655–662. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Ziora, K.; Oświęcimska, J.; Swiętochowska, E.; Szapska, B.; Wołkowska-Pokrywa, K.; Dyduch, A. RANKL/RANK/OPG System and Bone Status in Females with Anorexia Nervosa. Bone 2012, 50, 156–160. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK Signaling in Osteoclastogenesis and Bone Disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hanada, R.; Leibbrandt, A.; Hanada, T.; Kitaoka, S.; Furuyashiki, T.; Fujihara, H.; Trichereau, J.; Paolino, M.; Qadri, F.; Plehm, R.; et al. Central Control of Fever and Female Body Temperature by RANKL/RANK. Nature 2009, 462, 505–509. [Google Scholar] [CrossRef]
- Hanada, R.; Hanada, T.; Sigl, V.; Schramek, D.; Penninger, J.M. RANKL/RANK-Beyond Bones. J. Mol. Med. 2011, 89, 647–656. [Google Scholar] [CrossRef]
- Kichev, A.; Eede, P.; Gressens, P.; Thornton, C.; Hagberg, H. Implicating Receptor Activator of NF-κB (RANK)/RANK Ligand Signalling in Microglial Responses to Toll-Like Receptor Stimuli. Dev. Neurosci. 2017, 39, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.M.; Lu, S.H.; Zhang, Q.J.; Liu, H.Y.; Yang, R.C.; Cai, Y.L.; Han, Z.C. The Preliminary Study on in Vitro Differentiation of Human Umbilical Cord Blood Cells into Neural Cells. Chin. J. Hematol. 2003, 24, 484–487. [Google Scholar]
- Gutierrez, H.; Kisiswa, L.; O’Keeffe, G.W.; Smithen, M.J.; Wyatt, S.; Davies, A.M. Regulation of Neurite Growth by Tumour Necrosis Superfamily Member RANKL. Open Biol. 2013, 3, 120150. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Glasnović, A.; O’Mara, N.; Kovačić, N.; Grčević, D.; Gajović, S. RANK/RANKL/OPG Signaling in the Brain: A Systematic Review of the Literature. Front. Neurol. 2020, 11, 590480. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Nakagami, H.; Osako, M.K.; Kurinami, H.; Koriyama, H.; Zhengda, P.; Tomioka, H.; Tenma, A.; Wakayama, K.; Morishita, R. OPG/RANKL/RANK Axis Is a Critical Inflammatory Signaling System in Ischemic Brain in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 8191–8196. [Google Scholar] [CrossRef]
- Sakai, S.; Shichita, T. Inflammation and Neural Repair after Ischemic Brain Injury. Neurochem. Int. 2019, 130, 104316. [Google Scholar] [CrossRef]
- Guo, X.; Shi, W.; Lu, J.; Tang, P.; Li, R. Unraveling the Impact of Blood RANKL and OPG Levels on Alzheimer’s Disease: Independent of Bone Mineral Density and Inflammation. Alzheimer’s Dement. 2025, 11, e70044. [Google Scholar] [CrossRef]
- Haffner, D.; Leifheit-Nestler, M. Extrarenal Effects of FGF23. Pediatr. Nephrol. 2017, 32, 753–765. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Tang, W.; Jiang, X.; Rowe, D.W.; Quarles, L.D. Pathogenic Role of Fgf23 in Hyp Mice. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E38–E49. [Google Scholar] [CrossRef]
- Drew, D.A.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Fan, L.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. FGF-23 and Cognitive Performance in Hemodialysis Patients. Hemodial. Int. 2014, 18, 78–86. [Google Scholar] [CrossRef]
- Ursem, S.R.; Diepenbroek, C.; Bacic, V.; Unmehopa, U.A.; Eggels, L.; Maya-Monteiro, C.M.; Heijboer, A.C.; la Fleur, S.E. Localization of Fibroblast Growth Factor 23 Protein in the Rat Hypothalamus. Eur. J. Neurosci. 2021, 54, 5261–5271. [Google Scholar] [CrossRef]
- McGrath, E.R.; Himali, J.J.; Levy, D.; Conner, S.C.; Pase, M.P.; Abraham, C.R.; Courchesne, P.; Satizabal, C.L.; Vasan, R.S.; Beiser, A.S.; et al. Circulating Fibroblast Growth Factor 23 Levels and Incident Dementia: The Framingham Heart Study. PLoS ONE 2019, 14, e0213321. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.B.; Shah, N.H.; Mendez, A.J.; DeRosa, J.T.; Yoshita, M.; Elkind, M.S.; Sacco, R.L.; DeCarli, C.; Rundek, T.; Silverberg, S.; et al. Fibroblast Growth Factor 23 Is Associated with Subclinical Cerebrovascular Damage: The Northern Manhattan Study. Stroke 2016, 47, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Marebwa, B.K.; Adams, R.J.; Magwood, G.S.; Kindy, M.; Wilmskoetter, J.; Wolf, M.; Bonilha, L. Fibroblast Growth Factor23 Is Associated with Axonal Integrity and Neural Network Architecture in the Human Frontal Lobes. PLoS ONE 2018, 13, e0203460. [Google Scholar] [CrossRef]
- Hensel, N.; Schön, A.; Konen, T.; Lübben, V.; Förthmann, B.; Baron, O.; Grothe, C.; Leifheit-Nestler, M.; Claus, P.; Haffner, D. Fibroblast Growth Factor 23 Signaling in Hippocampal Cells: Impact on Neuronal Morphology and Synaptic Density. J. Neurochem. 2016, 137, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Oehmichen, J.; Van Aken, H.; Reuter, S.; Pavenstädt, H.J.; Meersch, M.; Unruh, M.; Zarbock, A. FGF23 Signaling Impairs Neutrophil Recruitment and Host Defense During CKD. J. Clin. Investig. 2016, 126, 962–974. [Google Scholar] [CrossRef]
- Laszczyk, A.M.; Nettles, D.; Pollock, T.A.; Fox, S.; Garcia, M.L.; Wang, J.; Quarles, L.D.; King, G.D. FGF-23 Deficiency Impairs Hippocampal-Dependent Cognitive Function. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Kuro, O.M. The FGF23 and Klotho System Beyond Mineral Metabolism. Clin. Exp. Nephrol. 2017, 21, 64–69. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan Communication by Exosomes, Adipose Tissue, and Adiponectin in Metabolic Syndrome. J. Clin. Investig. 2019, 129, 4041–4049. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, Z.X.; Liu, X.X.; Wan, M.D.; Liu, Y.W.; Jiao, B.; Liao, X.X.; Luo, Z.W.; Wang, Y.Y.; Hong, C.G.; et al. The Protective Effects of Osteocyte-Derived Extracellular Vesicles against Alzheimer’s Disease Diminished with Aging. Adv. Sci. 2022, 9, e2105316. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yang, L.; Wu, X.; Huang, W.; Yan, J.; Liu, S.; Sun, X.; Liu, K.; Lin, H.; Kuang, S.; et al. Evidences for B6C3-Tg (APPswe/PSEN1dE9) Double-Transgenic Mice between 3 and 10 Months as an Age-Related Alzheimer’s Disease Model. J. Mol. Neurosci. 2014, 53, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Howlett, D.R.; Bowler, K.; Soden, P.E.; Riddell, D.; Davis, J.B.; Richardson, J.C.; Burbidge, S.A.; Gonzalez, M.I.; Irving, E.A.; Lawman, A.; et al. Abeta Deposition and Related Pathology in an APP X PS1 Transgenic Mouse Model of Alzheimer’s Disease. Histol. Histopathol. 2008, 23, 67–76. [Google Scholar] [CrossRef]
- Shi, T.; Shen, S.; Shi, Y.; Wang, Q.; Zhang, G.; Lin, J.; Chen, J.; Bai, F.; Zhang, L.; Wang, Y.; et al. Osteocyte-Derived Sclerostin Impairs Cognitive Function During Ageing and Alzheimer’s Disease Progression. Nat. Metab. 2024, 6, 531–549. [Google Scholar] [CrossRef]
- Cararo-Lopes, M.M.; Mazucanti, C.H.Y.; Scavone, C.; Kawamoto, E.M.; Berwick, D.C. The Relevance of α-KLOTHO to the Central Nervous System: Some Key Questions. Ageing Res. Rev. 2017, 36, 137–148. [Google Scholar] [CrossRef]
- Chen, C.D.; Li, Y.; Chen, A.K.; Rudy, M.A.; Nasse, J.S.; Zeldich, E.; Polanco, T.J.; Abraham, C.R. Identification of the Cleavage Sites Leading to the Shed Forms of Human and Mouse Anti-Aging and Cognition-Enhancing Protein Klotho. PLoS ONE 2020, 15, e0226382. [Google Scholar] [CrossRef]
- Grøntvedt, G.R.; Sando, S.B.; Lauridsen, C.; Bråthen, G.; White, L.R.; Salvesen, Ø.; Aarsland, D.; Hessen, E.; Fladby, T.; Waterloo, K.; et al. Association of Klotho Protein Levels and KL-VS Heterozygosity with Alzheimer Disease and Amyloid and Tau Burden. JAMA Netw. Open 2022, 5, e2243232. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Wang, Q. Anti-Inflammatory Role of the Klotho Protein and Relevance to Aging. Cells 2024, 13, 1413. [Google Scholar] [CrossRef]
- Li, B.; Zhou, M.; Peng, J.; Yang, Q.; Chu, J.; Li, R.; Jiang, Y. Mechanism of the Fibroblast Growth Factor 23/α-Klotho Axis in Peripheral Blood Mononuclear Cell Inflammation in Alzheimer’s Disease. Immunol. Investig. 2022, 51, 1471–1484. [Google Scholar] [CrossRef]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-Catenin Signaling Is a Promising Therapeutic Strategy for Alzheimer’s Disease. Mol. Brain 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.Q.; Wei, M.D.; Wang, K.W.; Lan, Y.X.; Zhu, N.; Wang, Y. Nicotine Contributes to the Neural Stem Cells Fate against Toxicity of Microglial-Derived Factors Induced by Aβ via the Wnt/β-Catenin Pathway. Int. J. Neurosci. 2016, 126, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, M.; Meng, L.; Tian, Q.; Dong, Z. Osteoclasts Link Dysregulated Peripheral Degradation Processes and Accelerated Progression in Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 99, 773–785. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Rahbar, M.H.; Zhu, H.; Rowan, P.J.; Bambhroliya, A.B.; Savitz, S.I. Systematic Review and Meta-Analysis of Bone Marrow-Derived Mononuclear Cells in Animal Models of Ischemic Stroke. Stroke 2016, 47, 1632–1639. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Jiang, C.; Yu, L.; Wang, M.; Han, W.; Liu, L.; Wang, J. Bone Marrow Mononuclear Cell Transplantation Promotes Therapeutic Angiogenesis via Upregulation of the VEGF-VEGFR2 Signaling Pathway in a Rat Model of Vascular Dementia. Behav. Brain Res. 2014, 265, 171–180. [Google Scholar] [CrossRef]
- Kamiya, F.; Ueda, M.; Nito, C.; Kamiya, N.; Inaba, T.; Suda, S.; Saito, T.; Muraga, K.; Katayama, Y. Effect of Repeated Allogeneic Bone Marrow Mononuclear Cell Transplantation on Brain Injury Following Transient Focal Cerebral Ischemia in Rats. Life Sci. 2014, 95, 22–28. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Dos Santos Sampaio, M.; Santana Bastos Boechat, M.; Augusto Gusman Cunha, I.; Gonzaga Pereira, M.; Coimbra, N.C.; Giraldi-Guimarães, A. Neurotrophin-3 Upregulation Associated with Intravenous Transplantation of Bone Marrow Mononuclear Cells Induces Axonal Sprouting and Motor Functional Recovery in the Long Term after Neocortical Ischaemia. Brain Res. 2021, 1758, 147292. [Google Scholar] [CrossRef]
- Fujita, Y.; Ihara, M.; Ushiki, T.; Hirai, H.; Kizaka-Kondoh, S.; Hiraoka, M.; Ito, H.; Takahashi, R. Early Protective Effect of Bone Marrow Mononuclear Cells against Ischemic White Matter Damage through Augmentation of Cerebral Blood Flow. Stroke 2010, 41, 2938–2943. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Yu, L.; Li, N.; Wang, M.; Liu, X.; Zhang, D.; Han, W.; Zhou, C.; Wang, J. Preconditioning with VEGF Enhances Angiogenic and Neuroprotective Effects of Bone Marrow Mononuclear Cell Transplantation in a Rat Model of Chronic Cerebral Hypoperfusion. Mol. Neurobiol. 2016, 53, 6057–6068. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Terashima, T.; Katagi, M.; Ohashi, N.; Nozaki, K.; Tsuji, A. Bone Marrow-Derived Mononuclear Cells Ameliorate Neurological Function in Chronic Cerebral Infarction Model Mice via Improvement of Cerebral Blood Flow. Cytotherapy 2023, 25, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, J.; Yu, L.; Ou, C.; Liu, X.; Zhao, X.; Wang, J. Comparison of the Therapeutic Effects of Bone Marrow Mononuclear Cells and Microglia for Permanent Cerebral Ischemia. Behav. Brain Res. 2013, 250, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Lu, H.; Jiang, C.; Cui, X.; Yu, L.; Fu, X.; Li, Q.; Wang, J. CXCR4+CD45− BMMNC Subpopulation Is Superior to Unfractionated BMMNCs for Protection after Ischemic Stroke in Mice. Brain Behav. Immun. 2015, 45, 98–108. [Google Scholar] [CrossRef]
- Liem, N.T.; Huyen, T.L.; Huong, L.T.; Doan, N.V.; Anh, B.V.; Anh, N.T.P.; Tung, D.T. Outcomes of Bone Marrow Mononuclear Cell Transplantation for Neurological Sequelae Due to Intracranial Hemorrhage Incidence in the Neonatal Period: Report of Four Cases. Front. Pediatr. 2019, 7, 543. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Saino, O.; Okinaka, Y.; Kikuchi-Taura, A.; Takeuchi, Y.; Taguchi, A. Bone Marrow Mononuclear Cells Transplantation and Training Increased Transplantation of Energy Source Transporters in Chronic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105932. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.S., Jr.; Baumgartner, J.E.; Harting, M.T.; Worth, L.L.; Walker, P.A.; Shah, S.K.; Ewing-Cobbs, L.; Hasan, K.M.; Day, M.C.; Lee, D.; et al. Autologous Bone Marrow Mononuclear Cell Therapy for Severe Traumatic Brain Injury in Children. Neurosurgery 2011, 68, 588–600. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sane, H.M.; Kulkarni, P.P.; Gokulchandran, N.; Biju, H.; Badhe, P.B. Autologous Bone Marrow Mononuclear Cell Transplantation in Patients with Chronic Traumatic Brain Injury—A Clinical Study. Cell Regen. 2020, 9, 3. [Google Scholar] [CrossRef]
- Cox, C.S., Jr.; Hetz, R.A.; Liao, G.P.; Aertker, B.M.; Ewing-Cobbs, L.; Juranek, J.; Savitz, S.I.; Jackson, M.L.; Romanowska-Pawliczek, A.M.; Triolo, F.; et al. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells 2017, 35, 1065–1079. [Google Scholar] [CrossRef]
- Kanamaru, T.; Kamimura, N.; Yokota, T.; Nishimaki, K.; Iuchi, K.; Lee, H.; Takami, S.; Akashiba, H.; Shitaka, Y.; Ueda, M.; et al. Intravenous Transplantation of Bone Marrow-Derived Mononuclear Cells Prevents Memory Impairment in Transgenic Mouse Models of Alzheimer’s Disease. Brain Res. 2015, 1605, 49–58. [Google Scholar] [CrossRef]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Pai, S.; Kulkarni, P.; Ganwir, V.; Maheshwari, M.; Sharma, R.; Raichur, M.; Nivins, S.; et al. An Open-Label Proof-of-Concept Study of Intrathecal Autologous Bone Marrow Mononuclear Cell Transplantation in Intellectual Disability. Stem Cell Res. Ther. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zaverucha-do-Valle, C.; Gubert, F.; Bargas-Rega, M.; Coronel, J.L.; Mesentier-Louro, L.A.; Mencalha, A.; Abdelhay, E.; Santiago, M.F.; Mendez-Otero, R. Bone Marrow Mononuclear Cells Increase Retinal Ganglion Cell Survival and Axon Regeneration in the Adult Rat. Cell Transplant. 2011, 20, 391–406. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Pimentel-Coelho, P.M.; Mesentier-Louro, L.A.; Mendez, R.M.; Mello-Silva, J.P.; Cabral-da-Silva, M.C.; de Mello, F.G.; de Melo Reis, R.A.; Mendez-Otero, R. Trophic Activity Derived from Bone Marrow Mononuclear Cells Increases Peripheral Nerve Regeneration by Acting on Both Neuronal and Glial Cell Populations. Neuroscience 2009, 159, 540–549. [Google Scholar] [CrossRef]
- Leal, M.M.; Costa-Ferro, Z.S.; Souza, B.S.; Azevedo, C.M.; Carvalho, T.M.; Kaneto, C.M.; Carvalho, R.H.; Dos Santos, R.R.; Soares, M.B. Early Transplantation of Bone Marrow Mononuclear Cells Promotes Neuroprotection and Modulation of Inflammation after Status Epilepticus in Mice by Paracrine Mechanisms. Neurochem. Res. 2014, 39, 259–268. [Google Scholar] [CrossRef]
- do Prado-Lima, P.A.S.; Onsten, G.A.; de Oliveira, G.N.; Brito, G.C.; Ghilardi, I.M.; de Souza, E.V.; Dos Santos, P.G.; Salamoni, S.D.; Machado, D.C.; Duarte, M.M.F.; et al. The Antidepressant Effect of Bone Marrow Mononuclear Cell Transplantation in Chronic Stress. J. Psychopharmacol. 2019, 33, 632–639. [Google Scholar] [CrossRef]
- Costa-Ferro, Z.S.M.; do Prado-Lima, P.A.S.; Onsten, G.A.; Oliveira, G.N.; Brito, G.C.; Ghilardi, I.M.; Dos Santos, P.G.; Bertinatto, R.J.; da Silva, D.V.; Salamoni, S.D.; et al. Bone Marrow Mononuclear Cell Transplant Prevents Rat Depression and Modulates Inflammatory and Neurogenic Molecules. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 113, 110455. [Google Scholar] [CrossRef]
- Santhanam, L.; Liu, G.; Jandu, S.; Su, W.; Wodu, B.P.; Savage, W.; Poe, A.; Liu, X.; Alexander, L.M.; Cao, X.; et al. Skeleton-Secreted PDGF-BB Mediates Arterial Stiffening. J. Clin. Investig. 2021, 131, e147116. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis Stimulated by Elevated PDGF-BB in Subchondral Bone Contributes to Osteoarthritis Development. JCI Insight 2020, 5, e135446. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Wei, Z.; Fang, C.L.; Shen, K.; Qian, C.; Qi, C.; Li, T.; Gao, P.; Wong, P.C.; et al. Elevated PDGF-BB from Bone Impairs Hippocampal Vasculature by Inducing PDGFRβ Shedding from Pericytes. Adv. Sci. 2023, 10, e2206938. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory Mechanisms of Blood-Brain Barrier Damage in Ischemic Stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Kakaroubas, N.; Brennan, S.; Keon, M.; Saksena, N.K. Pathomechanisms of Blood-Brain Barrier Disruption in ALS. Neurosci. J. 2019, 2019, 2537698. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.N.; Berthiaume, A.A.; Faino, A.V.; McDowell, K.P.; Bhat, N.R.; Hartmann, D.A.; Shih, A.Y. Mild Pericyte Deficiency Is Associated with Aberrant Brain Microvascular Flow in Aged PDGFRβ+/− Mice. J. Cereb. Blood Flow Metab. 2020, 40, 2387–2400. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Fuchs, D.T.; Sheyn, J.; Wawrowsky, K.; Lahiri, S.; Black, K.L.; Koronyo-Hamaoui, M. Retinal Capillary Degeneration and Blood-Retinal Barrier Disruption in Murine Models of Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional Morphology of the Blood-Brain Barrier in Health and Disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Pederson, L.; Ruan, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Regulation of Bone Formation by Osteoclasts Involves Wnt/BMP Signaling and the Chemokine Sphingosine-1-Phosphate. Proc. Natl. Acad. Sci. USA 2008, 105, 20764–20769. [Google Scholar] [CrossRef]
- Garimella, R.; Tague, S.E.; Zhang, J.; Belibi, F.; Nahar, N.; Sun, B.H.; Insogna, K.; Wang, J.; Anderson, H.C. Expression and Synthesis of Bone Morphogenetic Proteins by Osteoclasts: A Possible Path to Anabolic Bone Remodeling. J. Histochem. Cytochem. 2008, 56, 569–577. [Google Scholar] [CrossRef]
- Meyers, E.A.; Kessler, J.A. TGF-β Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb. Perspect. Biol. 2017, 9, a022244. [Google Scholar] [CrossRef]
- Ji, J.; Wang, J.; Yang, J.; Wang, X.P.; Huang, J.J.; Xue, T.F.; Sun, X.L. The Intra-nuclear SphK2-S1P Axis Facilitates M1-to-M2 Shift of Microglia via Suppressing HDAC1-Mediated KLF4 Deacetylation. Front. Immunol. 2019, 10, 1241. [Google Scholar] [CrossRef]
- Takeshita, S.; Fumoto, T.; Matsuoka, K.; Park, K.A.; Aburatani, H.; Kato, S.; Ito, M.; Ikeda, K. Osteoclast-Secreted CTHRC1 in the Coupling of Bone Resorption to Formation. J. Clin. Investig. 2013, 123, 3914–3924. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, Y.; Chen, G.; Li, J.; Wang, B.; Lu, X. Potential Association of Bone Mineral Density Loss with Cognitive Impairment and Central and Peripheral Amyloid-β Changes: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2022, 23, 626. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Qiu, F.; He, J.; Chen, J. Cognitive Impairment and Risks of Osteoporosis: A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2023, 106, 104879. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, H.; Tao, Y.; Tan, J.; Chen, L.; Huang, H.; Chen, Y.; Li, Y.; Zhou, R. Alzheimer’s Disease Is Associated with Increased Risk of Osteoporosis: The Chongqing Aging Study. Curr. Alzheimer Res. 2016, 13, 1165–1172. [Google Scholar] [CrossRef]
- Li, R.; Miao, Z.; Liu, Y.; Chen, X.; Wang, H.; Su, J.; Chen, J. The Brain-Gut-Bone Axis in Neurodegenerative Diseases: Insights, Challenges, and Future Prospects. Adv. Sci. 2024, 11, e2307971. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Thal, D.R.; Tomé, S.O. The Central Role of Tau in Alzheimer’s Disease: From Neurofibrillary Tangle Maturation to the Induction of Cell Death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713.e1613. [Google Scholar] [CrossRef]
- Schwalbe, M.; Kadavath, H.; Biernat, J.; Ozenne, V.; Blackledge, M.; Mandelkow, E.; Zweckstetter, M. Structural Impact of Tau Phosphorylation at Threonine 231. Structure 2015, 23, 1448–1458. [Google Scholar] [CrossRef]
- Rani, L.; Mittal, J.; Mallajosyula, S.S. Effect of Phosphorylation and O-GlcNAcylation on Proline-Rich Domains of Tau. J. Phys. Chem. B 2020, 124, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Mayer, B.; Feldengut, S.; Schön, M.; Del Tredici, K. Sequence and Trajectory of Early Alzheimer’s Disease-Related Tau Inclusions in the Hippocampal Formation of Cases without Amyloid-β Deposits. Acta Neuropathol. 2025, 149, 50. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, S.; Medalsy, I.D.; Mandelkow, E.; Müller, D.J. The Fuzzy Coat of Pathological Human Tau Fibrils Is a Two-Layered Polyelectrolyte Brush. Proc. Natl. Acad. Sci. USA 2013, 110, E313–E321. [Google Scholar] [CrossRef] [PubMed]
- Moloney, C.M.; Lowe, V.J.; Murray, M.E. Visualization of Neurofibrillary Tangle Maturity in Alzheimer’s Disease: A Clinicopathologic Perspective for Biomarker Research. Alzheimer’s Dement. 2021, 17, 1554–1574. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid Precursor Protein Trafficking, Processing, and Function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Amyloid-β May Be Released from Non-Junctional Varicosities of Axons Generated from Abnormal Tau-Containing Brainstem Nuclei in Sporadic Alzheimer’s Disease: A Hypothesis. Acta Neuropathol. 2013, 126, 303–306. [Google Scholar] [CrossRef]
- Alrouji, M.; Alshammari, M.S.; Tasqeeruddin, S.; Shamsi, A. Interplay between Aging and Tau Pathology in Alzheimer’s Disease: Mechanisms and Translational Perspectives. Antioxidants 2025, 14, 774. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-Cell Transcriptomic Analysis of Alzheimer’s Disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Grubman, A.; Chew, G.; Ouyang, J.F.; Sun, G.; Choo, X.Y.; McLean, C.; Simmons, R.K.; Buckberry, S.; Vargas-Landin, D.B.; Poppe, D.; et al. A Single-Cell Atlas of Entorhinal Cortex from Individuals with Alzheimer’s Disease Reveals Cell-Type-Specific Gene Expression Regulation. Nat. Neurosci. 2019, 22, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.G.; Rogers, B.B.; Loupe, J.M.; Rodriguez-Nunez, I.; Roberts, S.C.; White, L.M.; Brazell, J.N.; Bunney, W.E.; Bunney, B.G.; Watson, S.J.; et al. Single Nucleus Multiomics Identifies ZEB1 and MAFB As Candidate Regulators of Alzheimer’s Disease-Specific Cis-Regulatory Elements. Cell Genom. 2023, 3, 100263. [Google Scholar] [CrossRef]
- Xiong, X.; James, B.T.; Boix, C.A.; Park, Y.P.; Galani, K.; Victor, M.B.; Sun, N.; Hou, L.; Ho, L.L.; Mantero, J.; et al. Epigenomic Dissection of Alzheimer’s Disease Pinpoints Causal Variants and Reveals Epigenome Erosion. Cell 2023, 186, 4422–4437.e21. [Google Scholar] [CrossRef]
- Chen, W.T.; Lu, A.; Craessaerts, K.; Pavie, B.; Sala Frigerio, C.; Corthout, N.; Qian, X.; Laláková, J.; Kühnemund, M.; Voytyuk, I.; et al. Spatial Transcriptomics and in Situ Sequencing to Study Alzheimer’s Disease. Cell 2020, 182, 976–991.e19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, W.M.; Andhey, P.S.; Swain, A.; Levy, T.; Miller, K.R.; Poliani, P.L.; Cominelli, M.; Grover, S.; Gilfillan, S.; et al. Human and Mouse Single-Nucleus Transcriptomics Reveal TREM2-Dependent and TREM2-Independent Cellular Responses in Alzheimer’s Disease. Nat. Med. 2020, 26, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Fertan, E.; Lam, J.Y.L.; Albertini, G.; Dewilde, M.; Wu, Y.; Akingbade, O.E.S.; Böken, D.; English, E.A.; De Strooper, B.; Klenerman, D. Lecanemab Preferentially Binds to Smaller Aggregates Present at Early Alzheimer’s Disease. Alzheimer’s Dement. 2025, 21, e70086. [Google Scholar] [CrossRef]
- Bayer, T.A. N-Truncated Aβ Starting at Position Four-Biochemical Features, Preclinical Models, and Potential as Drug Target in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 710579. [Google Scholar] [CrossRef]
- Roberts, M.; Sevastou, I.; Imaizumi, Y.; Mistry, K.; Talma, S.; Dey, M.; Gartlon, J.; Ochiai, H.; Zhou, Z.; Akasofu, S.; et al. Pre-Clinical Characterisation of E2814, a High-Affinity Antibody Targeting the Microtubule-Binding Repeat Domain of Tau for Passive Immunotherapy in Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 13. [Google Scholar] [CrossRef]
- Weinstock, M. Role of Oxidative Stress and Neuroinflammation in the Etiology of Alzheimer’s Disease: Therapeutic Options. Antioxidants 2025, 14, 769. [Google Scholar] [CrossRef]
- Akoury, E.; Pickhardt, M.; Gajda, M.; Biernat, J.; Mandelkow, E.; Zweckstetter, M. Mechanistic Basis of Phenothiazine-Driven Inhibition of Tau Aggregation. Angew. Chem. Int. Ed. 2013, 52, 3511–3515. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin Suppresses Soluble Tau Dimers and Corrects Molecular Chaperone, Synaptic, and Behavioral Deficits in Aged Human Tau Transgenic Mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef]
- Haroon, J.; Jordan, K.; Mahdavi, K.; Rindner, E.; Becerra, S.; Surya, J.R.; Zielinski, M.; Venkatraman, V.; Goodenowe, D.; Hofmeister, K.; et al. A Phase 2, Open-Label Study of Anti-Inflammatory NE3107 in Patients with Dementias. Medicine 2024, 103, e39027. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.L.; Ahlem, C.N.; Murphy, M.F. NM101 Phase III Study of NE3107 in Alzheimer’s Disease: Rationale, Design and Therapeutic Modulation of Neuroinflammation and Insulin Resistance. Neurodegener. Dis. Manag. 2021, 11, 289–298. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Nham, A.; Sherbaf, A.; Quach, D.; Yahya, E.; Ranburger, D.; Bi, X.; Baudry, M. Calpain-2 as a Therapeutic Target in Repeated Concussion-Induced Neuropathy and Behavioral Impairment. Sci. Adv. 2020, 6, eaba5547. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-Human TREM2 Induces Microglia Proliferation and Reduces Pathology in an Alzheimer’s Disease Model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef]
- Tan, Z.S.; Seshadri, S.; Beiser, A.; Zhang, Y.; Felson, D.; Hannan, M.T.; Au, R.; Wolf, P.A.; Kiel, D.P. Bone Mineral Density and the Risk of Alzheimer Disease. Arch. Neurol. 2005, 62, 107–111. [Google Scholar] [CrossRef]
- Loskutova, N.; Watts, A.S.; Burns, J.M. The Cause-Effect Relationship between Bone Loss and Alzheimer’s Disease Using Statistical Modeling. Med. Hypotheses 2019, 122, 92–97. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chuang, C.S.; Hung, C.H.; Lin, C.L.; Sung, F.C.; Tang, C.H.; Hsu, H.C.; Chung, C.J. Fracture as an Independent Risk Factor of Dementia: A Nationwide Population-Based Cohort Study. Medicine 2014, 93, e188. [Google Scholar] [CrossRef]
- Davoody, S.; Asgari Taei, A.; Khodabakhsh, P.; Dargahi, L. Mtor Signaling and Alzheimer’s Disease: What We Know and Where We Are? CNS Neurosci. Ther. 2024, 30, e14463. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, L. Alzheimer’s Disease Is an Important Risk Factor of Fractures: A Meta-Analysis of Cohort Studies. Mol. Neurobiol. 2017, 54, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Azargoonjahromi, A. The Duality of Amyloid-β: Its Role in Normal and Alzheimer’s Disease States. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, S.; Chen, Z.; Feng, F.; He, L.; Liu, B.; He, T.; Wang, X.; Chen, R.; Chen, Z.; et al. Amyloid β Peptide Promotes Bone Formation by Regulating Wnt/β-Catenin Signaling and the OPG/RANKL/RANK System. FASEB J. 2020, 34, 3583–3593. [Google Scholar] [CrossRef]
- Deng, A.F.; Wang, F.X.; Wang, S.C.; Zhang, Y.Z.; Bai, L.; Su, J.C. Bone-Organ Axes: Bidirectional Crosstalk. Mil. Med. Res. 2024, 11, 37. [Google Scholar] [CrossRef]
- Nasme, F.; Behera, J.; Tyagi, P.; Debnath, N.; Falcone, J.C.; Tyagi, N. The Potential Link between the Development of Alzheimer’s Disease and Osteoporosis. Biogerontology 2025, 26, 43. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.A.; Santos-Galindo, M.; Lagunas, N.; Azcoitia, I.; Garcia-Segura, L.M. Selective Estrogen Receptor Modulators as Brain Therapeutic Agents. J. Mol. Endocrinol. 2011, 46, R1–R9. [Google Scholar] [CrossRef]
- Jacobsen, D.E.; Samson, M.M.; Emmelot-Vonk, M.H.; Verhaar, H.J. Raloxifene Improves Verbal Memory in Late Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Menopause 2010, 17, 309–314. [Google Scholar] [CrossRef]
- Henderson, V.W.; Ala, T.; Sainani, K.L.; Bernstein, A.L.; Stephenson, B.S.; Rosen, A.C.; Farlow, M.R. Raloxifene for Women with Alzheimer Disease: A Randomized Controlled Pilot Trial. Neurology 2015, 85, 1937–1944. [Google Scholar] [CrossRef]
- Korol, D.L.; Pisani, S.L. Estrogens and Cognition: Friends or Foes?: An Evaluation of the Opposing Effects of Estrogens on Learning and Memory. Horm. Behav. 2015, 74, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Cibicková, L.; Palicka, V.; Cibicek, N.; Cermáková, E.; Micuda, S.; Bartosová, L.; Jun, D. Differential Effects of Statins and Alendronate on Cholinesterases in Serum and Brain of Rats. Physiol. Res. 2007, 56, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Chung, C.J.; Lin, C.L.; Sung, F.C.; Wu, T.N.; Kao, C.H. Increased Risk of Dementia in Patients with Osteoporosis: A Population-Based Retrospective Cohort Analysis. Age 2014, 36, 967–975. [Google Scholar] [CrossRef]
- Safer, U.; Safer, V.B.; Demir, S.O.; Yanikoglu, I. Effects of Bisphosphonates and Calcium Plus Vitamin-D Supplements on Cognitive Function in Postmenopausal Osteoporosis§. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 56–60. [Google Scholar] [CrossRef]
- Wu, P.H.; Lin, Y.T.; Chen, C.S.; Chiu, Y.W.; Tsai, J.C.; Kuo, P.L.; Hsu, Y.L.; Ljunggren, Ö.; Fellström, B.; Kuo, M.C. Associations of Bone Turnover Markers with Cognitive Function in Patients Undergoing Hemodialysis. Dis. Markers 2020, 2020, 8641749. [Google Scholar] [CrossRef]
- Chang, B.; Quan, Q.; Li, Y.; Qiu, H.; Peng, J.; Gu, Y. Treatment of Osteoporosis, with a Focus on 2 Monoclonal Antibodies. Med. Sci. Monit. 2018, 24, 8758–8766. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Gallelli, L.; Leo, A.; De Fazio, P.; Gallelli, P.; Russo, E.; De Sarro, G. Effects of Chronic Sodium Alendronate on Depression and Anxiety in a Menopausal Experimental Model. Pharmacol. Biochem. Behav. 2015, 129, 65–71. [Google Scholar] [CrossRef]
- Eimar, H.; Alebrahim, S.; Manickam, G.; Al-Subaie, A.; Abu-Nada, L.; Murshed, M.; Tamimi, F. Donepezil Regulates Energy Metabolism and Favors Bone Mass Accrual. Bone 2016, 84, 131–138. [Google Scholar] [CrossRef]
- Isik, A.T.; Soysal, P.; Usarel, C. Effects of Acetylcholinesterase Inhibitors on Balance and Gait Functions and Orthostatic Hypotension in Elderly Patients with Alzheimer Disease. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 580–584. [Google Scholar] [CrossRef]
- Kim, D.H.; Brown, R.T.; Ding, E.L.; Kiel, D.P.; Berry, S.D. Dementia Medications and Risk of Falls, Syncope, and Related Adverse Events: Meta-Analysis of Randomized Controlled Trials. J. Am. Geriatr. Soc. 2011, 59, 1019–1031. [Google Scholar] [CrossRef]
- Sato, T.; Enoki, Y.; Sakamoto, Y.; Yokota, K.; Okubo, M.; Matsumoto, M.; Hayashi, N.; Usui, M.; Kokabu, S.; Mimura, T.; et al. Donepezil Prevents RANK-Induced Bone Loss via Inhibition of Osteoclast Differentiation by Downregulating Acetylcholinesterase. Heliyon 2015, 1, e00013. [Google Scholar] [CrossRef]
- Choi, D.S.; Lee, H.J.; Shin, Y.I.; Lee, A.; Kim, H.G.; Kim, Y.H. Modulation of Cortical Activity by High-Frequency Whole-Body Vibration Exercise: An fNIRS Study. J. Sport Rehabil. 2019, 28, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Pallone, G.; Romagnoli, C.; Rinaldi, A.M.; Annino, G.; D’Arcangelo, G.; Tancredi, V. Whole Body Vibration Improves Brain and Musculoskeletal Health by Modulating the Expression of Tissue-Specific Markers: FNDC5 as a Key Regulator of Vibration Adaptations. Int. J. Mol. Sci. 2022, 23, 388. [Google Scholar] [CrossRef]
- Raval, A.P.; Schatz, M.; Bhattacharya, P.; d’Adesky, N.; Rundek, T.; Dietrich, W.D.; Bramlett, H.M. Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats. Int. J. Mol. Sci. 2018, 19, 2749. [Google Scholar] [CrossRef]
- Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Collado, P.S.; Almar, M.; Martinez-Florez, S.; de Paz, J.A.; González-Gallego, J.; Cuevas, M.J. Whole-Body Vibration Improves the Anti-Inflammatory Status in Elderly Subjects through Toll-Like Receptor 2 and 4 Signaling Pathways. Mech. Ageing Dev. 2015, 150, 12–19. [Google Scholar] [CrossRef]
- Chen, T.; Liu, W.B.; Ren, X.; Li, Y.F.; Li, W.; Hang, C.H.; Wang, Y.H. Whole Body Vibration Attenuates Brain Damage and Neuroinflammation Following Experimental Traumatic Brain Injury. Front. Cell Dev. Biol. 2022, 10, 847859. [Google Scholar] [CrossRef]
- Yan, J.G.; Zhang, L.L.; Agresti, M.; LoGiudice, J.; Sanger, J.R.; Matloub, H.S.; Havlik, R. Neural Systemic Impairment from Whole-Body Vibration. J. Neurosci. Res. 2015, 93, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Gao, W.; Lin, H.; Li, T.; Liu, T.C.Y.; Weng, X.; Yuan, Y. The Effects of Different Forms of Exercise During the Early Life on the Bone Microstructure of Ovariectomized Mice. Bone 2025, 192, 117364. [Google Scholar] [CrossRef] [PubMed]
- Minematsu, A.; Nishii, Y. Effects of Whole-Body Vibration on Bone Properties in Type 2 Diabetes Model Rats. Osteoporos. Sarcopenia 2024, 10, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, L.; Gao, C.; Zong, B. Therapeutic Effects of Whole-Body Vibration on Postmenopausal Women with Osteoporosis: A Systematic Review and Meta-Analysis. Braz. J. Med. Biol. Res. 2024, 57, e13996. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Ribeiro, F.C.; Sudo, F.K.; Drummond, C.; Assunção, N.; Vanderborght, B.; Tovar-Moll, F.; Mattos, P.; De Felice, F.G.; Ferreira, S.T. Cerebrospinal Fluid Irisin Correlates with Amyloid-β, BDNF, and Cognition in Alzheimer’s Disease. Alzheimer’s Dement. 2020, 12, e12034. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Ghaedi, K.; Megraw, T.L.; Curtiss, J.; Shirani Faradonbeh, M.; Vaziri, P.; Nasr-Esfahani, M.H. Does PGC1α/FNDC5/BDNF Elicit the Beneficial Effects of Exercise on Neurodegenerative Disorders? Neuromol. Med. 2016, 18, 1–15. [Google Scholar] [CrossRef]
- Zaidi, M.; Zaidi, S.; Yuen, T. Understanding Osteokine Biology. Cell Metab. 2024, 36, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.; Katam, K.; Suravajhala, P. Advancements in Multi-Omics Research to Address Challenges in Alzheimer’s Disease: A Systems Biology Approach Utilizing Molecular Biomarkers and Innovative Strategies. Front. Aging Neurosci. 2025, 17, 1591796. [Google Scholar] [CrossRef] [PubMed]
- Badia, I.M.P.; Wessels, L.; Müller-Dott, S.; Trimbour, R.; Ramirez Flores, R.O.; Argelaguet, R.; Saez-Rodriguez, J. Gene Regulatory Network Inference in the Era of Single-Cell Multi-Omics. Nat. Rev. Genet. 2023, 24, 739–754. [Google Scholar] [CrossRef]
- Batra, R.; Krumsiek, J.; Wang, X.; Allen, M.; Blach, C.; Kastenmüller, G.; Arnold, M.; Ertekin-Taner, N.; Kaddurah-Daouk, R. Comparative Brain Metabolomics Reveals Shared and Distinct Metabolic Alterations in Alzheimer’s Disease and Progressive Supranuclear Palsy. Alzheimer’s Dement. 2024, 20, 8294–8307. [Google Scholar] [CrossRef]
- Rahimzadeh, N.; Srinivasan, S.S.; Zhang, J.; Swarup, V. Gene Networks and Systems Biology in Alzheimer’s Disease: Insights from Multi-Omics Approaches. Alzheimer’s Dement. 2024, 20, 3587–3605. [Google Scholar] [CrossRef]
- Baldock, P.A.; Allison, S.J.; Lundberg, P.; Lee, N.J.; Slack, K.; Lin, E.J.; Enriquez, R.F.; McDonald, M.M.; Zhang, L.; During, M.J.; et al. Novel Role of Y1 Receptors in the Coordinated Regulation of Bone and Energy Homeostasis. J. Biol. Chem. 2007, 282, 19092–19102. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, Y.L.; Song, Z.Y.; Lin, S. Effects of Neuropeptide Y on Stem Cells and Their Potential Applications in Disease Therapy. Stem Cells Int. 2017, 2017, 6823917. [Google Scholar] [CrossRef]
- Farr, O.M.; Tsoukas, M.A.; Mantzoros, C.S. Leptin and the Brain: Influences on Brain Development, Cognitive Functioning and Psychiatric Disorders. Metabolism 2015, 64, 114–130. [Google Scholar] [CrossRef]
- Narita, K.; Kosaka, H.; Okazawa, H.; Murata, T.; Wada, Y. Relationship between Plasma Leptin Level and Brain Structure in Elderly: A Voxel-Based Morphometric Study. Biol. Psychiatry 2009, 65, 992–994. [Google Scholar] [CrossRef]
- Zhou, R.; Deng, J.; Zhang, M.; Zhou, H.D.; Wang, Y.J. Association between Bone Mineral Density and the Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 24, 101–108. [Google Scholar] [CrossRef]
- McGregor, G.; Harvey, J. Leptin Regulation of Synaptic Function at Hippocampal TA-CA1 and SC-CA1 Synapses: Implications for Health and Disease. Neurochem. Res. 2019, 44, 650–660. [Google Scholar] [CrossRef]
- Lieb, W.; Beiser, A.S.; Vasan, R.S.; Tan, Z.S.; Au, R.; Harris, T.B.; Roubenoff, R.; Auerbach, S.; DeCarli, C.; Wolf, P.A.; et al. Association of Plasma Leptin Levels with Incident Alzheimer Disease and MRI Measures of Brain Aging. JAMA 2009, 302, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; DePaoli, A.M.; Karalis, A.; Mantzoros, C.S. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Meloni, B.P.; Shi, T.; Bonser, A.; Papadimitriou, J.M.; Mastaglia, F.L.; Zhang, C.; Zheng, M.; Gao, J. The Potential Influence of Bone-Derived Modulators on the Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 69, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, M.J.; Labonté, A.; Picard, C.; Bowie, D.C.; Zetterberg, H.; Blennow, K.; Brinkmalm, A.; Villeneuve, S.; Poirier, J. Osteopontin: A Novel Marker of Pre-Symptomatic Sporadic Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, 6008–6031. [Google Scholar] [CrossRef]
- Fodor, D.; Bondor, C.; Albu, A.; Simon, S.P.; Craciun, A.; Muntean, L. The Value of Osteopontin in the Assessment of Bone Mineral Density Status in Postmenopausal Women. J. Investig. Med. 2013, 61, 15–21. [Google Scholar] [CrossRef]
- Luckhaus, C.; Mahabadi, B.; Grass-Kapanke, B.; Jänner, M.; Willenberg, H.; Jäger, M.; Supprian, T.; Fehsel, K. Blood Biomarkers of Osteoporosis in Mild Cognitive Impairment and Alzheimer’s Disease. J. Neural Transm. 2009, 116, 905–911. [Google Scholar] [CrossRef]
- Kumar, V.; Banerjee, A.; Roy, K. Breaking the Barriers: Machine-Learning-Based c-RASAR Approach for Accurate Blood-Brain Barrier Permeability Prediction. J. Chem. Inf. Model. 2024, 64, 4298–4309. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Modeling the Blood-Brain Barrier Permeability of Potential Heterocyclic Drugs via Biomimetic IAM Chromatography Technique Combined with QSAR Methodology. Molecules 2024, 29, 287. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Zeng, W.; Siramshetty, V.B.; Williams, J.; Kabir, M.; Hagen, N.; Padilha, E.C.; Wang, A.Q.; Mathé, E.A.; Xu, X.; et al. Development and Validation of PAMPA-BBB QSAR Model to Predict Brain Penetration Potential of Novel Drug Candidates. Front. Pharmacol. 2023, 14, 1291246. [Google Scholar] [CrossRef] [PubMed]
- Rejc, L.; Gómez-Vallejo, V.; Joya, A.; Moreno, O.; Egimendia, A.; Castellnou, P.; Ríos-Anglada, X.; Cossío, U.; Baz, Z.; Passannante, R.; et al. Longitudinal Evaluation of a Novel BChE PET Tracer as an Early in Vivo Biomarker in the Brain of a Mouse Model for Alzheimer Disease. Theranostics 2021, 11, 6542–6559. [Google Scholar] [CrossRef] [PubMed]
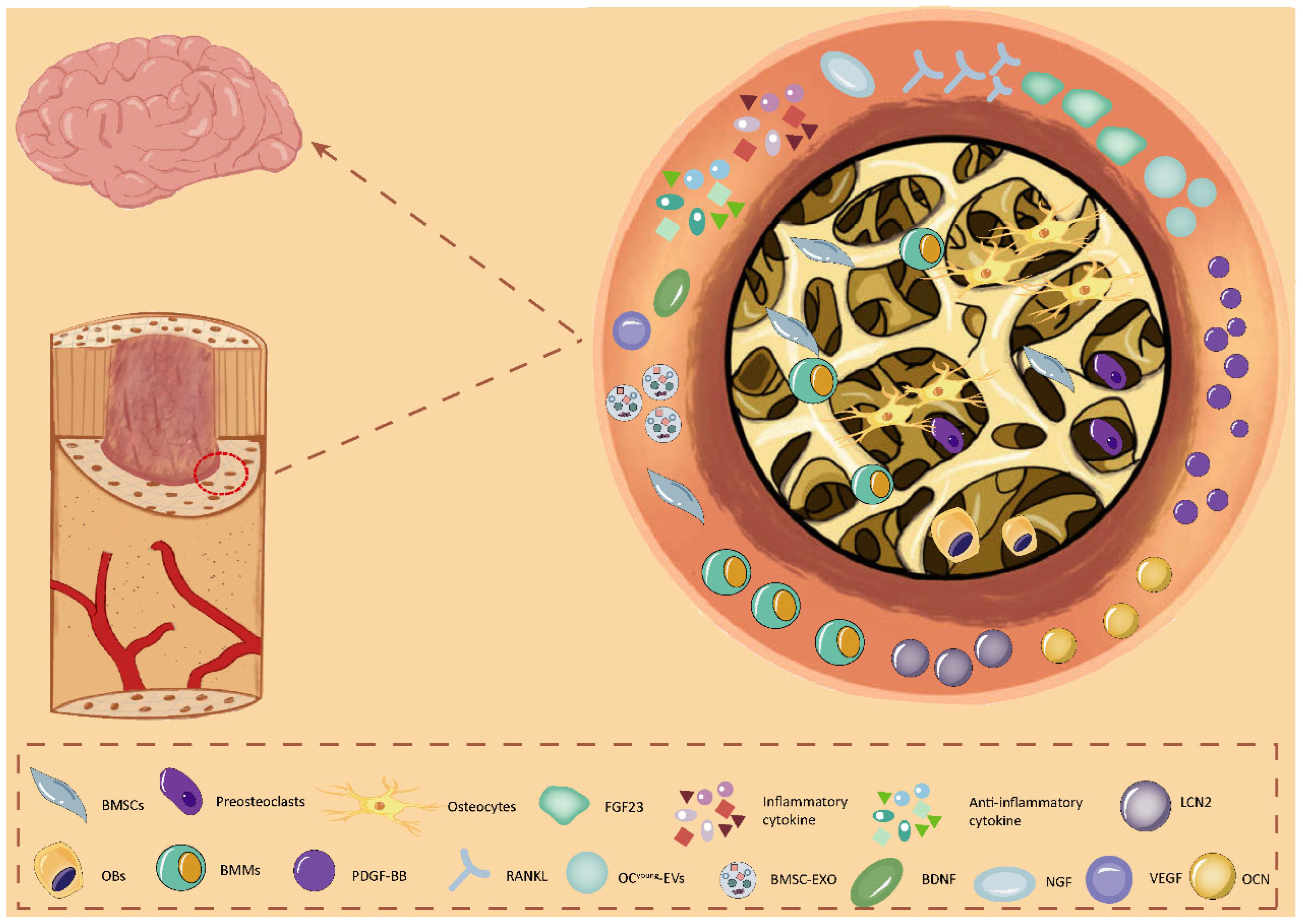





| Diseases | Methods | Effect | Mechanisms |
|---|---|---|---|
| AD | BMSC Transplant, BMSCs-EXO | Amelioration of hippocampal loss and enhancement of memory function in mice [15], Improvement of AD-like behavior in mice [72] | Inhibition of neuronal apoptosis, an increase in hippocampal superoxide dismutase activity, and a significant increase in protein levels of brain-derived neurotrophic factor in the hippocampus [15]. It regulates hippocampal glial cell activation, neuroinflammation, and BDNF [72]. BMSCs-EXO miR-146a can reduce NF-κB levels [80]. |
| Diabetic Cognitive Impairment | BMSC Transplant | Improvement of diabetes-induced cognitive dysfunction [23] | Repair of damaged neurons and astrocyte degeneration [23]. |
| Cerebral ischaemia | BMSCs-NGF | Promoting brain remodeling | Upregulation of neural-like cell differentiation and increased synaptophysin expression [36], downregulates the pro-inflammatory cytokine TNFα [42]. |
| MCAO | BMSCs-VEGF | Improvement in brain function and behavior | Promotes angiogenesis and improves blood flow recovery [46,48]. |
| TBI | BMSCs-EXO | Improves cognitive function and promotes neurological recovery | Reducing glutamate levels [67] promotes neurogenesis, regulates angiogenesis [16], and up-regulation of miR-181b activates the IL10/STAT3 pathway [75] and reduces neuroinflammation [16]. |
| SAH | BMSCs-EXO | Improves neurological function, reduces brain water content, and maintains BBB integrity [69] | Inhibition of NF-κB, activation of AMPK, attenuation of post-SAH inflammation, and neuroprotection [70]. miRNA-129-5p inhibits the anti-inflammatory and anti-apoptotic effects of the HMGB1-TLR4 pathway [69]. |
| PD | BMSCs-EXO | Reversal of rotational behavior and climbing speed in PD mice | Inhibition of the P38MAPK/P65NF-κB signaling cascade and thus the transcription of inflammation-related genes remodels the nigrostriatal inflammatory microenvironment and repairs DA nerve injury [71]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Q.; Cao, Y.; Zou, J.; Zhang, L. Bone-Derived Factors: Regulating Brain and Treating Alzheimer’s Disease. Biology 2025, 14, 1112. https://doi.org/10.3390/biology14091112
Guan Q, Cao Y, Zou J, Zhang L. Bone-Derived Factors: Regulating Brain and Treating Alzheimer’s Disease. Biology. 2025; 14(9):1112. https://doi.org/10.3390/biology14091112
Chicago/Turabian StyleGuan, Qiao, Yanting Cao, Jun Zou, and Lingli Zhang. 2025. "Bone-Derived Factors: Regulating Brain and Treating Alzheimer’s Disease" Biology 14, no. 9: 1112. https://doi.org/10.3390/biology14091112
APA StyleGuan, Q., Cao, Y., Zou, J., & Zhang, L. (2025). Bone-Derived Factors: Regulating Brain and Treating Alzheimer’s Disease. Biology, 14(9), 1112. https://doi.org/10.3390/biology14091112






