Functional Annotation and Comparative Analysis of Cytochrome P450 Protein Family Genes in Nine Chironomidae Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Annotation
2.2. Sequence Alignment, Conserved Motif Visualization
2.3. Classification of P450 Transporter Genes and Phylogenetic Analysis
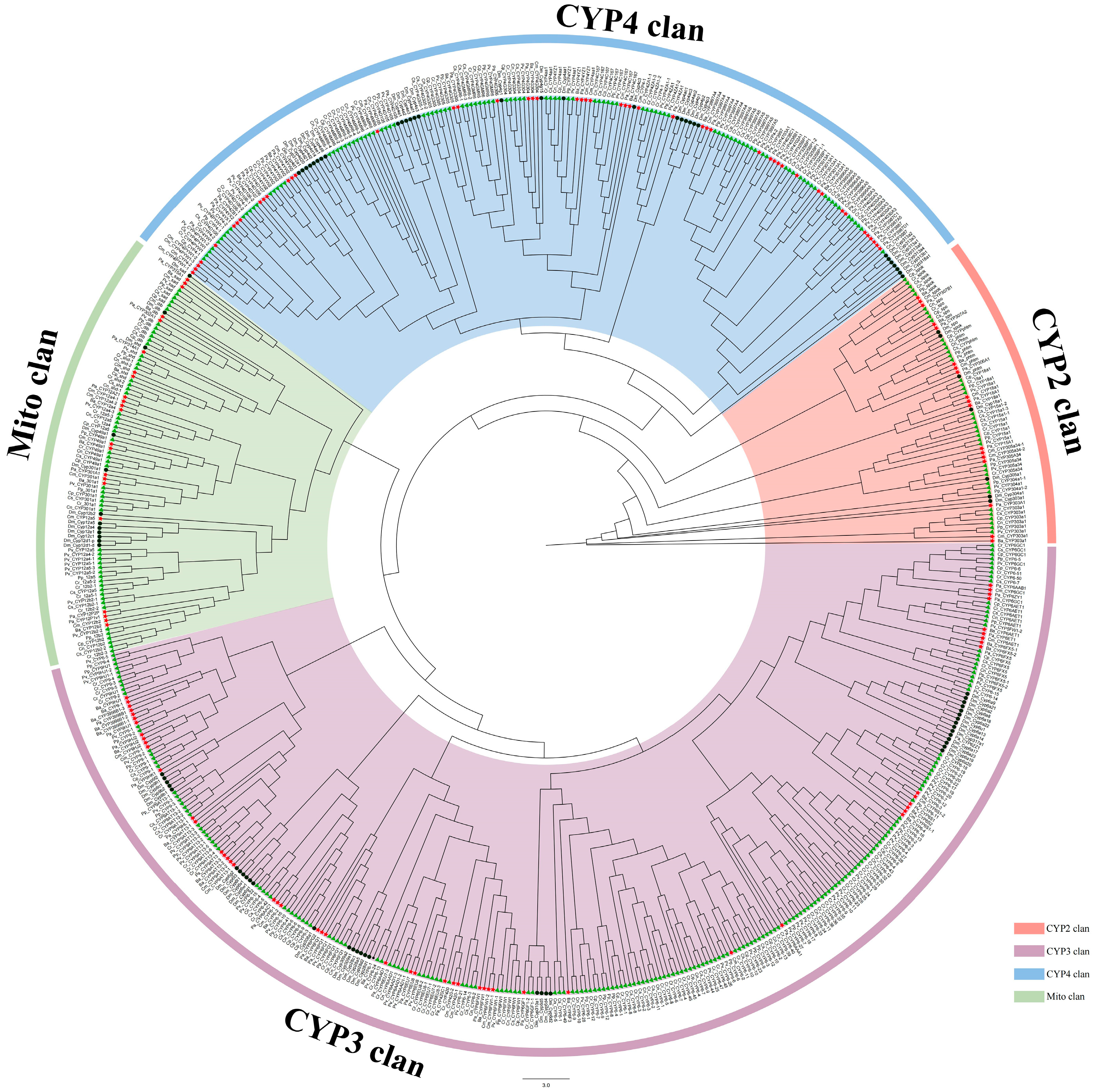
3. Results
3.1. Annotation and Classification of Predicted P450s in Eight Chironomidae Species
3.2. Conserved Domain Analysis
3.3. CYP2 Clan
3.4. CYP3 Clan
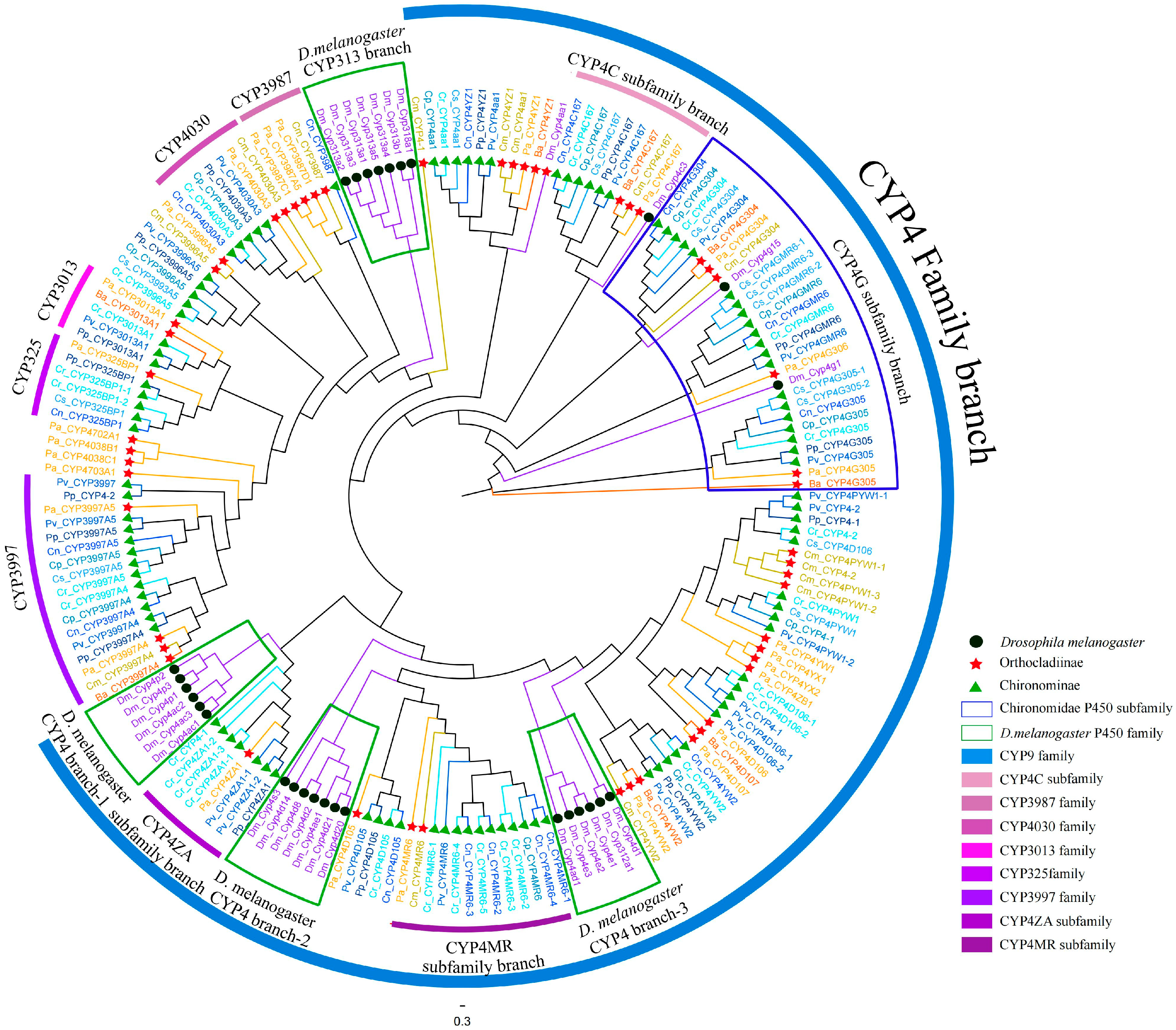
3.5. CYP4 Clan
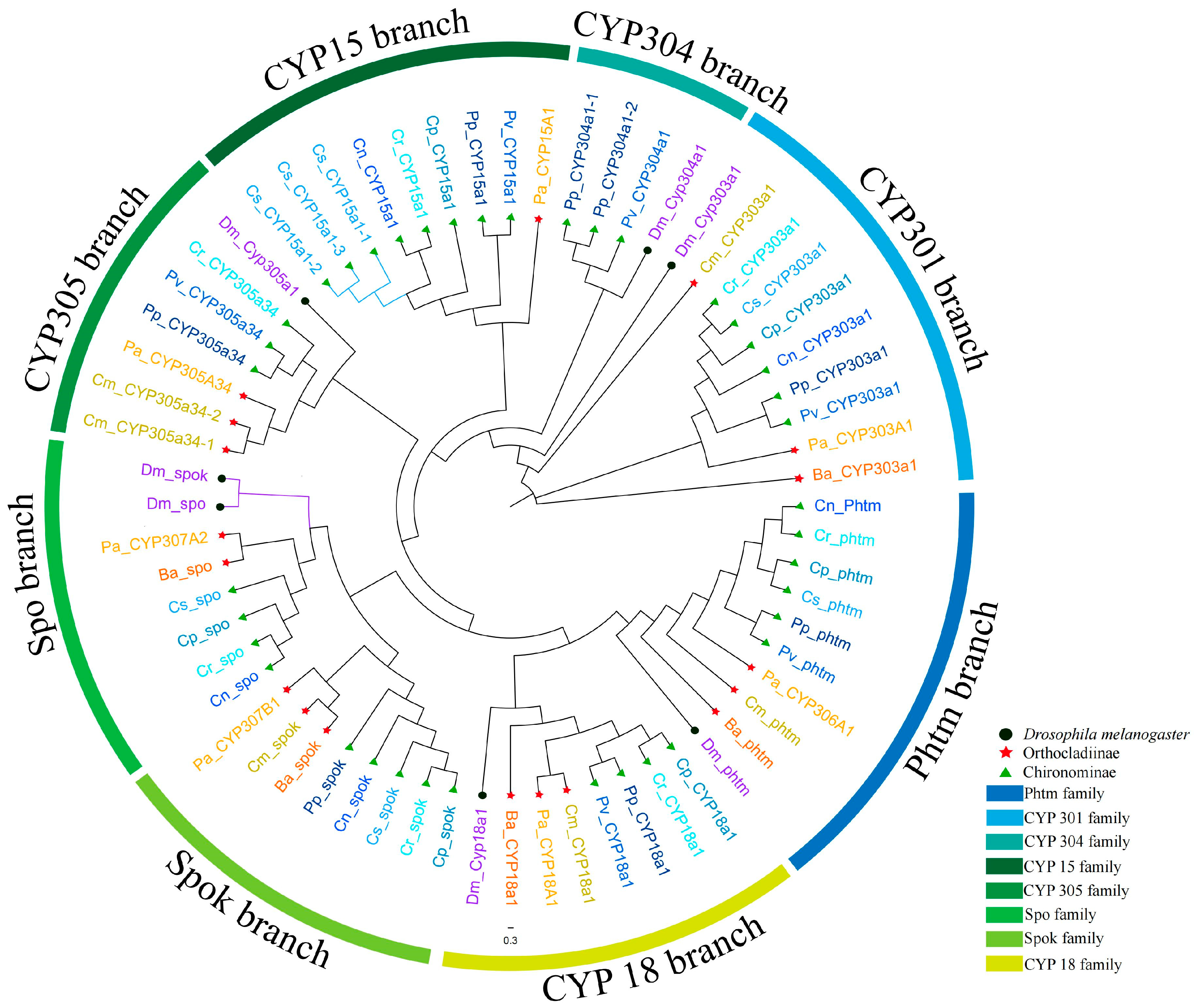
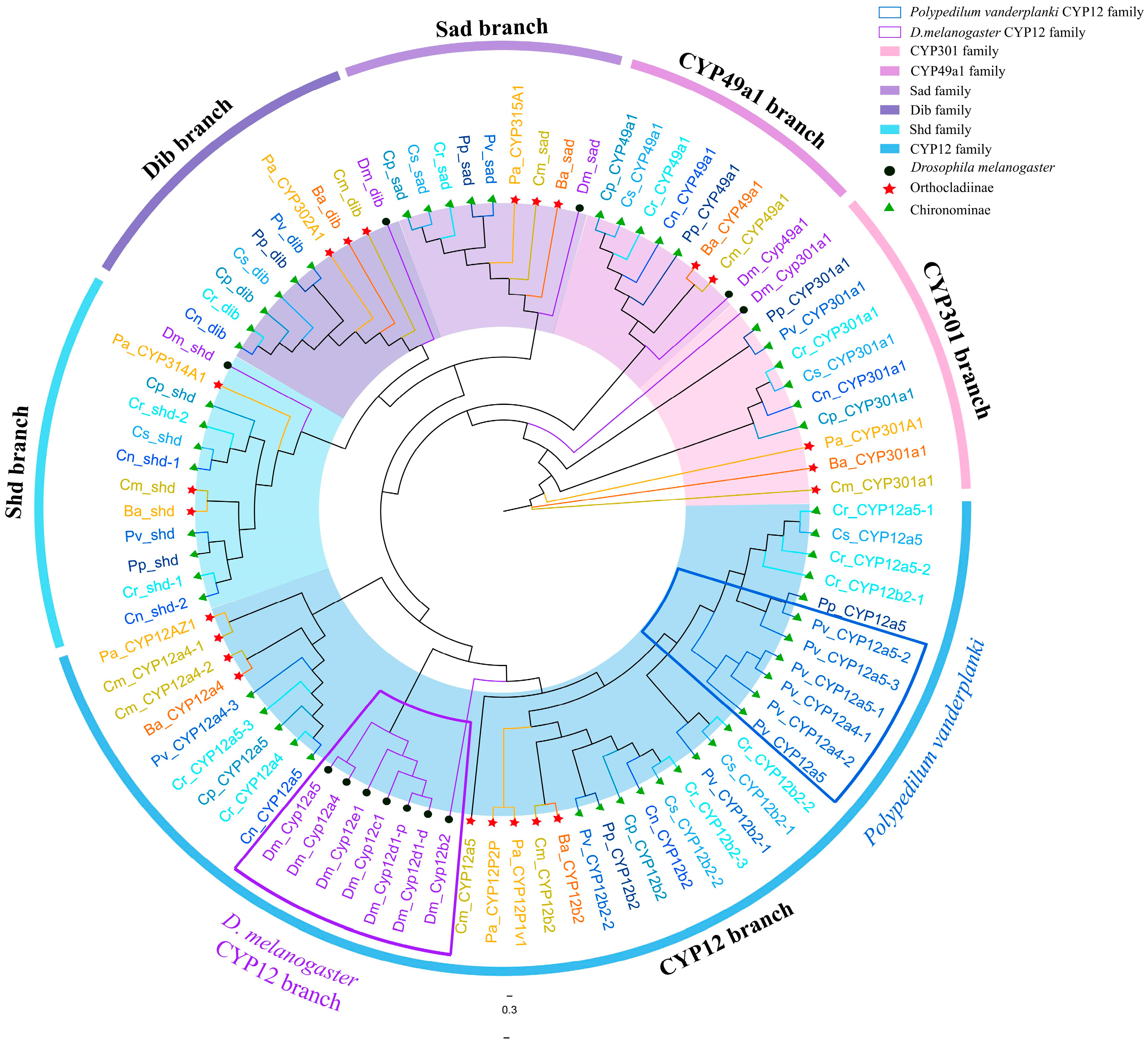
3.6. Mito Clan
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, reviews3003.1. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. 8—Insect CYP Genes and P450 Enzymes. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 236–316. [Google Scholar]
- Lamb David, C.; Lei, L.; Warrilow Andrew, G.S.; Lepesheva Galina, I.; Mullins Jonathan, G.L.; Waterman Michael, R.; Kelly Steven, L. The First Virally Encoded Cytochrome P450. J. Virol. 2009, 83, 8266–8269. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef]
- Nelson, D.R. Cytochrome P450 nomenclature, 2004. Methods Mol. Biol. 2006, 320, 1–10. [Google Scholar]
- Nelson, D.R. Metazoan cytochrome P450 evolution. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 121, 15–22. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T.; Feyereisen, R. Diversity and evolution of the P450 family in arthropods. Insect Biochem. Mol. Biol. 2020, 127, 103490. [Google Scholar] [CrossRef]
- Rewitz, K.F.; O’Connor, M.B.; Gilbert, L.I. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. 2007, 37, 741–753. [Google Scholar] [CrossRef]
- Helvig, C.; Koener, J.F.; Unnithan, G.C.; Feyereisen, R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc. Natl. Acad. Sci. USA 2004, 101, 4024–4029. [Google Scholar] [CrossRef]
- Helvig, C.; Tijet, N.; Feyereisen, R.; Walker, F.A.; Restifo, L.L. Drosophila melanogaster CYP6A8, an insect P450 that catalyzes lauric acid (omega-1)-hydroxylation. Biochem. Biophys. Res. Commun. 2004, 325, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Maïbèche-Coisne, M.; Nikonov, A.A.; Ishida, Y.; Jacquin-Joly, E.; Leal, W.S. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc. Natl. Acad. Sci. USA 2004, 101, 11459–11464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pandit, S.S.; Steppuhn, A.; Baldwin, I.T. Natural history-driven, plant-mediated RNAi-based study reveals CYP6B46’s role in a nicotine-mediated antipredator herbivore defense. Proc. Natl. Acad. Sci. USA 2014, 111, 1245–1252. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Scott, J.G.; Georghiou, G.P. Rapid development of high-level permethrin resistance in a field-collected strain of the house fly (Diptera: Muscidae) under laboratory selection. J. Econ. Entomol. 1985, 78, 316–319. [Google Scholar] [CrossRef]
- Scott, J.G.; Georghiou, G.P. Mechanisms responsible for high levels of permethrin resistance in the house fly. Pestic. Sci. 1986, 17, 195–206. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Shah, K.; Palli, S.R. Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genom. 2013, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34 Pt 6, 1252–1255. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, J.S.; Min, J.S.; Yoon, K.S.; Strycharz, J.P.; Johnson, R.; Mittapalli, O.; Margam, V.M.; Sun, W.; Li, H.M.; et al. Decreased detoxification genes and genome size make the human body louse an efficient model to study xenobiotic metabolism. Insect Mol. Biol. 2010, 19, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, N. Genome analysis of cytochrome P450s and their expression profiles in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS ONE 2011, 6, e29418. [Google Scholar] [CrossRef] [PubMed]
- Tijet, N.; Helvig, C.; Feyereisen, R. The cytochrome P450 gene superfamily in Drosophila melanogaster: Annotation, intron-exon organization and phylogeny. Gene 2001, 262, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.X.; Wang, D.F.; Ma, Y.P.; Zeng, L.L.; Meng, L.W.; Zhang, Q.; Dou, W.; Wang, J.J. Genome-wide and expression-profiling analyses of the cytochrome P450 genes in Bactrocera dorsalis (Hendel) and screening of candidate P450 genes associated with malathion resistance. Pest. Manag. Sci. 2020, 76, 2932–2943. [Google Scholar] [CrossRef]
- Papanicolaou, A.; Schetelig, M.F.; Arensburger, P.; Atkinson, P.W.; Benoit, J.B.; Bourtzis, K.; Castañera, P.; Cavanaugh, J.P.; Chao, H.; Childers, C.; et al. The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 2016, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Warren, W.C.; Beukeboom, L.W.; Bopp, D.; Clark, A.G.; Giers, S.D.; Hediger, M.; Jones, A.K.; Kasai, S.; Leichter, C.A.; et al. Genome of the house fly, Musca domestica L., a global vector of diseases with adaptations to a septic environment. Genome Biol. 2014, 15, 466. [Google Scholar] [CrossRef]
- Ai, J.; Zhu, Y.; Duan, J.; Yu, Q.; Zhang, G.; Wan, F.; Xiang, Z.-H. Genome-wide analysis of cytochrome P450 monooxygenase genes in the silkworm, Bombyx mori. Gene 2011, 480, 42–50. [Google Scholar] [CrossRef]
- Raghavendra, K.; Bp, N.R.; Gbks, P. Anopheles gambiae (Diptera: Culicidae) Cytochrome P450 (P450) Supergene Family: Phylogenetic Analyses and Exon-Intron Organization. Entomol. Ornithol. Herpetol. 2012, 1, 102. [Google Scholar]
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.J.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 2007, 316, 1718–1723. [Google Scholar] [CrossRef]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-Stranded RNA Uptake through Topical Application, Mediates Silencing of Five CYP4 Genes and Suppresses Insecticide Resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef]
- Bautista, M.A.M.; Miyata, T.; Miura, K.; Tanaka, T. RNA interference-mediated knockdown of a cytochrome P450, CYP6BG1, from the diamondback moth, Plutella xylostella, reduces larval resistance to permethrin. Insect Biochem. Mol. Biol. 2009, 39, 38–46. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of G-protein-coupled receptor-related genes in insecticide resistance of the mosquito, Culex quinquefasciatus. Sci. Rep. 2014, 4, 6474. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Zhou, C.; Ali, A.; Ali, S.; Wu, J. RNA interference in cytochrome P450 monooxygenase (CYP) gene results in reduced insecticide resistance in Megalurothrips usitatus Bagnall. Front. Physiol. 2023, 14, 1130389. [Google Scholar] [CrossRef]
- Martínez-Paz, P.; Morales, M.; Martínez-Guitarte, J.L.; Morcillo, G. Characterization of a cytochrome P450 gene (CYP4G) and modulation under different exposures to xenobiotics (tributyltin, nonylphenol, bisphenol A) in Chironomus riparius aquatic larvae. Comp. Biochem. Physiol. Part C. Toxicol. Pharmacol. 2012, 155, 333–343. [Google Scholar] [CrossRef]
- Tang, G.; Yao, J.; Li, D.; He, Y.; Zhu, Y.-C.; Zhang, X.; Zhu, K.Y. Cytochrome P450 genes from the aquatic midge Chironomus tentans: Atrazine-induced up-regulation of CtCYP6EX3 enhanced the toxicity of chlorpyrifos. Chemosphere 2017, 186, 68–77. [Google Scholar] [CrossRef]
- Londoño, D.K.; Siqueira, H.A.A.; Wang, H.; Sarath, G.; Lydy, M.J.; Siegfried, B.D. Cloning and expression of an atrazine inducible cytochrome P450, CYP4G33, from Chironomus tentans (Diptera: Chironomidae). Pestic. Biochem. Physiol. 2007, 89, 104–110. [Google Scholar] [CrossRef]
- Cao, C.W.; Sun, L.L.; Niu, F.; Liu, P.; Chu, D.; Wang, Z.Y. Effects of phenol on metabolic activities and transcription profiles of cytochrome P450 enzymes in Chironomus kiinensis larvae. Bull. Entomol. Res. 2016, 106, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chu, D.; Sun, L.; Cao, C. Cytochrome P450 CYP6EV11 in Chironomus kiiensis Larvae Involved in Phenol Stress. Int. J. Mol. Sci. 2018, 19, 1119. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, Y.; Xu, H.; Yan, C. Genome-Wide Identification of P450 Genes in Chironomid Propsilocerus akamusi Reveals Candidate Genes Involved in Gut Microbiota-Mediated Detoxification of Chlorpyrifos. Insects 2022, 13, 765. [Google Scholar] [CrossRef] [PubMed]
- Cranston, P.; Hardy, N.; Morse, G. A dated molecular phylogeny for the Chironomidae (Diptera). Syst. Entomol. 2011, 37, 172–188. [Google Scholar] [CrossRef]
- Martínez-Paz, P. Response of detoxification system genes on Chironomus riparius aquatic larvae after antibacterial agent triclosan exposures. Sci. Total Environ. 2018, 624, 1–8. [Google Scholar] [CrossRef]
- Kutsenko, A.; Svensson, T.; Nystedt, B.; Lundeberg, J.; Björk, P.; Sonnhammer, E.; Giacomello, S.; Visa, N.; Wieslander, L. The Chironomus tentans genome sequence and the organization of the Balbiani ring genes. BMC Genom. 2014, 15, 819. [Google Scholar] [CrossRef]
- Vicoso, B.; Bachtrog, D. Numerous transitions of sex chromosomes in Diptera. PLoS Biol. 2015, 13, e1002078. [Google Scholar] [CrossRef]
- Kaiser, T.S.; Poehn, B.; Szkiba, D.; Preussner, M.; Sedlazeck, F.J.; Zrim, A.; Neumann, T.; Nguyen, L.T.; Betancourt, A.J.; Hummel, T.; et al. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature 2016, 540, 69–73. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Li, R.; Zhao, C.; Pan, L.; Yan, C. A chromosome level genome assembly of Propsilocerus akamusi to understand its response to heavy metal exposure. Mol. Ecol. Resour. 2021, 21, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Nelson, D.R. The cytochrome p450 homepage. Hum. Genom. 2009, 4, 59–65. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Qin, P.; Zheng, H.; Tao, Y.; Zhang, Y.; Chu, D. Genome-Wide Identification and Expression Analysis of the Cytochrome P450 Gene Family in Bemisia tabaci MED and Their Roles in the Insecticide Resistance. Int. J. Mol. Sci. 2023, 24, 5899. [Google Scholar] [CrossRef]
- Sztal, T.; Chung, H.; Gramzow, L.; Daborn, P.J.; Batterham, P.; Robin, C. Two independent duplications forming the Cyp307a genes in Drosophila. Insect Biochem. Mol. Biol. 2007, 37, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Namiki, T.; Niwa, R.; Sakudoh, T.; Shirai, K.-i.; Takeuchi, H.; Kataoka, H. Cytochrome P450 CYP307A1/Spook: A regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 2005, 337, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Rewitz, K.F.; Shinoda, T.; Itoyama, K.; Petryk, A.; Rybczynski, R.; Jarcho, M.; Warren, J.T.; Marqués, G.; Shimell, M.J.; et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 2006, 298, 555–570. [Google Scholar] [CrossRef]
- Iga, M.; Kataoka, H. Recent studies on insect hormone metabolic pathways mediated by cytochrome P450 enzymes. Biol. Pharm. Bull. 2012, 35, 838–843. [Google Scholar] [CrossRef]
- Niwa, R.; Matsuda, T.; Yoshiyama, T.; Namiki, T.; Mita, K.; Fujimoto, Y.; Kataoka, H. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 2004, 279, 35942–35949. [Google Scholar] [CrossRef]
- Guittard, E.; Blais, C.; Maria, A.; Parvy, J.P.; Pasricha, S.; Lumb, C.; Lafont, R.; Daborn, P.J.; Dauphin-Villemant, C. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 2011, 349, 35–45. [Google Scholar] [CrossRef]
- Daimon, T.; Kozaki, T.; Niwa, R.; Kobayashi, I.; Furuta, K.; Namiki, T.; Uchino, K.; Banno, Y.; Katsuma, S.; Tamura, T.; et al. Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet. 2012, 8, e1002486. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, C.; Ishii, F.; Washidu, Y.; Ichikawa, A.; Tanaka, T.; Miura, K.; Shinoda, T. Expressional and functional analysis of CYP15A1, a juvenile hormone epoxidase, in the red flour beetle Tribolium castaneum. J. Insect Physiol. 2015, 80, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, W.; Gao, L.; Yan, Q.; Zhang, X.; Liu, M.; Zhou, S. miR-276 and miR-182013-5p modulate insect metamorphosis and reproduction via dually regulating juvenile hormone acid methyltransferase. Commun. Biol. 2024, 7, 1604. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Carvalho, R.A.; Oliphant, L.; Puinean, A.M.; Field, L.M.; Nauen, R.; Williamson, M.S.; Moores, G.; Gorman, K. Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2011, 20, 763–773. [Google Scholar] [CrossRef]
- Karunker, I.; Benting, J.; Lueke, B.; Ponge, T.; Nauen, R.; Roditakis, E.; Vontas, J.; Gorman, K.; Denholm, I.; Morin, S. Over-expression of cytochrome P450 CYP6CM1 is associated with high resistance to imidacloprid in the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2008, 38, 634–644. [Google Scholar] [CrossRef]
- Li, X.; Baudry, J.; Berenbaum, M.R.; Schuler, M.A. Structural and functional divergence of insect CYP6B proteins: From specialist to generalist cytochrome P450. Proc. Natl. Acad. Sci. USA 2004, 101, 2939–2944. [Google Scholar] [CrossRef]
- Rupasinghe, S.G.; Wen, Z.; Chiu, T.L.; Schuler, M.A. Helicoverpa zea CYP6B8 and CYP321A1: Different molecular solutions to the problem of metabolizing plant toxins and insecticides. Protein Eng. Des. Sel. 2007, 20, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, X.; Li, M.; Qiu, X. Functional characterization of CYP9J26, a cytochrome P450 associated with insecticide resistance in the arbovirus vector Aedes albopictus. Int. J. Biol. Macromol. 2025, 318, 145280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yu, R.; Wu, H.; Zhang, X.; Liu, Y.; Zhu, K.Y.; Zhang, J.; Ma, E. Identification and characterization of two CYP9A genes associated with pyrethroid detoxification in Locusta migratoria. Pestic. Biochem. Physiol. 2016, 132, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-G.; Ruan, Y.-W.; Gong, C.-W.; Xiang, X.; Xu, X.; Zhang, Y.-M.; Shen, L.-T. Transcriptome Analysis of Sogatella furcifera (Homoptera: Delphacidae) in Response to Sulfoxaflor and Functional Verification of Resistance-Related P450 Genes. Int. J. Mol. Sci. 2019, 20, 4573. [Google Scholar] [CrossRef]
- Wang, R.-L.; Staehelin, C.; Xia, Q.-Q.; Su, Y.-J.; Zeng, R.-S. Identification and Characterization of CYP9A40 from the Tobacco Cutworm Moth (Spodoptera litura), a Cytochrome P450 Gene Induced by Plant Allelochemicals and Insecticides. Int. J. Mol. Sci. 2015, 16, 22606–22620. [Google Scholar] [CrossRef]
- Arambourou, H.; Planelló, R.; Llorente, L.; Fuertes, I.; Barata, C.; Delorme, N.; Noury, P.; Herrero, Ó.; Villeneuve, A.; Bonnineau, C. Chironomus riparius exposure to field-collected contaminated sediments: From subcellular effect to whole-organism response. Sci. Total Environ. 2019, 671, 874–882. [Google Scholar] [CrossRef]
- Hinton, H. A new Chironomid from Africa, the larva of which can be dehydrated without injury. Proc. Zool. Soc. Lond. 2009, 121, 371–380. [Google Scholar] [CrossRef]
- Gusev, O.; Cornette, R.; Kikawada, T.; Okuda, T. Expression of heat shock protein-coding genes associated with anhydrobiosis in an African chironomid Polypedilum vanderplanki. Cell Stress. Chaperones 2011, 16, 81–90. [Google Scholar] [CrossRef]
- Pittendrigh, B.; Aronstein, K.; Zinkovsky, E.; Andreev, O.; Campbell, B.; Daly, J.; Trowell, S.; Ffrench-Constant, R.H. Cytochrome P450 genes from Helicoverpa armigera: Expression in a pyrethroid-susceptible and -resistant strain. Insect Biochem. Mol. Biol. 1997, 27, 507–512. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular Hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef]
- Balabanidou, V.; Kampouraki, A.; MacLean, M.; Blomquist, G.J.; Tittiger, C.; Juárez, M.P.; Mijailovsky, S.J.; Chalepakis, G.; Anthousi, A.; Lynd, A.; et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2016, 113, 9268–9273. [Google Scholar] [CrossRef]
- Yu, X.-M. LUOXWJWL-hYUS-r: Cytochrome P450 4X1:structure, distribution, regulation and function. Chin. J. Hosp. Pharm. 2018, 38, 1985–1988. [Google Scholar]
- Niwa, R.; Sakudoh, T.; Matsuya, T.; Namiki, T.; Kasai, S.; Tomita, T.; Kataoka, H. Expressions of the cytochrome P450 monooxygenase gene Cyp4g1 and its homolog in the prothoracic glands of the fruit fly Drosophila melanogaster (Diptera: Drosophilidae) and the silkworm Bombyx mori (Lepidoptera: Bombycidae). Appl. Entomol. Zool. 2011, 46, 533–543. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Unnithan, G.C.; Andersen, J.F.; Evans, P.H.; Murataliev, M.B.; Szabo, L.Z.; Mash, E.A.; Bowers, W.S.; Feyereisen, R. A cytochrome P450 terpenoid hydroxylase linked to the suppression of insect juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 12884–12889. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Unnithan, G.C.; Feyereisen, R. Terpenoid ω-hydroxylase (CYP4C7) messenger RNA levels in the corpora allata: A marker for ovarian control of juvenile hormone synthesis in Diploptera punctata. J. Insect Physiol. 2000, 46, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhuang, Z.; Wang, Y.; Xie, K.; Zhang, X.; Shen, Y.; Song, J.; Zhou, S. Nocturnin promotes NADH and ATP production for juvenile hormone biosynthesis in adult insects. Pest. Manag. Sci. 2025, 81, 3103–3111. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.-X.; Wang, W.-L.; Cao, Y.; Li, S.; Zhang, B.-X.; Li, S.-G. Identification of putative cytochrome P450 monooxygenase genes from the small white butterfly, Pieris rapae (Lepidoptera: Pieridae), and their response to insecticides. Arch. Insect Biochem. Physiol. 2018, 98, e21455. [Google Scholar] [CrossRef]
- Wan, P.-J.; Shi, X.-Q.; Kong, Y.; Zhou, L.-T.; Guo, W.-C.; Ahmat, T.; Li, G.-Q. Identification of cytochrome P450 monooxygenase genes and their expression profiles in cyhalothrin-treated Colorado potato beetle, Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2013, 107, 360–368. [Google Scholar] [CrossRef]
- Petryk, A.; Warren, J.T.; Marqués, G.; Jarcho, M.P.; Gilbert, L.I.; Kahler, J.; Parvy, J.P.; Li, Y.; Dauphin-Villemant, C.; O’Connor, M.B. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 2003, 100, 13773–13778. [Google Scholar] [CrossRef]
- Chávez, V.M.; Marqués, G.; Delbecque, J.P.; Kobayashi, K.; Hollingsworth, M.; Burr, J.; Natzle, J.E.; O’Connor, M.B. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 2000, 127, 4115–4126. [Google Scholar] [CrossRef]
- Warren, J.T.; Petryk, A.; Marqués, G.; Jarcho, M.; Parvy, J.-P.; Dauphin-Villemant, C.; O’Connor, M.B.; Gilbert, L.I. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2002, 99, 11043–11048. [Google Scholar] [CrossRef]
- Gilbert, L.I. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 2004, 215, 1–10. [Google Scholar] [CrossRef]
- Wan, P.J.; Jia, S.; Li, N.; Fan, J.M.; Li, G.Q. RNA interference depletion of the Halloween gene disembodied implies its potential application for management of planthopper Sogatella furcifera and Laodelphax striatellus. PLoS ONE 2014, 9, e86675. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Sakudoh, T.; Namiki, T.; Saida, K.; Fujimoto, Y.; Kataoka, H. The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by Prothoracicotropic hormone. Insect Mol. Biol. 2005, 14, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, K.; Yuan, Y.; Li, M.; Qiu, X. Identification and expression patterns of Halloween genes encoding cytochrome P450s involved in ecdysteroid biosynthesis in the cotton bollworm Helicoverpa armigera. Bull. Entomol. Res. 2017, 107, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-X.; Dou, W.; Li, C.-R.; Wang, J.-J. CYP314A1-dependent 20-hydroxyecdysone biosynthesis is involved in regulating the development of pupal diapause and energy metabolism in the Chinese citrus fruit fly, Bactrocera minax. Pest. Manag. Sci. 2022, 78, 3384–3393. [Google Scholar] [CrossRef]
- Sztal, T.; Chung, H.; Berger, S.; Currie, P.D.; Batterham, P.; Daborn, P.J. A Cytochrome P450 Conserved in Insects Is Involved in Cuticle Formation. PLoS ONE 2012, 7, e36544. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Zheng, X.W.; Liu, Y.J.; Fang, L.; Pan, Z.F.; Bao, M.H.; Huang, P. Effect of Oridonin on Cytochrome P450 Expression and Activities in HepaRG Cell. Pharmacology 2018, 101, 246–254. [Google Scholar] [CrossRef]
- Tang, G.; Yao, J.; Zhang, X.; Lu, N.; Zhu, K.Y. Comparison of gene expression profiles in the aquatic midge (Chironomus tentans) larvae exposed to two major agricultural pesticides. Chemosphere 2018, 194, 745–754. [Google Scholar] [CrossRef]


| Clans | Families | Propsilocerus akamusi | Belgica antarctica | Clunio marinus | Chironomus tentans | Chironomus tepperi | Chironomus riparius | Chironomus striatipennis | Polypedilum pembai | Polypedilum vanderplanki |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | CYP15 | 1 | 0 | 0 | 1 | 1 | 1 | 3 | 1 | 1 |
| CYP18 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | |
| CYP303 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| CYP304 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | |
| CYP305 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 1 | |
| CYP306 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| CYP307 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 0 | |
| 3 | CYP3998 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CYP420 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | |
| CYP6 | 15 | 11 | 8 | 18 | 15 | 64 | 16 | 17 | 38 | |
| CYP9 | 5 | 6 | 4 | 0 | 1 | 12 | 3 | 7 | 8 | |
| Clan3 (ungrouped) | 0 | 2 | 1 | 2 | 2 | 5 | 5 | 2 | 5 | |
| 4 | CYP325 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 0 |
| CYP3987 | 3 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| CYP3996 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | |
| CYP3997 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 3 | |
| CYP4030 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | |
| CYP4038 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CYP4 | 15 | 6 | 11 | 11 | 8 | 20 | 10 | 9 | 16 | |
| CYP4702 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CYP4703 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CYP3013 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | |
| Clan4 (ungrouped) | 0 | 1 | 6 | 4 | 4 | 23 | 9 | 7 | 16 | |
| Mito | CYP12 | 3 | 2 | 4 | 2 | 2 | 7 | 3 | 2 | 9 |
| CYP49 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | |
| CYP301 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| CYP302 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| CYP314 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | |
| CYP315 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | |
| total | 64 | 43 | 51 | 52 | 47 | 152 | 61 | 63 | 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhou, A.; Nie, J.; Shao, Z.; Nie, Z.; Zhang, Y.; Liu, C.; Yan, C.; Gao, S.; Wang, Y. Functional Annotation and Comparative Analysis of Cytochrome P450 Protein Family Genes in Nine Chironomidae Species. Biology 2025, 14, 1111. https://doi.org/10.3390/biology14091111
Liu W, Zhou A, Nie J, Shao Z, Nie Z, Zhang Y, Liu C, Yan C, Gao S, Wang Y. Functional Annotation and Comparative Analysis of Cytochrome P450 Protein Family Genes in Nine Chironomidae Species. Biology. 2025; 14(9):1111. https://doi.org/10.3390/biology14091111
Chicago/Turabian StyleLiu, Wenbin, Anmo Zhou, Jiaxin Nie, Ziming Shao, Zhe Nie, Yajin Zhang, Chunmian Liu, Chuncai Yan, Shaobo Gao, and Yiwen Wang. 2025. "Functional Annotation and Comparative Analysis of Cytochrome P450 Protein Family Genes in Nine Chironomidae Species" Biology 14, no. 9: 1111. https://doi.org/10.3390/biology14091111
APA StyleLiu, W., Zhou, A., Nie, J., Shao, Z., Nie, Z., Zhang, Y., Liu, C., Yan, C., Gao, S., & Wang, Y. (2025). Functional Annotation and Comparative Analysis of Cytochrome P450 Protein Family Genes in Nine Chironomidae Species. Biology, 14(9), 1111. https://doi.org/10.3390/biology14091111







