Simple Summary
Cotton produces two distinct types of seed fibers: short fuzz and long lint, with lint fiber initiation being the primary determinant of cotton yield as the world’s most important natural textile source. However, the genetic regulation underlying fiber initiation exhibits considerable complexity. This study characterized the mutant loci responsible for the fiberless (fuzzless–lintless, seeds without both lint and fuzz) phenotype in a naturally fiberless cotton mutant (fblSHZ), identifying duplicated GhMYB25like genes on homologous Chr A12 and Chr D12 as key regulators of both lint and fuzz fiber initiation. Genetic analysis revealed a dominant epistasis with the fuzz gene exerting dominance over the lint gene. Furthermore, the study demonstrated that these genes influence fiber initiation through multiple biological processes, particularly fatty acid metabolism. These findings provide fundamental insights into the genetic mechanisms governing cotton fiber initiation, offering valuable theoretical foundations for yield improvement in cotton breeding.
Abstract
Cotton fiber initiation determines the fiber yield, yet the genetic basis underlying lint and fuzz initiation has still not been fully uncovered. Here, map-based cloning was carried out to identify the fiberless mutant genes derived from a cross between Gossypium hirsutum acc. WT and a natural fiberless mutant, fblSHZ. The 12:3:1 segregation ratio in F2 populations (including 1848 and 3100 individuals that were developed in 2016 and 2018, respectively) revealed dominant epistasis, with the fuzz gene exerting dominance over the lint gene. Genetic linkage analysis revealed that GhMYB25like_A12 controls fuzz fiber initiation, while both GhMYB25like_A12 and GhMYB25like_D12 regulate lint fiber development. Sequencing analyses showed that the fblSHZ mutant exhibited a K104M mutation in the R2R3 domain of GhMYB25like_A12 and a transposable element insertion in GhMYB25like_D12, leading to fiberless seeds. Knockout of GhMYB25like_A12 produced fuzzless seeds, knockout of GhMYB25like_D12 led to no obvious change in seeds, and knockout of both (GhMYB25like_A12&D12) resulted in fiberless seeds. The 12:3:1 ratio reappeared in the F2 population developed from the GhMYB25like_A12&D12 mutated plants as female and Jin668 as the male, which further confirmed the genetic interaction observed in fblSHZ. RNA-seq analysis revealed that GhMYB25like regulates cotton fiber initiation through multiple pathways, especially fatty acid metabolism. This study elucidates the key genes and their genetic interaction mechanisms governing cotton fiber initiation, providing a theoretical foundation for genetic improvement of cotton fiber traits.
1. Introduction
Cotton fiber originates from the ovule epidermis, and most cultivated cotton varieties possess two types of fiber on the seed coat: the long spinnable lint fiber and the short fuzz that firmly adheres to the seed coat. Lint fiber initiates on or before the day of flowering, and fuzz fiber initiates at 4–5 days post-anthesis (DPA) [1]. The number of fiber cell initiations contributes to the lint percentage, a vital factor for cotton fiber yield. Identifying the genes involved in lint and fuzz initiation would provide insights into regulating cell patterning [2]. What is more, it is significant to breed varieties with higher yields of lint fiber in the future.
Cotton fiber initiation is complicated, and the genetics and molecular basis underlying lint and fuzz initiation remain totally uncharacterized. Fiber initiation shares a similar model of cell fate determination with Arabidopsis leaf trichomes in many ways [3,4]. Up to now, progress has been made in discovering genes regulating cotton fiber initiation. MYB-domain transcription factors (TFs) are vital in developing cotton fiber and leaf trichome [4,5,6]. The MYBMIXTA-Like (MML) TFs are crucial regulators of epidermal cell differentiation. Ten MYB-MIXTA-like homologs (GhMMLs) are all highly expressed during fiber initiation in cultivated cotton [6,7], which contains the typical protein motif AQWESARxxAExRLxRES [8].
Numerous studies have demonstrated that the MYB-MIXTA-like (MML) TFs are essential in cotton fiber development. MML3 (GhMYB25like) is especially expressed in the ovule epidermis and probably acts most upstream of the fiber initiation regulatory networks. RNA interference suppression of GhMYB25like led to fiberless seeds but normal trichomes elsewhere, which showed that GhMYB25like specifically promoted the initiation of fiber cells but not trichomes on cotton plants [4]. The small interfering RNAs (siRNAs) from bidirectional transcripts of GhMML3_A12 (GhMYB25like_A12) mediated self-cleavage, which caused extremely low expression of GhMML3_A12 and a fuzzless phenotype in N1 mutant [9]. Both sub-genome homologs of GbMML3 (GbMYB25like_A12 and GbMYB25like_D12) contributed to lint initiation, while only GbMYB25like_D12 promoted fuzz formation in a recessive manner in Gossypium barbadense [10]. The GhMML4_D12 was first reported as the lint fiber development gene (Li3); the downregulated expression of GhMML4_D12 in n2NSM (fuzzless–linted) plants resulted in a significant reduction in epidermal cell prominence and lint fiber production, but did not result in the lintless phenotype [11]. Recently, it was shown that GhMML3_D12 (GhMYB25like_D12) was also responsible for the n2 loci in n2NSM. Overexpression of GhMML3_D12 in n2NSM restored fuzz fiber development, while knockout of GhMML3_D12 in wild-type cotton resulted in a fuzzless–linted phenotype [12]. GhMML3_D12 was also reported as the candidate gene for the li3 locus of Xu142 fbl, and a retrotransposon was found in the second exon of GhMML3_D12, which was considered to be the real underlying mutation for li3 [13]. It could be shown that the MML3 TFs played a significant role in cotton fiber initiation. Still, it was disputable whether the function of the MML3 was on fuzz and lint initiation. Recently, the developmental trajectory starting from early differentiated fiber cells was reconstructed by scRNA-seq on cotton ovules, which showed that the high expression of GhMYB25like lasted until 3 DPA, and GhMYB25like not only participated in lint initiation but also in fuzz initiation [14]. Subsequently, it was reported that the dominant negative mutation in the GhMYB25like_A12 protein exerted its dominant negative effect by suppressing the activity of the normal GhMYB25like protein to reduce the lint and fuzz initiation in our previous study [15]. Thus, these studies have indicated the positive function of GhMYB25like on cotton fiber initiation; even so, it is difficult to articulate how GhMYB25like_A12 and GhMYB25like_D12 mediated fuzz and lint initiation.
In 2013, a fiberless mutant was found in the field of Cotton Research Institute, Shihezi Academy of Agriculture Science, Shihezi, Xinjiang, China (44° N, 86° E), and was named fblSHZ. In this study, we aim to (1) develop F2 segregation populations to uncover the genetic behavior of the fiberless trait; (2) identify the causal genes underlying this trait through map-based cloning approaches; and (3) functionally validate the roles of candidate genes through transgenic technology.
2. Materials and Methods
2.1. Plant Materials
After several rounds of selfing in Wuhan, Hubei province, China (30° N, 114° E), and in Sanya, Hainan, China (18° N, 109° E), the wild type (WT) and the fblSHZ were planted in the field of Huazhong Agricultural University, Wuhan, in May 2015, and F1 was made by crossing WT as the female parent and fblSHZ as the male parent. The F1 seeds were planted in Sanya, Hainan, in the winter, and were self-pollinated to develop an F2 mapping population. The F2 population, including 1848 individuals and their parents, was planted in the experimental field at Huazhong Agricultural University, Wuhan, in May 2016. Another larger F2 population with 3100 individuals was grown to fine-map the fblSHZ genes in the experimental field at Huazhong Agricultural University, Wuhan, in May 2018.
2.2. Agricultural Traits Evaluation of the fblSHZ
Twenty WT plants and twenty fblSHZ plants were selected to evaluate the plant height, branch number, and effective bolls. The epidermal hair on the stem, petiole, leaf, and petals was also compared to the WT and fblSHZ plants. The samples of 0 DPA ovules from WT and fblSHZ were harvested to observe the fiber initiation.
2.3. Genetic Mapping of the fblSHZ
The leaves of independent F2 plants were collected for DNA extraction via the modified CTAB method [16]. A total of 5152 SSR markers derived from a genetic map constructed from a BC1 population between G. barbadense acc. 3-79 and G. hirsutum cv. Emian22 was used to screen polymorphic markers [17]. The 102 recessive fiberless plants from the F2 populations planted in 2016 were used for the primary mapping of fblSHZ. All 286 recessive fiberless plants from the F2 populations planted in 2016 and 2018 were used for fine mapping of fblSHZ. The leaves of 30 individuals with fuzzy–linted plants and 30 individuals with fiberless plants were mixed, respectively, to build fuzzy–linted bulk and fiberless bulk from the F2 population in 2016 for the linkage markers screening. The two parents were sequenced by Illumina sequencing to exploit molecular markers to narrow down the mapping region. The clean sequencing reads were mapped to the G. hirsutum reference TM-1 genome [18] by the BWA v0.2.0 software [19], and the SNPs and InDels (Insertion/Deletion) from the sequenced data of WT and fblSHZ were identified using the Genome Analysis Toolkit (GATK v4.3.0.0) software [20]. The SNPs located within the candidate region were used for Kompetitive Allele-Specific PCR (KASP) markers development, and the method of KASP markers development and genotyping followed our previous studies [21]. The genetic linkage analysis of the target fblSHZ gene was performed by Mapmaker3.0, and the genetic map was obtained by the QTL IciMapping v4.1.0.0 software [22].
2.4. Gene Cloning, Vector Construction, and Cotton Transformation
The primers of the candidate genes were designed based on the TM-1 reference genome [18] by Primer Premier v5.00 software. The full length of GhMYB25like_A12 and GhMYB25like_D12 was amplified from WT and fblSHZ, and ligated into the pGEM-T Easy cloning vector (Biotech Co. Ltd., Promega, Beijing, China); the positive clones were sequenced by Wuhan Tsingke Biotechnology Co., Ltd. (Wuhan, China). The final sequences were analyzed with DNAMAN v6 software. The CRISPR-Cas9-mediated gene editing vector for GhMYB25like_D12 was constructed by infusion reactions, and the standard methodology was followed from the previous study [23]. The gene editing vector was then introduced into G. hirsutum acc. Jin668 by Agrobacterium tumefaciens-mediated transformation. Transgenic cotton lines were grown in the greenhouse (28–35 °C by day and 20–25 °C by night) under a 16/8 h light/dark cycle. All the primers used for vector construction are listed in Table S1.
2.5. On-Target Analysis of Gene-Edited Plants
The T0 transgenic plants were screened by PCR analysis using Cas9 primers (Table S1). High-throughput tracking of mutations (Hi-TOM) was performed to identify the mutated alleles in T1 transgenic lines [24]. Firstly, the targeted regions were amplified by PCR using site-specific primers. Then, barcode primers were used to add barcodes to the first-round PCR products. The products of all the samples were mixed in equal amounts and purified to perform next-generation sequencing (NGS). Last of all, the NGS data was analyzed using the Hi-Tom platform (http://hi-tom.net/hi-tom/ accessed on 28 July 2025).
2.6. Scanning Electron Microscopy (SEM)
Ovule samples were collected at 0 DPA from the WT, fblSHZ, Jin668, and transgenic plants. The samples were immediately fixed in 2.5% (v/v) glutaraldehyde at 4 °C. Following fixation, they were dehydrated through a graded ethanol series, transferred to amyl acetate, and then dried to the critical point. Fiber initiation was observed and photographed with a JSM-6390/LV SEM (Jeol, Tokyo, Japan).
2.7. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
All the samples used for RNA extraction were collected from the experimental field at 9–10 a.m. on a sunny day. The −3 DPA, −1 DPA, 0 DPA, +1 DPA, and +3 DPA ovules were harvested and immediately frozen in liquid nitrogen and then stored at −80 °C. Total RNA was extracted using the RNA prep Pure Plant Kit (TIANGEN Biotech, Beijing, China). A total of 3 μg RNA for each sample was reverse transcribed into cDNA using M-MLV reverse transcriptase (Promega). The cDNA was used as a template for RT-qPCR using the 7500 Real-Time PCR System (Applied Biosystems), and GhUBQ7 (Ghir_A11G011460) was used as the internal reference. Three repetitions of each sample were applied. All the primers used for RT-qPCR are listed in Table S1.
2.8. Subcellular Localization
The full-length CDS of GhMYB25like was amplified from both WT and fblSHZ, then independently inserted into the pGWB741 vectors with GFP fused to the N-terminal via Gateway BP and LR recombination reactions (Invitrogen, America). The 35S::GFP-GhMYB25like construct was transiently expressed in tobacco epidermal cells following Agrobacterium-mediated transfection. Nicotiana benthamiana was grown in 16/8 h light/dark conditions under white, fluorescent light at 25 °C. GFP fluorescence was detected under a confocal microscope (Olympus FV1200, Tokyo, Japan) after 48 h following Agrobacterium transfection. All the primers used for vector constructions are listed in Table S1.
2.9. Transcriptome Sequencing
The −3 DPA, 0 DPA, and +1 DPA ovules from Jin668 and Cr-GhMYB25like_At&Dt (GhMYB25like_A12 and GhMYB25like_D12 were both mutated) CRISPR-Cas9-edited lines were used for RNA extraction. RNA-seq was performed using the Illumina HiSeq 2000 system. The clean RNA-seq reads were mapped to the TM-1 reference genome [18] by HISAT version 2.0 [25]. FeatureCounts were used to calculate the transcript levels of annotated genes [26]. Differentially expressed genes (DEGs) were determined using the threshold of p ≤ 0.05 and the absolute value of the log2fold change ≥ 1.
3. Results
3.1. Phenotype and Inheritance of the Fiberless Mutant fblSHZ
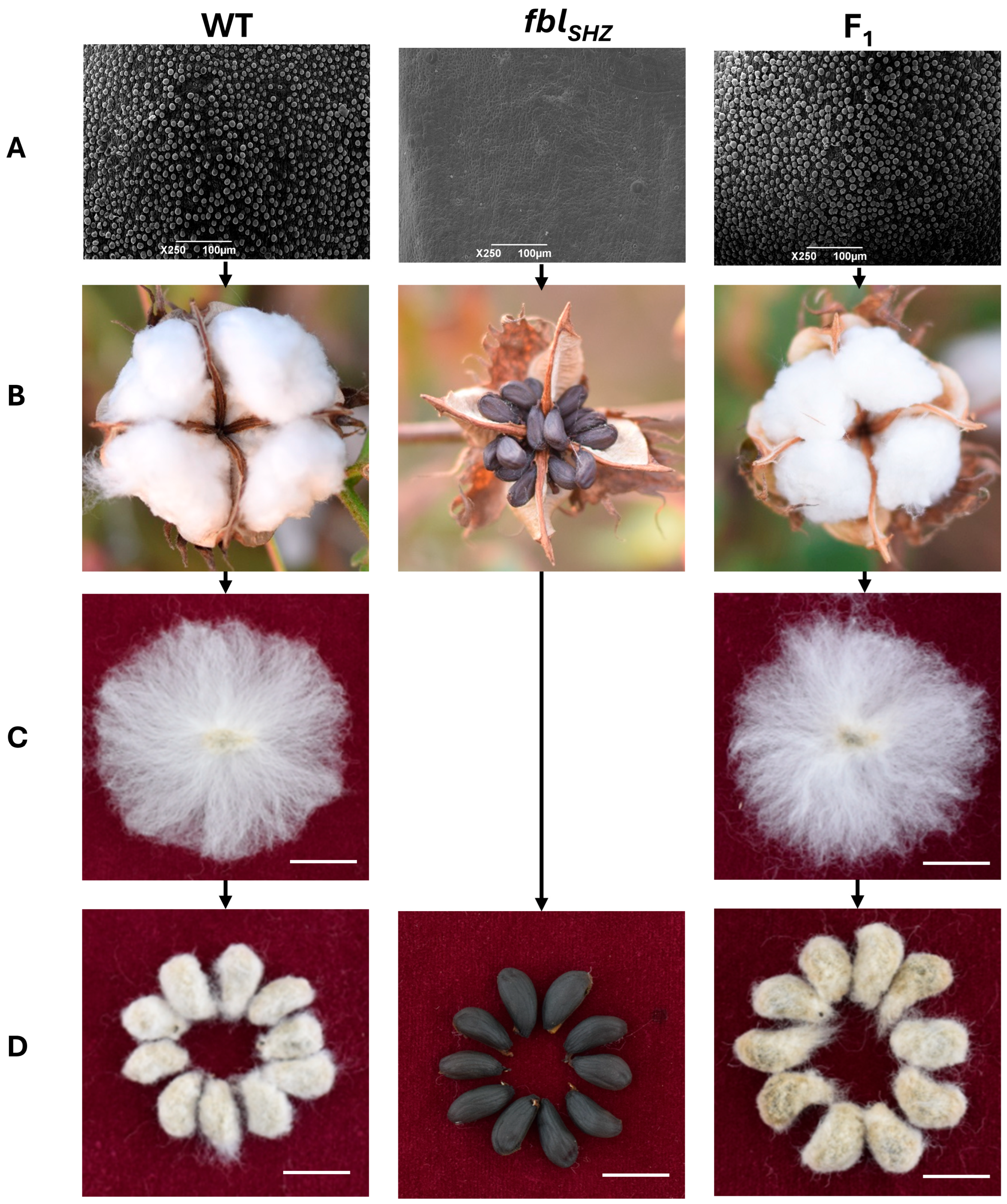
To assess potential developmental differences, the plant height, branch number, and effective bolls were tested from 20 WT plants and 20 fblSHZ plants; no statistically significant differences were found in these agronomic traits between WT and fblSHZ. Moreover, the epidermal hair on the stem, petiole, leaf, and petal was also compared between the WT and fblSHZ plants, and fewer trichomes were present in fblSHZ than in WT (Figure S1). To investigate fiber cell initiation, scanning electron microscopy (SEM) was performed on 0 DPA ovule epidermis from the WT, fblSHZ, and F1 hybrid plants. It revealed that the fiber cells of WT and F1 protuberated normally from the surface of the ovule, while no fiber cells protruded on the surface of the ovule in fblSHZ (Figure 1).
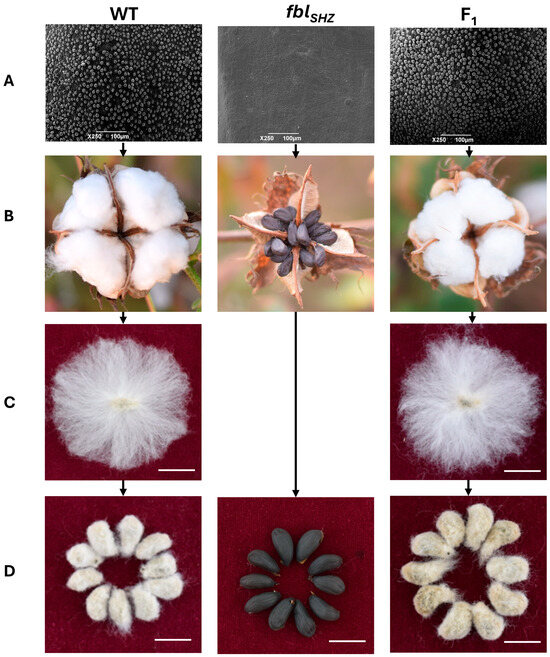
Figure 1.
Phenotypic observation of cotton lint and fuzz fiber in WT, fblSHZ, and F1: (A) The ovules at 0 DPA were observed by electron microscopy. Bars = 100 µm. (B) Phenotypic observation of three materials during boll opening. (C,D) Seed phenotype after combing and ginning of fibers. Bars = 1 cm.
To fine-map the fblSHZ gene, two F2 mapping populations (fblSHZ × WT) of 1848 and 3100 individuals were developed in 2016 and 2018, respectively. Three phenotypes appeared in the F2 populations (Figure S2), including 1428 fuzzy–linted plants, 318 less/no fuzzy but linted plants, and 102 fiberless plants in the population of 2016, and the segregation ratio was 12:3:1 (χ2 12:3:1 = 5.19); and 1900 fuzzy–linted plants, 550 less fuzzy but normal linted plants, and 184 fiberless plants in the population of 2018, which also occurred with 12:3:1 segregation ratio (χ2 12:3:1 = 3) (Table S2). These results showed that two loci conferred the fiberless trait in fblSHZ, and the fuzz fiber was epistatic to the lint fiber.
3.2. Genetic Mapping of the fblSHZ Gene
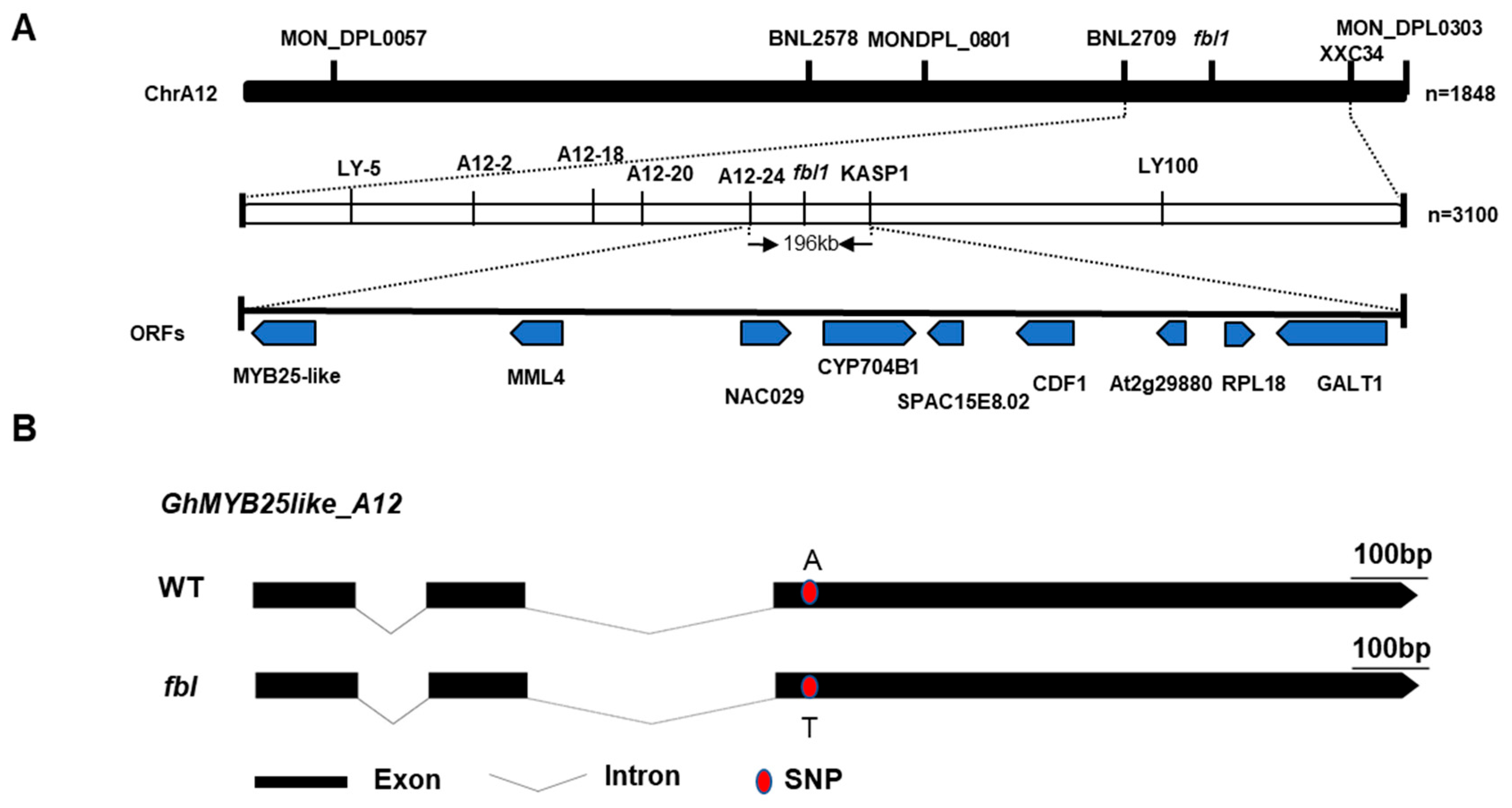
To primarily map the fblSHZ gene, markers were selected at every 10 cM distance from the 5152 markers in the genetic map constructed by a BC1 population of G. barbadense acc. 3-79 and G. hirsutum cv. Emian22, and 30 polymorphic SSR markers between WT and fblSHZ were screened out (Table S3). To identify markers linked with fblSHZ, bulked segregant analysis was performed using the fuzzy–linted (WT) and fiberless (fblSHZ) pools. A total of 102 recessive individuals from the F2 population in 2016 were used for genotyping and linkage analysis. Chi-square test of the linkage relationship between SSR markers and fblSHZ indicated that the SSR markers from Chr A12, BNL2709, MON_DPL0303, BNL2578, MON_DPL0801, and MON_DPL0057 were linked to fblSHZ (Table S3). Thus, fblSHZ was anchored to Chr A12 between BNL2709 and MON_DPL0801 with genetic distances of 1.8 cM and 35.2 cM, respectively. To narrow down the interval further, 75 SSR markers between the region of BNL2709 and MON_DPL0801 were developed, and only the SSR marker XXC_34 was linked to fblSHZ, with a genetic distance of 5.2 cM. Thus, the fblSHZ locus was mapped between SSR markers BNL2709 and XXC_34 (Figure 2A). According to the sequence variations between WT and fblSHZ, 100 InDel markers and 10 KASP markers in the candidate region were developed to narrow down the mapping region further, and 7 InDel markers and 1 KASP marker were polymorphic between the two parents. The mapping region was further narrowed down to a 196 kb region flanked by the Indel marker A12-24 and the KASP marker KASP1 using the 291 recessive individuals from the two F2 populations, with genetic distances of 1.03 cM and 0.86 cM, respectively (Figure 2A). Within this region, nine putative open reading frames (ORFs) were found, including the MYBMIXTA-like (MML) transcriptional factors, GhMML3_A12/GhMYB25like_A12 [4,9] and GhMML4_A12 (MYB106), which was the homology from the Dt genome (GhMML4_D12) [11]. The others were NAC transcription factor 29 (NAC29), Cytochrome P450 704B1 (CYP704B1), uncharacterized endoplasmic reticulum membrane protein C16E8.02 (SPAC16E8.02), cyclic dof factor 1 (CDF1), uncharacterized protein At2g29880 (At2g29880), 50S ribosomal protein L18 (RPL18), and beta-1,3-galactosyltransferase GALT1 (GALT1) (Table S4). Given that our previous study proved that GhMYB25like_A12 is responsible for fuzz fiber development [15], and numerous studies have indicated that GhMYB25like acts as a hub in a regulatory network that controls both lint and fuzz initiation [4,9,12], it was identified as the candidate gene for fblSHZ.
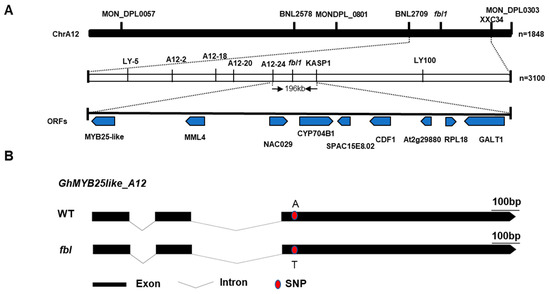
Figure 2.
Map-based cloning of fblSHZ: (A) Map-based cloning of the fblSHZ locus. It was first mapped on Chr A12 between the markers BNL2709 and XXC34 using an F2 generation with 1848 individuals. It was further fine-mapped to a region between markers A12-24 and KASP1 using 3100 individuals. The mapping area was narrowed down to a 196 kb genomic interval, including nine predicted genes: MYB25like, MML4, NAC029, CYP704B1, SPAC16E8.02, CDF1, At2g29880, RPL18, and GALT1. (B) The gene structure of the GhMYB25like_A12 between WT and fblSHZ.
3.3. Cloning of the fblSHZ Gene
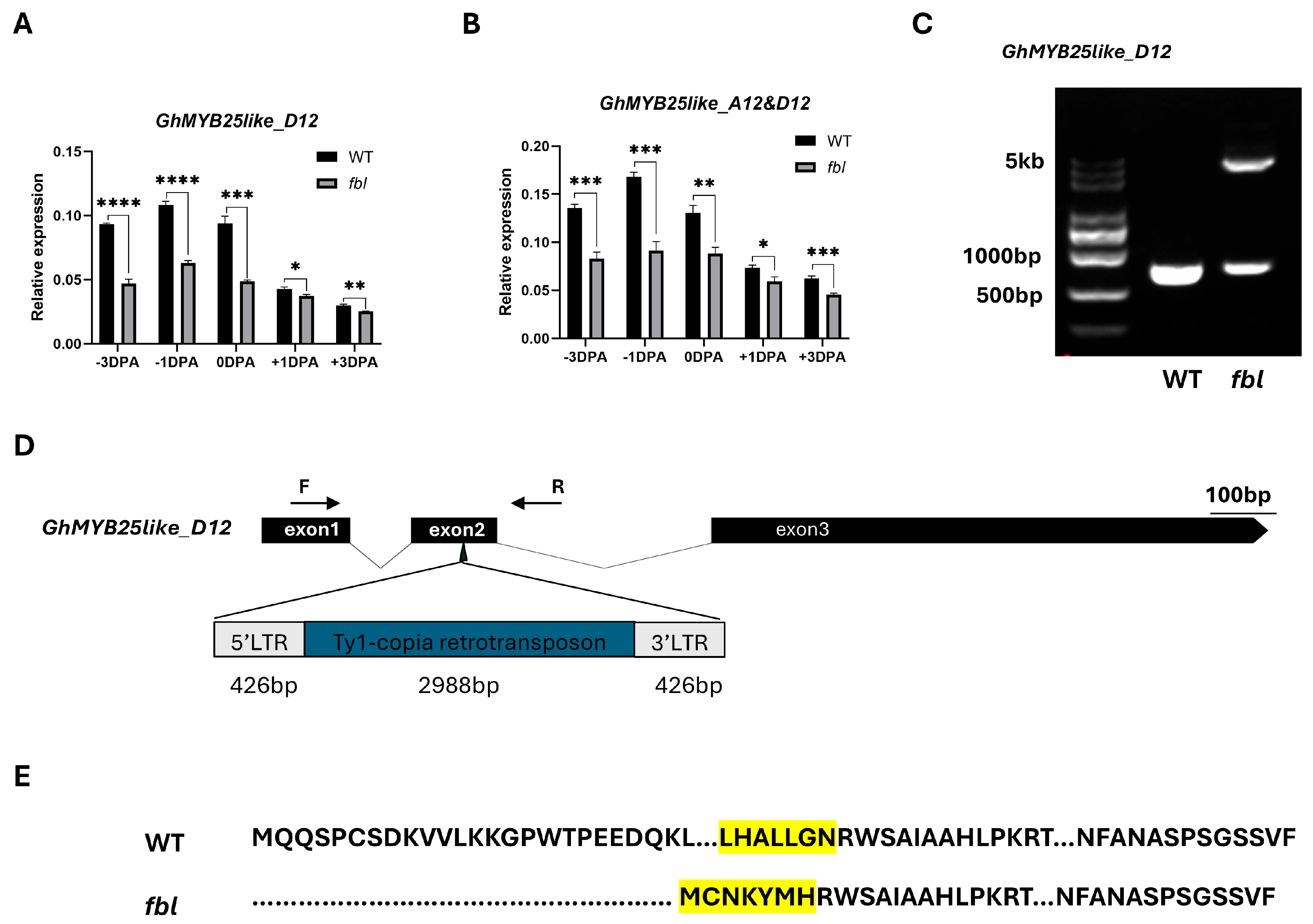
The full-length genomic sequences of the candidate gene GhMYB25like_A12 and the homologs from the Dt sub-genomes GhMYB25like_D12 were amplified from WT and fblSHZ. One SNP located on the R2R3 domain in GhMYB25like_A12 was found to cause one nonsynonymous mutation from A to T (Figure 2B), causing an amino acid mutation from lysine to methionine (K104M) (Figure S3A). As the highly homologous sequences between the At and Dt genomes, only eight amino acids changed between GhMYB25like_A12 and GhMYB25like_D12 in the reference genome (Figure S3B). Specific primers were designed to analyze the expression of GhMYB25like_D12, revealing significantly lower transcript levels in fblSHZ compared with the WT plants during critical fiber initiation stages (Figure 3A); the expression analysis of both GhMYB25like_A12 and GhMYB25like_D12 using nonspecific primers demonstrated consistently reduced transcript accumulation in fblSHZ (Figure 3B). The resequencing data of GhMYB25like_D12 from WT and fblSHZ were aligned visually, and an obvious gap was found in fblSHZ (Figure S4A); the same result was found in transcript sequence data (Figure S4B). In addition, it displayed a longer PCR product than the expected length in fblSHZ than in WT (Figure 3C). A 3840 bp insertion fragment was found in the second exon of GhMYB25like_D12 in fblSHZ by Sanger sequencing (Figure 3D). Further sequence analysis indicated that the insertion fragment was a putative Ty1/copia long terminal repeat (LTR) retrotransposon (named GhMYB25like_D12_TE hereafter). The components of a typical Ty1/copia element, such as LTRs, gag, and pol, could be found in GhMYB25likeD12 in fblSHZ. The retrotransposon consists of a 426 bp 5′-LTR, a 2988-bp internal region, and a 426 bp 3′-LTR (Figure 3D). Interestingly, the insertion of GhMYB25like_D12_TE generated a novel transcript in fblSHZ (Figure 3E), encoding 286 amino acids in fblSHZ, with the C-terminal 280 amino acids being the same as the amino acids in WT (Figure S5). Since retrotransposon insertions often interfere with transcriptional regulation, the aberrant transcript structure in fblSHZ presumably underlies the downregulation of GhMYB25like_D12 during fiber initiation.
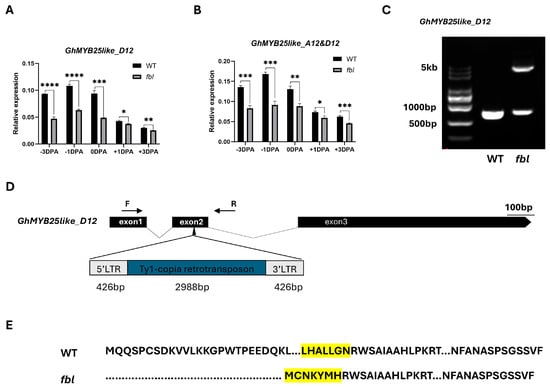
Figure 3.
A TE insertion was observed in GhMYB25like_Dt in fblSHZ. The relative expression of GhMYB25like_D12 (A) and GhMYB25like (B) in the WT and fblSHZ ovules during fiber initiation at −3, −1, 0, +1, +3 DPA, as determined by RT-qPCR. The GhUBQ7 (Ghir_A11G011460) was used as the internal reference. Error bars represent ± SD. Significance was calculated using a t-test (**** p < 0.0001; *** p < 0.001; ** p < 0.01; * p < 0.05). (C) A retrotransposon insertion in the gene body of GhMYB25like_D12 in fblSHZ. The primers used for retrotransposon amplification were labeled by F and R, which were shown in (D). (D) Gene structure of the GhMYB25like_D12 in WT and fblSHZ. The retrotransposon was inserted in the third exon of GhMYB25like_D12. (E) The varieties of protein sequences between WT and fblSHZ. The retrotransposon insertion in GhMYB25like_D12 led to a new protein in fblSHZ, and the highlight codon means the conserved regions between WT and fblSHZ.
In conclusion, the fiberless phenotype in the fblSHZ mutant should be caused by the nonsynonymous mutation in the R2R3 domain of GhMYB25like_A12 and a TE insertion in the second exon of GhMYB25like_D12, which may impair the DNA binding activation to targets and interrupt the gene function, respectively.
3.4. Functional Verification of GhMYB25like
To determine whether the amino acid variations in GhMYB25like_A12 between WT and fblSHZ affect protein localization, a subcellular localization assay was performed in N. benthamiana leaves, which showed that they were located in the cell nucleus and the variations did not influence the protein location (Figure S6). To comprehend the function of GhMYB25like_A12 and GhMYB25like_D12 on fiber initiation, specific sgRNAs with the CRISPR-Cas9 system were used to create mutant lines (the Cr_GhMYB25like_A12 line was used in our previous study [15], and the Cr_GhMYB25like_D12 line was generated in this study) (Figures S7 and S8). The transgenic plants showed that when GhMYB25like_A12 was specifically knocked out (Cr-GhMYB25like_A12 mutant line), no fuzz was found on the cotton seed coat compared with Jin668 (Figure 4); what is more, the trichome on the surface of the stem, petiole, leaf, and petal was reduced obviously (Figure S9); when GhMYB25like_D12 was independently knocked out (Cr-GhMYB25like_D12 mutant line), no significant difference was found on cotton seeds (Figure 4); it showed glabrous on cotton seed when both GhMYB25like_A12 and GhMYB25like_D12 were knocked out simultaneously (Cr-GhMYB25like_A12&D12 mutant line), and no trichomes were covered on the surface of the stem, petiole, leaf, and petal (Figure S9).

Figure 4.
Phenotypic observation of cotton lint and fuzz fiber in GhMYB25like mutant lines: (A) Fiber phenotype in Jin668, GhMYB25like_A12 mutant line, GhMYB25like_D12 mutant line, and GhMYB25like_A12&D12 (GhMYB25like) mutant line. Bars = 1 cm. (B) Seed phenotype after combing of fibers. Bars = 1 cm. (C) Seed phenotype after ginning of fibers. Bars = 1 cm.
3.5. The Fuzz Gene Was Dominant Epistatic to the Lint Gene
To uncover the phenotypes of the mutant lines further, an F2 segregation population was developed from a cross between the Cr-GhMYB25like_A12&D12 mutant line as the female parent and Jin668 as the male parent. A total of 289 individuals were obtained, and the three phenotypes reappeared in the F2 populations, including 226 fuzzy–linted plants, 51 less/no fuzzy but linted plants, and 12 fiberless individuals; the segregation ratio was 12:3:1 (χ2 = 0.27) (Table S5), which revealed the dominant epistatic of fuzz gene to lint gene. The results showed that GhMYB25like_A12 mainly regulated the fuzz initiation, while GhMYB25like_A12 and GhMYB25like_D12 were responsible for the lint fiber initiation.
3.6. GhMYB25like Is Involved in Regulating Fiber Initiation in Multiple Pathways
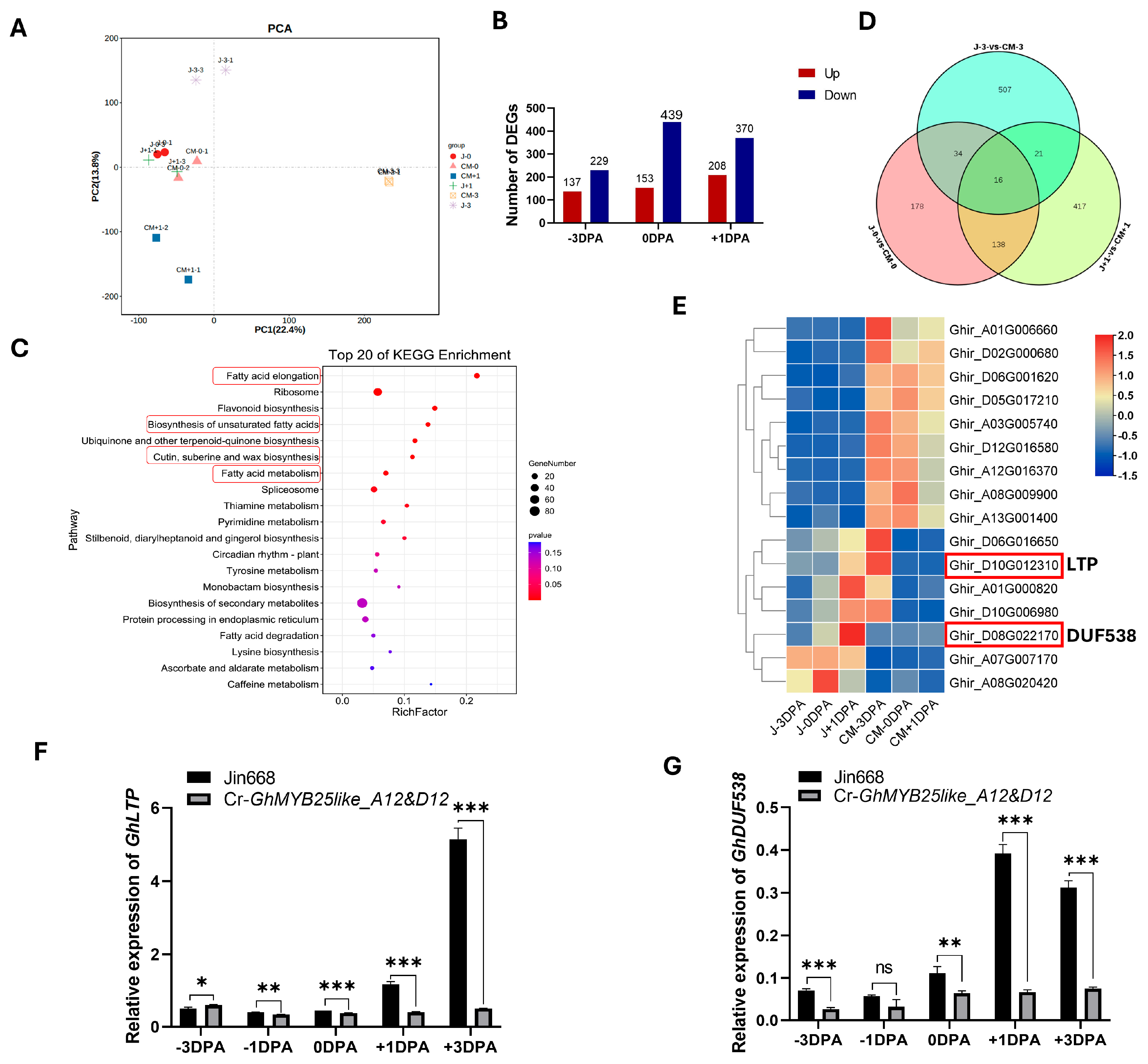
To understand the possible metabolic pathway and target genes of GhMYB25like during cotton fiber initiation, the −3 DPA, 0 DPA, and +1 DPA ovules from the Jin668 and Cr-GhMYB25like_A12&D12 mutant lines were used for RNA-seq analysis, and PCA analysis is shown in Figure 5A. Based on the transcriptome data, 578, 366, and 592 differentially expressed genes (DEGs) were found in −3 DPA, 0 DPA, and +1 DPA, respectively (Figure 5B). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was used to categorize function annotations for the DEGs, and these DEGs were mainly enriched in fatty acid metabolism, flavonoid biosynthesis, cutin, suberine, and wax biosynthesis (Figure 5C), suggesting that a series of genes regulated networks through different pathways to initiate cotton fiber. Only 16 genes performed significant expression levels among all the DEGs during the three stages (Figure 5D), including the nonspecific lipid-transfer protein LTP and the unknown protein DUF538 (Figure 5E).
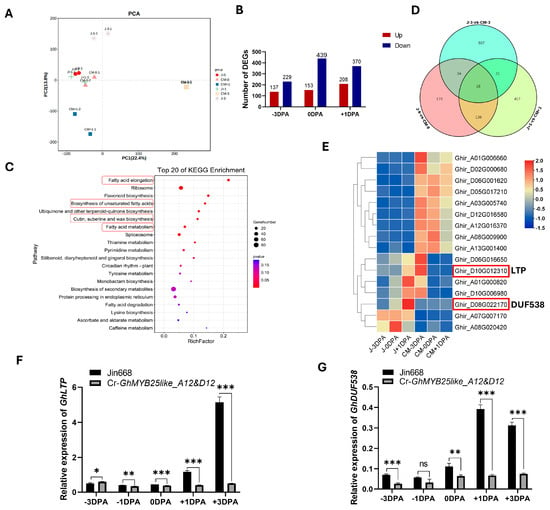
Figure 5.
RNA-seq analysis of GhMYB25like mutant line during the fiber initiation at −3, 0, and +1 DPA ovules: (A) The PCA analysis. (B) The DEGs in three stages of fiber initiation in Cr-GhMYB25like_A12&D12 mutant lines. (C) The top 20 of the KEGG enrichment analysis, and the red frames indicates the iterms of fatty acid related metabolism. (D) Venn diagram of the specific genes at the −3 DPA, 0 DPA, and +1 DPA ovule in the Jin668 and GhMYB25like mutant lines. (E) The heatmap of 16 DEGs in three stages. The relative expression of GhLTP (F) and GhDUF538 (G) in the Jin668 and GhMYB25like mutant lines during fiber initiation at −3, −1, 0, +1, +3 DPA, as determined by RT-qPCR. The GhUBQ7 (Ghir_A11G011460) was used as the internal reference. Error bars represent ± SD. Significance was calculated using a t-test (*** p < 0.001; ** p < 0.01; * p < 0.05; ns, no signifacance).
In this study, GhLTP exhibited extremely low expression levels in the Cr-GhMYB25like_A12&D12 mutant line during cotton fiber initiation (Figure 5F), which revealed that GhMYB25like might target the GhLTP protein to regulate cotton fiber initiation directly or indirectly. The transcript level of GhDUF538 was also sharply decreased in the Cr-GhMYB25like_A12&D12 mutant line (Figure 5G), which implied its role in cotton fiber initiation.
4. Discussion
4.1. The Duplicated GhMYB25like Genes Regulate Cotton Lint and Fuzz Fiber Initiation
According to previous reports, GhMYB25like had been identified as the fuzz and lint loci in different fiber mutants through a map-based cloning method: the dominant fuzzless locus N1 was controlled by the GhMML3_A12 (GhMYB25like_A12) [9], the less mutant phenotype with lint and no fuzz mutant was induced by the dominant negative mutation GhMYB25like_AthapT [15]; the lint fiber locus was indeterminate in different mutants, GhMML4 and GhMYB25like_D12 were considered as the genes that regulate the initial development of lint in fuzzless–linted mutant n2NSM [11,12]; GhMYB25like_D12 was also reported as the li3 in Xu142 fl mutant, a retrotransposon insertion in the second exon of GhMYB25like_D12, which decreased its expression and resulted in the lintless–fuzzless phenotype [13]. Here, a nonsynonymous mutation A/T with a K104M mutation in the R2R3 domain of GhMYB25like_A12 was identified in the fblSHZ mutant, which was inconsistent with previous studies [4,9], while it was consistent with the variation in the recent study [15]. A retrotransposon insertion in the second exon of GhMYB25like_D12, which generated a novel but decreased transcription. The two variations may lead to the fiberless phenotype in the fblSHZ mutant.
In this study, GhMYB25like_A12 and GhMYB25like_D12 were identified as the fuzz and lint initiation genes in the fblSHZ mutant. It presented fuzzless–linted, fuzzy–linted, and fiberless seeds when GhMYB25like_A12, GhMYB25like_D12, and GhMYB25like_A12&D12 were knocked out by the CRISPR-Cas9 system, respectively. These results revealed that GhMYB25like_A12 regulates fuzz initiation, while GhMYB25like_A12 and GhMYB25like_D12 coordinately control lint development. These results confirmed that GhMYB25like from At and Dt genomes played similar but different roles in cotton fuzz and lint initiation.
Our previous study elucidated the intricate functional mechanism of GhMYB25like, demonstrating that the mutant GhMYB25like protein acts as a dominant-negative regulator by inhibiting the activity of its wild-type counterpart during lint and fuzz initiation. Further investigation revealed that the degree of interference with normal GhMYB25like function correlated with the extent of R2R3 MYB binding domain preservation in the truncated mutant protein generated through gene editing. Specifically, mutant proteins retaining larger portions of the R2R3 domain exhibited stronger inhibitory effects on the activity of wild-type GhMYB25like, consequently more severely impairing fiber initiation [15]. The especially knocked out GhMYB25like_D12 caused almost normal lint and fuzz fiber, which may be caused by no R2R3 domain preservation (Figure S8). A recent study demonstrated that the specific knockout of the GhMYB25like_D12 locus resulted in fuzzless seeds, whose phenotypic effects were identical to those observed in the GhMYB25like_A12 mutant line. Notably, the editing target was situated in the third exon of GhMYB25like_D12, yet the mutant protein retained an intact R2R3 DNA binding domain [12]. In summary, the regulation mechanism of GhMYB25like_A12 and GhMYB25like_D12 on cotton fuzz and lint initiation was intricate in the reported study.
4.2. The Dominant Epistasis of the Fiberless Mutant in Cotton
There are usually two or more copies of each gene located in the A and D sub-genome chromosomes in the G. hirsutum (AD)1 genome, representing homoeologous or duplicate copies of the diploid ancestral species A and D genomes [11]. GhMYB25like_A12 shares high homology to GhMYB25like_D12, and there are only eight nonsynonymous differences between GhMYB25like_A12 and GhMYB25like_D12. In the previous study, RNA interference suppression of GhMYB25like led to a fiberless phenotype, with no change in trichomes elsewhere [4]. On account of the high homology of GhMYB25like from At and Dt genomes, the fiberless seeds in RNAi transgenic cotton may be caused by the suppression of GhMYB25like_A12 and GhMYB25like_D12 simultaneously. In this study, knocking out GhMYB25like (both GhMYB25like_A12 and GhMYB25like_D12) through the CRISPR-Cas9 system resulted in fiberless seeds and mostly glabrous elsewhere, compared with Jin668, and the phenotype of seeds was inconsistent with the previous study [4]. The especially knocked out GhMYB25like_A12 caused fuzzless–linted seeds but normal trichomes elsewhere in our previous research [15]; it showed that GhMYB25like_A12 was mainly responsible for fuzz production but not trichomes on plants elsewhere, which was consistent with previous studies [9]. The especially knocked out GhMYB25like_D12 caused normal lint and fuzz fiber, resulting in no conspicuous change on the cotton plant elsewhere. In addition to this, the segregation ratio in the F2 segregation population developed by the cross of GhMYB25like mutant line and Jin668 was consistent with the result of the segregation ratio in the F2 segregation population, which was developed from a cross between WT and fblSHZ. No fuzzy–lintless plants appeared in the F2 populations, which indicated that the fuzz gene was dominant epistatic to the lint gene, which was also proved by the previous research [11,12].
Based on current evidence, it was reasonable for us to speculate that two potential regulatory pathways for lint fiber initiation existed, with GhMYB25like_A12 and GhMYB25like_D12 serving as core transcriptional regulators. The observation that the targeted knockout of GhMYB25like_D12 did not produce conspicuous phenotypic alterations in cotton seeds suggests potential functional compensation by GhMYB25like_A12. This compensatory mechanism may involve GhMYB25like_A12 regulating downstream targets normally controlled by GhMYB25like_D12. In summary, the regulatory mechanism underlying fuzz and lint fiber initiation mediated by GhMYB25like homologs was intricate, and further investigation is needed.
4.3. GhMYB25like Regulates Cotton Fiber Initiation Through Multiple Pathways
As the key regulator in the regulatory network of fiber initiation, GhMYB25like regulates both lint and fuzz initiation by regulating the expression of various initiation-related genes. Some key TFs were identified in the downstream of GhMYB25like. The silencing of GhMYB109, homologous to Arabidopsis GL1, substantially reduced fiber production in transgenic cotton [2]. Silencing of GhMYB25 caused a short fiber phenotype [2,3]. Silencing of GhHD-1 resulted in retarded fiber initiation, and all three TFs located downstream of GhMYB25like in the TF-mediated networks of fiber initiation [27]. Only the silencing of GhMYB25like led to a fiberless phenotype [4], which showed the significant regulation of GhMYB25like on cotton fiber initiation.
According to the transcriptome analysis, GhMYB25like might regulate cotton fiber initiation through multiple pathways, with particularly significant involvement in fatty acid metabolism. In this study, two DEGs, nsLTP and DUF538, were further analyzed. The nonspecific lipid transfer proteins (nsLTPs) abundantly exist in plant tissues. The proteins are characterized by a tunnel-like hydrophobic cavity, which makes them suitable for binding and transporting various lipids [28]. They are deemed to function for the shuttling of lipids between membranes and across the cytoplasm and regulating the beta-oxidation of fatty acids in glyoxysomes and intracellular fatty acid pools [29]. The plant nsLTPs play a role in diverse activities, including cotton fiber development [30,31]. The DUF538 (domain of unknown function) protein family consists of proteins widely distributed in land plants but not in animals or yeasts [32,33]. It was reported that a pair of DUF538 proteins, SVB and SVBL, played roles in the transcriptional regulation of trichome development, modulating plant growth and trichome development through the transcriptional regulation of GL1, the key TF of trichome initiation in Arabidopsis [34]. One GhDUF538 protein was recently defined as a fiber cell cluster with the fiber marker genes (GhMYB25, GhMML9, and GhHD1) verified by situ hybridization assays [14]. The two genes downregulated significantly in GhMYB25like mutants during fiber initiation, suggesting they may function as direct or indirect downstream targets in the GhMYB25like mediated regulatory network controlling fiber initiation. To further elucidate this regulatory relationship, direct molecular validation approaches should be employed, including LUC reporter assays, Y1H (Yeast One-Hybrid), EMSA (Electrophoretic Mobility Shift Assay), or ChIP-qPCR (Chromatin Immunoprecipitation quantitative PCR).
5. Conclusions
This study demonstrated that the duplicated GhMYB25like genes (GhMYB25like_A12 and GhMYB25like_D12) on homologous chromosomes coordinately regulate cotton fiber initiation, with GhMYB25like_A12 primarily controlling fuzz initiation and both homologs regulating lint formation in the fiberless mutant fblSHZ; the fuzz gene was dominant epistasis over the lint gene. A K104M mutation in GhMYB25like_A12 and a TE insertion in GhMYB25like_D12 collectively led to the fiberless seeds in fblSHZ. GhMYB25like regulated fiber initiation primarily through modulation of fatty acid metabolic pathways. The study deciphers the laws of inheritance of fiberless mutants in cotton on the genetic level, advancing our understanding of the role of gene duplication in fiber initiation, which could guide us to develop different varieties of fuzz/lint to meet the multiple demands of the textile industry.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology14080983/s1, Table S1. All primers were used in this study. Table S2. Phenotypic statistics of the F2 populations in the field. Table S3. Chi-Squared test of linkage relationship between SSRs and fblSHZ. Table S4. The candidate genes in the fblSHZ mapping locus. Table S5. Phenotypic statistics of the F2 population developed by the WT and GhMYB25like_A12&D12 mutant line. Figure S1. Phenotypic observation of cotton plants in WT and fblSHZ. Figure S2. Phenotypic observation of cotton lint and fuzz fiber in F2 populations. Figure S3. The protein sequence of GhMYB25like. Figure S4. The genome and transcript sequences visual alignment of GhMYB25like_D12 in WT and fblSHZ. Figure S5. The protein sequence alignment of GhMYB25like_D12 between WT and fblSHZ. Figure S6. Subcellular localization assay of GhMYB25like. Figure S7. Characterization of cotton CRISPR mutant lines on GhMYB25like. Figure S8. The protein sequences of the GhMYB25like in CRISPR-Cas9 mutant lines. Figure S9. Phenotypic observation of cotton plants in GhMYB25like CRISPR mutant lines.
Author Contributions
Methodology, Z.L. and Y.L. (Yu Le); validation and investigation, X.X., Z.X., M.C. and Y.L. (Yuanxue Li); data curation and visualization, C.F.; writing—original draft preparation, Y.L. (Yu Le); writing—review and editing, C.Y. and Z.L.; project administration and supervision, Z.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Development Fund for Xinjiang Talents XL202403-02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data used in this study can be accessed at Science Data Bank: https://www.scidb.cn/anonymous/bnVVajZi (accessed on 13 June 2025), https://www.scidb.cn/anonymous/VXp5QVZi (accessed on 13 June 2025), and https://www.scidb.cn/anonymous/WVZSalli (accessed on 13 June 2025).
Acknowledgments
The computations in this article were run on the bioinformatics computing platform of the National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CDS | Coding Sequence |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeptides-Cas9 |
| CTAB | Hexadecyltrimethylammonium bromide |
| DEG | Differentially Expressed Gene |
| DPA | Days Post-Anthesis |
| GATK | Genome Analysis Toolkit |
| GFP | Green Fluorescent Protein |
| Hi-Tom | High-Throughput Tracking of Mutations |
| InDel | Insertion/Deletion |
| KASP | Kompetitive Allele-Specific PCR |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MML | MYB-MIXTA-like |
| NGS | Next-Generation Sequencing |
| PCR | Polymerase Chain Reaction |
| RNA-seq | RNA sequencing |
| RT-qPCR | Real-Time quantitative PCR |
| SEM | Scanning Electron Microscopy |
| SNP | Single Nucleotide Polymorphism |
| SSR | Simple Sequence Repeat |
| TE | Transposable Element |
| TF | Transcription Factor |
| WT | Wild Type |
References
- Lang, A.G. The origin of lint and fuzz hairs of cotton. J. Agric. Res. 1938, 56, 507–521. [Google Scholar]
- Pu, L.; Li, Q.; Fan, X.; Yang, W.; Xue, Y. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics 2008, 180, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Wu, Y.; Yang, Y.; Llewellyn, D.J.; Dennis, E.S. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 2009, 59, 52–62. [Google Scholar] [CrossRef]
- Walford, S.-A.; Wu, Y.; Llewellyn, D.J.; Dennis, E.S. GhMYB25-like: A key factor in early cotton fibre development. Plant J. 2011, 65, 785–797. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.W.; Yu, N.; Li, C.H.; Luo, B.; Gou, J.Y.; Wang, L.J.; Chen, X.Y. Control of plant trichome development by a cotton fiber MYB gene. Plant Cell 2004, 16, 2323–2334. [Google Scholar] [CrossRef]
- Bedon, F.; Ziolkowski, L.; Walford, S.A.; Dennis, E.S.; Llewellyn, D.J. Members of the MYBMIXTA-like transcription factors may orchestrate the initiation of fiber development in cotton seeds. Front. Plant Sci. 2014, 5, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Wan, Q.; Guan, X.; Yang, N.; Wu, H.; Pan, M.; Liu, B.; Fang, L.; Yang, S.; Hu, Y.; Ye, W.; et al. Small interfering RNAs from bidirectional transcripts of GhMML3_A12 regulate cotton fiber development. New Phytol. 2016, 210, 1298–1310. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Yuan, Y.M.; Stiller, W.; Jia, Y.H.; Wang, P.P.; Pan, Z.E.; Du, X.M.; Llewellyn, D.; Wilson, I. Genetic dissection of the fuzzless seed trait in Gossypium barbadense. J. Exp. Bot. 2018, 69, 997–1009. [Google Scholar] [CrossRef]
- Wu, H.; Tian, Y.; Wan, Q.; Fang, L.; Guan, X.; Chen, J.; Hu, Y.; Ye, W.; Zhang, H.; Guo, W.; et al. Genetics and evolution of MIXTA genes regulating cotton lint fiber development. New Phytol. 2018, 217, 883–895. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Li, J.; Chen, J.; Dai, F.; Tian, Y.; Hu, Y.; Zhu, Q.H.; Zhang, T. Two duplicated GhMML3 genes coordinately control development of lint and fuzz fibers in cotton. Plant Commun. 2025, 6, 101281. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Zhu, S.; Fang, S.; Zhao, L.; Guo, Y.; Wang, J.; Yuan, L.; Lu, Y.; Liu, F.; et al. A retrotransposon insertion in GhMML3_D12 is likely responsible for the lintless locus li3 of tetraploid cotton. Front. Plant Sci. 2020, 11, 593679. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, M.; Li, W.; Xu, M.; Shao, L.; Liu, Y.; Zhao, G.; Liu, Z.; Xu, Z.; You, J.; et al. Single-cell RNA-seq reveals fate determination control of an individual fibre cell initiation in cotton (Gossypium hirsutum). Plant Biotechnol. J. 2022, 20, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.N.; Le, Y.; Sun, M.L.; Xu, J.W.; Qin, Y.; Men, S.; Ye, Z.X.; Tan, H.Z.; Hu, H.Y.; You, J.Q.; et al. A dominant negative mutation of GhMYB25-like alters cotton fiber initiation, reducing lint and fuzz. Plant Cell 2024, 36, 2759–2777. [Google Scholar] [CrossRef]
- Paterson, A.H.; Brubaker, C.L.; Wendel, J.F. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol. Biol. Rep. 1993, 11, 122–127. [Google Scholar] [CrossRef]
- Li, X.; Jin, X.; Wang, H.; Zhang, X.; Lin, Z. Structure, evolution, and comparative genomics of tetraploid cotton based on a high-density genetic linkage map. DNA Res. 2016, 23, 283–293. [Google Scholar] [CrossRef]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, C.; Zhu, D.; Le, Y.; Wang, N.; Li, Y.; Zhang, X.; Lin, Z. Fine-mapping and candidate gene analysis of qFL-c10-1 controlling fiber length in upland cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 2022, 135, 4483–4494. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Sun, L.; Ma, Y.; Xu, J.; Liang, S.; Deng, J.; Tan, J.; Zhang, Q.; Tu, L.; et al. High efficient multisites genome editing in allotetraploid cotton (Gossypium hirsutum) using CRISPR/Cas9 system. Plant Biotechnol. J. 2018, 16, 137–150. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Jiao, X.; Zhang, H.; Song, L.; Li, Y.; Gao, C.; Wang, K. Hi-TOM: A platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 2019, 62, 1–7. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Walford, S.A.; Wu, Y.; Llewellyn, D.J.; Dennis, E.S. Epidermal cell differentiation in cotton mediated by the homeodomain leucine zipper gene, GhHD-1. Plant J. 2012, 71, 464–478. [Google Scholar] [CrossRef]
- Salminen, T.A.; Blomqvist, K.; Edqvist, J. Lipid transfer proteins: Classification, nomenclature, structure, and function. Planta 2016, 244, 971–997. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Cheng, P.T.; Peng, P.; Lyu, P.C.; Sun, Y.J. Lipid binding in rice nonspecific lipid transfer protein-1 complexes from Oryza sativa. Protein Sci. 2009, 13, 2304–2315. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Yao, H.; Wang, J.; Wang, J.; Xue, H.; Zuo, K. GhLTPG1, a cotton GPI-anchored lipid transfer protein, regulates the transport of phosphatidylinositol monophosphates and cotton fiber elongation. Sci. Rep. 2016, 6, 26829. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Shang, X.; He, Q.; Zhu, L.; Li, W.; Song, X.; Guo, W. LIPID TRANSFER PROTEIN4 regulates cotton ceramide content and activates fiber cell elongation. Plant Physiol. 2023, 193, 1816–1833. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A. DUF538 protein superfamily is predicted to be chlorophyll hydrolyzing enzymes in plants. Physiol. Mol. Biol. Plants 2016, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Yoshikawa, M.; Kamada, A.; Ohtsuki, T.; Uchida, A.; Nakayama, K.; Satoh, H. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in embryophyta. J. Plant Physiol. 2013, 170, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Sharma, O.; Nguyen, P.H.T.; Hartono, C.D.; Kanehara, K. A pair of DUF538 domain-containing proteins modulates plant growth and trichome development through the transcriptional regulation of GLABRA1 in Arabidopsis thaliana. Plant J. 2021, 108, 992–1004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).