Phytochemical Profiling, Antioxidant Capacity, and α-Amylase/α-Glucosidase Inhibitory Effects of 29 Faba Bean (Vicia faba L.) Varieties from China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Materials and Extraction

2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. In Vitro Antioxidant Capacity
2.6. In Vitro Enzyme Inhibitory Effects
2.7. UPLC-QTOF-MS Analysis
2.8. HPLC Analysis
2.9. Statistics Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Content in 29 Faba Bean Varieties
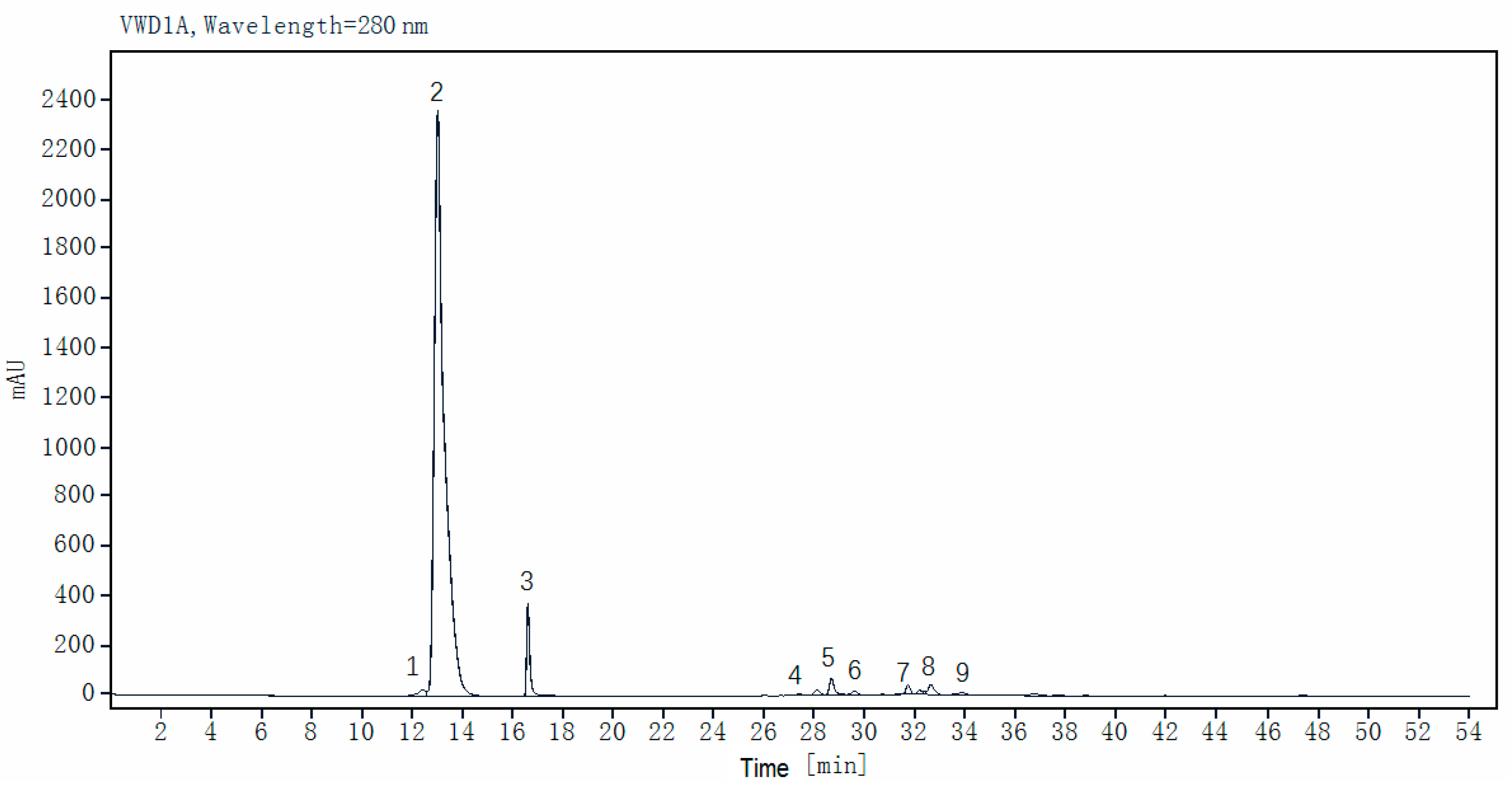
3.2. Preliminary Identification of Phenolic Compounds in Faba Beans
3.3. Quantification Analysis of Phenolic Compounds in 29 Faba Bean Varieties
3.4. In Vitro Antioxidant Capacity of 29 Faba Bean Varieties
3.5. In Vitro Enzyme Inhibitory Effects of 29 Faba Bean Varieties
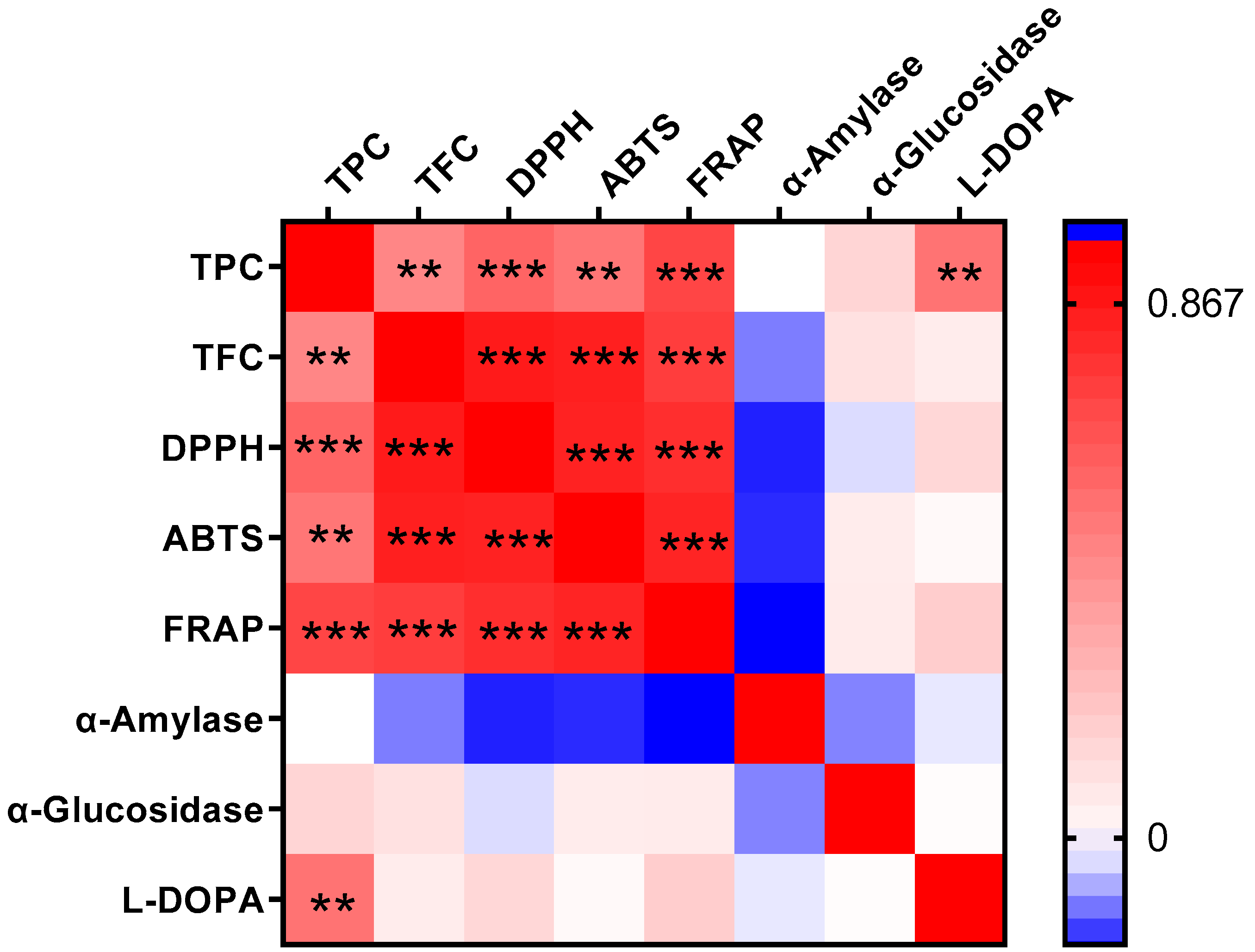
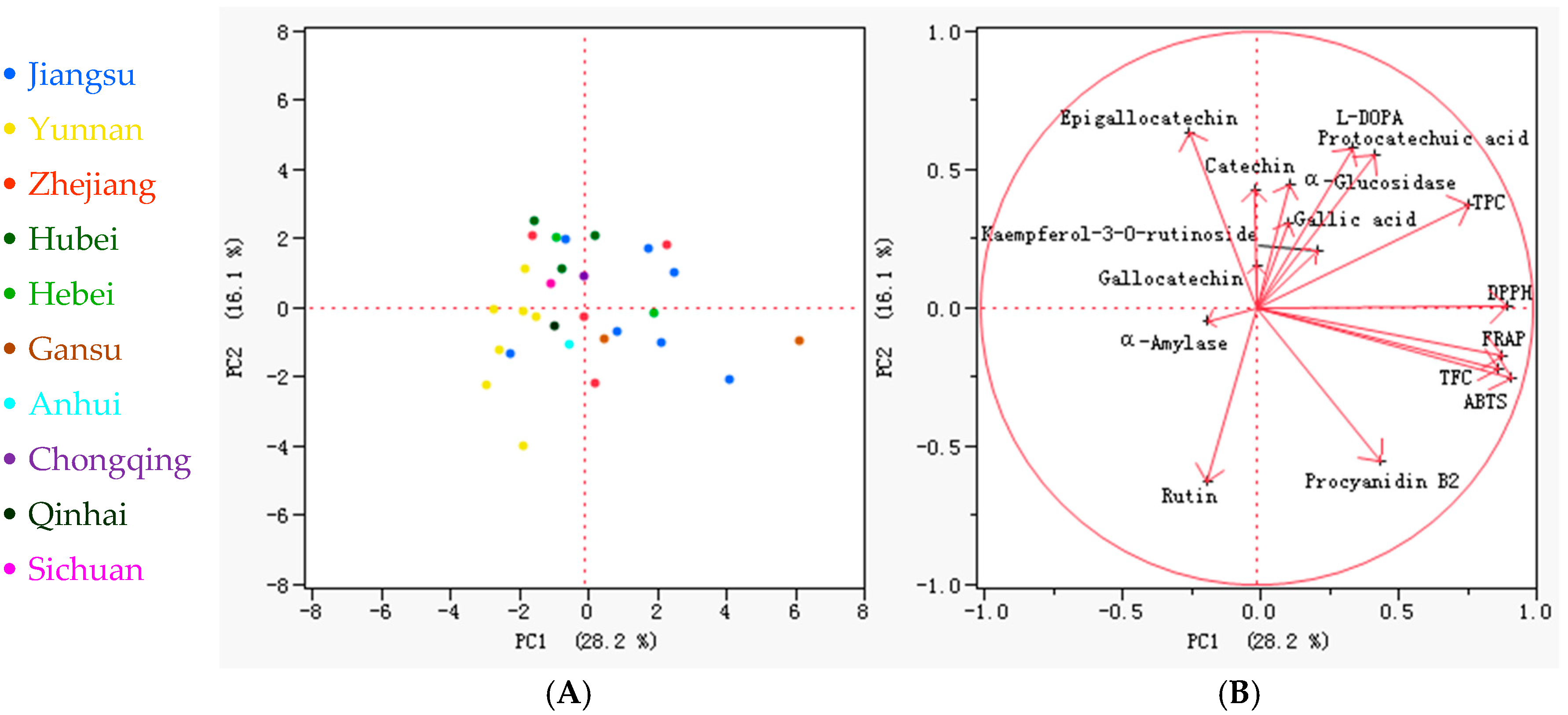
3.6. Correlation Among Phenolics and Bioactivities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and phytochemical content of high-protein crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef]
- Alam, T.; Najam, L. Faba-bean antioxidant and bioactive composition: Biochemistry and functionality. In Faba Bean: Chemistry, Properties and Functionality; Bangar, S.P., Dhull, S.B., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Kim, D.-G.; Kim, J.M.; Kang, K.-Y.; Lee, M.-K.; Hong, M.J.; Kim, J.-B.; Eom, S.H.; Kang, S.-Y.; Ha, B.-K.; et al. Phytochemical compounds and antioxidant activity in the grain of selected faba bean (Vicia faba) genotypes. Plant Breed. Biotechnol. 2018, 6, 65–73. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Shetty, K. Microwave-induced stimulation of L-DOPA, phenolics and antioxidant activity in fava bean (Vicia faba) for Parkinson’s diet. Process Biochem. 2004, 39, 1775–1784. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, P.; Shetty, K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem. 2002, 37, 1247–1256. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänan, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Tsukaguchi, R.; Hasebe, M.; Shibue, K.; Hamasaki, A. Diabetic striatopathy: Hyperglycemic chorea/ballism successfully treated with L-dopa. J. Diabetes Investig. 2024, 15, 1524–1527. [Google Scholar] [CrossRef]
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.L.; Walsh, K.B.; Naiker, M. Phenolic profiles of ten Australian faba bean varieties. Molecules 2021, 26, 4642. [Google Scholar] [CrossRef]
- Skovbjerg, C.K.; Angra, D.; Robertson-Shersby-Harvie, T.; Kreplak, J.; Keeble-Gagnère, G.; Kaur, S.; Ecke, W.; Windhorst, A.; Nielsen, L.K.; Schiemann, A.; et al. Genetic analysis of global faba bean diversity, agronomic traits and selection signatures. Theor. Appl. Genet. 2023, 136, 114. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kim, D.-G.; Lee, M.-K.; Kim, J.M.; Hong, M.J.; Kang, K.-Y.; Eom, S.H.; Kang, S.-Y.; Kim, J.-B.; Kwon, S.-J. Fatty acid composition, isoflavone and L-3,4-dihydroxyphenylalanine (L-dopa) contents in different parts of faba bean (Vicia faba) genotypes. Plant Breed. Biotechnol. 2017, 5, 314–324. [Google Scholar] [CrossRef][Green Version]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food. Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Wang, B.; Wang, H.; Zhang, Y.; Wang, W.; Yu, L. Flavonoids analysis in citrus peels by UPLC-Q-TOF-MS/MS and its antioxidant and anti-inflammation activity. Food Chem. Adv. 2024, 5, 100853. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- El-Beltagy, A.E.; Alharthi, S. Free radical scavenging activity of some legumes hulls extract and its efficacy on oil oxidative stability. J. AOAC Int. 2021, 104, 472–478. [Google Scholar] [CrossRef]
- García, A.R.H.; Avila, D.S.A.; Paredes, J.M.V.; Roa, R.I.L.; Preciado, A.H.M. Pithellobium dulce (Roxb.) Benth. fruit flour intake enhances short-chain fatty acid production and glucose metabolism in BALB/c Mice. Pol. J. Food Nutr. Sci. 2025, 75, 79–94. [Google Scholar] [CrossRef]
- Saito, Y.; Nishi, S.; Koaze, H.; Hironaka, K.; Kojima, M. Antioxidant and inhibitory activity on α-amylase and α-glucosidase in legume polyphenols. J. Jpn. Soc. Food Sci. Technol. 2007, 54, 563–567. [Google Scholar] [CrossRef]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.L.; Niu, L.Y.; Li, D.J.; Yu, H.; Huang, W.Y. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Sansenya, S.; Nanok, K. A-glucosidase, A-amylase inhibitory potential and antioxidant activity of fragrant black rice (Thai coloured rice). Flavour Fragr. J. 2020, 35, 376–386. [Google Scholar] [CrossRef]
- Suo, W.Y.; Wang, W.Z.; Li, D.J.; Wu, H.H.; Liu, H.Y.; Huang, W.Y.; Ma, Y.H. Optimization of ultrasonic-enzymatic-assisted extraction of flavonoids from sea buckthorn (Hippophae rhamnoides L.) pomace: Chemical composition and biological activities. Foods 2025, 14, 1656. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Xie, J.Y.; Xie, J.H.; Chen, Y.; Hu, X.; Yu, Q. Puriffcation, composition and activity of bound polyphenols from mung bean coat dietary fiber. Food Res. Int. 2022, 162, 111997. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y.B. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Baginsky, C.; Peña-Neira, Á.; Cáceres, A.; Hernández, T.; Estrella, I.; Morales, H.; Pertuzé, R. Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Chai, Z.; Herrera-Balandrano, D.D.; Yu, H.; Beta, T.; Zeng, Q.L.; Zhang, X.X.; Tian, L.L.; Niu, L.Y.; Huang, W.Y. A comparative analysis on the anthocyanin composition of 74 blueberry cultivars from China. J. Food Compos. Anal. 2021, 102, 104051. [Google Scholar] [CrossRef]
- Alcaraz-Mármol, F.; Nuncio-Jauregui, N.; Garcia-Sanchez, F.; Martinez-Nicolas, J.J.; Hernandez, F. Characterization of twenty pomegranate (Punica granatum L.) cultivars grown in Spain: Aptitudes for fresh consumption and processing. Sci. Hortic. 2017, 219, 152–160. [Google Scholar] [CrossRef]
- Valente, I.M.; Maia, M.R.G.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Profiling of phenolic compounds and antioxidant properties of European varieties and cultivars of Vicia faba L. pods. Phytochemistry 2018, 152, 223–229. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Joët, T.; Salmona, J.; Laffargue, A.; Descroix, F.; Dussert, S. Use of the growing environment as a source of variation to identify the quantitative trait transcripts and modules of co-expressed genes that determine chlorogenic acid accumulation. Plant Cell Environ. 2010, 33, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Schwarz, L.J.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Cereal phenolic contents as affected by variety and environment. Cereal Chem. 2018, 95, 589–602. [Google Scholar] [CrossRef]
- Zeng, Q.L.; Dong, G.Q.; Tian, L.L.; Wu, H.; Ren, Y.J.; Tamir, G.; Huang, W.Y.; Yu, H. High altitude is beneficial for antioxidant components and sweetness accumulation of rabbiteye blueberry. Front. Plant Sci. 2020, 11, 573531. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhang, C.L.; Chen, D.L. Changes in phenolics, vitamin C and antioxidant capacity during development of different cultivars of jujube fruits. Food Sci. 2015, 36, 94–98. [Google Scholar]
- Zuk, M.; Szperlik, J.; Hnitecka, A. Temporal biosynthesis of flavone constituents in flax growth stages. Plant Physiol. Biochem. 2019, 142, 234–245. [Google Scholar] [CrossRef]
- Yehmed, J.; Tlahig, S.; Mohamed, A.; Yahia, H.; Lachiheb, B.; Ben Yahia, L.; Loumerem, M. Nutritional and phytochemical profiling of Vicia faba L. var. Minor seeds: A multifaceted exploration of natural antioxidants and functional food potential. Appl. Biochem. Biotechnol. 2024, 196, 8471–8492. [Google Scholar] [CrossRef]
- Song, Y.L.; Gong, T.; Xiang, Y.Y.; Mikkelsen, M.; Wang, G.B.; Edden, R.A.E. Single-dose L-dopa increases upper brainstem GABA in Parkinson’s disease: A preliminary study. J. Neurol. Sci. 2021, 422, 117309. [Google Scholar] [CrossRef] [PubMed]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Varela, A.; Guillamón, E.; Pedrosa, M.M.; Muzquiz, M. Content and distribution of vicine, convicine and L-DOPA during germination and seedling growth of two Vicia faba L. varieties. Eur. Food Res. Technol. 2008, 227, 1537–1542. [Google Scholar] [CrossRef]
- Crepon, K.; Marget, P.; Peyronnt, C.; Carrouee, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Hu, J.; Kwon, S.J.; Park, J.J.; Landry, E.; Mattinson, D.S.; Gang, D.R. LC-MS determination of L-DOPA concentration in the leaf and flower tissues of six faba bean (Vicia faba L.) lines with common and rare flower colors. Funct. Foods Health Dis. 2015, 5, 243–250. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, A.R.; Han, H. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control 2013, 29, 112–120. [Google Scholar] [CrossRef]
- Li, X.X.; Bai, Y.X.; Jin, Z.Y.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT-Food Sci. Technol. 2022, 153, 112455. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Proença, C.; Freitas, M.; Araújo, A.N.; Silva, A.M.S.; Fernandes, E. 2-Styrylchromones as inhibitors of α-amylase and α-glucosidase enzymes for the management of type 2 diabetes mellitus. Med. Chem. Res. 2024, 33, 600–610. [Google Scholar] [CrossRef]
- Moussou, N.; Corzo-Martínez, M.; Sanz, M.L.; Zaidi, F.; Montilla, A.; Villamiel, M. Assessment of Maillard reaction evolution, prebiotic carbohydrates, antioxidant activity and a-amylase inhibition in pulse fours. J. Food Sci. Technol. 2017, 54, 890–900. [Google Scholar] [CrossRef]
- Oluwajuyitan, T.D.; Kadam, D.; Aluko, R.E. Amino acid profile, peptide size distribution, antioxidant effect, and enzyme inhibitory activities of fava bean (Vicia fava) protein hydrolyzates. Int. J. Food Prop. 2025, 28, 2466575. [Google Scholar] [CrossRef]
- Mojica, L.; Meyer, A.; Berhow, M.A.; González de Mejía, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Randhir, R.; Kwon, Y.-I.; Shetty, K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innov. Food Sci. Emerg. Technol. 2008, 9, 355–364. [Google Scholar] [CrossRef]
- Pinnen, F.; Cacciatore, I.; Cornacchia, C.; Sozio, P.; Iannitelli, A.; Costa, M.; Pecci, L.; Nasuti, C.; Cantalamessa, F.; Di Stefano, A. Synthesis and study of L-dopa-glutathione codrugs as new anti-parkinson agents with free radical scavenging properties. J. Med. Chem. 2007, 50, 2506–2515. [Google Scholar] [CrossRef]
- Farag, M.A.; Naser, A.A.F.; Zayed, A.; El-Dine, M.S.G. Comparative insights into four major legume sprouts efficacies for diabetes management and its complications: Untargeted versus targeted NMR biochemometrics approach. Metabolites 2023, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suarez, M.J. Broad bean and pea by-products as sources of fibre-rich ingredients: Potential antioxidant activity measured in vitro. J. Sci. Food Agric. 2012, 92, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Vega, A.; Drash, A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 1985, 75, 921–927. [Google Scholar] [CrossRef] [PubMed]
| Faba Bean Varieties | Origin a | TPC (mg GAE/g DW) | TFC (mg RE/g DW) | DPPH (µmol TE/g DW) | ABTS (µmol TE/g DW) | FRAP (mmol FE/g DW) | Inhibition Rate of α-Amylase (%) | Inhibition Rate of α-Glucosidase (%) |
|---|---|---|---|---|---|---|---|---|
| Baihuahudou | Yunnan | 1.05 ± 0.10 | 2.24 ± 0.02 | 7.92 ± 0.04 | 2.15 ± 0.05 | 11.89 ± 0.16 | 22.68 ± 0.70 | 10.71 ± 0.90 |
| Chenghu 21 | Sichuan | 1.40 ± 0.03 | 2.04 ± 0.07 | 8.91 ± 0.56 | 3.13 ± 0.66 | 14.66 ± 0.45 | 43.24 ± 1.20 | 10.44 ± 0.90 |
| Chenghudabai | Hebei | 1.38 ± 0.08 | 1.92 ± 0.08 | 8.77 ± 0.39 | 2.78 ± 0.14 | 14.59 ± 0.29 | 31.19 ± 0.50 | 14.86 ± 0.60 |
| Chuzao 1 | Yunnan | 1.26 ± 0.01 | 1.78 ± 0.07 | 8.42 ± 0.67 | 2.08 ± 0.06 | 12.27 ± 0.53 | 32.62 ± 0.40 | 9.08 ± 0.10 |
| Cican 1 | Zhejiang | 1.24 ± 0.01 | 2.13 ± 0.02 | 11.47 ± 0.04 | 3.42 ± 0.27 | 16.12 ± 0.42 | 24.89 ± 0.70 | 8.74 ± 0.50 |
| Dingxuancan 3 | Jiangsu | 1.62 ± 0.05 | 3.81 ± 0.06 | 15.52 ± 0.41 | 4.76 ± 0.16 | 18.40 ± 0.31 | 37.15 ± 1.00 | 4.26 ± 0.70 |
| Edou 1103 | Hubei | 1.58 ± 0.02 | 2.22 ± 0.02 | 8.20 ± 0.59 | 3.64 ± 0.41 | 16.54 ± 0.77 | 23.53 ± 0.00 | 8.77 ± 0.20 |
| Edou 1208 | Hubei | 1.40 ± 0.03 | 1.80 ± 0.08 | 8.39 ± 0.52 | 2.69 ± 0.45 | 15.03 ± 0.54 | 39.86 ± 0.80 | 13.41 ± 0.60 |
| Fengdou 11 | Yunnan | 1.24 ± 0.06 | 1.47 ± 0.06 | 8.82 ± 0.32 | 2.49 ± 0.23 | 13.07 ± 0.05 | 16.00 ± 0.10 | 9.65 ± 0.40 |
| Fengdou 28 | Yunnan | 1.36 ± 0.03 | 1.99 ± 0.04 | 9.60 ± 0.22 | 3.17 ± 0.05 | 13.33 ± 0.46 | 36.32 ± 0.60 | 12.16 ± 0.70 |
| Jianlixiacandou | Hubei | 1.34 ± 0.06 | 1.87 ± 0.09 | 9.51 ± 0.17 | 2.89 ± 0.05 | 14.08 ± 0.72 | 24.17 ± 0.60 | 6.29 ± 0.20 |
| Jingdou 5 | Yunnan | 1.12 ± 0.06 | 2.19 ± 0.07 | 9.78 ± 0.51 | 2.95 ± 0.02 | 11.82 ± 0.36 | 40.77 ± 0.30 | 7.23 ± 0.80 |
| Jizhangcan 5 | Hebei | 1.62 ± 0.01 | 2.81 ± 0.05 | 12.21 ± 0.11 | 3.89 ± 0.15 | 16.64 ± 0.64 | 29.81 ± 0.50 | 9.24 ± 0.10 |
| Lican 3 | Zhejiang | 1.08 ± 0.09 | 2.69 ± 0.02 | 11.56 ± 0.13 | 4.49 ± 0.16 | 16.12 ± 0.50 | 15.17 ± 0.40 | 7.97 ± 0.20 |
| Lican 7 | Zhejiang | 1.29 ± 0.08 | 2.22 ± 0.06 | 7.50 ± 0.46 | 3.06 ± 0.08 | 12.59 ± 0.09 | 22.78 ± 0.80 | 19.54 ± 0.90 |
| Lincan 13 | Gansu | 1.71 ± 0.06 | 4.05 ± 0.04 | 16.32 ± 0.31 | 5.85 ± 0.20 | 21.38 ± 0.68 | 21.67 ± 0.20 | 22.05 ± 0.30 |
| Lincan15 | Gansu | 1.30 ± 0.03 | 2.36 ± 0.09 | 10.08 ± 0.25 | 3.47 ± 0.17 | 16.79 ± 0.48 | 27.79 ± 0.70 | 8.24 ± 0.00 |
| Qidou 2 | Jiangsu | 1.25 ± 0.05 | 2.44 ± 0.01 | 9.52 ± 0.29 | 2.94 ± 0.05 | 12.40 ± 0.55 | 23.86 ± 0.80 | 16.05 ± 0.40 |
| Qingcan 25 | Qinghai | 1.27 ± 0.03 | 2.09 ± 0.05 | 9.55 ± 0.12 | 2.62 ± 0.12 | 14.41 ± 0.40 | 23.45 ± 0.70 | 12.10 ± 0.40 |
| Tongcanxian 20 | Jiangsu | 1.48 ± 0.07 | 3.35 ± 0.05 | 13.34 ± 0.36 | 4.64 ± 0.04 | 19.20 ± 0.79 | 36.83 ± 0.60 | 9.66 ± 0.50 |
| Tongcanxian 21 | Jiangsu | 1.14 ± 0.06 | 2.47 ± 0.06 | 8.76 ± 0.48 | 3.12 ± 0.11 | 12.18 ± 0.54 | 40.77 ± 0.20 | 12.09 ± 0.30 |
| Wancan 1 | Anhui | 1.14 ± 0.08 | 2.39 ± 0.14 | 9.92 ± 0.48 | 4.21 ± 0.24 | 13.77 ± 0.14 | 30.21 ± 0.30 | 6.47 ± 0.10 |
| Yican 4 | Yunnan | 1.25 ± 0.10 | 2.16 ± 0.06 | 7.24 ± 0.35 | 2.77 ± 0.25 | 14.00 ± 0.16 | 43.33 ± 0.90 | 18.34 ± 0.30 |
| Yican 6 | Yunnan | 1.38 ± 0.08 | 1.68 ± 0.04 | 7.51 ± 0.13 | 2.93 ± 0.39 | 14.51 ± 0.42 | 37.80 ± 0.60 | 5.68 ± 0.50 |
| Yucan 1 | Chongqing | 1.44 ± 0.10 | 2.62 ± 0.11 | 10.28 ± 0.30 | 3.17 ± 0.05 | 13.01 ± 0.64 | 37.95 ± 1.40 | 7.84 ± 0.30 |
| Zhecan 1 | Zhejiang | 1.81 ± 0.03 | 2.84 ± 0.10 | 12.18 ± 0.28 | 4.51 ± 0.23 | 17.45 ± 0.67 | 28.42 ± 0.30 | 17.27 ± 0.70 |
| Zhongjiang changxiu | Jiangsu | 1.78 ± 0.02 | 2.81 ± 0.09 | 12.36 ± 0.07 | 3.69 ± 0.08 | 17.55 ± 0.71 | 19.82 ± 0.40 | 8.99 ± 0.50 |
| 2016-831 | Jiangsu | 1.65 ± 0.07 | 2.32 ± 0.02 | 13.28 ± 0.27 | 3.69 ± 0.18 | 15.91 ± 0.44 | 33.81 ± 0.30 | 11.09 ± 0.10 |
| P16-06-3 | Jiangsu | 1.39 ± 0.01 | 2.78 ± 0.07 | 11.70 ± 0.64 | 4.40 ± 0.03 | 16.63 ± 0.21 | 28.29 ± 0.40 | 8.92 ± 0.20 |
| Compound Identification | Mode | tR (min) | Formula | Expected m/z | Found at m/z | Error (ppm) | Major Fragment Ions (m/z) |
|---|---|---|---|---|---|---|---|
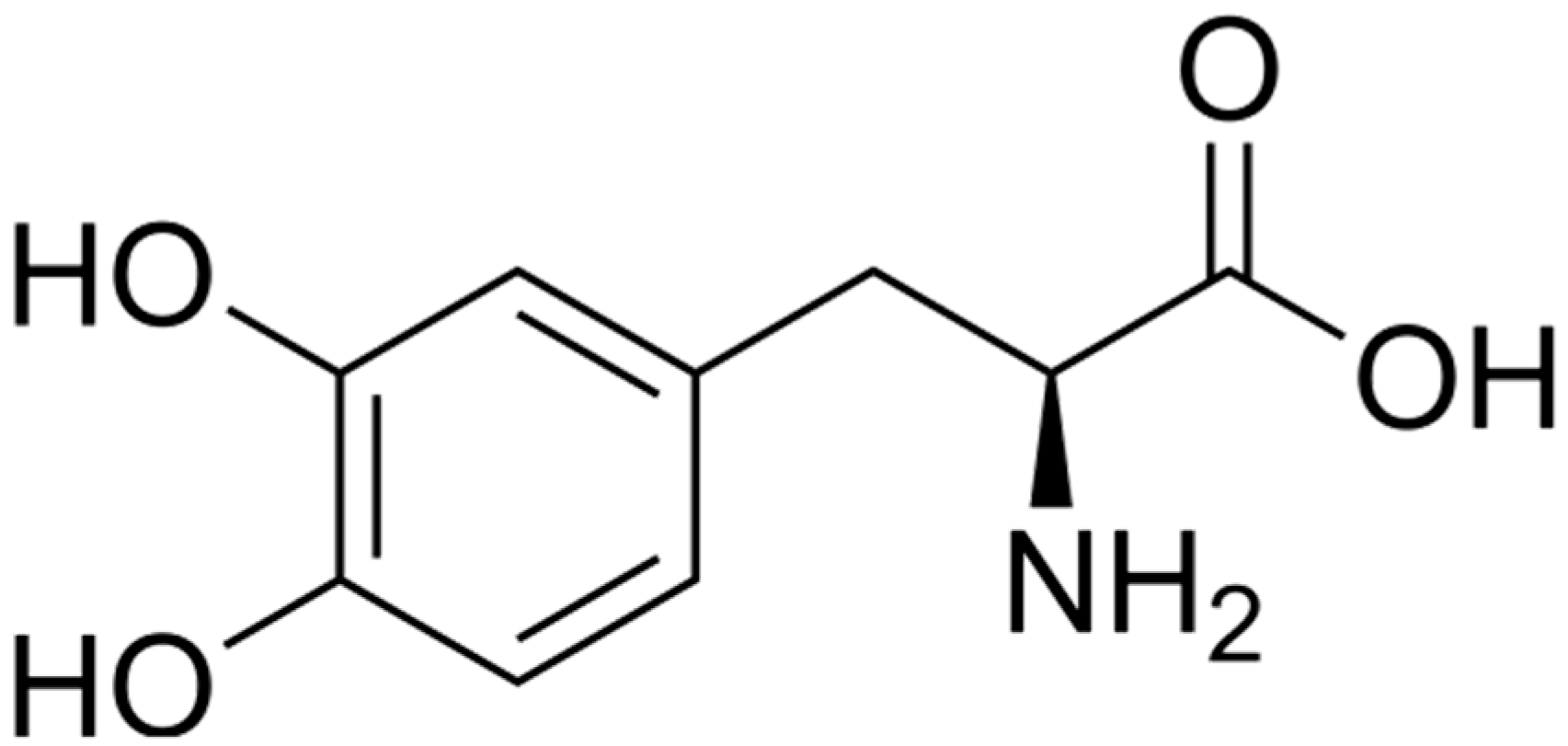
| L-3,4-dihydroxyphenylalanine (L-DOPA) | [M + H]+ | 1.77 | C9H11NO4 | 198.0761 | 198.0761 | 0.0 | 77.0419 |
| Phenolic acids and derivatives | |||||||
| Gallic acid | [M − H]– | 3.38 | C7H6O5 | 169.0143 | 169.0143 | 0.3 | 125.0239, 79.0218 |
| Cinnamic acid | [M + H]+ | 4.16 | C9H8O2 | 149.0597 | 149.0598 | 0.5 | 77.0424 |
| [M − H]– | 4.17 | C9H8O2 | 147.0452 | 147.0453 | 0.7 | 103.0547, 77.0401 | |
| Coumaric acid | [M + H]+ | 6.26 | C9H8O3 | 165.0546 | 165.0542 | −2.8 | 147.0445, 77.0439 |
| p-Hydroxybenzoic acid | [M − H]– | 7.32 | C7H6O3 | 137.0244 | 137.0247 | 2.0 | 93.0356, 65.0422 |
| Protocatechuic acid | [M − H]– | 7.42 | C7H6O4 | 153.0193 | 153.0198 | 3.1 | 109.0292 |
| p-Coumaric acid | [M − H]– | 9.08 | C9H8O3 | 163.0401 | 163.0403 | 1.2 | 119.0508, 93.0359 |
| Ferulic acid | [M − H]– | 9.75 | C10H10O4 | 193.0506 | 193.0514 | 3.9 | 178.0264134.0379 |
| Flavonoids and derivatives | |||||||
| Gallocatechin | [M + H]+ | 5.67 | C15H14O7 | 307.0812 | 307.0810 | −1.0 | 163.0379, 139.0397 |
| [M − H]– | 5.67 | C15H14O7 | 305.0667 | 305.0664 | −0.9 | 179.0352, 125.0247 | |
| Epigallocatechin | [M − H]– | 6.73 | C15H14O7 | 305.0667 | 305.0667 | 0.1 | 179.0344, 125.0248, 109.0301 |
| [M + H]+ | 6.74 | C15H14O7 | 307.0812 | 307.0812 | 0.0 | 139.0392 | |
| Quercetin-3-O-glucoside | [M + H]+ | 6.89 | C21H20O12 | 465.1028 | 465.1024 | −0.8 | 303.0501 |
| Procyanidin B2 | [M − H]– | 6.94 | C30H26O12 | 577.1352 | 577.1347 | −0.9 | 425.0873, 407.1035, 289.0719, 287.0552, 161.0243, 125.0249 |
| [M + H]+ | 6.95 | C30H26O12 | 579.1497 | 579.1491 | −1.1 | 561.1401, 427.1035, 291.0845, 139.0395 | |
| Quercetin-3-O-rutinoside (rutin) | [M + H]+ | 7.32 | C27H30O16 | 611.1607 | 611.1581 | −4.1 | 465.1019, 303.0468 |
| Catechin | [M + H]+ | 7.40 | C15H14O6 | 291.0863 | 291.0859 | −1.5 | 139.0400, 123.0459 |
| Quercetin-3-β-sophoroside | [M + H]+ | 7.77 | C27H30O17 | 627.1556 | 627.1543 | −2.0 | 465.1007, 303.0496 |
| Epicatechin | [M − H]– | 7.93 | C15H14O6 | 289.0718 | 289.0720 | 0.8 | 245.0825, 203.0712, 161.0610, 151.0405, 125.0254, 109.0308 |
| [M + H]+ | 7.95 | C15H14O6 | 291.0863 | 291.0863 | −0.2 | 139.0400 | |
| Kaempferol-3-O-rutinoside | [M − H]– | 8.34 | C27H30O15 | 593.1512 | 593.1505 | −1.1 | 447.0932, 285.0405 |
| Quercetin-7-rhamnoside | [M + H]+ | 8.42 | C21H20O11 | 449.1078 | 449.108 | 0.4 | 303.0521, 257.0513 |
| Isorhamnetin-3-O-neohespeidoside | [M + H]+ | 8.44 | C28H32O16 | 625.1763 | 625.1756 | −1.1 | 317.0661 |
| Kaempferol-3,7-L-dirhamnoside (Kaempferitrin) | [M + H]+ | 8.86 | C27H30O14 | 579.1709 | 579.1710 | 0.3 | 433.1140, 287.0531 |
| Myricetin-3-O-rhamnoside (Myricitrin) | [M − H]– | 8.95 | C21H20O12 | 463.0882 | 463.0870 | −2.7 | 317.0275, 271.0240 |
| Cyanidin-3-O-glucoside | [M + H]+ | 9.60 | C21H20O11 | 449.1078 | 449.1080 | 0.3 | 287.0534 |
| Quercetin-3-O-rhamnoside (Quercitrin) | [M + H]+ | 10.32 | C21H20O11 | 449.1078 | 449.1083 | 1.0 | 303.0508 |
| Myricetin | [M − H]– | 10.45 | C15H10O8 | 317.0303 | 317.0297 | −1.8 | 151.0023 |
| Quercetin | [M − H]– | 11.80 | C15H10O7 | 301.0354 | 301.0354 | 0.0 | 178.9977, 151.0044, 121.0301 |
| Faba Bean Varieties | L-DOPA (mg/g DW) | Gallic Acid (mg/100 g DW) | Protocatechuic Acid (mg/100 g DW) | Gallocatechin (mg/100 g DW) | Epigallocatechin (mg/100 g DW) | Catechin (mg/100 g DW) | Procyanidin B2 (mg/100 g DW) | Rutin (mg/100 g DW) | Kaempferol-3-O-Rutinoside (mg/100 g DW) |
|---|---|---|---|---|---|---|---|---|---|
| Baihuahudou | 14.18 ± 0.19 | 2.62 ± 0.03 | 8.63 ± 0.45 | 1.03 ± 0.01 | 20.98 ± 0.69 | 2.30 ± 0.42 | 1.43 ± 0.01 | 1.96 ± 0.02 | 1.57 ± 0.01 |
| Chenghu 21 | 16.01 ± 0.57 | 2.92 ± 0.01 | 13.28 ± 0.13 | 2.05 ± 0.03 | 17.60 ± 0.02 | 3.15 ± 0.02 | 0.31 ± 0.02 | 4.59 ± 0.01 | 0.43 ± 0.01 |
| Chenghudabai | 16.86 ± 0.46 | 2.87 ± 0.04 | 15.15 ± 0.14 | 2.35 ± 0.43 | 18.13 ± 0.26 | 2.95 ± 0.07 | 0.27 ± 0.00 | 2.32 ± 0.01 | 0.37 ± 0.01 |
| Chuzao 1 | 13.39 ± 0.31 | 2.53 ± 0.01 | 10.31 ± 0.05 | 1.43 ± 0.02 | 13.35 ± 0.31 | 1.90 ± 0.62 | 0.30 ± 0.02 | 8.23 ± 0.00 | 0.39 ± 0.01 |
| Cican 1 | 13.90 ± 0.35 | 3.48 ± 0.08 | 8.80 ± 0.27 | 0.79 ± 1.36 | 20.18 ± 0.46 | 3.89 ± 0.12 | 1.28 ± 0.01 | 2.90 ± 0.21 | 0.64 ± 0.01 |
| Dingxuancan 3 | 17.08 ± 0.20 | 3.15 ± 0.08 | 12.18 ± 0.38 | 2.74 ± 0.08 | 14.41 ± 0.01 | 1.97 ± 0.29 | 3.96 ± 0.01 | 5.56 ± 0.02 | 1.11 ± 0.03 |
| Edou 1103 | 15.68 ± 0.25 | 3.50 ± 0.02 | 13.92 ± 0.14 | 1.45 ± 0.16 | 18.76 ± 0.36 | 4.36 ± 0.50 | 0.26 ± 0.02 | 1.91 ± 0.01 | 0.69 ± 0.01 |
| Edou 1208 | 15.19 ± 0.34 | 3.25 ± 0.01 | 13.95 ± 0.09 | 2.08 ± 0.07 | 23.57 ± 0.01 | 3.94 ± 0.13 | 0.30 ± 0.00 | 2.78 ± 0.01 | 0.48 ± 0.01 |
| Fengdou 11 | 12.99 ± 0.27 | 2.96 ± 0.01 | 10.21 ± 0.06 | 1.52 ± 0.28 | 14.88 ± 0.16 | 1.61 ± 0.24 | 0.24 ± 0.00 | 4.59 ± 0.10 | 0.31 ± 0.02 |
| Fengdou 28 | 14.10 ± 0.43 | 2.87 ± 0.01 | 11.33 ± 0.05 | 3.86 ± 0.53 | 16.96 ± 0.29 | 2.32 ± 0.45 | 0.31 ± 0.00 | 5.14 ± 0.01 | 0.51 ± 0.03 |
| Jianlixiaocandou | 16.70 ± 0.71 | 2.11 ± 0.01 | 14.70 ± 0.16 | 1.56 ± 0.02 | 20.96 ± 0.03 | 2.64 ± 0.11 | 1.59 ± 0.01 | 2.67 ± 0.01 | 1.68 ± 0.01 |
| Jingdou 5 | 12.88 ± 0.13 | 3.31 ± 0.08 | 7.72 ± 0.31 | 1.09 ± 0.01 | 16.08 ± 0.07 | 2.28 ± 0.01 | 5.47 ± 0.01 | 8.31 ± 0.01 | 0.27 ± 0.02 |
| Jizhangcan 5 | 16.07 ± 0.27 | 3.75 ± 0.08 | 11.14 ± 0.24 | 4.94 ± 0.03 | 14.46 ± 0.07 | 3.55 ± 0.11 | 2.84 ± 0.30 | 3.90 ± 0.10 | 0.81 ± 0.02 |
| Lican 3 | 13.07 ± 0.19 | 3.15 ± 0.06 | 8.04 ± 0.54 | 0.83 ± 0.70 | 13.38 ± 1.33 | 3.33 ± 0.49 | 1.36 ± 0.01 | 2.90 ± 0.05 | 0.38 ± 0.01 |
| Lican 7 | 14.54 ± 0.13 | 3.86 ± 0.05 | 9.48 ± 0.55 | 4.01 ± 0.02 | 17.53 ± 0.04 | 6.08 ± 0.04 | 0.93 ± 0.00 | 1.57 ± 0.01 | 0.46 ± 0.00 |
| Lincan 13 | 15.28 ± 0.18 | 2.89 ± 0.11 | 15.06 ± 0.25 | 1.23 ± 0.03 | 14.08 ± 0.13 | 2.12 ± 0.23 | 3.61 ± 0.01 | 5.05 ± 0.02 | 1.52 ± 0.02 |
| Lincan15 | 14.65 ± 0.21 | 2.98 ± 0.15 | 14.54 ± 0.15 | 2.32 ± 0.02 | 11.55 ± 0.10 | 2.32 ± 0.09 | 1.67 ± 0.02 | 3.20 ± 0.02 | 0.79 ± 0.01 |
| Qidou 2 | 16.86 ± 0.20 | 3.15 ± 0.07 | 12.11 ± 0.30 | 1.64 ± 0.02 | 19.73 ± 0.01 | 3.64 ± 0.03 | 1.34 ± 0.01 | 2.06 ± 0.01 | 2.16 ± 0.01 |
| Qingcan 25 | 14.06 ± 0.09 | 2.94 ± 0.15 | 12.35 ± 0.12 | 2.38 ± 0.02 | 17.68 ± 0.08 | 1.99 ± 0.40 | 2.12 ± 0.01 | 5.91 ± 0.02 | 1.84 ± 0.02 |
| Tongcanxian 20 | 12.49 ± 0.29 | 2.90 ± 0.09 | 11.33 ± 0.17 | 1.03 ± 0.06 | 17.04 ± 0.08 | 3.74 ± 0.07 | 1.21 ± 0.01 | 2.71 ± 0.01 | 0.35 ± 0.01 |
| Tongcanxian 21 | 11.96 ± 0.08 | 2.80 ± 0.07 | 7.21 ± 0.37 | 0.00 ± 0.00 | 14.53 ± 0.02 | 5.10 ± 0.02 | 1.22 ± 0.00 | 2.06 ± 0.00 | 0.25 ± 0.01 |
| Wancan 1 | 14.18 ± 0.02 | 2.87 ± 0.06 | 12.13 ± 0.28 | 3.09 ± 0.01 | 18.38 ± 0.03 | 3.17 ± 0.01 | 2.65 ± 0.01 | 4.19 ± 0.01 | 0.45 ± 0.01 |
| Yican 4 | 14.75 ± 0.35 | 4.60 ± 0.02 | 12.12 ± 0.11 | 2.10 ± 0.34 | 18.11 ± 0.03 | 2.20 ± 0.00 | 0.20 ± 0.01 | 4.86 ± 0.01 | 0.45 ± 0.01 |
| Yican 6 | 15.57 ± 0.44 | 3.74 ± 0.01 | 12.35 ± 0.10 | 3.91 ± 0.03 | 16.40 ± 0.07 | 2.79 ± 0.02 | 0.20 ± 0.03 | 7.92 ± 0.02 | 0.38 ± 0.01 |
| Yucan 1 | 16.42 ± 0.31 | 3.45 ± 0.01 | 12.11 ± 0.48 | 2.54 ± 0.01 | 19.09 ± 0.01 | 3.58 ± 0.04 | 2.05 ± 0.01 | 2.86 ± 0.41 | 1.30 ± 0.02 |
| Zhecan 1 | 14.54 ± 0.25 | 3.95 ± 0.04 | 12.43 ± 0.12 | 0.01 ± 0.00 | 19.84 ± 0.13 | 4.37 ± 0.02 | 1.09 ± 0.01 | 1.67 ± 0.02 | 0.37 ± 0.01 |
| Zhongjiang changxiu | 17.93 ± 0.21 | 3.60 ± 0.05 | 13.14 ± 0.42 | 3.52 ± 0.04 | 14.56 ± 1.44 | 3.41 ± 0.57 | 1.80 ± 0.01 | 2.36 ± 0.00 | 0.17 ± 0.00 |
| 2016-831 | 16.47 ± 0.17 | 3.21 ± 0.07 | 16.37 ± 0.19 | 1.05 ± 0.02 | 18.68 ± 0.07 | 2.43 ± 0.05 | 1.01 ± 0.01 | 2.23 ± 0.02 | 1.68 ± 0.05 |
| P16-06-3 | 12.49 ± 0.37 | 3.18 ± 0.07 | 11.28 ± 0.07 | 1.45 ± 0.38 | 17.10 ± 0.24 | 3.66 ± 0.14 | 1.20 ± 0.06 | 2.84 ± 0.02 | 0.76 ± 0.02 |
| Sample | DPPH (µmol TE/g) | ABTS (µmol TE/g) | FRAP (mmol FE/g) | α-Amylase IC50 (μmol/L) | α-Glucosidase IC50 (μmol/L) |
|---|---|---|---|---|---|
| L-DOPA | 3.66 ± 0.39 | 3.21 ± 0.33 | 9.75 ± 0.01 | 24.87 ± 2.21 | 16.66 ± 0.69 |
| Positive control | 3050.00 ± 83.89 | 4271.11 ± 3.85 | 13.50 ± 0.33 | 0.54 ± 0.08 | 1.24 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Z.; Mei, C.; Sun, W.; Yuan, X.; Wang, J.; Huang, W. Phytochemical Profiling, Antioxidant Capacity, and α-Amylase/α-Glucosidase Inhibitory Effects of 29 Faba Bean (Vicia faba L.) Varieties from China. Biology 2025, 14, 982. https://doi.org/10.3390/biology14080982
Li Y, Wang Z, Mei C, Sun W, Yuan X, Wang J, Huang W. Phytochemical Profiling, Antioxidant Capacity, and α-Amylase/α-Glucosidase Inhibitory Effects of 29 Faba Bean (Vicia faba L.) Varieties from China. Biology. 2025; 14(8):982. https://doi.org/10.3390/biology14080982
Chicago/Turabian StyleLi, Ying, Zhihua Wang, Chengkai Mei, Wenqi Sun, Xingxing Yuan, Jing Wang, and Wuyang Huang. 2025. "Phytochemical Profiling, Antioxidant Capacity, and α-Amylase/α-Glucosidase Inhibitory Effects of 29 Faba Bean (Vicia faba L.) Varieties from China" Biology 14, no. 8: 982. https://doi.org/10.3390/biology14080982
APA StyleLi, Y., Wang, Z., Mei, C., Sun, W., Yuan, X., Wang, J., & Huang, W. (2025). Phytochemical Profiling, Antioxidant Capacity, and α-Amylase/α-Glucosidase Inhibitory Effects of 29 Faba Bean (Vicia faba L.) Varieties from China. Biology, 14(8), 982. https://doi.org/10.3390/biology14080982







